B3 Carbon Dioxide
King Lit Wong, Ph.D.
Johnson Space Center Toxicology Group
Biomedical Operations and Research Branch
Houston, Texas
Physical and Chemical Properties
Carbon dioxide is an odorless and colorless gas (Sax, 1984).
|
Synonym: |
Carbonic anhydride |
|
Formula: |
CO2 |
|
CAS number: |
124389 |
|
Molecular weight: |
44 |
|
Boiling point: |
Not applicable |
|
Melting point: |
Sublime at-78°C |
|
Vapor pressure: |
Not applicable |
|
Conversion factors at 25°C, 1 atm: |
1 ppm = 1.80 mg/m3 1 mg/m3 = 0.56 ppm |
Occurrence and Use
CO2 normally exists in the atmosphere at 0.03% (Morey and Shattuck, 1989). In a Danish study, the maximal CO2 concentrations inside 14 town-hall buildings (6 had natural and 8 had mechanical ventilation) were measured to be 0.05-0.13% (Skov et al., 1987). Wang (1975) reported that the CO2 concentration inside a university auditorium built up to about 0.06-0.09% during a lecture. CO2 is not used in space shuttles, but it will be used as a fire extinguishant in the space station.
Metabolism is a source of CO2 in spacecraft, and thermodegradation of organic materials is a potential source of CO2 (Coleman et al., 1968; Terrill et al., 1978; Wooley et al., 1979). Humans produce CO2 via oxidative metabolism of carbohydrates, fatty acids, and amino acids; the production rate is dependent on the caloric expenditure of the individual (Baggott, 1982; Diamondstone, 1982; LeBaron, 1982; Olson, 1982). A young adult male produces about 22,000 me of CO2 per day (Baggott, 1982). For a 70-kg adult doing light work in spaceflight, the amount of CO2 exhaled was estimated to be 500 L/d (Clamann, 1959). The amount of CO2 exhaled by a group of normal male subjects, aged 18-45, inside a steel chamber was measured at 469 L/d per person (Consolazio et al., 1947). During a 7-d shuttle mission with seven crew members, the mean CO2 concentration in the cabin was about 2 mm Hg, which was equivalent to 0.26% in an atmosphere of 760 mm Hg, with a 5-h peak of 9 mm Hg or 1.2% (NASA, 1984).
Pharmacokinetics and Metabolism
When inhaled, CO2 freely penetrates cellular membranes (Baggott, 1982). The diffusion rate of CO2 through the alveolar membrane into blood is about 20 times that of O2 (West, 1979). CO2 is carried in blood in three forms, the bicarbonate being the major form. Ninety percent of the CO2 in blood reacts with water, under the catalysis of carbonic anhydrous inside the erythrocytes, to form carbonic acid, which in turn is ionized to bicarbonate (Baggott, 1982). This reaction also takes place in serum in the absence of carbonic anhydrous, but it proceeds much more slowly than with catalysis (Baggott, 1982).
The other two forms of CO2 transport in blood are relatively minor. About 5% of the CO2 in blood is dissolved in serum and cytoplasm (Baggott, 1982). The solubility of CO2 in water is approximately 20 times that of O2, so that CO2 dissolved in plasma is a more important form of transport in blood than dissolved O2 (West, 1979). CO2 is present in blood in the third form as carbamino compounds, which are formed from the reaction of CO2 with uncharged amino groups in hemoglobin (Baggott, 1982). The carbamino form accounts for about 5 % of the CO2 in blood (Baggott, 1982).
Normally, CO2 is eliminated from the body via exhalation. A healthy man exhales CO2 at about 220 mL/min at rest and 1,650 mL/min during moderate exercise (Cotes, 1979, pp. 266, 276, 384).
The CO2-bicarbonate system functions as the major buffering system in blood (Baggott, 1982). In acidosis, an individual is exposed to a high concentration of CO2. Hyperventilation increases the CO2 exhalation, which raises the pH in blood (Baggott, 1982). In alkalosis, the individual will hypoventilate to reduce CO2 exhalation and the kidney will excrete bicarbonate ions into the urine, both of which lower the pH in blood (Baggott, 1982).
Toxicity Summary
Acute and Short-Term Toxicity
Miscellaneous Signs and Symptoms
Both hearing and vision can be impaired by CO2. A 6-min exposure to 6.1-6.3% CO2 resulted in a 3-8% decrease in hearing threshold in six human subjects (Gellhorn and Spiesman, 1935). For CO2 exposures of six human subjects lasting 5-22 min, 3-4% CO2 was the threshold for causing slight hearing impairment and 2.5% was the no-observed-adverse-effect level (NOAEL) (Gellhorn and Spiesman, 1934, 1935). Because the amount of hearing impairment produced by about 6% CO2 is very small and because the SMACs are expected to be much lower than 6%, hearing impairment is not considered in setting the SMACs for CO2. Acute exposures to 6% CO2 affected vision by reducing visual intensity discrimination in 1-2 min (Gellhorn, 1936) and by causing visual disturbances in several hours in an unspecified number of men (Schulte, 1964).
CO2 exposures can cause other symptoms, such as tremor, discomfort, dyspnea, headache, and intercostal pain. Tremor was produced in human subjects exposed to 6% CO2 for several hours (number of subjects unknown) (Schulte, 1964) or 7-14% CO2 for 10-20 min (12 subjects) (Sechzer et al., 1960). Exposures of six volunteers to 6% CO2 for 20.5-22 min led to discomfort (Gellhorn and Spiesman, 1935).
Dyspnea
Available data indicate that acute exposures to CO2 at concentrations higher than 3% definitely could produce dyspnea. For instance, White et al. (1952) found that, in a 16-min exposure to 6% CO2 in O2, 19 of 24 volunteers had slight or moderate dyspnea, and the dyspneic sensation was severe in the remaining five subjects. A 17-32 min exposure of 16 human subjects to 4-5 % CO2 (Schneider and Truesdale, 1922) or a 2.5-10 min exposure to 7.6% CO2 (Dripps and Comroe, 1947) resulted in dyspnea.
There were conflicting data on whether 2.8-3% CO2 would cause dyspnea. On one hand, Menn et al. (1970) found that, in a 30-min exposure to 2.8% CO2, dyspnea was detected in three of eight human subjects during maximal exercise, but not during half-maximal or two-thirds-maximal exercises. On the other hand, Sinclair et al. (1971) showed that a 1-h or 15- to 20-d exposure of four volunteers to 2.8% CO2 failed to produce any dyspnea during steady strenuous exercise. However, Schulte (1964) reported that an exposure to CO2 at concentrations as low as 2% for several hours resulted in dyspnea on exertion in an unknown number of human subjects. In the study conducted by Menn et al., 1.1% CO2 failed to cause dyspnea in eight subjects even during maximal exercise in 30 min. There were also conflicting data on CO2's dyspneic effect in resting subjects. Brown (1930a) showed that 3.2% CO2 or 2.5-2.8% CO2 did not produce dyspnea in five resting human subjects. In contrast, Schulte (1964) reported that an exposure to 3% CO2 for several hours resulted in dyspnea even at rest, without specifying the number of human subjects on which he based his conclusion. The bulk of the data indicate that the NOAEL for CO2 exposures based on dyspnea appears to be 2.8% because astronauts will engage in moderate, but not maximal, exercise.
Headaches
In addition to dyspnea, acute CO2 exposures could produce headaches. Without specifying the size of population he based his conclusion on, Schulte (1964) reported that human subjects exposed to 2% or 3% CO2 for several hours developed headaches on mild exertion; the
headache was more severe at 3% CO2 than 2%. Sinclair et al. (1971) showed that a 1-h exposure of four human subjects to 2.8% CO2 resulted in occasional mild headaches during strenuous steady-state exercise. Menn et al. (1970) found that mild-to-moderate frontal headaches developed in six of eight human subjects exposed to 3.9% CO2 for 30 min while doing two-thirds-maximal exercise. A similar exposure to 1.1% or 2.8% CO2 failed to cause headaches (Menn et al., 1970). Therefore, there is conflicting evidence whether 2.8% CO2 produces headaches during exertion.
In a comparison of the data on exercising subjects (Schulte, 1964; Menn et al., 1970; Sinclair et al., 1971) and on subjects at rest (Schneider and Truesdale, 1922; Brackett et al., 1965), CO2 appears to cause more headaches at a lower concentration during exercise than at rest. White et al. (1952) showed that, soon after a 16-min exposure of 24 subjects to 6% CO2, one developed a severe headache and nine developed mild headaches of very short durations. In a study of five or six resting human subjects conducted by Brown (1930a), an exposure to 3.2% CO2 in 13.4% O2 for several hours produced headache and giddiness, but an exposure to 2.5-2.8% CO2 in 14.6-15% O2 was devoid of any symptoms. Schneider and Truesdale (1922) showed that, in 16 resting volunteers exposed to 1-8% CO2 for 17-32 min, headaches developed only at a CO2 concentration of 5 % or more and the headache could be intense. In a study by Brackett et al. (1965), 7% CO2 caused mild headache in approximately seven resting volunteers in 40-90 min.
CO2 exposures do not cause headaches immediately. Menn et al. (1970) reported that headaches mostly developed near the end of a 30-min exposure to 3.9% CO2 while the subjects were performing two-thirds-maximal exercise. Glatte et al. (1967a) found that, in a 5-d exposure to 3% CO2, mild-to-moderate throbbing frontal headaches were detected in four of seven human subjects in the first day. A similar response was found in human subjects exposed to 4% CO2 (Glatte et al., 1967b; Menn et al., 1968). The headaches usually began in the first few hours of exposure.
The headaches produced by CO2 are not long lasting. In a 30-min exposure to 3.9% CO2, the headaches disappeared an hour after the exposure (Menn et al., 1970). In human subjects exposed to 3% or 4% CO2 for 5 d, they recovered from the headaches in 3 d (Glatte et al., 1967b; Menn et al., 1968). Menn et al. (1970) postulated that the
headaches are caused by CO2-induced dilation of cerebral blood vessels (Patterson et al., 1955). The disappearance of the headaches soon after an acute exposure or disappearing beginning on the third day of a 5-d exposure suggests, as another possibility, that the headaches are due to CO2-induced acidosis.
As discussed above, it is not certain whether 2.8% CO2 could cause headaches. Similarly, there is conflicting evidence on 2% CO2. Without specifying the size of the study population, Schulte (1964) reported that headaches were detected in human subjects exposed to 2% CO2 for several hours on mild exertion. In contrast, Radziszewski et al. (1988) showed that a 30-d exposure of six human subjects to 2% CO2 rarely produced headaches, even when they exercised.
Intercostal Pain
Acute CO2 exposures can produce intercostal pain. Menn et al. (1970) reported that a 30-min exposure to 2.8% CO2 caused intercostal muscle pain during maximal exercise in two of eight human subjects. They did not report any intercostal pain in the subjects during two-thirds- or half-maximal exercise. However, Sinclair et al. (1971) showed that a 1-h exposure to 2.8% CO2 failed to produce intercostal muscle pain in four volunteers during steady strenuous exercise. It is possible that the test subjects in Sinclair's study did not exercise maximally during the exposure to 2.8% CO2, so that they did not experience the intercostal pain that was reported by those in Menn's study. Menn et al. failed to detect intercostal muscle pain in eight human subjects exposed to 1.1% CO2 for 30 min even during maximal exercise. Because astronauts will not be exercising maximally in the spacecraft, 2.8% is chosen as the NOAEL for intercostal muscle pain resulting from acute CO2 exposures.
Acid-Base Balance
An exposure to CO2 at concentrations much higher than the normal value of 0.03% increases the pCO2 in blood (Mines, 1981). The increased pCO2 in blood lowers the blood pH, although the lowering is
reduced somewhat by the bicarbonate and protein buffers in blood (Mines, 1981). Acidosis is known to occur in humans after a 1-h exposure to 2.8% CO2 (Sinclair et al., 1971). Both the CO2 absorption and acidosis happen very rapidly. During a 1-h exposure of volunteers to 7% CO2, the arterial pCO2 and HCO3 concentrations were raised, while the arterial plasma pH dropped from 7.40 to 7.30 as early as 10 min into the exposure (Brackett et al., 1965). These arterial parameters remained at a plateau from min 10-60 during the CO2 exposure. The decreases in arterial plasma pH in humans resulting from acute CO2 exposures are tabulated as follows.
TABLE 3-1 Arterial pH Decreases After Acute CO2 Exposures
|
Concentration, % |
Exposure Duration |
Arterial pH Drop |
Reference |
|
1.5 |
1 d |
0.05 |
Schaefer, 1963b |
|
2 |
2 h |
0 |
Guillerm and Radziszewski, 1979 |
|
2 |
2-3 d |
0.01 |
Guillerm and Radziszewski, 1979 |
|
2.8 |
1 d |
0.02 |
Glatte et al., 1967a |
|
3.0 |
6-24 h |
0.025 |
Sinclair et al., 1969 |
|
7 |
10-60 min |
0.10 |
Brackett et al., 1965 |
|
10 |
10-60 min |
0.22 |
Brackett et al., 1965 |
Electrolyte Levels
Messier et al. (1976) reported some electrolyte changes in 7-15 human subjects in 57-d submarine patrols, the atmosphere of which was maintained at 0.8-1.2% CO2, 19-21% O2, and CO at <25 ppm. On the first day of a patrol, the plasma levels of calcium decreased, with no change in plasma phosphorus levels, but the erythrocyte level of calcium increased.
Respiratory System
The most obvious effect of CO2 exposures is increased alveolar ventilation, which is not a toxic effect per se, but it and other physiological
changes inducible by CO2 will be described in the Toxicity Summary. If O2 is maintained at a constant concentration, alveolar ventilation of humans varies linearly with the CO2 concentration at ventilation up to about 60 L/min (Cotes, 1979, pp. 149, 258, 363). The amounts of ventilatory increase during an acute exposure of normal human subjects to CO2 at various concentrations are summarized in Table 3-2.
The hyperventilatory response is due mainly to a tidal volume increase, although the respiratory rate was found to increase in one study but not in another (Schaefer, 1963b; Glatte et al., 1967a; Guillerm and Radziszewski, 1979). The hyperventilatory response to inhaled CO2 is triggered by CO2's effect on chemoreceptors in the brain and the carotid chemoreceptors (Cotes, 1979, pp. 149, 258, 363; Phillipson et al., 1981). When the CO2 exposure terminates, residual hyperventilation helps to lower the pCO2 in blood, and thus the hyperventilation plays a role in restoring the normal blood pH.
Three studies show that human subjects acclimate somewhat to the hyperventilatory effect of CO2 (Chapin et al., 1955; Schaefer, 1958; Radziszewski et al., 1988). The alveolar ventilation at rest was 15.1 L/min shortly after an exposure to 3% CO2 began, but it was lowered to 12.9 L/min near the end of the 78-h exposure (Chapin et al., 1955). Schaefer (1958) also reported acclimation to CO2's ventilatory effect. He presented evidence that diving instructors, who had held their breaths daily for long durations under water (resulting in CO2 accumulation in their bodies), showed a smaller hyperventilatory response toward acute CO2 challenges than other volunteers who were not accustomed to CO2 retention. Some of the data from Radziszewski et al. (1988), summarized in Table 3-2 showed that the hyperventilatory response to CO2 was diminished about one fifth at 24 h compared with 2 h in a continuous CO2 exposure.
Some evidence indicates that CO2 can stimulate or depress ventilation depending on the concentration. As mentioned above, CO2 stimulates respiration at a concentration as low as 1%. CO2 at concentrations higher than 8% has been reported to depress respiration in humans (Cotes, 1979, pp. 149, 258, 363). However, a 3.8-min exposure of human subjects to 10.4% CO2 is known to stimulate respiration (Dripps and Comroe, 1947). So the exact CO2 concentration required to consistently depress respiration is unknown and it might be much higher than 8%.
TABLE 3-2 Hyperventilatory Responses to Acute CO2 Exposures
|
Concentration, %. |
Exposure No. |
Increase in Minimum Volume, % Duration |
(mean ± SD) |
Reference |
|
0.5-0.6 |
5 |
10 min |
14 ± 4 |
Campbell et al., 1913 |
|
0.5 |
6 |
24 h |
—a |
Radziszewski et al., 1988 |
|
1 |
16 |
17-32 min |
32 |
Schneider and Truesdale, 1922 |
|
1 |
6 |
24 h |
19 |
Radziszewski et al., 1988 |
|
2 |
16 |
17-32 min |
80 |
Schneider and Truesdale, 1922 |
|
2 |
6 |
2 h |
60 |
Radziszewski et al., 1988 |
|
2 |
6 |
24 h |
45 |
Radziszewski et al., 1988 |
|
2.1-2.5 |
3 |
10 min |
63 ±13 |
Campbell et al., 1913 |
|
2.2 |
3 |
10 min |
36 ±21 |
Eldridge and Davis, 1959 |
|
2.5 |
3 |
10-20 min |
30 ± 9 |
Brown et al., 1948 |
|
2.5 |
9 |
≈20 min |
33 ± 21 |
Tashkin and Simmons, 1972 |
|
3 |
16 |
17-32 min |
148 |
Schneider and Truesdale, 1922 |
|
3 |
5 |
2 h |
70 |
Radziszewski et al., 1988 |
|
3 |
5 |
24 h |
50 |
Radziszewski et al., 1988 |
|
3.8 |
5 |
2 h |
160 |
Radziszewski et al., 1988 |
|
3.8 |
5 |
24 h |
130 |
Radziszewski et al., 1988 |
|
4 |
16 |
17-32 min |
208 |
Schneider and Truesdale, 1922 |
|
4.2 |
3 |
10 min |
184 ± 110 |
Eldridge and Davis, 1959 |
|
4.3 |
5 |
2 h |
240 |
Radziszewski et al., 1988 |
|
4.3 |
5 |
24 h |
180 |
Radziszewski et al., 1988 |
|
5 |
3 |
10-20 min |
130 ± 30 |
Brown et al., 1948 |
|
5 |
9 |
≈20 min |
91 ± 60 |
Tashkin and Simmons, 1972 |
|
5 |
16 |
17-32 min |
309 |
Schneider and Truesdale, 1922 |
|
5.7-6.1 |
5 |
10 min |
413 ± 57 |
Campbell et al., 1913 |
|
5.9 |
7 |
5 min |
184 |
Brown, 1930a |
|
6 |
3 |
20.5-22 min |
203 |
Brown, 1930a |
|
6 |
23 |
16 min |
200 |
White et al., 1952 |
|
6 |
16 |
17-32 min |
419 |
Schneider and Truesdale, 1922 |
|
7 |
16 |
17-32 min |
512 |
Schneider and Truesdale, 1922 |
|
7.5 |
3 |
10-20 min |
474 ± 242 |
Brown et al., 1948 |
|
7.5 |
9 |
≈20 min |
269 ± 123 |
Tashkin and Simmons, 1972 |
|
8 |
16 |
17-32 min |
640 |
Schneider and Truesdale, 1922 |
|
8.8 |
5 |
7-10 min |
228 |
Brown, 1930a |
|
10 |
9 |
≈20 min |
456 ± 189 |
Tashkin and Simmons, 1972 |
|
12.4 |
7 |
0.75-2 min |
153 |
Brown, 1930a |
|
a Statistically not significant. |
||||
Exposures to CO2 are also known to affect lung functions. CO2 inhalation for 2 h at 5% or 7.5% decreased specific airway conductance in volunteers, but 2.5% CO2 did not change the conductance (Tashkin and Simmons, 1972). A 120% increase in the total lung resistance was detected in human subjects who inhaled 8% CO2 in 19% O2 for 3-6 min (Nadel and Widdicombe, 1962).
There are no data on the structural effect of CO2 on the lungs of human beings. However, Schaefer and his colleagues reported that acute exposures to CO2 injured the lungs of guinea pigs (Niemoeller and Schaefer, 1962; Schaefer et al., 1964a). In some of the guinea pigs exposed to 15% CO2 in 21% O2, Schaefer's group detected subpleural atelectasis, an increase of lamellar bodies in alveolar lining cells, congestion, edema, and hemorrhage in the lungs in 1 or 6 h (Schaefer et al., 1964a). When the exposure was extended to 1 or 2 d, they reported that hyaline membranes were seen in the lungs, in addition to the pulmonary injuries seen at 1 and 6 h. As the exposure was further extended to 7 or 14 d, they described a decline in incidences of atelectasis, edema, hemorrhages, and hyaline membranes in the lung. In that 1964 study, Schaefer's group looked at a total of six time points, with 4-14 guinea pigs exposed to CO2 per time point. However, they used only 13 guinea pigs as controls, and they did not specify how many control guinea pigs were sacrificed per time point. That means, on the average, only two control guinea pigs were sacrificed at each time point and that is grossly inadequate. In another study, Niemoeller and Schaefer (1962) reported that CO2 exposures at 1.5 % or 3 % could produce similar lung injuries as 15% CO2. In this study, the same problem existed. They used only four control guinea pigs in the 1.5%-CO2 experiment in which a group of exposed guinea pigs was examined at four time points. Similarly, in the 3%-CO2 experiment, they used seven guinea pigs to control for examinations of exposed guinea pigs at five time points. Consequently, their findings that CO2 exposures produced lung injuries in guinea pigs might not be reliable. Therefore, their findings in the lungs of guinea pigs (Niemoeller and Schaefer, 1962; Schaefer et al., 1964a) are disregarded in setting SMACs.
Cardiovascular System
CO2 exposures are known to affect the heart and the circulatory sys-
tem. A 17-32-min exposure of humans to 1% or 2% CO2 is known to cause slight increases of systolic and diastolic pressure (Schneider and Truesdale, 1922). In another human study, a 15-30-min exposure to 5% or 7% CO2 caused increases in blood pressure and cerebral blood flow and a decrease in cerebrovascular resistance (Kety and Schmidt, 1948). In the same study, no change in cardiac output was detected, but in another study, a 4-25-min exposure of volunteers to 7.5% CO 2 increased the cardiac output and blood pressure (Grollman, 1930). In addition to changing the cardiac output, CO2 can increase the heart rate. A 10-15-min exposure to 5.4% CO2 or a 4-25-min exposure to 7.5% CO2 increased the pulse rate in humans (Grollman, 1930; Schaefer, 1958).
Acute CO2 exposures can result in some EKG changes. Nodal and atrial premature systoles, premature ventricular contractions, inversion of P waves, low P waves, and increased T-wave voltage were observed in psychiatric patients exposed to 30% CO2 in 70% O2 for 38 s (MacDonald and Simonson, 1953). Similarly, McArdle (1959) exposed psychiatric patients to 30% CO2 in 70% O2 for 10-15 breaths, and he detected acidosis, marked increases in systolic and diastolic pressures, atrial extrasystoles, atrial tachycardia (but no ventricular extrasystole), increased P-wave voltage, low or inverted P waves, spiked T waves with a broad base, increased T-wave voltage, slight increases in PR intervals and QRS intervals, and a marked increase in the QT interval, which was the most consistent finding. The fact that it took only 35-45 breaths of the mixture of 30% CO2 in 70% O2 to produce narcosis in these patients suggests that the CO2 concentration used was very high.
In CO2 exposures at lower concentrations, lower incidences of abnormal cardiac rhythm result. For instance, in human subjects breathing 7-14% CO2, balance O2, for 10-20 min at rest, premature nodal contraction was detected in only 2 of 27 subjects (versus 0 of 27 before the exposure) and premature ventricular contraction was found in only 3 of 27 subjects (versus 1 of 27 before the exposure) (Sechzer et al., 1960).
At even lower CO2 concentrations, only minor EKG changes were produced without any abnormal rhythm. In human subjects, a 6-8 min exposure to 6% CO2 depressed the amplitude of the QRS complex and T wave, but there were no T-wave inversions or changes in the S-T segment (Okajima and Simonson, 1962). These EKG changes were more severe in men of about 60 years of age than in men in their twenties. In volunteers doing moderate or maximal exercise while exposed
to 2.8% or 3.9% CO2 for 30 min, Menn et al. (1970) found no significant increase in premature atrial or ventricular contractions over the incidences normally seen in exercising individuals in room air.
These data indicate that, in acute exposures, CO2 can produce clinically unimportant abnormal cardiac rhythm at a fairly high concentration of 7-14% and requires a very high concentration of 30% to produce atrial tachycardia. Therefore, CO2's EKG effects are not used in setting the SMACs for CO2.
The mechanism of EKG changes produced by CO2 is unknown. Altschule and Sulzbach (1947) postulated that the CO2-induced EKG changes were due to CO2-induced acidosis because the changes were seen with acidosis in a 45-90-min exposure of two patients at 5 % CO2 in 95 % O2 and the changes disappeared within 30 min of terminating the CO2 exposure.
Nervous System
Exposures to CO2 at the appropriate concentrations could cause CNS depression. Consolazio et al. (1947) discovered a decrease in hand-arm steadiness, but no change in the ability to compute, translate, check numbers, and discriminate pitch and loudness in four volunteers exposed to 5-6.75% CO2 in 19.2% O2 for 37 h. Schulte reported that an exposure of an unspecified number of human subjects to 5% CO2 for several hours produced CNS depression (Schulte, 1964). An exposure of fighter pilots to 5% CO2 for a unspecified duration degraded their performance in landing maneuvers, such as lengthened flight time between gear down and touch down and unacceptable increases in touch-down sink rates (Wamsley et al., 1969). Therefore, these studies indicate that 5% CO2 is depressive to the CNS.
Brown (1930a) conducted a study with five human subjects in a static exposure chamber for 8 h with the CO2 concentration measured at 4.1 % and 5.3% at the end of the fourth and seventh hours, respectively. Brown showed that the number of numbers canceled in a cancellation test dropped 24% at the end of the seventh hour when the CO2 concentration was 5.3% with 21% O2. Using the data provided by Brown, the 24% reduction is found to be statistically significant from the pre-exposure number. However, Brown commented that the reduction was not
serious deterioration. It should be noted that the same CO2 exposure caused no changes on the scores in Army Alpha intelligence and arithmetic tests, attention, and muscular coordination (Brown, 1930a). Whether the 24% reduction in the cancellation test score was due to the concentration of CO2 at 5.3% in the exposure chamber or due to boredom from confinement in the exposure chamber is unknown because there was no sham-exposed control group.
Data on CNS depression resulting from exposure to CO2 at concentrations below 5% were inconsistent. In the study of five human volunteers conducted by Brown, the number of numbers canceled in the cancellation test decreased by 13% at the end of the fourth hour when the atmosphere contained 4.1% CO2 in 21% O2 (Brown, 1930a). Although the 13% reduction is statistically significant (based on a paired t test using Brown's data), Brown did not consider it a serious deterioration. There were no effects observed in the Army Alpha intelligence and arithmetic tests, attention, and muscular coordination (Brown, 1930a). Because Brown did not have a sham-exposed control group in assessing the CNS effects of CO2, interpretation of Brown's CNS data is difficult.
Schaefer et al. (1958, 1959, 1963a) reported that some crew members on board a German submarine that contained 3-3.5% CO2 in 1517% O2, suffered impaired attentiveness in a 2-mo underwater patrol in World War II. Nevertheless, as noted by Glatte et al. (1967b) and Menn et al. (1968), the submarine atmosphere was not tightly controlled, so that simultaneous exposure of the crew to other contaminants, such as carbon monoxide, could not be ruled out. It is, therefore, possible that the CNS depression suffered by the crew was due to the relatively low oxygen concentration, carbon monoxide, or certain organic solvents instead of the 3-3.5% CO2. Brown's test produced giddiness and headache in four human subjects exposed to 3.2% CO2 for several hours (Brown, 1930b). Unfortunately, the subjects were also exposed to a relatively low O2 concentration of 13.4%, which makes interpretation of the finding of giddiness difficult.
In contrast to the findings of Schaefer et al. and Brown, other evidence shows that exposures to CO2 in the range of 2-4% do not depress the CNS. For instance, Glatte et al. showed that a 5-d exposure of seven human subjects to 3% or 4% CO2 failed to influence hand steadiness, vigilance, auditory monitoring, memory, and arithmetic and problem solving performance (Glatte et al., 1967a,b; Menn et al., 1968).
Storm and Giannetta (1974) showed that there were no changes in aiming ability, closure flexibility, visualization, perceptual speed, and number facility in 12 human volunteers who were tested everyday during a 2-w exposure to 4% CO2. Schulte (1964) reported no mental depression in subjects exposed to 2% or 3% CO2 for a few hours. The number of subjects is not known. From these data, the NOAEL for CNS depression is estimated to be 4%.
At a CO2 concentration higher than 5 %, CO2's effect on the CNS is not purely depressive. Restlessness and dizziness have been detected in human subjects exposed to 7.5% CO2 for 15 min, 10% CO2 for 15-25 min, and 10.4% CO2 for 3.8 min (Dripps and Comroe, 1947; Schaefer, 1963a; Brackett et al., 1965). Some studies have shown that acute exposures to CO2 at high concentrations produced purely depressive signs and symptoms. For instance, unconsciousness was detected in human subjects exposed to 10% CO2 for several hours (Schulte, 1964) and drowsiness and near stupor were found in individuals who had inhaled 12.4% CO2 for 0.75-2 min (Brown, 1930b). In contrast, some investigators have presented evidence that CO2 exposures excite the CNS. Psychomotor excitation, eye flickering, myoclonic twitches, increased muscle tone, and restlessness were produced by exposures to 10% CO2 for 1.5 min and 15% CO2 for 3 min in a study by Lambertsen (1971).
At even higher CO2 concentrations, the CNS effects of CO2 are mostly depressive. Unconsciousness was the predominant finding in human subjects exposed to 17% CO2 in 17.3% O2 for 20-52 s (Aero Medical Association, 1953) or 18.6% CO2 in 17% O2 for <2 min (Dalgaard et al., 1972). Of course, at such high CO2 concentrations, it is difficult to separate the CO2 effect from the hypoxic effect. The CNS effects of acute CO2 exposures are summarized in Table 3-3.
At CO2 concentrations much higher than 17%, the CNS depression could result in death. Several workers in a ship carrying fish were found dead in the holding tank with a CO2 concentration at 20-22% (Dalgaard et al., 1972). Similarly, CO2 could be the cause of death in some fires. Gormsen et al. (1984) examined the causes of death in fire victims. They concluded that CO2 poisoning or oxygen deficiency or both is the second most common cause of death, carbon monoxide poisoning being the most common.
TABLE 3-3 CNS Effects Resulting from Acute CO2 Exposures
|
Concentration, % |
CNS Effects |
Reference |
|
1.5 |
No effect |
Schaefer, 1959 |
|
3-4.5 |
No effect or marginal depression |
Glatte et al., 1967a; Deitrick et al., 1948; Brown, 1930a |
|
5 |
Depression |
Wamsley et al., 1969; Consolazio et al., 1947 |
|
6 |
Subjective feelings of speech and movement difficulties that did not exist when determined objectively |
White et al., 1952 |
|
7.5-15 |
Mixture of depression and excitation |
Brown, 1930a; Dripps and Comroe, 1947; Lambertsen, 1971 |
|
> 17 |
Unconsciousness |
Aero Medical Association, 1953; Dalgaard et al., 1972 |
Kidneys
CO2 exposures might produce physiological changes in the kidney. A 30-min exposure of human subjects to 5% CO2 produced increases in renal blood flow, glomerular filtration rate, and renal venous pressure, as well as decreased renal vascular resistance (Yonezawa, 1968). These physiological changes in the kidney probably represent renal compensation for the CO2-induced acidosis because the plasma HCO3- level was increased. Due to their innocuous nature, the SMACs are not set to prevent these renal physiological changes.
Male Reproductive System
Acute CO2 exposures might affect some of the mature cell types in the testis of laboratory animals. Vandemark et al. (1972) showed that a 4- or 8-h exposure to 2.5% CO2 resulted in a disappearance of mature spermatids in rats. The disappearance was apparently due to sloughing of mature spermatids and Sertoli cells in the seminiferous tubules, resulting in cellular debris in the lumen. The degenerative change
showed a concentration response, with the testis responding more to 5% CO2 and even more at 10%. The testicular degenerative change was reversible because the testis appeared completely normal histologically 36 h after the CO2 exposure. Although acute CO2 exposures could affect the mature spermatids and Sertoli cells in the rat, they did not affect the weight of the testis and seminal vesicles. The response in the testis to CO2 was somewhat affected by the exposure duration. An acute exposure to 2.5%, 5%, or 10% CO2 did not affect the testis in 1 or 2 h, but it caused a similar degree of sloughing of mature spermatids in 4 or 8 h.
Even though Vandemark et al. (1972) found no testicular changes immediately after a 1-h exposure to 2.5% CO2, it does not mean that the 1-h exposure absolutely would not cause any testicular change. It is possible that had the rats been sacrificed at a later time rather than immediately after exposure, some testicular degeneration could show up. However, the key point is that the testicular degeneration produced by acute CO2 exposures is transient, with complete structural recovery in 36 h (Vandemark et al., 1972). Therefore, the 1-h and 24-h SMACs are not set according to CO2's testicular toxicity.
Finally, Mukherjee and Singh (1967) observed spermatozoa with smaller head and midpiece in the vas deferens of mice exposed alternatively to 2 h of 36% CO2 in 13.4% O2 and 0.5 h of air for a total of 6 h. In another experiment, they exposed male mice to about 4 h of CO2 at the same concentration per day (two CO2 exposure periods of 2 h each separated by an air exposure of 0.5 h) for 6 d. The fertility was reduced in these male mice. However, the meaning of Mukherjee and Singh's findings is uncertain due to the very high CO2 concentration and very low O2 concentration used.
Intestine and Spleen
Other than injuring the lung and testes, there was a report that acute exposures of guinea pigs to high concentrations of CO2 might also damage other tissues. Schaefer et al. (1971) present evidence that in 1-d exposure of guinea pigs to 15 % CO2 led to hemorrhages in the intestine and the spleen. Unfortunately, as discussed above, due to an inadequate number of control animals used in that study, it appeared that
they did not adequately control their experiments. That casts doubt on the meaning of their positive findings. As a result, the SMACs are not set based on any intestinal or splenic end point.
Subchronic and Chronic Toxicity
Dyspnea and Intercostal Pain
Acute CO2 exposures could cause headaches, dyspnea, and intercostal muscle pain, especially during exercise or exertion. However, Sinclair et al. (1971) showed that a 15-20 d exposure to 2.8% CO2 failed to produce any dyspnea or intercostal muscle pain in four human subjects, who performed, twice daily, 45-min of continuous steady state exercises on a bicycle ergometer at a low, moderate, or heavy level. Radziszewski and his colleagues reported no dyspnea or intercostal pain in six human subjects who were exposed to 2% CO2 for 30 d or 2.9% for 8 d and who performed, twice a week, a 10-min exercise with a bicycle ergometer at a 150-watt workload (Guillerm and Radziszewski, 1979; Radziszewski et al., 1988).
Headaches
Subchronic CO2 exposures are known to produce headaches at a concentration of 3% or higher. In a 30-d exposure of six human volunteers to CO2 conducted by Radziszewski et al. (1988), the subjects rarely developed headaches to 2% CO2, but slight headaches were detected to 2.9% CO2. Sinclair et al. (1969, 1971) showed that four subjects exposed to 2.8% CO2 for 15-30 d or 3.9% CO2 for 11 d occasionally developed mild headaches during heavy exertion, but the headaches disappeared after the first day of exposure. Glatte et al. (1967a,b) and Menn et al. (1968) reported that, in the 5-d exposure to 3% or 4% CO2, mild-to-moderate throbbing frontal headaches started to appear in the first day in about 60% of seven human subjects and the headaches disappeared in the third day. They claimed that the headaches were not severe enough to interfere with normal activities. However, because 75% of the subjects who were inflicted with headaches felt that the
headache was sufficiently prominent to request an analgesic, headaches are considered in setting the long-term SMACs.
The above data are summarized in Table 3-4 to provide a glimpse of the CO2's concentration-response relationship based on headaches developed in repetitive CO2 exposures.
TABLE 3-4 Data on CO2-Induced Headaches
|
Concentration, % |
Exposure Duration |
Intensity of Headache |
Reference |
|
2 |
30 d |
Rare headache even during exercise |
Radziszewski et al., 1988 |
|
2.8 |
15-30 d |
Occasional mild transient headache during heavy exertion |
Sinclair et al., 1969, 1971 |
|
2.9 |
30 d |
Slight headache |
Radziszewski et al., 1988 |
|
3 |
5 d |
Mild-to-moderate throbbing frontal headache that disappeared in 2 d |
Glatte et al., 1967; Menn et al., 1968 |
|
3.8 |
30 d |
Intense and annoying headache |
Radziszewski et al., 1988 |
|
3.9 |
11 d |
Occasional mild transient headache during heavy exertion |
Sinclair et al., 1969, 1971 |
|
4 |
5 d |
Mild-to-moderate throbbing frontal headache that disappeared in 2 d |
Glatte et al., 1967a; Menn et al., 1968 |
|
4.3 |
30 d |
Intense and annoying headache |
Radziszewski et al., 1988 |
Nervous System
As discussed in the Acute Toxicity subsection, it is questionable whether acute CO2 exposures at less than 5% cause CNS depression. Similarly, there are conflicting data on the CNS effects of subchronic CO2 exposures at 3-5%. Schaefer (1949a,b) reported that, in an 8-d exposure of human subjects to 3% CO2, mild excitement (euphoria, troubled sleep with frequent dreams and nightmares) was seen in d 1, followed by inattentiveness, erratic behavior, exhaustion, and confusion in d 2-8. However, no behavioral changes were found by Glatte et al.
in seven human volunteers exposed to 3 % or 4 % CO2 for 5 d (Glatte et al., 1967a,b; Menn et al., 1968).
Although Schaefer (1949a,b) found that human subjects suffered motor skill impairment on d 2-8 of an 8-d exposure to 3 % CO2, Glatte et al. (1967a,b), Menn et al. (1968), and Storm and Giannetta (1974) did not find any psychomotor impairment in volunteers exposed to 3 % or 4% CO2. Glatte et al. exposed seven volunteers to 3% or 4% CO2 for 5 d, and Storm and Giannetta exposed 12 volunteers to 4% CO2 for 2 w. Both Glatte et al. and Storm and Giannetta used a battery of tests called the Repetitive Psychometric Measures, which tested hand steadiness, visualization, the arithmetical ability to add, the ability to find four-letter words in rows of letters, the speed of canceling letters in a row of letters, and the speed of perception. Glatte et al. also tested the subjects' ability to solve arithmetical problems involving multiplication and memory, compensatory tracking maneuvers, pitch, roll, and yaw maneuvers, simple visual vigilance (monitoring the on and off of a light), complex auditory monitoring, and memory (counting and remembering the number of flashes of a light for 1-min periods, as well as listening and remembering combinations of letters and numbers). Neither Glatte et al. nor Storm and Giannetta found any effect on these tests with exposures to 3% or 4% CO2. Due to the extensive psychometric testing done by these two groups of investigators in the Air Force, an exposure of humans to 3% CO2 is not likely to impair the CNS or the motor ability.
The data available indicate that a subchronic CO2 exposure at less than 2% definitely has no CNS impairment effect. In a summary report of a 42-d study of 23 human volunteers exposed to 1.5% CO2 rin a submarine, Schaefer (1961) reported that there were no effects on immediate memory, problem-solving abilities, a letter-canceling test, the Minnesota Manual Dexterity test, a complex coordination test, the McQuarrie test of mechanical ability, strength, visual accommodation, visual acuity, depth perception, and pitch discrimination. There were, however, moderate increases in anxiety, apathy, increased uncooperativeness, a desire to leave, and increased sexual desire. In a study sponsored by NASA, Jackson et al. (1972) showed that there were no changes in psychomotor performances, as determined with a Langley Complex Coordinator, in four human volunteers continuously exposed
to CO2 for 90 d (0.6% CO2 in the first 46 d and 0.8% in the remainder).
Acid-Base Balance
Similar to acute CO2 exposures, subchronic CO2 exposures also lower the blood pH. Table 3-5 summarizes the effect of CO2 exposure on the acid-base balance of human subjects. The amount of plasma pH drop caused by CO2 varied somewhat with the CO2 exposure concentration. In a subchronic exposure of human subjects to CO2 at 5 mm Hg (equivalent to 0.7% at sealevel) or 1.5% CO2, a plasma pH drop of 0.05 unit was detected in the first 20 or so days (Schaefer, 1963b; Messier et al., 1971). However, Guillerm and Radziszewski (1979) showed that a 3-d exposure to 2% CO2 lowered the plasma pH by 0.01 unit. In comparison, Glatte et al. (1967a) found that an exposure of seven humans to CO2 at 21 mm Hg (equivalent to 2.8% at sealevel) lowered the plasma pH by 0.02 unit in 2-3 d and 0.01 unit in 4-5 d, but these pH drops were not statistically significant.
TABLE 3-5 Plasma pH Decreases During CO2 Exposures
|
Concentration, % |
No. |
Exposure Duration |
Plasma pH Drop |
Reference |
|
0.85 |
15 |
56 d |
—a |
Peck, 1971 |
|
0.7 |
12 |
3-24 d |
0.05 |
Messier et al., 1971 |
|
0.7 |
12 |
31-38 d |
— |
Messier et al., 1971 |
|
1 |
15 |
44 d |
0.02 |
Pingree, 1977 |
|
1.5 |
21 |
1-20 d |
0.05 |
Schaefer, 1958 |
|
1.5 |
21 |
24-42 d |
— |
Schaefer, 1958 |
|
2 |
6 |
3 d |
0.01 |
Guillerm and Radziszewski, 1979 |
|
2 |
6 |
8-30 d |
— |
Guillerm and Radziszewski, 1979 |
|
2.8 |
7 |
1-5 d |
0.01-0.02 |
Glatte et al., 1967a |
|
3.9 |
3-4 |
1-2 d |
0.02 |
Sinclair et al., 1969 |
|
3.9 |
3-4 |
5 d |
— |
Sinclair et al., 1969 |
|
a Not significant. |
||||
During subchronic hypercapnia, the kidney compensates for the acidosis by increasing the secretion of H+ in urine and conserving HCO3+ (Kryger, 1981). The renal compensation of the acidosis is, however, rather slow; it takes days before its effect is manifested (Kryger, 1981). The data summarized in Table 3-5 show that the body compensated for the respiratory acidosis in 5-8 d in two studies (Sinclair et al., 1969; Guillerm and Radziszewski, 1979) and in about 30 d in two other studies (Schaefer, 1963a; Messier et al., 1971).
The CO2-induced acid-base change in animals is similar to that in humans. Schaefer et al. (1964a) reported that the arterial pCO2 increased maximally as early as 1 h into a 14-d exposure of guinea pigs to 15% CO2. The arterial pCO2 remained higher than controls all through the 14-d CO2 exposure period, but it gradually declined with time starting at 1 h, which was the first sampling point in that study (Schaefer et al., 1964). Schaefer et al. (1964) showed that the time course of arterial pH changes in guinea pigs exposed to 15% CO2 followed that of pCO2 quite closely. Barbour and Seevers (1943) showed that, in rats exposed to 11% CO2 for 17 d, the arterial pH dropped below the pre-exposure level as early as 0.5 h (the first sampling point) into the CO2 exposure. Starting at 0.5 h, the arterial pH gradually rose, but it remained lower than the pre-exposure level throughout the 17-d exposure.
Electrolyte Levels
Similar to acute CO2 exposures, subchronic CO2 exposures might also change the electrolyte levels in the body. The data on plasma total calcium levels and urinary total calcium excretion gathered in humans are summarized in Table 3-6.
In a 57-d submarine-patrol study, with an atmosphere of 0.8-1.2% CO2 and less than 25-ppm CO, Messier et al. (1976) detected that calcium levels decreased in plasma, but increased in erythrocytes, in 7-15 human subjects. Because there were no changes in the parathyroid hormone and calcitonin plasma levels, the calcium changes detected by Messier et al. were not due to parathyroid hormone or calcitonin. A
TABLE 3-6 CO2-Induced Calcium Changes
|
Concentration, % |
Exposure No. |
Plasma Calcium Duration |
Urinary Calcium Level |
Excretion |
Reference |
|
0.5 |
6 |
13 d |
Not measured |
No change |
Davies et al., 1978a |
|
|
4 |
90 d |
d 1-53: No change |
No higher than the range of normal |
Jackson et al., 1972; Schaefer, 1979 |
|
0.6 |
d 54-90:4% decrease |
||||
|
0.8 |
|||||
|
0.7 |
15 |
49 d |
d 5:3% increase d 12, 19: No change d 26:2% increase d 33: No change d 40:5% increase d 47:3% increase |
w 1: No change w 2-7:24-37% decrease |
Gray et al., 1973 |
|
0.65 |
14 |
56 d |
Not measured |
2: No change d 9, 17:35-42% decrease d 30: No decrease d 42, 56:23-40% decrease |
Peck, 1971 |
|
1 |
7 |
57 d |
d 7-57:10% decrease |
d 1-57:30-40% decrease |
Messier et al., 1976 |
|
1.5 |
20 |
42 d |
d 3-21:6% decrease d 30-42: No decrease |
d 1-42:47% decrease |
Schaefer et al., 1963b |
|
3 |
7 |
5 d |
No change |
No change |
Glatte et al., 1967a |
group in the United Kingdom's Institute of Naval Medicine also discounted the role of a reduction in vitamin D, which promotes intestinal calcium absorption, because the reduction in urinary calcium excretion occurred fairly rapidly (Davies and Morris, 1979).
Similarly, Schaefer et al. (1963b) found that, in 20 human subjects exposed to 1.5% CO2 for 42 d, the plasma calcium levels were lowered in the first 3 w of the exposure, but they returned to the pre-exposure levels in the last 3 w. Taking the data from a 90-d study performed by Jackson et al. (1972) under NASA's sponsorship, paired t-tests show
that there was no significant change in the serum calcium levels in four human volunteers exposed to a median CO2 concentration of 0.6% in the first 53 d. The serum calcium levels, however, dropped 4% in 54-90 d when the median CO2 concentration was at 0.8 %.
There were, however, other studies that tended to dispute that CO2 has any effect on the plasma level of calcium. For instance, Glatte et al. (1967a) found no changes in the plasma and urinary calcium levels in humans exposed to 3% CO2 for 5 d. Davies et al. (1978a) also found no changes in the urinary excretion of calcium, phosphorus, sodium, potassium, and magnesium in humans exposed to 0.5% CO2 for 13 d. Davies et al. (1978b) showed that the reduction in urinary excretion of minerals found by other investigators might be artifacts of urine collection methodology.
To further complicate the picture, Schaefer et al. (1979a,b) found that subchronic exposures of guinea pigs to CO2 also resulted in time-dependent changes in plasma levels of calcium, but in an opposite direction compared with the human data of Schaefer et al. and Messier et al. discussed above. In guinea pigs exposed to 1 % CO2 for 6 w, Schaefer et al. (1979a) detected increases in the plasma calcium levels in w 6, but the plasma calcium levels did not differ from the control in w 1-4. In another study conducted by Schaefer et al. (1979b) with guinea pigs exposed to 0.5% CO2 for 8 w, no change in plasma calcium levels was detected in w 4 and an increase was detected in w 8. Because the data indicate that CO2 increases the plasma calcium levels in guinea pigs (Schaefer et al., 1979a,b), but CO2 either decreases or causes no change in the plasma calcium levels in humans (Schaefer et al., 1963b; Glatte et al., 1967a; Messier et al., 1976), the meaning of these data gathered from guinea pigs is doubtful.
Gray et al. (1973) reported that the serum levels of calcium, magnesium, and inorganic phosphorus were raised during a 7-w exposure of 15 submariners exposed to 0.7% CO2. During the CO2 exposure, the urinary excretions of these three electrolytes were reduced. Because the inverse relationship between the serum levels of calcium and urinary excretion of calcium also existed during the pre-exposure period, Gray et al. admitted that the renal handling of calcium in these subjects was unusual. Gray et al. speculated that, when the 15 submariners took part in the 7-w exposure, they had not completely recovered from a 3-w
exposure to 1 % CO2 in a submarine patrol 3 m earlier. That cast doubt on whether the findings of Gray et al. in the 7-w study were representative of the responses in normal individuals exposed to CO2.
The effect of CO2 on urinary excretion of calcium has also been studied. In the 20 human subjects exposed to 1.5% CO2 for 42 d, Schaefer et al. (1963b) found that the amount of calcium excreted in the urine per day was reduced by about 45-50% throughout the CO2 exposure. Since the daily urine volume was reduced by only about one-third during the 42-d CO2 exposure, that means the calcium concentration in the urine must have been reduced during the CO2 exposure (Schaefer et al., 1963). Schaefer et al. reported that the urine pH dropped in the first 23 d of CO2 exposure, but it returned to the pre-exposure value in the last 19 d of the exposure. Similarly, Messier et al. (1976) showed that the amount of calcium excreted in the urine per day was lower in the 7-15 human subjects in a 57-d submarine patrol exposed to about 1% CO2. However, Messier et al. failed to find any changes in the daily urine volume during the CO2 exposure. So one can infer that the concentration of calcium in the urine was reduced during the 57-d exposure to 1 % CO2. Unlike the finding of Schaefer et al., Messier et al. detected that the urine pH was elevated during the CO2 exposure. Davies et al. (1978a) exposed six men to fresh air for 9 d followed by 0.5% CO2 for 13 d with intensive physical training during the chamber stay. They found no changes in the daily urine volume and the urinary and fecal excretions of calcium.
There were two studies in which the amounts of urinary calcium excretion were reported, but the investigators were silent about the daily urine volume. The first study was a 90-d study sponsored by NASA in which four volunteers were exposed to a median CO2 concentration of 0.7% (Jackson et al., 1972). Schaefer (1979) displayed urinary data gathered in that 90-d study. According to the data, the amounts of daily urinary calcium excretion, averaged among the four volunteers, ranged from 95 mg to 170 mg in the 90-d exposure. Unfortunately, Schaefer did not present the pre-exposure data. Nevertheless, the 90-d exposure to 0.7% CO2 most likely did not increase the urinary calcium excretion because the values were all below the maximum limit of urinary calcium excretion of, 250 mg/d determined by Pak et al. (1985, 1989) in at least 77 normal volunteers without kidney stones. The second study was conducted by Peck on 15 healthy male Navy servicemen
in a 56-d submarine patrol (Peck, 1971). These 15 men were exposed to a mean CO2 concentration of 0.85%, with a range of 0.72% to 0.95 %. Peck did not measure the pre-exposure daily urinary calcium excretion, but all values measured during the entire 56 d fell within normal limits.
The results of the studies conducted by Messier et al. (1976) and Schaefer et al. (1963b) did not agree on CO2's effect on phosphorus levels. Messier et al. showed that a 57-d exposure to 1% CO2 caused no change in the plasma phosphorus levels in seven human subjects during the exposure, but it reduced urinary excretion of phosphorus. In contrast, Schaefer et al. found that the plasma phosphorus levels were raised during a 42-d exposure of 20 human subjects to 1.5% CO2, and that, while the urinary phosphorus excretion was increased in the first 2 d, it declined with time so that it was lower than the pre-exposure level in the last 3 w of the CO2 exposure. In 15 men exposed to 0.7% CO2 for 52 d, Gray et al. (1973) reported that the urinary excretion of phosphorus was raised in d 1-2, but it was below the control value from d 3-52. The serum phosphorus levels in these 15 subjects were raised from d 5-47. However, Glatte et al. (1967a) did not detect any change in the plasma and urinary levels of phosphorus in seven human subjects exposed to 3% CO2 for 5 d. Similarly, Davies et al. (1978a) found no changes in urinary and fecal excretion of phosphorus in six men exposed to 0.5% CO2 for 13 d. The sum findings of these studies seem to indicate that CO2's effect on the body levels of phosphorus varies.
Messier et al. (1976) also found increased sodium in the plasma every week during the 57-d submarine-patrol study with exposure to 0.81.2% CO2. A decrease in the plasma levels of potassium started to appear in the third week. Opposite changes in the erythrocyte levels of these electrolytes were detected.
Bones and Kidneys
The changes in plasma calcium levels discovered by Schaefer et al. (1979a) in guinea pigs seem to be related somewhat to renal calcification, which might be assessed either histologically or biochemically. Histological evidence of renal calcification, in the form of focal calcification primarily in tubules in the renal cortex, was presented by
Schaefer et al. in guinea pigs exposed to 1.5% CO2 for 35-42 d and in rats exposed to 1.5% CO2 for 35 or 91 d. Meessen (1948) also showed renal tubular necrosis with calcification in rabbits exposed to 4.5% CO2 for 13 d. The presence of renal calcification can be determined biochemically by measuring the renal calcium concentration. Schaefer et al. (1979a,b) defined renal calcification as any rise in renal calcium concentration larger than 25%. An 8-w exposure of guinea pigs to 0.5% CO2 resulted in an increase in the plasma calcium level and a larger than 25 % rise in the renal calcium levels with no change in bone calcium levels in w 8 (but not in w 4-6) (Schaefer et al., 1979b). Similarly, in a 6-w exposure of guinea pigs to 1% CO2, Schaefer et al. found an increase in plasma calcium levels and a decrease in bone calcium levels in w 1 and 6, as well as a larger than 25 % rise in the renal calcium levels in w 2-6 (Schaefer et al., 1979a). These changes in the calcium levels in the bone, plasma, and kidneys support the theory that CO2-induced renal calcification in these animals was due to the mobilization of calcium from the bone.
There is no evidence of subchronic CO2 exposures causing renal calcification in humans. In guinea pigs, CO2-induced renal calcification appeared to be associated with a rise in plasma calcium level (Schaefer et al., 1979a,b, 1980). In contrast, subchronic exposures to CO2 at about 1-3% are known to decrease or cause no change in plasma calcium levels in humans (Schaefer et al., 1963b; Glatte et al., 1967a; Messier et al., 1976). It is, therefore, doubtful that subchronic exposures to CO2 at low concentrations produce renal calcification in humans. As a result, the renal calcification data gathered in animals are not relied on in setting the SMACs of CO2. The unchanged or lower plasma calcium levels in human subjects exposed to CO2 (Schaefer et al., 1963b; Glatte et al., 1967a; Messier et al., 1976) also discount the possibility of CO2 causing bone demineralization.
Tansey et al. (1979) compared the medical records of the crew in over 1000 Polaris submarine patrols in two periods: 1963-1967 and 1968-1973. Each patrol lasted about 60 d with a crew of 140. Data on submariners in these two periods are summarized in Table 3-7 as follows.
The CO2 concentration onboard was higher in 1963-1967 than in
TABLE 3-7 Information on Submarine Patrolsa
1968-1973, with the concentration higher than 1% CO2 70-90% of the time in 1963-1967, but less than 20% of the time in 1968-1973. Tansey et al. did not report any pre-1963 CO2 concentration data. Citing data from other studies, Tansey et al. reported that the CO2 concentrations onboard submarines were 0.9% to 1.2% in 1966-1967 and that they were 0.8% to 0.9% in 1968-1971. The rate of crewmen taking sick leave on board due to ureteral calculi in the 1963-1967 period was almost twice that in the 1968-1973 period. The number of workdays lost to ureteral calculi, after normalization by the number of man-days, was three times higher in 1963-1967 than in 1968-1973.
The question is whether the larger number of workdays lost to ureteral calculi in 1963-1967 was due to the higher CO2 concentrations onboard. In other words, could subchronic CO2 exposures cause ureteral calculi in humans? Three lines of reasoning tend to cast doubt that the larger number of workdays lost to ureteral calculi was caused by subchronic CO2 exposures. First, Tansey et al. admitted that the submarine atmosphere contained contaminants such as CO2, CO, hydrocarbons, and aerosols in low concentrations. Even though the CO2 concentrations in 1963-1967 appeared to be higher than those in 1968-
1973, there were no data on the concentrations of hydrocarbons and aerosols in these two periods. Although Tansey et al. did not measure the CO concentration, they presented data gathered by others showing that CO concentrations in the submarine declined about 50% from 1961 to 1969, and they declined another 50% from 1969 to 1972 (Tansey et al., 1979). These declines in CO concentrations illustrate the possibility that, other than reductions in CO2 and CO concentrations in the two periods studied, there could be reductions in the concentrations of other air contaminants onboard the Polaris submarines. The difference in the number of workdays lost to genitourinary diseases between 1963-1967 and 1968-1973 might be due to an air contaminant other than CO2.
The second reason is that kidney stone formation is affected by a number of risk factors, such as the oxalate contents of food (Schwille and Herrmann, 1992), hypocitraturia (Goldberg et al., 1989; Hofbauer et al., 1990), low urine volume (Thun and Schober, 1991), low testosterone concentration in urine (van Aswegen et al, 1989), and dietary protein intakes (Breslau et al., 1988; Trinchieri et al., 1991). There could be dietary differences in oxalate contents or protein intakes in these two periods and the dietary differences could play a role in causing the difference in the rate of workdays lost to ureteral calculi.
The third reason, which is the most convincing one, relates to the mechanism of nephrolithiasis formation in humans. According to Coe and Favus (1987), 75% to 85% of kidney stones are calcium oxalate and calcium phosphate stones. Calcium phosphate stones usually consist of hydroxyapatite. Urinary excretion of calcium is the major risk factor for calcium stone formation (Wasserstein et al., 1987; Goldberg et al., 1989). Urinary stones are usually formed when calcium salts become supersaturated in the urine (Coe and Favus, 1987). Since CO2 exposures have been shown to either lower or not change urinary calcium excretion (Schaefer et al., 1963b; Glatte et al., 1967a; Davies et al., 1978a) and to reduce the urinary calcium concentration (Schaefer et al., 1963b; Messier et al., 1976), the chance of CO2 exposures causing calcium supersaturation is low. In addition, the lower urinary pH detected in CO2 exposures (Schaefer et al., 1963b; Radziszewski et al., 1976) disfavors kidney stone formation because the solubility of calcium oxalate is independent of pH and the deposition of apatite and octocalcium phosphate is disfavored in acidic urine (Coe and Favus, 1987). Therefore, it is highly unlikely that CO2 exposures produce kidney stones in humans.
Tansey et al. (1979) stated that the rate of ureteral calculi aboard the 1968-1973 submarine patrols was ''exactly the same as that for the general population.'' It stands to reason that even if all the ureteral calculi cases detected in the 1968-1973 submarine patrols were due to 0.80.9% CO2 in the submarine, exposures to 0.8-0.9% CO2 does not increase the risk of ureteral calculi in human subjects. It will be shown in the latter part of this document that the long-term SMACs for CO2 are recommended at 0.7% based on other toxic end points. So the long-term SMACs will not be associated with any increased risk of ureteral calculi according to the submarine patrol data of Tansey et al.
Schaefer et al. (1980) showed that an 8-w exposure of guinea pigs to 1% CO2 increased the CO2 content in the bone, in the fourth to the eighth week, with the increases in the sixth and eighth weeks due mainly to an increase in the bicarbonate contents of the bone. Commensurate with the bicarbonate content increase in the bone, they saw an increase in plasma calcium levels in the sixth and eighth week of the CO2 exposure. Schaefer et al. hypothesized that the increase in plasma calcium levels was due to CO2 binding to the bone and releasing calcium from the bone in guinea pigs. As a result of this hypothesis, several scientists in NASA raised their concerns on the potential of CO2 in releasing calcium from the bone of astronauts in the space station and causing kidney stones. However, according to an analysis of this potential given below, it is unlikely that their concerns would become reality.
First, CO2's effects on calcium in human beings differ from those in guinea pigs. All available data showed that continuous CO2 exposures lasting from 13 to 90 d at concentrations ranging from 0.5% to 1.5% either lowered or did not change urinary calcium excretion in human subjects (Schaefer et al., 1963b; Jackson et al., 1972; Gray et al., 1973; Messier et al., 1976; Davies et al., 1978a). Four studies showed that exposures of volunteers to 0.6-3% CO2 lasting from 5 to 90 d either decreased or did not change the plasma calcium levels (Schaefer et al., 1963b; Glatte et al., 1967a; Jackson et al., 1972; Messier et al., 1976). Only one study by Gray et al. (1973) showed that a 7-w exposure of 15 submariners to 0.7% CO2 increased the serum level of calcium. However, because these submariners excreted less calcium in the urine, Gray et al. admitted that the increase in serum calcium levels in these submariners was an anomaly. All in all, because plasma calcium levels are usually not raised in humans exposed to CO2, it is unlikely that CO2 would displace calcium from bones in humans.
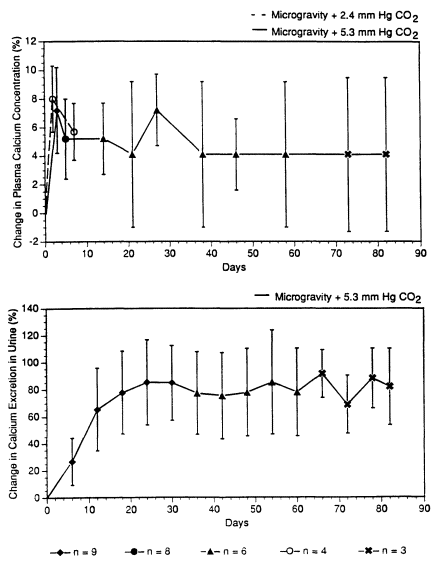
Because bone demineralization is associated with calcium changes in astronauts in space (Whedon et al., 1977; Leach and Rambaut, 1977, pp. 204-216), it is of interest to examine the CO2 effect, if any, on calcium in space. Calcium data, in means and standard deviations, gathered by Leach and Rambaut (1977), Whedon et al. (1977), and Whedon (1984) in three Skylab missions are plotted by the solid lines in Figure 3-1. According to Hopson et al. (1974), the CO2 partial pressures in Skylab missions ranged from 4.8 to 5.5 mm Hg, with a mean of 5.3 mm Hg, time-weighted average (TWA). The Skylab data showed that, in three to nine astronauts exposed to microgravity and CO2 at 5.3 mm Hg for up to 82 d, the plasma calcium levels increased 4-5% and the daily urinary calcium excretion increased 60-80% starting from d 12 (Figure 3-1). Vogel (1975) reported bone losses in three of the nine Skylab crew members. Because immobilization bed-rest studies performed by Donaldson et al. (1970) and Deitrick et al. (1948) showed increases in urinary calcium excretion of approximately the same level as that seen in the Skylab crew, the increase in urinary calcium excretion detected in Skylab missions was associated with bone demineralization in microgravity. The exposure to CO2 at 5.3 mm Hg in these Skylab astronauts probably played no role in the calcium changes. These Skylab data also showed that both the plasma calcium levels and urinary calcium excretion in space missions lasting up to 84 d were quite stable once a plateau was reached. The plasma calcium levels reached a plateau at about 5 d, and the urinary calcium excretion reached a plateau in about 20 d.
To prove that CO2 exposures in spacecraft play no important role in calcium changes in astronauts, calcium data from a space mission with CO2 concentrations at much less than 5.3 mm Hg, but of a similar duration as Skylab missions, is needed as control data. Unfortunately, there is no such "control" mission. Inflight calcium plasma data are, nevertheless, available from the Spacelab 2 mission, which lasted for 8 d with a CO2 partial pressure of 2.4 mm Hg, TWA (Shih, 1987). So the plasma calcium data of Spacelab 2, reported by Morey-Holton et al. (1988), reflect the plasma calcium concentrations in four astronauts who stayed for several days in microgravity with less CO2 than those in Skylab. The inflight calcium plasma data from the Spacelab 2 mission are plotted by the dashed line in Figure 3-1. Compared with the plasma calcium data of Skylab missions in Figure 3-1 (the solid line), the plas-
ma calcium concentration in microgravity appeared to be independent of CO2 for at least 7 d. This lends credence to the belief that CO2 exposures in spacecraft do not seem to play a major role in causing the calcium changes in microgravity. It should be noted that partial pressures of 5.3 mm Hg and 2.4 mm Hg are equivalent to concentrations of 0.7% and 0.3%, respectively, in an atmosphere of 760 mm Hg.
Respiratory System
Similar to acute CO2 exposures, subchronic CO2 exposures could also cause hyperventilation. Table 3-8 shows the amounts of ventilatory increase attained, after a plateau has been reached, in human subjects exposed to CO2 for more than a day. The sum of the data shows that it takes at least 1 % CO2 to increase, with statistical significance, the minute volume after the hyperventilatory response reaches a plateau after the first few hours in a subchronic exposure. At 0.5% CO2, the slight increase in minute volume at the plateau was masked by the physiological noise (Radziszewski et al., 1988).
In subchronic CO2 exposure, the hyperventilatory response could diminish somewhat in human subjects after the first few days of exposure, indicative of a reduced sensitivity to CO2's stimulation on respiration. Pingree (1977) showed that, in a 44-d exposure of 15 human subjects to 1% CO2, the minute volume increased about 30% on the fourth day, but it returned to the control value starting on the eighth day. In contrast, Schaefer (1963b) reported that, at exposure to 1.5% CO2, the respiratory ventilation was raised about 30% in normal volunteers throughout a 42-d exposure.
However, in a 30-d exposure of humans to 2% CO2, the minute volume increase was diminished about one-third after 9 d of exposure and remained constant in the remaining 21 d of the CO2 exposure (Guillerm and Radziszewski, 1979). Similarly, in a 30-d exposure to 2.7% CO2, the minute volume increase was reduced after 4 d and the minute volume increase remained constant from d 5 to d 14 (Clark et al., 1971). On the thirtieth day of exposure to 2.7% CO2, the hyperventilatory response recovered fully, so that the minute volume equaled that in the first day of CO2 exposure (Clark et al., 1971).
TABLE 3-8 Hyperventilatory Responses to CO2 Exposures
|
Concentration, % |
Exposure No. |
Minimum Volume Duration |
Increase, % |
Reference |
|
1 |
15 |
4 d |
30 |
Pingree, 1977 |
|
1 |
15 |
8-40 d |
0 |
Pingree, 1977 |
|
1.5 |
21 |
1-42 d |
30 |
Schaefer et al., 1963a; Schafer, 1963b |
|
2 |
6 |
9-30 d |
44 |
Guillerm and Radziszewski, 1979 |
|
2.8 |
7 |
5 d |
25 |
Glatte et al., 1967a |
|
3.9 |
3-4 |
3-11 d |
130 |
Sinclair et al., 1969 |
The reduction in CO2 hyperventilatory response during subchronic exposures appeared to occur sooner at higher CO2 exposure concentrations. In an 11-d exposure of humans to 3.9% CO2, the hyperventilatory response was diminished about one-third after two days of exposure (Sinclair et al., 1969). Another piece of evidence that humans developed reduced sensitivity toward CO2's hyperventilatory effect was obtained by Schaefer (1963b). Schaefer showed that, after 35-40 d of continuous exposure of human subjects to 1.5% CO2, the subjects did not increase their minute volume upon a 15-min challenge with 5 % CO2 as much as they did before the subchronic exposure to 1.5% CO2.
An Air Force study showed that a 5-d exposure of seven human volunteers to 3 % CO2 resulted in no changes in maximum breathing capacity, vital capacity, and 1-s vital capacity (Glatte et al., 1967a). It is of interest that several studies done by the Navy indicate that subchronic CO2 exposures might affect lung function. Schaefer et al. showed that a 42-d exposure to 1.5% CO2 increased the anatomic dead space of the lung by about 40% and the physiologic dead space by 60% in 20-21 human subjects (Schaefer et al., 1963a; Schaefer, 1963b). An exposure of human subjects to 0.8-0.9% CO2 raised the physiological dead space 50-60% in 20 d, which returned to normal soon after the exposure, indicating that the effect was reversible (Gude and Schaefer, 1969). The data on CO2-induced increase in physiological dead space are not relied on in setting the SMACs. This is because the size of the decrease in physiological dead space caused by CO2 exposures is similar to that
caused by aging in a normal individual going from age 20 years to age 40 (Cotes, 1979, pp. 149, 358, 363).
There is no evidence of subchronic CO2 exposures causing lung injuries in humans. However, based on electron microscopic studies of guinea pigs, subchronic CO2 exposures are known to cause changes in type II pneumocytes. Schaefer et al. (1979b) reported, in guinea pigs exposed to 1 % CO2, increases in the size and number of type II pneumocytes, increases in the size and number of osmiophilic lamellar bodies inside type II pneumocytes, and clustering of 2-4 type II pneumocytes starting after 4 w of exposure (Douglas et al., 1979). These ultrastructural changes were also observed after 6 w of exposure. In comparison, an 8-w exposure to 0.5% CO2 failed to cause any change in type II pneumocytes (Schaefer et al., 1979b). Schaefer et al. hypothesized that the proliferation of type II pneumocytes was a compensatory reaction to CO2's impairment on type I pneumocytes (Douglas et al., 1979).
However, there was no evidence that type I pneumocytes were damaged by CO2 (Schaefer et al., 1979b; Douglas et al., 1979). So there seems to be no support for the hypothesis of Schaefer et al. The type II pneumocyte changes probably represent a metabolic adaptation of the lung to CO2 challenges because, among the alveolar lining cells, type II pneumocytes are the more metabolically active cell type (West, 1979). Since type II pneumocytes are thicker than type I pneumocytes (West, 1979), a potential adverse consequence of type II pneumocyte proliferation is impaired gas exchanges. Due to the fact that there was no difference between the arterial pO2 in the guinea pigs with CO2-induced type II pneumocyte changes and the control guinea pigs (Douglas et al., 1979), the proliferation of type II pneumocytes caused by 1% CO2 in guinea pigs did not impair gas exchanges.
Another potential consequence of type II pneumocyte proliferation is the higher amount of lung surfactants that are synthesized by type II pneumocytes (Wright and Clements, 1987). Lung surfactants have been postulated to perform three functions: to help maintain a low lung compliance, to stabilize alveoli, and to reduce the chance of pulmonary edema (Notter and Finkelstein, 1984). Quite a bit is known about the biological effects of a lack of lung surfactants via studies of respiratory distress syndromes, but practically nothing is known about the biological effects of a higher than usual amount of lung surfactants. The only dose-response information gathered in a recent literature search is that,
in the treatment of premature infants with respiratory distress syndrome, increasing the dose of surfactant given intratracheally by 300% up to 400 mg/kg body weight could improve the treatment (Gortner et al., 1990; Dunn et al., 1990). Since premature infants are deficient in lung surfactants to begin with, the dose-response data obtained from these infants probably do not reflect the biological effects of a higher than usual amount of lung surfactant in normal subjects. However, However, Douglas et al., 1979 showed that exposure to 1% CO2 increased the number and size of lamellar bodies by only 30-50% in type II pneumocytes of guinea pigs. Assuming that the amount of lung surfactants secreted by type II pneumocytes in these guinea pigs was also increased by 30-50%, increases of such magnitude are not expected to have any harmful effect in the lung because any resultant decreases in surface tension would be of little clinical significance.
By considering the potential effects on gas exchanges and lung surfactants, it is safe to assume that the type II pneumocyte changes caused by subchronic exposures to 1% CO2 are functionally insignificant. Therefore, type II pneumocyte changes are not a toxic end point used in setting SMACs for CO2.
Finally, it should be noted that hyaline membranes and distended alveoli and alveolar ducts were seen in rabbits exposed to 4.5% CO2 for 13 d by Meessen (1948). However, Meessen did not use a control group in the study, so the meaning of the findings is unclear.
Cardiovascular System
As discussed above, acute CO2 exposures produced clinically significant arrhythmia in human subjects only at very high concentrations (30%). All the subchronic studies with EKG evaluations were performed with CO2 concentrations of 4% or less and there are conflicting data on whether these concentrations of CO2 cause arrhythmia. Glatte et al. (1967a) found no EKG problems in individuals exposed to 3% or 4% CO2 for 5 d, in which they exercised an hour daily and were monitored with a 12-lead EKG. Sinclair et al. (1971) showed no increase in premature ventricular contractions in individuals exposed to 2.8% CO2 for 15-20 d during near-maximal or maximal exercises. In another report, Sinclair et al. (1969) stated that a few individuals exposed to
3.9% CO2 for 11 d or 2.7% CO2 for 30 d developed "ectopic foci activities," presumably premature ventricular contractions (PVCs), during exercises. However, some of the ectopic foci were associated with exercises when breathing air (Sinclair et al., 1969). In addition, the ectopic foci activities during CO2 breathing did not show a concentration-response relationship. The data of Glatte et al. and Sinclair et al. seem to suggest that subchronic exposures to 3-4% CO2 are devoid of arrhythmia effects. In contrast, in two French studies, an exposure of human subjects to 2.9% or 3.8% CO2 for 8 or 9 d resulted in extrasystoles (PVCs), but no extrasystoles were detected in a 30-d exposure to 1% or 1.9% CO2 (Radziszewski et al., 1988; Guillerm and Radziszewski, 1979). Because extrasystoles are of little clinical significance (Massie and Sokolow, 1990), CO2's SMACs are not set based on the EKG effects of CO2.
Subchronic CO2 exposures might affect heart morphology. In a 7-d exposure of guinea pigs to 15% CO2, fat deposition in the myocardium was detected in d 7, but not at 1 h or d 1 (Schaefer et al., 1971). Other than fat deposition, there were no other changes in cardiac histology. According to the investigators, the experiment "failed to demonstrate any signs of myocardial damage in guinea pigs exposed for periods up to 7 days to 15% CO2" (Schaefer et al., 1971). The fat deposition probably represents only a metabolic change in the heart and not any serious damage. For comparison, no cardiac histopathology was found in rats exposed to 8% CO2 for 32 d (Pepelko, 1970). Due to the relatively minor nature of the myocardial changes, these findings are not relied on in setting the SMACs.
Structural Effects on Other Tissues
Other than affecting the kidney and lungs, subchronic CO2 exposures might affect the liver. In rabbits exposed to 4.5% CO2 in 21% O2 for 13 d, necrosis was seen scattered throughout the liver lobules (Meessen, 1948). Unfortunately, no control group was used in this study, so its results are not relied on in setting SMACs. A 32-d exposure of rats to 8% CO2 failed to cause any histological lesions in the liver, lungs, kidneys, adrenals, spleen, thyroid, and heart (Pepelko, 1970). Similarly, Schaefer et al. (1971) showed that exposures of guinea pigs to 3% CO2 for 42 d or 15% CO2 in 21% O2 for 7 d failed to produce any histopa-
thology in the liver. Schaefer et al. however, found a decrease in glycogen granules and an increase in fat granules in the liver of guinea pigs exposed to 3% CO2 for 7 d. The granular changes recovered in 1 d after the end of the exposure. These changes in the granules were interpreted by the investigators to reflect functional changes in liver metabolism. Because these changes are not actual damages, they are not relied on in setting SMACs.
As mentioned above, 4- or 8-h exposures of CO2 are known to produce injuries in the testis of rats (Vandemark et al., 1972). However, it is unclear whether subchronic CO2 exposures could damage the testis. In a study without an adequate number of control animals, Schaefer et al. (1971) observed in the testis a marked reduction of mature spermatocytes with a concomitant increase in the precursor cells of spermatocytes in guinea pigs exposed to 15% CO2 for 2 d. When the exposure was extended to 7 d, multinucleated giant cells were observed in the testis. Because on the average only about two control animals were examined per time point, it is not certain whether the testicular changes observed by Schaefer et al. in the 15% CO2 group were due to CO2 or whether they were artifacts. Nevertheless, some of the data gathered by Schaefer et al. in that subchronic study are of value in setting the SMACs. Schaefer et al. (1971) reported that the testes of the guinea pigs and rats exposed to 3% CO2 for 42 d or to 1.5% CO2 for 6 mo appeared normal histologically, it can be concluded that a subchronic exposure to 3 % CO2 is not toxic to the testis.
Hematological Changes
Guillerm and Radziszewski (1979) reported that a 10% reduction in hematocrit and a 9% reduction in red blood cell count were detected in six human subjects exposed to 2% CO2 for 16-30 d. Because they failed to observe these reductions in humans exposed to 4% CO2, they discounted hypercapnia as the cause of the hematological changes. Instead, they hypothesized that prolonged confinement might be the cause. Similarly, the Navy found that prolonged hypercapnia might not always produce hematological changes. Wilson and Schaefer (1979) showed that, in Polaris submarine patrols with CO2 levels maintained between 0.7% and 1.2% and a CO level between 15 and 20 ppm, the hematological responses in smokers differed from that in nonsmokers.
In nine smokers in the patrol, the red blood cell count increased by 12% and the hematocrit increased by 4% on the sixth day, but not on the 32nd and 52nd day. However, these two hematological parameters did not change in 11 nonsmokers in the patrol on the sixth, 32nd, and 52nd day. Because astronauts will not be allowed to smoke cigarettes in spacecraft and most of them are nonsmokers, the hematological data are not relied on in setting CO2 SMACs.
Carcinogenicity
No traditional carcinogenic bioassay has been known to be conducted with CO2. However, Goldsmith et al. (1980) discovered that infusion of humidified 99.99% CO2 into the peritoneal cavity of 4- to 6-mo-old BALB/c mice for 10-12 d led to lymphoma after a latent period of about 8 mo (the incidence in the air-exposed control group was 0% and that in the CO2-exposed control group was about 60%) and a doubling of the incidence of pulmonary adenocarcinoma (from 15% in the control group to about 30% in the CO2-exposed group). Due to the highly artificial nature of the CO2 exposure, the practical meaning of the tumorigenic findings is uncertain.
Epidemiological Data
Only one epidemiological study involving CO2 was found. In a criteria document for CO2, NIOSH cited, an unpublished report submitted to NIOSH by the United States Brewers Association, Inc. (Riley and Barnea-Bromberger, 1976). The report concerned the acid-base effect of CO2 exposures in brewery workers. In these workers, exposed to 1.1% CO2 TWA with 3-min excursions up to 8%, the blood HCO3- levels did not differ from the control values (Riley and Barnea-Bromberger, 1976).
Genetic Toxicity
No genotoxic data of CO2 have been found.
Developmental Toxicity
An exposure of rabbits to 10-13% CO2 for 4-10 h on d 2 or 3 between d 7 and d 12 of pregnancy resulted in congenital hypoplasias in the vertebral column (Grote, 1965). The value of this teratogenic study was limited because only three pregnant rabbits were exposed to CO2. In another teratogenic study, 71 pregnant rats were exposed to 6% CO2 in 20% O2 for 24 h between d 5 and d 21 of pregnancy (Haring, 1960). More increased incidences of cardiac and skeletal malformations were detected in the CO2-exposed group than in the control group (Haring, 1960).
Interaction with Other Chemicals
Only one report of synergism involving CO2 was found. Levin et al. (1987) reported that the amount of acidosis produced by a combined exposure of rats to 5 % CO2 and 2500 ppm CO was larger than that produced by either agent alone. The addition of CO2 to an exposure atmosphere containing CO decreased the mean survival time of rats, compared with rats exposed to only CO (Rodkey and Collison, 1979). This potentiation of CO's lethal effect by CO2 is thought to be due to CO2's hyperventilatory effect (Rodkey and Collison, 1979). Indeed, co-exposures of rats to 5% CO2 in 2500 ppm CO are known to increase the rate of COHb rise in blood compared with CO exposures alone (Levin et al., 1987). However, it should be noted that co-exposures to 5% CO2 in CO do not always result in potentiation of CO's toxicity. For instance, exposure to CO2 at 5% did not potentiate the incapacitation effect of 3500 ppm CO on the rat (Hartzell and Switzer, 1985). Finally, just as other substances might potentiate the effect of CO2, the reverse is also true. A subcutaneous injection of naloxone at 5 mg/kg has been shown to increase the hyperventilatory response to CO2 in rats because naloxone displaced endogenous endorphins from central opioid receptors (Isom and Elshowihy, 1982).
In addition to interacting with CO, CO2 is known to interact with NO2. Exposures of rats to 200 ppm NO2 for 30 min resulted in an increase in methemoglobin level in blood (Levin et al., 1989). A co-exposure with 5 % CO2 led to a larger increase in methemoglobin than with 200 ppm NO2 alone (Levin et al., 1989).
TABLE 3-9 Toxicity Summary
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
0.5 |
30 d |
Human |
No acidosis, hyperventilation, or symptoms; no changes in urinary excretion of potassium, sodium, or calcium |
Radziszewski et al., 1988 |
|
0.6 in d 1-46, 0.8 in d 47-90 |
90 d |
Human |
No change in serum calcium level on d 1-53, but decreases accompanied with increases in serum phosphorus on d 54-90; no changes in hematological indices and psychomotor performance |
Jackson et al., 1972 |
|
0.7 |
7 w |
Human |
Higher serum levels of calcium, magnesium, and inorganic phosphorus; lower urinary excretions of calcium, magnesium, and inorganic phosphorus; lower urinary excretion of acids except in w 3 and 4 when it was higher than pre-exposure level |
Gray et al., 1973 |
|
0.8-0.9 |
20 d |
Human |
Physiological dead space increased by 50-60%, which returned to normal soon after the exposure |
Gude and Schaefer, 1969 |
|
0.85-1.2 |
57 d |
Human |
In plasma, increase in sodium, decreases in K+ and Ca++, but no change in phosphorus; increased Mg++ only on d 51; decrease in C1- in w 5-7; decreases in pH, increases in pCO2 and bicarbonate in w 4 with complete recovery by d 51; in urine, phosphorus and hydroxyproline decreased in w 1-3; Ca++ decreased in w 1-3, increased in w 4-5, then decreased in w 6-9; no change in parathyroid or calcitonin activity |
Messier et al., 1976 |
|
1 |
17-32 min |
Human |
Alveolar ventilation increased by 24%; slight increases of systolic and diastolic blood pressure |
Schneider and Truesdale, 1922 |
|
1 |
30 d |
Human (n = 1) |
Acidosis; increases in blood pCO2 and respiratory ventilation; no change in performance in physical exercise |
Zharov et al., 1963 |
|
1 |
30 d |
Human |
Hyperventilation; no symptoms; no changes in urinary excretion of potassium, sodium, or calcium; increased arterial pO2 |
Radziszewski et al., 1988 |
|
1 or 2 |
30 min |
Human |
No symptoms during exercises at two-thirds maximum or maximum oxygen consumption |
Menn et al., 1970 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
1.1 TWA with excursions up to 8% for 3 min |
8 h/d, 5 d/w |
Human (workers) |
No change in blood HCO31- levels |
Riley and Barnea-Bromberger, 1976 |
|
1.5 |
10-15 min |
Human |
Increases in respiratory rate and tidal volume; lower respiratory rate increase and higher tidal volume increase in instructors than in other individuals |
Schaefer, 1958 |
|
1.5 |
15 h/d, 6 d |
Human (n = 1) |
Impairment in night vision sensitivity and green color sensitivity; all other visual functions were normal |
Weitzman and Kinney, 1969 |
|
1.5 |
42 d |
Human |
Alveolar ventilation increased by 8%; ventilatory response to 5% CO2 challenges decreased at end of w 6; anatomic dead space of lung increased; O2 consumption increased in first 2 w; plasma Ca++ and phosphorus followed changes in plasma pH; uncompensated respiratory acidosis in first 3 w (decrease in blood pH, urine pH, urinary HCO3- excretion, and CO2 exhalation); compensated respiratory acidosis in last 3 w (normal blood pH, increases in urine pH, urinary HCO3- excretion, and CO2 exhalation); no effects on weight, pulse rate, blood pressure, oral temperature, adaptation to darkness, visual acuity, visual accommodation, depth perception, pitch discrimination, manual dexterity, strength, coordination, immediate memory, and letter-canceling, problem-solving, and mechanical abilities; apathy, increased sexual desire, desire to leave, and uncooperativeness |
Schaefer, 1961a,b, 1963b,c; Schaefer et al., 1963a,b, 1964b |
|
1.8 or 3.5 |
11-40 min |
Human |
No changes in oxygen consumption, pulse rate, and cardiac output; increase in respiratory ventilation |
Grollman, 1930 |
|
1.9 |
N.S.b (until subjects could not exercise further) |
Human |
Compared with exercising in normal air, 45% higher ventilation when doing submaximal exercise, but exposure did not increase ventilation further when doing maximal exercise (O2 consumption was even lower) |
Luft et al., 1974 |
|
2 |
30 d |
Human |
No acidosis, headache, or change in psychomotor performance; hyperventilation (more at 2 h than 24 h); good ability to exercise |
Radziszewski et al., 1988 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
2 |
17-32 min |
Human |
Alveolar ventilation increased by 50%; slight increases of systolic and diastolic blood pressure |
Schneider and Truesdale, 1922 |
|
2 |
Several h |
Human |
Headache and dyspnea on mild exertion |
Schulte, 1964 |
|
2 |
30 d |
Human (n = 1) |
Acidosis; increases in blood pCO2 and respiratory ventilation; deterioration in performance in physical exercise |
Zharov et al., 1963 |
|
2.5 |
2 h |
Human |
No changes in specific airway conductance |
Tashkin and Simmons, 1972 |
|
2.5-2.8% CO2 in 14.6-15% O2 |
Several h |
Human |
No giddiness, headache, dyspnea, or drop in body temperature |
Brown, 1930b |
|
2.7 |
30 d |
Human |
Mild headache only on d 1; hyperventilation diminished after d 1 |
Sinclair et al., 1969 |
|
2.8 |
1 h or 15-20 d |
Human |
Acidosis; abilities to exercise moderately or heavily did not change; during exercise, occasional mild headaches, but no dyspnea, intercostal muscle pain, or EKG changes; no difference between acute and subchronic CO2 exposures |
Sinclair et al., 1971 |
|
2.8 or 3.9 |
30 min |
Human |
Intercostal muscle pain and respiratory difficulties during exercises at two-thirds maximum or maximum oxygen consumption; ability to do heavy exercise impaired; mild-to-moderate frontal headache at 3.9% CO2 occurred near end of exercise; no significant increases in premature atrial or ventricular contractions usually seen with exercise in normal atmosphere |
Menn et al., 1970 |
|
2.9 |
8 d |
Human |
Acidosis and hyperventilation at 2 and 24 h; slight headache; extrasystoles during exercise; no change in psychomotor performance |
Radziszewski et al., 1988 |
|
3 |
Several h |
Human |
Dyspnea even at rest, headache (more severe than at 2% CO2), and diffuse sweating |
Schulte, 1964 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
3 |
78 h |
Human (n = 2) |
Acclimation in the ventilatory effect of CO2; minute volume 15.1 L/min at the start and 12.9 L/min near end of exposure |
Chapin et al., 1955 |
|
3 |
5 d |
Human |
Very slight acidosis on d 1-3; raised arterial pCO2 and serum HCO3- levels on d 3-5; respiratory ventilation increased by 15-60%, which was easily tolerated; mild-to-moderate headaches in 4/7 subjects on d 1-2; no changes in vital capacity and 1-s vital capacity, psychomotor functions (hand steadiness, vigilance, auditory monitoring, memory, arithmetic, and problem solving); no changes in urinary levels of Ca++, phosphorus, K+, Na+, NH3, and titratable acidity or in serum levels of Ca++, phosphorus, K+, Na+, alkaline phosphatase, SGOT, SGPT, direct bilirubin, and indirect bilirubin; no change in the ability to exercise moderately for 1 h daily; no EKG problems |
Glatte et al., 1967a |
|
3 |
8 d |
Human |
A slight state of excitement on d 1 (euphoria, troubled sleep with frequent dreams and nightmares), followed by slight depression of the nervous systems in the remainder of the exposure (inattentiveness, erratic behavior, exhaustion, confusion, and decreased manual skills); uncompensated respiratory acidosis in the first 3 d; acidosis then compensated by increases in plasma HCO31- level, urinary excretion of acid, and alkali retention by kidneys; when the subjects performed moderate work, the tidal volume decreased and the respiratory rate increased, leading to higher O2 update and CO2 excretion |
Schaefer, 1949ab |
|
3.0-3.5 |
1.2 min |
Human |
Small loss in hearing threshold |
Gellhorn and Spiesman, 1934, 1935 |
|
3.2% CO2 in 13.4% O2 |
Several h |
Human |
Giddiness and headache; no dyspnea or drop in body temperature |
Brown, 1930b |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
3.3 |
10-15 min |
Human |
Increases in respiratory rate and tidal volume (diving instructors and other subjects responded similarly); CNS-depression decrease in flicker-fusion threshold and increase in latent time of alpha blocking after light stimulus; increases in blood sugar and oxygen consumption; decrease in the eosinophil count |
Schaefer, 1958, 1963a |
|
3.8 |
9 |
Human |
Acidosis and hyperventilation at 2 and 24 h; intense and headaches and gastralgia; extrasystoles during exercise; limited exercise capacity; no change in psychomotor performance |
Radziszewski et al., 1988 |
|
3.9 |
5 or 11 d |
Human |
Acidosis on d 1-4; arterial and CSF pH returned to normal on d 5; mild headaches on d 1 only; hyperventilation (decreased in magnitude on d 2) |
Sinclair et al., 1969 |
|
4 |
5 d |
Human |
Acidosis; tidal volume almost doubled but no change in respiratory rate; mild-to-moderate throbbing frontal headaches beginning in the first few hours but none by d 3 |
Menn et al.. 1968 |
|
4 |
11 d |
Human |
Alveolar ventilation increased by 200% on day 1 but dropped to 150% after day 1; increased pCO2 in arterial blood and cerebral spinal fluid |
Clark et al., 1971 |
|
4 |
2 w |
Human |
No change in hand-eye coordination, complex tracking performance, and problem-solving ability |
Storm and Giannetta, 1974 |
|
4.3 |
1 d |
Human |
Acidosis and hyperventilation at 2 and 24 h; intense and annoying headaches and gastralgia; not able to exercise; no change in psychomotor performance |
Radziszewski et al., 1988 |
|
4-5 |
17-32 min |
Human |
Dyspnea |
Schneider and Truesdale, 1922 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
4-5 |
≈4 h |
Human |
Body temperature dropped 1°F; deterioration in performance in cancellation test; no effects on Army alpha intelligence test, arithmetic test, muscular coordination, and attention |
Brown, 1930b |
|
4.7 |
Several h |
Human |
Headache and dyspnea |
Brown, 1930b |
|
4.7% CO2, balance O2 |
15 min |
Human (emphysema patients) |
Alveolar ventilation increased by <100% (increased by 150-300% in normal subjects) |
Tenney, 1954 |
|
5 |
N.S. |
Human (fighter pilots, n = 2) |
Significant degradation in pilot performance during landing; lengthened flight time between gear down and touch down, and unacceptable increase in touch-down sink rates |
Wamsley et al., 1969 |
|
5 |
30 min |
Human |
Increased renal blood flow, glomerular filtration rate, and renal vascular resistance; increase in plasma HCO3- level but no increase in NA+, K+, and CI- levels |
Yonezawa, 1968 |
|
5 |
17-32 min |
Human |
Headache, dizziness, hiccoughing |
Schneider and Truesdale, 1922 |
|
5 or 7 |
15-30 min |
Human |
Increases in blood pressure and cerebral blood flow; decrease in cerebrovascular resistance; no changes in cardiac output or cerebral oxygen consumption |
Kety and Schmidt, 1948 |
|
5% in 95% O2 |
45-90 min |
Human (n = 2 psychotic patients) |
Arterial pH dropped to 6.9; A-V nodal beats; increases in amplitude of R and T waves; raise or depression of S-T segment; inverted T waves; EKG changes disappeared 30 min after exposure |
Altschule and Sulzbach, 1947 |
|
5, 7.5 or 10 |
2 h |
Human |
Decreased specific airway conductance |
Tashkin and Simmons, 1972 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
5-6.75 |
37 h |
Human |
Headache; increased respiratory ventilation; soreness of respiratory musculature; 10-bpm increase in heart rate; slight decrease in hand-arm steadiness; no changes in blood pressure, auditory discrimination, hand-eye coordination, or abilities to stand still, walk 1-in rail, or compute, translate, and check numbers |
Consolazio et al., 1947 |
|
5 |
Several h |
Human |
CNS depression |
Schulte, 1964 |
|
5.4 or 7.5 |
10-15 min |
Human |
Increases in respiratory rate and tidal volume; diving instructors responded less than other subjects; increases in blood sugar and oxygen consumption; decrease in eosinophil count; increase in pulse rate at 7.5% CO2 |
Schaefer, 1958 |
|
6 |
1-2 min |
Human |
Decreased visual intensity discrimination |
Gellhorn, 1936 |
|
6 |
6-8 min |
Human |
More decreases in amplitude of QRS complex and T wave in older men (aged 61 y) than in younger men (aged 23 y); no change in S-T segments; no T inversion |
Okajima and Simonson, 1962 |
|
6 |
16 min |
Human |
Dyspnea; headache; sweating; hyperventilation; subjective feeling of speech difficulty (speech understandable) and of movement difficulty; slightly slower rate of card sorting but no change in card-sorting error rate |
White et al., 1952 |
|
6 |
20.5-22 min |
Human |
Considerable discomfort but tolerable; 9% rise in systolic pressure; 7% rises in diastolic pressure and pulse rate |
Brown, 1930b |
|
6 |
Several h |
Human |
Visual disturbances and tremors |
Schulte, 1964 |
|
7% CO2, 93% O2 |
3 min |
Human |
Tidal volume, respiratory rate, and ventilation increased by 140, 50, and 250%, respectively |
Sullivan and Yu, 1983 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
7% CO2, 93% O2 |
5 min |
Human |
Tidal volume, respiratory rate, and ventilation increased by 150, 60, and 290%, respectively |
Sullivan and Yu, 1983 |
|
7 |
60 min |
Human |
Arterial pCO2, H+, and HCO3- levels increased in 10 min of exposure and remained at a plateau at min 10-60; arterial Na+ level increased by < 1%; no changes in arterial K+, C1-, and phosphate levels; mild headache and burning of eyes |
Brackett et al., 1965 |
|
7-14% CO2, balance O2 |
10-20 min |
Human |
Headache, moaning, belligerent complaining, coughing, restlessness, sweating, twitching, tremor, amnesia, unconsciousness; increased respiratory ventilation, arterial pressure, heart rate, and plasma concentrations of epinephrine, norepinephrine, and corticosteroids; premature nodal contraction (2/27 subjects vs 0/27 before exposure) and premature ventricular contraction (3/27 subjects vs 1/27 before exposure) on EKG |
Sechzer et al., 1960 |
|
7.5% CO2 in 16% O2 |
3.25-6 min |
Human |
Considerable discomfort, but tolerable; 24% and 20% rises in systolic and diastolic pressures; 10% rise in pulse rate |
Brown, 1930b |
|
7.5 |
4-25 min |
Human |
Increases in pulse rate. cardiac output, blood pressure. and respiratory ventilation |
Grollman, 1930 |
|
7.5 |
15 min |
Human |
Headache, dizziness, restlessness, and dyspnea |
Schaefer. 1963a |
|
7.6 |
2.5-10 |
Human |
Dyspnea, dizziness, headache, head fullness, sweating, and increases in respiratory ventilation and systolic and diastolic pressures |
Dripps and Comroe, 1947 |
|
8% CO2, 19% O2 |
3-6 min |
Human |
Total lung resistance increased by 120%; no change in static lung compliance |
Nadel and Widdicombe, 1962 |
|
8 |
17-32 min |
Human |
Tolerance limit |
Schneider and Truesdale, 1922 |
|
8.8% CO2 in 39% O2 |
7-10 min |
Human |
Approaching tolerance limit; 22% and 13% rises in systolic and diastolic pressures, respectively, and 13% rise in pulse rate |
Brown, 1930b |
|
10 |
1.5 min |
Human |
Eye flickering, myoclonic twitches, and psychomotor excitation |
Lambertsen, 1971 |
|
10 |
15-25 min |
Human |
Restlessness, confusion, and listlessness |
Brackett et al., 1965 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
10 |
N.S. |
Human |
Unconsciousness |
Schulte, 1964 |
|
10.4 |
3.8 min |
Human |
Dizziness, dyspnea, headache, head fullness, restlessness, hyperventilation, unconsciousness, and rises in systolic and diastolic pressures |
Dripps and Comroe, 1947 |
|
10.4% CO2 in 14.4% O2 |
1-2.25 min |
Human |
33% and 38% rises in systolic and diastolic pressure, respectively, and 19% rise in pulse rate |
Brown, 1930b |
|
12.4% |
0.75-2 min |
Human |
Dizziness, drowsiness, near stupor, dyspnea, head fullness, sweating, flushing sensation, sense of impending collapse, throat irritation, and slight choking sensation; 1/7 subjects collapsed; no nausea or throbbing of temples; 55% and 26% rises in systolic and diastolic pressures, respectively, and 13% rise in pulse rate |
Brown, 1930b |
|
15 |
3 min |
Human |
Eye flickering, myoclonic twitches, psychomotor excitation, increased muscle tone, sweating, flushing, dilated pupils, leg flexion, torsion spasms, and restlessness |
Lambertsen, 1971 |
|
17% CO2, 17.3% O2 |
20-52 s |
Human |
Unconsciousness |
Aero Medical Association, 1953 |
|
18.6, 17 O2 |
<2 min |
Human |
Dullness, unconsciousness, cyanosis, and throbbing headache |
Haldane and Smith, 1892 |
|
20-22% CO2, ca. 16% O2 |
N.S. |
Human (workers) |
Death; survivors experienced unconsciousness, cyanosis, sluggish reflexes, rattling respiration, and motor unrest |
Dalgaard et al., 1972 |
|
20% CO2, 80% O2 |
3 min |
Human |
Eye flickering, myoclonic twitches, psychomotor excitation, increased muscle tone, sweating, flushing, dilated pupils, leg flexion, torsion spasms, restlessness, tonic and tonic-clonic seizures |
Lambertsen, 1971 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
30% CO2, 70% O2 |
38 s |
Human (patients in psychiatry) |
Narcosis; extrasystoles, premature atrial and nodal beats, atrial tachycardia, and supraventricular tachycardia on EKG |
MacDonald and Simonson, 1953 |
|
30% CO2, 70% O2 |
50-52 s |
Human (patients in psychiatry) |
Unconsciousness and extrasystoles on EKG; consciousness regained 110 s after exposure |
Friedlander and Hill, 1954 |
|
30% CO2, 70% O2 |
3 min |
Human |
Eye flickering, myoclonic twitches, psychomotor excitation, increased muscle tone, sweating, flushing, dilated pupils, leg flexion, torsion spasms, restlessness, tonic and tonic-clonic seizures; unconsciousness within 2 min |
Lambertsen, 1971 |
|
30% CO2, 70% O2 |
N.S. (10-15 breaths) |
Human (patients in psychiatry) |
Auricular extrasystoles, auricular tachycardia, increased P-wave voltage, low or inverted P waves, spiked T waves with a broad base, increased T-wave voltage, slight increases in PR intervals and QRS intervals, and marked increase in QT interval: marked increases in systolic and diastolic pressure; acidosis; no ventricular extrasystole |
McArdle, 1959 |
|
0.5 |
4 w |
Guinea pig |
No effects on body weight; calcium levels in kidney, bone, or plasma; type II pneumocyte cell size; and number of lamellar bodies in type II pneumocytes |
Schaefer et al.. 1979b |
|
0.5 |
8 w |
Guinea pig |
Increased calcium levels in kidneys and plasma; no significant effects on bone calcium level, body-weight gain, type II pneumocyte cell size, and the number of lamellar bodies in type II pneumocytes |
Schaefer et al., 1979b |
|
1 |
1 w |
Guinea pig |
Acidosis; Ca++ increased in kidney; Ca++ and phosphorus increased in plasma; Ca++ and phosphorus decreased in bone; no change in body weight gain |
Schaefer et al., 1979a |
|
1 |
1 or 2 w |
Guinea pig |
Acidosis; no change in arterial pO2, pCO2, or HCO3- level; no change in appearance of pneumocytes, alveolar macrophages, ciliated epithelial cells and Clara cells of terminal bronchioles, and endothelial cells in the lung under the electron microscope |
Douglas et al., 1979 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
1 |
2 or 4 w |
Guinea pig |
Acidosis; Ca++ increased in kidney; Ca++ and phosphorus levels in plasma did not differ from the control levels; Ca++ and phosphorus levels in bone did not differ from the control levels; no changes in body weight gain |
Schaefer et al., 1979a |
|
1 |
3 w |
Guinea pig |
No change in arterial pH and pO2; increased arterial pCO2; decreased arterial HCO3-; no change in the appearance of pneumocytes, alveolar macrophages, ciliated epithelial cells and Clara cells of terminal bronchioles, and endothelial cells in the lung under the electron microscope |
Douglas et al., 1979 |
|
1 |
4 w |
Guinea pig |
Acidosis; increased arterial pCO2; no change in arterial pO2 and HCO3; marked increases in the size and number of type II pneumocytes; clustering of 2-4 type II pneumocytes together; the changes in type II pneumocytes remained 2 or 4 w after exposure; no changes in the other cell types in lung under electron microscopy |
Douglas et al., 1979 |
|
1 |
6 w |
Guinea pig |
No changes in arterial pH, pO2, pCO2, and HCO3-; marked increases in the size and number of type II pneumocytes; clustering of 2-4 type II pneumocytes; no changes in other cell types in lung under electron microscopy |
Douglas et al.. 1979 |
|
1 |
6 w |
Guinea pig |
No acidosis; Ca++ increased in kidney; Ca++ and phosphorus increased in plasma; Ca++ and phosphorus decreased in bone; no changes in body weight gain |
Schaefer et al., 1979a |
|
1-2 |
30 min |
Rat |
Respiratory frequency and ventilation increased by 30-40% |
Lai et al., 1978 |
|
1-2 |
5 h |
Mouse |
All animals died |
Zink and Reinhardt, 1975 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
1-5 |
24-42 d |
Guinea pig |
No lowering in blood pH; subpleural atelectasis in lung; no hyaline membranes or edema; atelectasis disappeared 2 w after the end of exposure |
Niemoeller and Schaefer, 1962 |
|
1.5 |
1-14w |
Guinea pig |
Depressed body weight gain |
Schaefer et al., 1971 |
|
1.5 |
1 w |
Guinea pig |
No histopathology in liver, heart, testes, spleen, and pancreas |
Schaefer et al., 1971 |
|
1.5 |
35-42 d |
Guinea pig |
Histological signs of focal renal tubular calcification in the cortex; the incidence increased with exposure duration; the calcification remained 27 d after exposure; no histological signs of renal calcification during the first 2 w of exposure |
Schaefer et al., 1979a |
|
1.5 |
6 mo |
Guinea pig |
No lowering in blood pH; subpleural atelectasis in lung; no hyaline membranes or edema |
Niemoeller and Schaefer, 1962 |
|
2.5 |
1 or 2 h |
Rat |
No changes in testicular histology and weight |
Vandemark et al., 1972 |
|
2.5 |
4 or 8 h |
Rat |
Sloughing of spermatid and Sertoli cells into seminiferous tubule lumen; no mature spermatids in the tubule; no change in testicular weight; return to normal 36 h after exposure |
Vandemark et al., 1972 |
|
3 |
1 h |
Guinea pig |
Lowered blood pH: plasma GOT level doubled; plasma GPT level unchanged |
Schaefer et al.. 1971 |
|
3 |
1 d |
Guinea pig |
Lowered blood pH; no changes in plasma GOT and GPT levels |
Schaefer et al., 1971 |
|
3 |
1 d |
Guinea pig |
Reduced body weight; increased kidney and testicular weight; no changes in liver, lung, thyroid, spleen, thymus, and adrenal weight |
Schaefer et al., 1971 |
|
3 |
1-7 d |
Guinea pig |
Blood pH dropped more in guinea pigs than in rats; blood bicarbonate levels increased in rats, not in guinea pigs |
Schaefer et al., 1971 |
|
3 |
2 d |
Guinea pig |
Blood pH lowered to 7.27; subpleural atelectasis and edema in lung; no hyaline membranes in lung |
Niemoeller and Schaefer, 1962 |
|
3 |
3d |
Guinea pig |
Reduced body and thymus weight; increased lung and testicular weight; no changes in kidney, liver, spleen, thyroid, and adrenal weight |
Schaefer et al., 1971 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
3 |
4 d |
Guinea pig |
Blood pH lowered to 7.31; subpleural atelectasis and hyaline membranes in lung; no edema in lung |
Niemoeller and Schaefer, 1962 |
|
3 |
4 d |
Guinea pig |
Blood pH lowered to 7.32; increase in lung/body-weight ratio; no change in surface tension of lung extracts; increase in abnormal lamellar bodies in alveolar lining cells, subpleural atelectasis, edema, hyaline membranes, and phagocytic pneumocytes in lung |
Schaefer et al., 1964a |
|
3 |
7 d |
Guinea pig |
No changes in blood pH and plasma levels of GOT and GPT; depletion of glycogen vacuoles and increase in fat vacuoles in liver; no significant liver histopathology; a small incidence of hyaline membranes in lung; increase in zymogen granules in pancreas; no testicular histopathology |
Schaefer et al., 1971 |
|
3 |
21 d |
Guinea pig |
Lower body-weight gain; increased adrenal weight and decreased spleen weight; no changes in liver, kidney, lung, thymus, thyroid, and testicular weight. Glycogen granules depleted in liver on 7 d were restored on 21 d; no testicular histopathology |
Schaefer et al., 1971 |
|
3 |
42 d |
Guinea pig |
No lowering of blood pH; subpleural atelectasis and hyaline membranes in lung; no edema in lung |
Niemoeller and Schaefer, 1962 |
|
3 |
42 d with 2 w recovery |
Guinea pig |
No lowering of blood pH; no subpleural atelectasis, hyaline membranes, or edema in lung |
Niemoeller and Schaefer, 1962 |
|
3 |
93 d |
Monkey |
No changes in body weight, blood glucose, hemoglobin, hematocrit, total leukocyte count, serum levels of Ca++, C1-, phosphate, blood urea nitrogen, serum bilirubin and cholesterol levels, and RCB sedimentation rate |
Stein et al., 1959 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
45 |
13 d |
Rabbit |
Distended alveoli and alveolar ducts with hyaline membranes and leukocytic infiltration in lung; Necrosis scattered throughout the liver lobules. Necrotic renal tubular epithelium with calcium incrustation in the cortical-medullary zone; tubular lumen were obstructed with calcium casts |
Meessen, 1948 |
|
5 |
1 or 2 h |
Rat |
No changes in testicular histology and weight |
Vandemark et al., 1972 |
|
5 |
4 or 8 h |
Rat |
Sloughing of spermatid and Sertoli cells into seminiferous tubule lumen; no mature spermatids in the tubule; no change in testicular weight; return to normal 36 h after exposure |
Vandemark et al., 1972 |
|
8 |
32 d |
Rat |
Reduced body-weight gain; increased heart/body-weight and kidney/body-weight ratios; no change in liver/body-weight and lung/body-weight ratios; increase in eosinophils, decreased hematocrit, and no change in reticulocyte percent in blood; no histological changes in spleen, thyroid, liver, kidney, adrenals, heart, and lungs |
Pepelko, 1970 |
|
10 |
1 or 2 h |
Rat |
No change in testicular histology and weight |
Vandemark et al., 1972 |
|
10 |
4 or 8 h |
Rat |
Sloughing of spermatic and Sertoli cells into seminiferous tubule lumen; no mature spermatids in the tubule; no change in testicular weight; return to normal 36 h after exposure |
Vandemark et al., 1972 |
|
10 |
4 d |
Rat |
No death or CNS depression |
Barbour and Seevers, 1943 |
|
10 |
30 d |
Rat |
Hyperventilation; body-weight loss and reduced food intake; marked reticulocytosis, but no changes in hemoglobin level, RBC count, and white-blood-cell count |
Barbour and Seevers, 1943 |
|
11 |
2.5 to 5 h |
Rat |
O2 consumption reduced |
Barbour and Seevers, 1943 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
15 |
1 h |
Guinea pig |
Blood pH decreased to 7.00; increases in blood corticosteroids and free fatty acids; decreases in adrenal epinephrine and adrenal cholesterol |
Schaefer et al., 1968 |
|
15 |
1 or 6 h |
Guinea pig |
Blood pH decreased to 7.01-7.09; increase in lung/body-weight ratio; no change in surface tension of lung extracts; increase of lamellar bodies in alveolar lining cells, subpleural atelectasis, congestion, edema, and hemorrhage in lung; no hyaline membranes; increase in phagocytic pneumocytes at 6 h but not at 1 h |
Schaefer et al., 1964a |
|
15 |
6 h |
Guinea pig |
Blood pH decreased to 7.10; increases in blood corticosteroids and free fatty acids; decrease in adrenal epinephrine |
Schaefer et al., 1968 |
|
15 |
6 h or 1 d |
Guinea pig |
Reduction in oxygen partial pressure required to half saturate the blood; decreases in blood pH and 2,3-diphosphoglycerate |
Messier and Schaefer, 1971 |
|
15 |
1 d |
Guinea pig |
Blood pH decreased to 7.10; body weight decreased by ≈ 10%; increase in adrenal/body-weight ratio; decreases in thymus/body-weight and spleen/body-weight ratios; reduction in lymphocyte count; no change in total white-blood-cell count; increases in blood corticosteroids and free fatty acids; decreases in adrenal epinephrine and adrenal cholesterol |
Schaefer et al., 1968 |
|
15 |
1 d |
Guinea pig |
Blood pH decreased to 7.10; increases in lung/body-weight ratio and surface tension of lung extracts; increase in abnormal lamellar bodies in alveolar lining cells, subpleural atelectasis, congestion, edema, hemorrhage, hyaline membranes, and phagocytic pneumocytes in lung |
Schaefer et al., 1964a |
|
15 |
1 d |
Guinea pig |
Intestinal hemorrhage that disappeared after 3 or 4 d of exposure; congestion and hemorrhages of spleen |
Schaefer et al., 1971 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
15 |
1-7 d |
Guinea pig |
Blood pH decreased more in guinea pigs than in rats; blood bicarbonate level increased on 1 d in rats but not in guinea pigs |
Schaefer et al., 1971 |
|
15 |
2 d |
Guinea pig |
A marked reduction of mature spermatocytes in testes |
Schaefer et al., 1971 |
|
15 |
3 d |
Guinea pig |
Blood pH decreased to 7.3; body weight decreased by ≈ 10%; increase in adrenal/body-weight ratio; decrease in thymus/body-weight ratio; no change in spleen/body-weight ratio; reduced lymphocyte count; no changes in total white-blood-cell count and adrenal cholesterol; increase in free fatty acids in blood |
Schaefer et al., 1968 |
|
15 |
8 h/d, 7 d |
Guinea pig |
Blood pH decreased to 7.11; increase in blood corticosteroids; decrease in adrenal epinephrine |
Schaefer et al., 1968 |
|
15 |
7 d |
Guinea pig |
No significant changes in blood pH and body weight; increase in adrenal/body-weight and decrease in thymus/body-weight ratios; no change in spleen/body-weight ratio; no change in lymphocyte and total white-blood-cell counts; no change in corticosteroids and free fatty acids in blood or epinephrine in adrenal |
Schaefer et al.. 1968 |
|
15 |
7 d |
Guinea pig |
No decrease in blood pH; no changes in lung/body-weight ratio and surface tension of lung extracts: subpleural atelectasis, congestion. increases in phagocytic pneumocytes in lung; no edema, hemorrhage, hyaline membranes or abnormal lamellar bodies in alveolar lining cells in lung |
Schaefer et al.. 1964a |
|
15 |
7 d |
Guinea pig |
No change in oxygen partial pressure required to half saturate the blood decreases and 2,3-diphosphoglycerate in RBC; lowered blood pH |
Messier and Schaefer, 1971 |
|
15 |
7 d |
Guinea pig |
Multinucleated giants cells in testes; fat deposition in myocardium (not seen on 1 d); increase in zymogen granules in pancreas; congestion and hemorrhage in spleen; decreased blood pH; no changes in SGOT and SGPT levels |
Schaefer et al., 1971 |
|
Concentration, % |
Exposure Duration |
Species |
Effects |
Reference |
|
15 |
14 d |
Guinea pig |
No decrease in blood pH; no changes in lung/body-weight ratio and surface tension of lung extracts; congestion and increase in phagocytic pneumocytes in lung; no subpleural atelectasis, edema, hemorrhage, or abnormal lamellar bodies in alveolar lining cells in lung |
Schaefer et al., 1964a |
|
15 |
42 d |
Guinea pig |
No change in adrenal cholesterol content, lymphocyte and total white-blood-cell counts |
Schaefer et al., 1968 |
|
15.8 |
3 h |
Dog |
Gastrointestinal bleeding, multiple ulcerations, marked dilatation of arterioles and capillaries in the GI tract, markly reduced platelet counts, and increased clotting time |
DeBellis et al., 1968 |
|
16 |
2 d |
Guinea pig |
Distended alveoli and alveolar ducts with hyaline membranes and leukocytic infiltration in lung; necrosis scattered throughout the liver lobules; Necrotic renal tubular epithelium with calcium incrustation in the cortical-medullary zone; tubular lumen were obstructed with calcium casts; irreversible degenerative changes of ganglion cells in cerebral cortex, basal ganglia, and brain stem |
Meessen, 1948 |
|
20 |
4 d |
Rat |
80% mortality associated with pulmonary edema and sanguinous exudate; CNS depression |
Barbour and Seevers, 1943 |
|
25 |
36 h |
Rat |
100% mortality associated with pulmonary edema and sanguinous exudate; CNS depression |
Barbour and Seevers, 1943 |
|
25 |
N.S. |
Rabbit |
Transient convulsions followed by marked CNS depression |
Barbour and Seevers, 1943 |
|
30 |
N.S. |
Rat |
Narcosis developed immediately |
Barbour and Seevers, 1943 |
TABLE 3-10 Exposure Limits Set by Other Organizations
|
Organization |
Concentration, ppm |
|
ACGIH's TLV |
5000 (TWA) |
|
ACGIH's STEL |
30,000 |
|
OSHA's PEL |
5000 (TWA) |
|
NIOSH's REL |
10,000 (TWA) 30,000 (ceiling) |
|
NIOSH's IDLH |
50,000 |
|
Navy's 90-d limit |
5000a |
|
Navy's 24-h limit |
40,000 |
|
Navy's 1-h limit |
40,000 |
|
a According to the Navy (1988), long-term exposures at 5000-8000 ppm probably have no significant health effect. TLV = threshold limit value. TWA = time-weighted average. STEL = short-term exposure limit. PEL = permissible exposure limit. REL = recommended exposure limit. IDLH = immediately dangerous to life and health. |
|
TABLE 3-11 Spacecraft Maximum Allowable Concentrations
|
Duration |
ppm |
mg/m3 |
Target Toxicity |
|
1 h |
13,000 |
23,400 |
CNS depression, visual disturbance |
|
24 h |
13,000 |
23,400 |
CNS depression, visual disturbance |
|
7 da |
7000 |
12,600 |
Hyperventilation |
|
30 d |
7000 |
12,600 |
Hyperventilation |
|
180 d |
7000 |
12,600 |
Hyperventilation |
|
a There was no 7-d SMAC. Space-shuttle flight rules require mission termination at 2% or above and flight surgeon's evaluation at 1-2% (NASA, 1988). |
|||
Rationale for Acceptable Concentrations
To set the SMACs, guidelines developed by a subcommittee of the Committee of Toxicology are consulted (NRC, 1992). First, an acceptable concentration (AC) is estimated for each relevant toxic end point based on data gathered from an exposure of the appropriate duration.
The lowest AC is then selected as the SMAC for that exposure duration.
The finding by Zink and Reinhardt (1975) that an exposure to 1-2% CO2 for 5 h killed all the mice was not used in setting the SMACs. The reason is that no mortality was found in human subjects exposed to 2% or 2.7% CO2 for 30 d (Zharov et al., 1963; Sinclair et al., 1969). The mortality finding of Zink and Reinhardt on the mouse is obviously of no value in setting exposure limits for humans.
In humans, subchronic CO2 exposures are known to either decrease the plasma levels of calcium and phosphorus (Schaefer, 1961a, 1963a,b; Schaefer et al., 1963a,b, 1964a; Messier et al., 1976) or cause no change (Glatte et al., 1967a). One group of investigators even suggested that the decrease in urinary excretion of calcium and phosphorus during subchronic CO2 exposures could be an artifact (Davies et al., 1978a,b). Therefore, subchronically CO2 has no or only weak effects on calcium and phosphorus levels. Even if CO2 does affect calcium and phosphorus plasma levels, the effects are opposite to those seen in space missions (Schaefer et al., 1963b), so the long-term SMACs are not set to prevent the calcium and phosphorus changes.
Visual Impairments, Tremors, and CNS Depression
An exposure of human subjects to 6% CO2 for several hours produced visual disturbances and tremors (Schulte, 1964). Similarly, visual intensity discrimination was also found to be reduced in a 1-2 min exposure of human subjects to 6% CO2 (Gellhorn, 1936). Both visual impairments and tremors are toxic end points that should be prevented because they might interfere with the astronauts in dealing with a contingency.
Several studies showed that the NOAEL for visual impairments and tremors is about 3-4%. Both Storm and Giannetta (1974) and Glatte et al. (1967a) used a battery of tests, called Repetitive Psychometric Measures, to evaluate the effects of CO2 on vision, hand steadiness, and CNS functions. Storm and Giannetta exposed 12 volunteers to 4% CO2 for 2 w and they detected no visual or tremor problems in the volunteers. Glatte et al. found that a 5-d exposure of seven subjects to 3% CO2 had no effects on vision and hand steadiness.
There are other investigators who used methods other than the Repet-
itive Psychometric Measures to evaluate the effects of CO2. For instance, Radziszewski et al. (1988) did not find any visual problems or tremors in six human subjects exposed to 2.9% CO2 for 30 d. Similarly, Sinclair et al. (1971) found that 2.8% CO2 did not produce visual problems or tremors in four human subjects after an exposure of 1 h or 15-20 d. Menn et al. (1970) also failed to detect visual problems or tremors in eight human subjects exposed to 2.8% CO2 for 30 min.
The mechanisms of CO2-induced visual disturbances and tremors are unknown. It is possible that they are related to the effect of CO2 induced acidosis on the eye or the nervous system. Because there are indications that acidosis develops rapidly during CO2 exposures, the visual impairments and tremors are assumed not to increase in severity with exposure duration. In rats, an exposure to 11% CO2 resulted in acidosis as soon as 0.5 h into the exposure (the earliest blood pH determination in that study) and the blood pH gradually rose afterward (Barbour and Seevers, 1943). During a 1-h exposure of human subjects to 7% CO2, the arterial plasma pH decreased as early as 10 min into the exposure and stayed constant from min 10-60 (Brackett et al., 1965). Due to the rapid development of acidosis and the fact that there is no evidence that the visual problems and tremors are exposure-duration dependent, the same AC is derived for an exposure lasting 1 h, 24 h, 7 d, 30 d, or 180 d.
As concluded in the Toxicity Summary section, 5 % CO2 causes mild CNS depression in acute exposures. Based on a 5-d exposure of seven human subjects conducted by Glatte et al. (1967a) and a 2-w exposure of 12 human subjects done by Storm and Giannetta (1974), 3% CO2 is the NOAEL for the CNS effect of CO2. Because the same method, Repetitive Psychometric Measures, was used to evaluate CO2's effects on vision, hand steadiness, and CNS functions, the data of Glatte et al. and Storm and Giannetta are combined in deriving the ACs for the prevention of visual disturbances, tremors, and CNS impairment.
1-h, 24-h, 7-d, 30-d, and 180-d AC based on visual disturbances, tremors, and CNS depression
= NOAEL x 1/small n factor
= NOAEL x (square root of n)/10
= 3% x (square root of (7 + 12))/10
= 3% x 0.44
= 1.3%.
Headaches
Acute CO2 exposures might also produce other symptoms, such as headaches, dyspnea, and intercostal muscle pain, especially during exercise or exertion (Schulte, 1964; Glatte et al., 1967b; Menn et al., 1968, 1970). Because CO2-induced headaches are usually transient (Glatte et al., 1967a; Sinclair et al., 1969; Glatte et al., 1967b; Menn et al., 1968; Mines, 1981) and because Radziszewski et al. showed that an exposure to 2% CO2 rarely produced headaches in 30 d in six human subjects who exercised twice weekly for 10 min each at a 150-watt workload (Radziszewski et al., 1988; Guillerm and Radziszewski, 1979), 2% CO2 is chosen to be the ACs for headaches.
Dyspnea and Intercostal Pain
CO2 has been shown to produce dyspnea and intercostal pain during exercise or exertion (Schulte, 1964; Menn et al., 1970). Menn et al. showed that exposure to 2.8% CO2 did not produce dyspnea and intercostal pain in eight human subjects in 30 min. Similarly, Sinclair et al. (1971) did not detect dyspnea and intercostal pain in four human subjects who were exposed to 2.8% CO2 for 1 h or 15-20 d. These individuals performed a 45-min continuous steady state exercise at low, moderate, or heavy load once during the 1-h exposure and twice daily during the 15-20 d of CO2 exposure. Radziszewski and his colleagues reported no dyspnea or intercostal pain in six human subjects exposed to 2.9% CO2 and who exercised at a 150-watt workload for 10 min twice a week (Radziszewski et al., 1988). Because there is no evidence that the production of dyspnea and intercostal pain by hypercapnia is time-dependent, the data of Menn et al. and Sinclair et al. are combined in deriving the 1-h and 24-h ACs for dyspnea and intercostal pain. The 1-h and 24-h ACs are derived without any adjustment for the small number of human subjects used since a large safety margin is not needed in short-term contingencies, in which astronauts can tolerate a little bit of dyspnea or intercostal pain on exertion for a short time.
1-h and 24-h ACs for dyspnea and intercostal pain
= 30-min or 1-h NOAEL
= 2.8%.
In deriving the 7-d, 30-d, and 180-d ACs for dyspnea and intercostal pain, the data of Sinclair et al. (1971) and Radziszewski et al. (1988) are combined. Because the six subjects in the study of Radziszewski et al. did not develop dyspnea and intercostal pain when exposed to 2.9% CO2, the NOAEL of 2.8% obtained by Sinclair et al. should also be a NOAEL for these six subjects.
7-d, 30-d, and 180-d ACs for dyspnea and intercostal pain
= 15-20 d NOAEL x 1/small n factor
= 15-20 d NOAEL x (square root of n)/10
= 2.8% x (square root of (4 + 6))/10
= 2.8% x 0.32
= 0.9%.
Hyperventilation
The NRC subcommittee on SMACs advised NASA to consider the hyperventilatory effect in setting CO2's SMACs. Because the 1-h and 24-h SMACs are designed for contingencies, it is acceptable for the astronauts to tolerate some hyperventilation. Therefore, the 1-h and 24-h SMACs are not set based on CO2-induced hyperventilation.
The hyperventilatory effect is considered in establishing the 7-d, 30-d, and 180-d SMACs. Unlike other end points, the acceptable concentrations for a 7-d, 30-d, or 180-d exposure are not set at levels that will prevent any hyperventilation. Doing otherwise will be too conservative because CO2-induced hyperventilation is not harmful per se. Under most situations, CO2-induced hyperventilation is a protective response for the body when oxygen is displaced by CO2 at an abnormally high level. The situation is different, however, in spacecraft. Since oxygen is artificially maintained in spacecraft at a level sufficiently high for metabolic needs, CO2-induced hyperventilation is of lesser importance in spacecraft than on earth. In setting the acceptable CO2 concentrations, toxic effects secondary to CO2-induced hyperventilation should be taken into consideration. There are three potential secondary effects of CO2-induced hyperventilation:
- Discomfort associated with extreme hyperventilation.
- Impairment of the ability to exercise or take on heavy workload.
- Increased inhalation of airborne toxicants.
Consideration of the discomfort associated with extreme hyperventilation overlaps somewhat with that of the miscellaneous symptoms, such as dyspnea and intercostal muscle pain, discussed above. The levels set to prevent dyspnea and intercostal muscle pain will not be repeated here. Sinclair et al. in an Air force study stated that, ''with the exception of occasional mild headaches and awareness of increased ventilation during the first 24 hours'' of a 30-d exposure of four subjects to 2.8% CO2 or a 11-d exposure of another four subjects to 3.9% CO2, the humans subjects tolerated the hypercapnia "without apparent difficulty" (Sinclair et al., 1969). In a French study, there was no report of any symptoms or complaints in six human volunteers exposed for 30 d to 2% CO2, which increased the minute volume by 45% in d 8-30 (Radziszewski et al., 1988; Guillerm and Radziszewski; 1979). So the Air Force and French studies indicate that humans can tolerate 2-3.9% CO2; the low end of the range, 2%, appears to be a prudent choice as the NOAEL based on tolerability. The number of test subjects used in the Air Force and French studies are pooled, 4 + 4 + 6 = 14, in calculating acceptable concentrations based on tolerability.
7-d, 30-d, and 180-d ACs based on tolerability
= NOAEL x 1/small n factor
= NOAEL x (square root of n)/10
= 2% x (square root of 14)/10
= 2% x 0.37
= 0.7%.
Another factor to be considered is whether a subchronic exposure to CO2 will impair the ability of astronauts to exercise daily, which is very important in conditioning the muscle in microgravity. The French group showed that, in a 1-d exposure of five human subjects to 4.3% CO2, the subjects were unable to exercise (Radziszewski et al., 1988). In a 9-d exposure to 3.8% CO2, five subjects could exercise only at a limited capacity (Radziszewski et al., 1988). In contrast, in a 30-d exposure of six volunteers to 2% CO2, the volunteers had good ability to exercise (Radziszewski et al., 1988), and their oxygen consumption
rate, respiratory rate, and heart rate during the 10-min exercise at a 150-watt workload did not differ from performance of the same exercise in the normocapnic control period (Guillerm and Radziszewski, 1979). The only small change was that their minute volumes were raised about 17% when they were breathing 2% CO2 than air (Guillerm and Radziszewski, 1979). Similarly, the Air Force group showed that there was no reduction in the ability of four human subjects to perform 45-min of steady state exercise at low, moderate, or heavy workload during a 15-20 d exposure to 2.8% CO2 (Sinclair et al., 1971). The oxygen consumption rate and heart rate of the subjects during these exercises stayed the same in hypercapnic or normocapnic condition. The minute volumes during these exercises when they were breathing 2.8% CO2 were about 20% higher than that when breathing air. The Glatte et al. (1967a) Air Force study showed that six out of seven human volunteers easily tolerated daily 1-h moderate exercises at a 100-watt workload during a 5-d exposure to 3% CO2. Glatte et al. reported that the lone human volunteer who could not tolerate the exercises was of so small a statute that the exercise bike could not be lowered sufficiently to fit him. Therefore, the exercise data of this man can be ignored. The data from the French and Air Force studies (Glatte et al., 1967a; Sinclair et al., 1971; Radziszewski et al., 1988) showed that a subchronic exposure to 2-3% CO2 should have no adverse impact on the ability of astronauts to exercise or work, so the number of test subjects used in these studies are pooled, 6 + 4 + 6 = 16, in estimating the acceptable concentrations based on the ability to exercise.
7-d, 30-d, and 180-d ACs based on exercise ability
= NOAEL x 1/small n factor
= NOAEL x (square root of n)/10
= 2% x (square root of 16)/10
= 2% x 0.40
= 0.8%.
The final factor worth considering is the increased inhalation of airborne toxicants caused by CO2-induced hyperventilation. Based on the consideration of the subjective feeling of tolerability and the exercise ability, the acceptable concentration is about 0.7%. Radziszewski et al. (1988) exposed six volunteers to 0.5% and 1% CO2 and they reported
that 0.5% CO2 failed to produce significant increase in the minute volume, while 1% CO2 produced a 19% hyperventilation in the first day of exposure. In 15 human subjects exposed to 1% CO2 for 44 d, Pingree (1977) showed that the minute volume rose 30% in d 4, but it returned to the control value on the eighth day. Judging from these data of Radziszewski et al. and Pingree, the AC of 0.7% will increase inhalation of airborne toxicants by less than 30% in the first few days of CO2 exposure. A less than 30% increase is small compared with the potential respiratory ventilatory increase caused by moderate or heavy workload. According to the NRC Committee on Toxicology, a man inhales 7.5 L/min at rest and 20 L/min at light activity (NRC, 1992). NRC assumes that a man inhales 20 m3/d, which is approximately equivalent to the amount of air inhaled in 10 h of rest and 14 h of light activity per day (NRC, 1992). The minute volume at light activity is about 170% higher than that at rest. According to the data of Sinclair et al. (1971), the minute volumes at moderate and heavy workload are 590% and 940% higher than that at rest. Therefore, the less-than-30% increase in minute volume in the first few days of an exposure to 0.7% CO2 would be less than the exercise-induced minute volume increase if the astronauts were to engage in any moderate or heavy exercises. Especially because the less-than-30% increase in CO2-induced minute volume will disappear in a few days, in the long run there will not be any significant increase in the inhalation of airborne toxicants.
From the analysis with these three secondary effects of hyperventilation, 0.7% CO2 is selected to be the acceptable concentration for a 7-d, 30-d, or 180-d exposure based on hyperventilation.
Airway Resistance Increases
In humans, acute CO2 exposures to 5% or 7.5% CO2 in 2 h might result in increased airway resistance, and increased total lung resistance, without any lung compliance change, in exposure to 8% CO2 in 3-6 min (Nadel and Widdicombe, 1962; Tashkin and Simmons, 1972). Theoretically, increased total lung resistance could be caused by an increase in tissue resistance in the lung or airway resistance. Although an increased tissue resistance could be produced by CO2 because CO2 is known to raise the surface tension of the alveolar extract (Schaefer et
al., 1964a), the increased total lung resistance detected in the human subjects exposed to 8% CO2 for 3-6 min (Nadel and Widdicombe, 1962) was most probably not due to any raised tissue resistance. This is because tissue resistance normally contributes only 20% of the total lung resistance, with airway resistance contributing the remaining 80% in young adults (West, 1979). As a result, the CO2-induced increase in total lung resistance was probably due mostly to increased airway resistance. The finding by Nadel and Widdicombe (1962) that an acute exposure to 8% CO2 increased the total lung resistance without changing the lung compliance supports the notion that CO2 increases the total lung resistance by increasing the airway resistance. Therefore, a CO2 concentration that does not increase airway resistance should also prevent any increases in total lung resistance. According to Tashkin and Simmons (1972), a 2-h exposure of nine human subjects to 2.5% CO2 did not change the airway resistance. Glatte et al. (1967a) found that the forced expiratory volume in 1 s failed to change in seven volunteers during a 5-d exposure to 3% CO2. Therefore, 3% CO 2 is considered the NOAEL for increases in airway resistance. Because a large safety margin is not needed in short-term contingencies, no adjustment is made for using a NOAEL based on only seven human subjects in deriving the short-term ACs.
1-h and 24-h ACs based on airway resistance increases
=5-d NOAEL
=3%.
The increase in airway resistance has been postulated to be due to the local action of hypercapnia in the larynx (Cotes, 1979, pp. 149, 258, 363). Being a local reaction on the upper airway, it is not expected to increase in severity with time of exposure. Therefore, the 5-d NOAEL of 3% is used to derive the ACs for 7-d, 30-d, or 180-d exposures without any time adjustment.
7-d, 30-d, and 180-d ACs based on lung mechanics changes
= 5-d NOAEL x 1/small n factor
= 5-d NOAEL x (square root of n)/10
= 3% x (square root of 7)/10
= 3% x 0.26
= 0.8%.
Testes
Although an acute exposure of rats to CO2 at a concentration as low as 2.5% was found to cause the sloughing of mature spermatids and Sertoli cells in the testis at 4 or 8 h (but not at 1 or 2 h) (Vandemark et al., 1972), both the 1-h and 24-h SMACs are not set based on testicular toxicity. This is because the testicular morphology recovered completely in these rats in 36 h after the 8-h CO2 exposure (Vandemark et al., 1972). So the only potential functional deficit is a 1- or 2-d period of temporarily reduced fertility within a week of the acute CO2 exposure at 2.5%. Such a temporary reduced fertility is acceptable, considering that the 1-h and 24-h SMACs are aimed at emergency situations.
Although there is no solid proof that subchronic CO2 exposures cause testicular injuries, for prudence' sake, it is assumed that CO2 is toxic to the testis subchronically. This is because acute CO2 exposures could injure the testis, albeit only temporarily (Vandemark et al., 1972). If the CO2 exposure duration is extended from acute to subchronic, it is possible that the testicular injury will persist. Even though the study of Schaefer et al. (1971) was not adequately controlled, the absence of testicular damage in guinea pigs and rats exposed to 3 % CO2 for 42 d suggests that 3% could be treated as a NOAEL for subchronic exposures. The NRC Subcommittee on SMACs advised NASA not to apply the traditional interspecies factor of 10 with CO2's testicular toxicity because they felt that the toxicity is due to the acidosis and they did not think that the testes in humans will be more sensitive than the testes in rodents toward CO2-induced acidosis.
7-d, 30-d, and 180-d AC based on testicular toxicity
= 42-d NOAEL in the rodent studies
= 3%.
Establishment of SMACs
The ACs based on these toxic end points are summarized in Table 3-12. By comparing the ACs, the 1-h and 24-h SMACs are set at 1.3% (9.9 torr), while the 7-d, 30-d, and 180-d SMACs are all established at 0.7% (5.3 torr). For comparison purpose, it should be noted that it has been the Navy's position that "[h]uman exposures have been safely
conducted in atmospheres containing up to 5 torr CO2, for up to 90 days. Such exposures are therefore considered safe at this time" (Naval Submarine Medical Research Laboratory, 1982).
Finally, it should be pointed out that potential influences of microgravity-induced physiological changes on the acceptable concentrations are not needed in setting the SMACs for CO2. Even though microgravity-induced hypercalciuria is a risk factor for kidney stone formation (Pak et al., 1989), CO2's SMACs need not be adjusted for hypercalciuria because CO2 exposures are not known to increase urinary calcium excretion in human subjects (in fact CO2 exposures decreased urinary calcium excretion in human subjects) (Messier et al., 1976; Schaefer et al., 1963b).
TABLE 3-12 End Points and Acceptable Concentrations
|
|
Uncertainty factors |
Acceptable Concentrations, % |
|||||||
|
End Point |
Exposure Data |
Species and Reference |
Species |
Small n |
1 h |
24 h |
7 d |
30 d |
180 d |
|
Visual impairment, tremor, CNS depression |
NOAEL at 3%, 24 h/d, 5 d or 2 w |
Human (n = 7, 12) (Glatte et al., 1967; Storm and Giannetta, 1974) |
— |
10/(sq. rt. of 19) |
1.3 |
1.3 |
1.3 |
1.3 |
1.3 |
|
Headache |
NOAEL at 2%, 24 h/d, 30 d |
Human (n = 6) (Radziszewski et al., 1988; Guillerm and Radziszewski, 1979) |
— |
— |
2 |
2 |
2 |
2 |
2 |
|
Dyspnea, intercostal pain |
NOAEL at 2.8%, 0.5 or 1 h |
Human (n = 8, 4) (Menn et al., 1970; Sinclair et al., 1971) |
— |
— |
2.8 |
2.8 |
— |
— |
— |
|
|
NOAEL at 2.8%, 15 or 20 d |
Human (n = 4, 6) (Sinclair et al., -1971; Radziszewski et al., 1988) |
— |
10/(sq. rt. of 10) |
— |
— |
0.9 |
0.9 |
0.9 |
|
Airway resistance increases |
NOAEL at 3%, 24 h/d, 5 d |
Human (n = 7) (Glatte et al., 1967) |
— |
— |
3 |
3 |
— |
— |
— |
|
|
NOAEL at 3%, 24 h/d, 5 d |
Human (n = 7) (Glatte et al., 1967) |
— |
10/(sq. rt. of 10) |
— |
— |
0.8 |
0.8 |
0.8 |
|
Hyperventilation |
|
||||||||
|
Tolerability |
NOAEL at 2%, 24 h/d, 11 or 30 d |
Human (n = 4, 4, 6) (Sinclair et al., 1969; Radziszewski et al., 1988 Guillerm and Radziszewski, 1979) |
— |
10/(sq. rt. of 14) |
— |
— |
0.7 |
0.7 |
0.7 |
|
Exercise impairment |
NOAEL at 2%, 24 h/d, 5, 15, or 30 d |
Human (n = 6, 4, 6) (Glatte et al., 1967; Sinclair et al., 1971; Radziszewski et al., 1988) |
— |
10/(sq. rt. of 16) |
— |
— |
0.8 |
0.8 |
0.8 |
|
Testicular injury |
NOAEL at 3%, 24 h/d, 42 d |
Rat and guinea pig (Schaefer et al., 1971) |
1 |
— |
— |
— |
3 |
3 |
3 |
|
SMAC |
|
1.3 |
1.3 |
0.7 |
0.7 |
0.7 |
|||
References
Aero Medical Association, Committee on Aviation Toxicology. 1953. Pp. 6-9, 31-39, 52-55, 74-79, and 110-115 in Aviation Toxicology: An Introduction to the Subject and a Handbook of Data. New York: The Blakiston Co.
Altschule, M.D., and W.M. Sulzbach. 1947. Tolerance of the human heart to acidosis: Reversible changes in RS-T interval during severe acidosis caused by administration of carbon dioxide. Am. Heart J. 33:458-463.
Baggott, J. 1982. Gas transport and pH regulation. Pp. 1098-1101, 1114, 1120-1123 in Textbook of Biochemistry with Clinical Correlations, T.M. Devlin, ed. New York: John Wiley & Sons.
Barbour, J.H., and M.H. Seevers. 1943. A comparison of the acute and chronic toxicity of carbon dioxide with especial reference to its narcotic action. J. Pharmacol. Exp. Ther. 78:11-21.
Brackett, N.C., Jr., J.J. Cohen, and W.B. Schwartz. 1965. Carbon dioxide titration curve of normal man: Effect of increasing degrees of acute hypercapnia on acid-base equilibrium. New Engl. J. Med. 272:6-12.
Breslau, N.A., L. Brinkley, K.D. Hill, and C.Y. Pak. 1988. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J. Clin. Endocrinol. Metab. 66:140-146.
Brown, E.W. 1930a. The physiological effects of high concentrations of carbon dioxide. U.S. Naval Med. Bull. 28:721-934.
Brown, E.W. 1930b. The value of high oxygen in preventing the physiological effects of noxious concentrations of carbon dioxide. U.S. Naval Med. Bull. 32:523-553.
Brown, E.B., G.S. Campbell, M.N. Johnson, A. Hemingway, and M.B. Visscher. 1948. Changes in response to inhalation of CO2 before and after 24 hours of hyperventilation in man. J. Appl. Physiol. 1:333-338.
Bungo, M.W. 1989. The cardiopulmonary system. Pp. 197-199 in Space Physiology and Medicine, A.E. Nicogossian, ed. Philadelphia: Lea & Febiger
Campbell, J.M.H., C.G. Douglas, J.S. Haldane, and F.G. Hodgson. 1913. The response of the respiratory centre to carbonic acid, oxygen, and hydrogen ion concentration. J. Physiol. 46:301-318.
Chapin, J.L., A.B. Otis, and H. Rahn. 1955. Changes in the Sensitiv-
ity of the Respiratory Center in Man After Prolonged Exposure to 3% CO2. Pp. 250-254 in WADC Tech. Rep. No. 55-357, Wright-Patterson Air Force Base, Dayton, Ohio.
Clamann, H.G. 1959. Some metabolic problems of space flight. Fed. Proc. 18:1249-1255.
Clark, J.M., R.D. Sinclair, and B.E. Welch. 1971. Rate of acclimatization to chronic hypercapnia in man . Pp 399-408 in Underwater Physiology, C.J. Lambertsen, ed. New York: Academic Press.
Coe, F.L., and M.J. Favus. 1987. Nephrolithiasis. Pp. 1211-1215 in Harrison's Principles of Internal Medicine, E. Braunwald, K.J. Isselbacher, R.G. Petersdorf, J.D. Wilson, J.B. Martin, and A.S. Fauci, eds. New York: McGraw-Hill.
Coleman, W.E., L.D. Scheel, R.E. Kupel, and R.L. Larkin. 1968. The identification of toxic compounds in the pyrolysis products of polytetrafluoroethylene (PTFE). Am. Ind. Hyg. Assoc. J. 29:33-40.
Consolazio, W.B., M.B. Fisher, N. Pace, L.J. Pecora, and A.R. Behnke. 1947. Effects on man of high concentrations of carbon dioxide in relation to various oxygen pressures during exposures as long as 72 hours. Am. J. Physiol. 151:479-503.
Cotes, J.E. 1979. Lung function. In Assessment and Application in Medicine. Oxford, U.K.: Blackwell Scientific Publications.
Dalgaard, J.B., F. Dencker, B. Fallentin, P. Hansen, B. Kaempe, J. Steensberg, and P. Wilhardt. 1972. Fatal poisoning and other health hazards connected with industrial fishing. Br. J. Ind. Med. 29:307-316.
Davies, D.M., J.E.W. Morris, D.J. Smith, and R.J. Pethybridge. 1978a. Mineral metabolism and circadian patterns of renal excretion in man in a closed environment study involving hypercapnia and abnormal physical activity. INM Rep. No. 25/78. Institute of Naval Medicine, Gosport, Alverstoke, U.K.
Davies, D.M., W.M. Edmondstone, A. Bishop, and J.E.W. Morris. 1978b. Urinary mineral excretion in men on a long submarine patrol and effects upon it of the oral administration of Vitamin D. INM Rep. No. 26/78. Institute of Naval Medicine, Gosport, Alverstoke, U.K.
Davies, D.M. and J.E.W. Morris. 1979. Carbon dioxide and vitamin D effects on calcium metabolism in nuclear submariners: A review. Undersea Biomed. Res. 6(Suppl.):S71-S80.
DeBellis, J., H. Constantine, and M. Stein. 1968. Effect of acute hypercapnia on gastrointestinal blood loss. Fed. Proc. 28:509.
Deitrick, J.E., G.D. Whedon, and E. Shorr. 1948. Effects of immobilization upon various metabolic and physiologic functions of normal men . Am. J. Med. 4:3-36.
Diamondstone, T.I. 1982. Amino acid metabolism I. P. 545 in Textbook of Biochemistry with Clinical Correlations, T.M. Devlin, ed. New York: John Wiley & Sons.
Donaldson, C.L., S.B. Hulley, J.M. Vogel, R.S. Hattner, J.H. Bayes, and D.E. McMillan. 1970. Effect of prolonged bed rest on bone mineral. Metabolism 19:1071-1084.
Douglas, W.H.J., K.E. Schaefer, A.A. Messier, and S.M. Pasquale. 1979. Proliferation of pneumocyte II cells in prolonged exposure to 1% CO2. Undersea Biomed. Res. (Submarine Suppl.):S135-S142.
Dripps, R.D., and J.H. Comroe, Jr. 1947. The respiratory and circulatory response of normal man to inhalation of 7.6 and 10.4 percent CO2 in a comparison of the maximal ventilation produced by severe muscular exercise, inhalation of CO2 and maximal voluntary hyperventilation. Am. J. Physiol. 149:43-51.
Dunn, M.S., A.T. Shennan, and F. Possmayer. 1990. Single-versus multiple-dose surfactant replacement therapy in neonates of 30 to 36 weeks' gestation with respiratory distress syndrome. Pediatrics 86: 564-571.
Eldridge, F., and J.M. Davis. 1959. Effect of mechanical factors on respiratory work and ventilatory responses to CO2. J. Appl. Physiol. 14:721-726.
Fisch, C. 1988. Electrocardiography and vectorcardiography. Pp. 180-219 in Heart Disease: A Textbook of Cardiovascular Medicine, E. Braunwald, ed. Philadelphia: W.B. Saunders.
Friedlander, W.J., and T. Hill. 1954. EEG changes during administration of carbon dioxide. Dis. Nerv. Syst. 15:71-75.
Gellhorn, E. 1936. Effect of O2 lack, variations in CO2 content of inspired air, and hyperpnea on visual intensity discrimination. Am. J. Physiol. 115:679-684.
Gellhorn, E., and I. Spiesman. 1934. Influence of variations of O2 and CO2 tension in inspired air upon hearing. Proc. Soc. Exp. Biol. Med. 32:46-47.
Gellhorn, E., and I. Spiesman. 1935. Influence of hyperpnea and of
variations of O2- and CO2-tension in inspired air upon hearing. Am. J. Physiol. 112:519-528.
Glatte, H.A., G.J. Motsay, and B.E. Welch. 1967a. Carbon Dioxide Tolerance Studies. Rep. No. SAM-TR-67-77. U.S. Air Force School of Aerospace Medicine, Brooks Air Force Base, San Antonio, Tex.
Glatte, H.A., B.O. Hartman, and B.E. Welch. 1967b. Nonpathologic hypercapnia in man. Pp. 110-129 in Lectures in Aerospace Medicine. 6th Series. Rep. No. SAM-TR-68-116. U.S. Air Force School of Aerospace Medicine, Brooks Air Force Base, San Antonio, Tex.
Goldberg, H., L. Grass, R. Vogl, A. Rapoport, and D.G. Oreopoulos. 1989. Urine citrate and renal stone disease. Can. Med. Assoc. J. 141:217-221.
Goldsmith, A.E., G.F. Ryan, and A.B. Joseph. 1980. Metabolic carcinogenesis: Induction of murine lymphoma by CO2-treatment in vivo and in vitro. Jpn. J. Med. Sci. Biol. 33:7-18.
Gortner, L., F. Pohlandt, P. Bartmann, and B. Disse. 1990. Treatment of respiratory distress syndrome in very small premature infants with bovine surfactant. Monatsschr. Kinderheilkd. 138:8-12.
Gray, S.P., J.E.W. Morris, and C.J. Brooks. 1973. Renal handling of calcium, magnesium, inorganic phosphate and hydrogen ions during prolonged exposure to elevated carbon dioxide concentrations . Clin. Sci. Mol. Med. 45:751-764.
Grote, W. 1965. [Disturbances of embryonic development at elevated CO2 and O2 partial pressure and at reduced atmospheric pressure.] Z. Morphol. Anthropol. 56:165-194.
Gormsen, H., N. Jeppesen, and A. Lund. 1984. The causes of death in fire victims. Forensic Sci. Int. 24:107-111.
Grollman, A. 1930. Physiological variations in the cardiac output of man. IX. The effect of breathing carbon dioxide, and of voluntary forced ventilation on the cardiac output of man. Am. J. Physiol. 94: 287-299.
Gude, J.K., and K.E. Schaefer. 1969. The Effects on Respiratory Dead Space of Prolonged Exposure to a Submarine Environment. Rep. No. 587. Submarine Medical Research Laboratory, Naval Submarine Medical Center, Groton, Connecticut.
Guillerm, R., and E. Radziszewski. 1979. Effects on man of 30-day exposure to a PICO2 of 14 torr (2%): Application to exposure limits.
Undersea Biomed. Res. (Submarine Suppl.):S91-S114.
Haldane, J., and J.L. Smith. 1892. The physiological effects of air vitiated by respiration. J. Pathol. Bacteriol. 1:168-186.
Haring, O.M. 1960. Cardiac malformations in rats induced by exposure of the mother to carbon dioxide during pregnancy. Circ. Res. 8:1218-1227.
Hartzell, G., and W.G. Switzer. 1985. On the toxicities of atmospheres containing both carbon monoxide and carbon dioxide. J. Fire Sci. 3:307-309.
Hofbauer, J., K. Hobarth, and O. Zechner. 1990. The significance of citrate excretion and calcium/citrate quotients in urine in patients with calcium calculi. Z. Urol. Nephrol. 83:597-602.
Hopson, G.D., J.W. Littles, and W.C. Patterson. 1974. MSFC Skylab Thermal and Environmental Control System Mission Evaluation. Rep. No. NASA TM X-64822. National Aeronautics and Space Administration, Washington, D.C.
Huntoon, C.L., P.C. Johnson, and N.M. Cintron. 1989. Hematology, immunology, endocrinology, and biochemistry. Pp. 222-239 in Space Physiology and Medicine, A.E. Nicogossian, ed. Philadelphia: Lea & Febiger.
Isom, G.E., and R.M. Elshowihy. 1982. Naloxone-induced enhancement of carbon dioxide stimulated respiration. Life Sci. 31:113-118.
Jackson, J.K., J.R. Wamsley, M.S. Bonura, and J.S. Seeman. 1972. Pp. 34, 48-50 in Program Operational Summary: Operational 90 Day Manned Test of a Regenerative Life Support. Rep. No. NASA CR-1835. National Aeronautics and Space Administration, Washington, D.C.
Johnston, R.F. 1959. The syndrome of carbon dioxide intoxication: Its etiology, diagnosis, and treatment. Univ. Mich. Med. Bull. 25: 280-292.
Kety, S.S., and C.F. Schmidt. 1948. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J. Clin. Invest. 27:484-492.
Kryger, M.H. 1981. Respiratory failure 2: Carbon dioxide. Pp. 205-219 in Pathophysiology of Respiration, M.H. Kryger, ed. New York: John Wiley & Sons.
Lai, Y.-L., Y. Tsuya, and J. Hildebrandt. 1978. Ventilatory responses to acute CO2 exposure in the rat. J. Appl. Physiol. 45:611-618.
Lambertsen, C.J. 1971. Therapeutic gases: Oxygen, carbon dioxide, and helium. Ch. 55 in Drill's Pharmacology in Medicine, J.R. DiPalma, ed. New York: McGraw-Hill.
Leach, C.S., and P.C. Rambaut. 1977. Biochemical responses of the Skylab crewmen: An overview. In Biomedical Results from Skylab, R.S. Johnston and L.F. Dietlein, eds. National Aeronautics and Space Administration, Washington, D.C.
LeBaron, F.N. 1982. Lipid metabolism I. P. 473 in Textbook of Biochemistry with Clinical Correlations, T.M. Devlin, ed. New York: John Wiley & Sons.
Levin, B.C., M. Paabo, J.L. Gurman, S.E. Harris, and E. Braun. 1987. Toxicological interactions between carbon monoxide and carbon dioxide. Toxicology 47:135-164.
Levin, B.C., M. Paabo, L. Highbarger, and N. Eller. 1989. Synergistic Effects of Nitrogen Dioxide and Carbon Dioxide Following Acute Inhalation Exposures in Rats. Society of the Plastics Industry, Inc. Available from the National Technical Information Services, Springfield, Va., Doc. No. PB89-214779.
Luft, U.C., S. Finkelstein, and J.C. Elliott. 1974. Respiratory gas exchange, acid-base balance, and electrolytes during and after maximal work breathing 15 mm Hg PICO2. Pp. 282-293 in Topics in Environmental Physiology and Medicine: Carbon Dioxide and Metabolic Regulations, G. Nahas and K.E. Schaefer, eds. New York: Springer-Verlag.
MacDonald, F.M., and E. Simonson. 1953. Human electrocardiogram during and after inhalation of thirty percent carbon dioxide. J. Appl. Physiol. 6:304-310.
McArdle, L. 1959. Electrocardiographic studies during the inhalation of 30 per cent carbon dioxide in man. Br. J. Anaesth. 31:142-151.
McCarthy, D.S. 1981. Airflow obstruction. P. 16 in Pathophysiology of Respiration, M.H. Kryger, ed. New York: John Wiley & Sons.
Massie, B.M., and M. Sokolow. 1990. Heart and great vessels. Pp. 267-271 in Current Medical Diagnosis and Treatment in 1990, S.A. Schroeder, M.A. Krupp, L.M. Tierney, Jr., and S.J. McPhee, eds. Norwalk, Conn.: Appleton and Lange.
Meessen, H. 1948. Chronic carbon dioxide poisoning experimental studies. Arch. Pathol. 45:36-40.
Menn, S.J., R.D. Sinclair, and B.E. Welch. 1968. Response of Normal Man to Graded Exercise in Progressive Elevations of CO2. Rep.
No. SAM-TR-68-116. Aerospace Medical Division, USAF School of Aerospace Medicine, Brooks Air Force Base, San Antonio, Tex.
Menn, S.J., R.D. Sinclair, and B.E. Welch. 1970. Effect of inspired pCO2 up to 30 mm Hg on response of normal man to exercise. J. Appl. Physiol. 28:663-61.
Messier, A.A., and K.E. Schaefer. 1971. The effect of chronic hypercapnia on oxygen affinity and 2,3-diphosphoglycerate. Resp. Physiol. 12:291-296.
Messier, A.A., E. Heyder, and K.E. Schaefer. 1971. Effect of 90-Day Exposure to 1% CO2 on Acid-Base Status of Blood. Rep. No. 655. U.S. Naval Submarine Medical Center, Submarine Base, Groton, Conn.
Messier, A.A., E. Heyder, W.R. Braithwaite, C. McCluggage, A. Peck, and K.E. Schaefer. 1976. Calcium, magnesium, and phosphorus metabolism, and parathyroid-calcitonin function during prolonged exposure to elevated CO2 concentrations on submarines. Undersea Biomed. Res. 6(Suppl.):S57-S70.
Mines, A.H. 1981. Pp. 91-99 in Respiratory Physiology. New York: Raven Press.
Mitsuda, H., S. Ueno, H. Mizuno, H. Fujikawa, K. Konaka, and C. Fukada. 1982. Effects of various molecular oxygen levels in mixed gas on acute respiratory insufficiency induced with carbon dioxide inhalation in rats. Kankyo Kagaku Sogo Kenkyusho Nenpo 2:35-46.
Morey, P.R. and D.E. Shattuck. 1989. Role of ventilation in the causation of building-associated illness. Occup. Med. State of the Art Rev. 4:625-642.
Morey-Holton, E.R., H.K. Schnoes, H.F. DeLuca, M.E. Phelps, R.F. Klein, R.H. Nissenson, and C.D. Arnaud. 1988. Vitamin D metabolites and bioactive parathyroid hormone levels during Spacelab 2. Aviat. Space Environ. Med. 59:1038-1041.
Mukherjee, D.P., and S.P. Singh. 1967. Effect of increased carbon dioxide in inspired air on the morphology of spermatozoa and fertility of mice. J. Reprod. Fert. 13:165-167.
Nadel, J.A. and J.G. Widdicombe. 1962. Effect of changes in blood as tensions and carotid sinus pressure on tracheal volume and total lung resistance to airflow. J. Physiol. 163:13-33.
NASA. 1984. PPCO2 history for STS 51-D. P. 4.3.7-7 in Shuttle Operations Data Book. Doc. No. JSC 08934. NASA, Johnson Space Center, Houston, Tex.
NASA. 1988. PPCO2 Constraint. Flight Rule No. 13-8. NASA, Johnson Space Center, Houston, Tex.
Naval Submarine Medical Research Laboratory. 1982. Position Paper: The Toxic Effects of Chronic Exposures to Low Levels of Carbon Dioxide. Rep. No. 973. Naval Submarine Medical Center, Naval Submarine Base, Groton, Conn.
Niemoeller, H., and K.E. Schaefer. 1962. Development of hyaline membranes and atelectasis in experimental chronic respiratory acidosis. Proc. Soc. Exp. Biol. Med. 110:804-808.
Notter, R.H., and J.N. Finkelstein. 1984. Pulmonary surfactant: An interdisciplinary approach. J. Appl. Physiol. 57:1613-1624.
NRC. 1992. Guidelines for Developing Spacecraft Maximum Allowable Concentrations for Space Station Contaminants. Washington, D.C.: National Academy Press.
Okajima, M., and E. Simonson. 1962. Effect of breathing six percent carbon dioxide on ECG changes in young and older healthy men. J. Gerontol. 17:286-288.
Olson, M.S. 1982. Bioenergetics and oxidative metabolism. P. 279 in Textbook of Biochemistry with Clinical Correlations, T.M. Devlin, ed. New York: John Wiley & Sons.
Pak, C.Y.C., C. Skurla, and J.A. Harvey. 1985. Graphic display of urinary risk factors for renal stone formation. J. Urol. 134:867-870.
Pak, C.Y.C., K. Hill, N.M. Cintron, and C. Huntoon. 1989. Assessing applicants to the NASA flight program for their renal stone-forming potential. Aviat. Space Environ. Med. 60:157-161.
Patterson, J.L., H. Heyman, L.L. Battery, and R.W. Ferguson. 1955 Threshold of response of the cerebral vessels of man to increases in blood carbon dioxide. J. Clin. Invest. 34:1857-1864.
Peck, A.S. 1971. The Time Course of Acid-Base Balance While on FBM Patrol. Rep. No. 675. Naval Submarine Medical Center, Naval Submarine Base, Groton, Conn.
Pepelko, W.E. 1970. Effects of hypoxia and hypercapnia, singly and combined, on growing rats. J. Appl. Physiol. 28:646-651.
Phillipson, E.A., G. Bowes, E.R. Townsend, J. Duffin, and J.D. Cooper. 1981. Carotid chemoreceptors in ventilatory responses to changes in venous CO2 load. J. Appl. Physiol. 51:1398-1403.
Pingree, B.J.W. 1977. Acid-base and respiratory changes after prolonged exposure to 1% carbon dioxide. Clin. Sci. Mol. Med. 52:67-74.
Radziszewski, E., R. Guillerm, R. Badre, and C. Abran. 1976. Cinetique de la compensation de l'acidose respiratoire induite par l'hypercapnie chronique experimentale chez l'homme. Bull. Eur. Physiopathol . Resp. 12:-100.
Radziszewski, E., L. Giacomoni, and R. Guillerm. 1988. Effets physiologiques chez l'homme du confinement de longue duree en atmosphere enrichie en dioxyde de carbone. Pp. 19-23 in Proceedings of the Colloquium on Space and Sea. European Space Agency, Brussels, Belgium.
Redding, R.A., T. Arai, W.H.J. Douglas, H. Tsurutani, and J. Oven. 1975. Early changes in lungs of rats exposed to 70% O2. J. Appl. Physiol. 38:136-142.
Riley, R.L., and B. Barnea-Bromberger. 1976. Acid-Base Changes in Blood of Brewery Workers Exposed to CO2. An unpublished report cited by NIOSH in Criteria for a Recommended Standard. Occupational Exposure to Carbon Dioxide, Rep. No. NIOSH-76-194. National Institute for Occupational Safety and Health, Cincinnati, Ohio.
Rodkey, F.L., and H.A. Collison. 1979. Effects of oxygen and carbon dioxide on carbon monoxide toxicity. J. Combust. Toxicol. 6: 208-212.
Sax, I. 1984. P. 640 in Dangerous Properties of Industrial Materials. New York: Van Nostrand Reinhold.
Schaefer, K.E. 1949a. [Influence exerted on the psyche and the excitatory processes in the peripheral nervous system under long-term effects of 3% CO2.] Pfluegers Arch. Gesamte. Physiol. Menschen Tiere 251:716-725.
Schaefer, K.E. 1949b. [Respiratory and acid-base balance during prolonged exposure to a 3% CO2 atmosphere.] Pfluefers Arch. Gesamte. Physiol. Menschen. Tiere. 251:689-715.
Schaefer, K.E. 1958. Effects of Carbon Dioxide as Related to Submarines and Diving Physiology. Memo. Rep. No. 58-11. Naval Medical Research Laboratory, New London, Conn.
Schaefer, K.E. 1959. Experiences with submarine atmospheres. J. Aviat. Med. 30:350-359.
Schaefer, K.E. 1961a. Blood pH and pCO2 homeostasis in chronic respiratory acidosis related to the use of amine and other buffers. Ann. N.Y. Acad. Sci. 92:401-413.
Schaefer, K.E. 1961b. A concept of triple tolerance limits based on chronic carbon dioxide toxicity studies. Aerospace Med. 32:197-204.
Schaefer, K.E. 1963a. The effects of CO2 and electrolyte shifts on the central nervous system. Pp. 101-123 in Selective Vulnerability of the Brain in Hypoxemia, J.P. Schade, ed. Oxford, U.K.: Blackwell Scientific Publications.
Schaefer, K.E. 1963b. Respiratory adaptation to chronic hypercapnia. Ann. N.Y. Acad. Sci. 109:772-782.
Schaefer, K.E. 1963c. Acclimatization to low concentration of carbon dioxide. Ind. Med. Surg. 32:11-13.
Schaefer, K.E. 1979. Physiological stresses related to hypercapnia during patrols on submarines. Undersea Biomed. Res. 6(Suppl.): S15-S47.
Schaefer, K.E., B.J. Hastings, C.R. Carey, and G. Nichols, Jr. 1963a. Respiratory acclimatization to carbon dioxide. J. Appl. Physiol. 18: 1071-1078.
Schaefer, K.E., G. Nichols, Jr., and C.R. Carey. 1963b. Calcium phosphorus metabolism in man during acclimatization to carbon dioxide. J. Appl. Physiol. 18:1079-1084.
Schaefer, K.E., M.E. Avery, and K. Bensch. 1964a. Time course of changes in surface tension and morphology of alveolar epithelial cells in CO2-induced hyaline membrane disease. J. Clin. Invest. 43:2080-2093.
Schaefer, K.E., G. Nichols, Jr., and C.R. Carey, 1964b. Acid-base balance and blood and urine electrolytes of man during acclimatization to CO2. J. Appl. Physiol. 19:48-58.
Schaefer, K.E., N. McCabe and J. Withers. 1968. Stress response in chronic hypercapnia. Am. J. Physiol. 214:543-548.
Schaefer, K.E., H. Niemoeller, A. Messier, E. Heyder, and J. Spencer. 1971. Chronic CO2 Toxicity: Species Difference in Physiological and Histopathological Effects. Rep. No. 656. Naval Submarine Medical Research Laboratory, Groton, Conn.
Schaefer, K.E., S.M. Pasquale, A.A. Messier, and H. Niemoeller. 1979a. CO2-induced kidney calcification. Undersea Biomed. Res. (Submarine Suppl.):S143-S153.
Schaefer, K.E., W.H.J. Douglas, A.A. Messier, M.L. Shea, and P.A.
Gohman. 1979b. Effect of prolonged exposure to 0.5% CO2 on kidney calcification and ultrastructure of lungs. Undersea Biomed. Res. (Submarine Suppl.):S155-S161.
Schaefer, K.E., S. Pasquale, A.A. Messier, and M. Shea. 1980. Phasic changes in bone CO2 fractions, calcium, and phosphorus during chronic hypercapnia. J. Appl. Physiol. 48:802-811.
Schwille, P.O., and U. Herrmann. 1992. Environmental factors in the pathophysiology of recurrent idiopathic calcium urolithiasis (RCU), with emphasis on nutrition. Urol. Res. 20:72-83.
Schneider, E.C., and D. Truesdale. 1922. The effects on the circulation and respiration of an increase in the carbon dioxide content of the blood in man. Am. J. Physiol. 63:155-175.
Schulte, J.H. 1964. Sealed environments in relation to health and disease. Arch. Environ. Health 8:438-452.
Sechzer, P.H., L.D. Egbert, H.W. Linde, D.Y. Cooper, R.D. Dripps, and H.L. Price. 1960. Effect of CO2 inhalation on arterial pressure, ECG and plasma catecholamines and 17-OH corticosteroids in normal man. J. Appl. Physiol. 15:454-458.
Shih, K.C. 1987. P. C-34 in SL-2 Subsystem and Verification Flight Instrumentation Data. Doc. No. TM-SEAD-87004. MacDonald Douglas Huntsville, Huntsville, Ala.
Sinclair, R.D., J.M. Clark, and B.E. Welch. 1969. Carbon dioxide tolerance levels for space cabins. Proceedings of the Fifth Annual Conference on Atmospheric Contamination in Confined Spaces, Sept. 16-18, Wright-Patterson Air Force Base, Dayton, Ohio.
Sinclair, R.D., J.M. Clark, and B.E. Welch. 1971. Comparison of physiological responses of normal man to exercise in air and in acute and chronic hypercapnia. Pp. 409-417 in Underwater Physiology, C.J. Lambertsen, ed. New York: Academic Press.
Skov, P., O. Valbjorn, and the Danish Climate Study Group (DISG) 1987. The ''sick'' building syndrome in the office environment: The Danish town hall study. Environ. Int. 13:339-349.
Stein, S.N., H.E. Lee, J.H. Annegers, S.A. Kaplan, and D.G. McQuarrie. 1959. The effects of prolonged inhalation of hypernormal amounts of carbon dioxide. I. Physiological effects of 3 percent CO2 for 93 days upon monkeys. Pp. 527-536 in Research Report NM 24 01 00.01.01, Vol. 17. Naval Medical Research Institute, Bethesda, Md.
Strachova, Z., and F. Plum. 1973. Reproducibility of the rebreathing carbon dioxide response test using an improved method. Am. Rev. Resp. Dis. 107:864-869.
Storm, W.F., and C.L. Giannetta. 1974. Effects of hypercapnia and bedrest on psychomotor performance. Aerosp. Med. 45:431-433.
Sullivan, T.Y., and P.-L. Yu. 1983. Airway anesthesia effects on hypercapnic breathing pattern in humans. J. Appl. Physiol. 55:368-376.
Tansey, W.A., J.M. Wilson, and K.E. Schaefer. 1979. Analysis of health data from 10 years of Polaris submarine patrols. Undersea Biomed. Res. 6(Suppl.):S217-S246.
Tashkin, D.P., and D.H. Simmons. 1972. Effect of carbon dioxide breathing on specific airway conductance in normal and asthmatic subjects. Am. Rev. Resp. Dis. 106:729-739.
Tenney, S.M. 1954. Ventilatory response to carbon dioxide in pulmonary emphysema. J. Appl. Physiol. 6:477-484.
Terrill, J.B., R.R. Montgomery, and C.F. Reinhardt. 1978. Toxic gases from fires. Science 200:1343-1347.
Thomas, J.A. 1991. Toxic responses of the reproductive system. Pp. 484-520 in Cassarett and Doull's Toxicology: The Basic Science of Poisons, M.O. Amdur, J. Doull, and C.D. Klaassen, eds. New York: Pergammon Press.
Thun, M.J., and S. Schober. 1991. Urolithiasis in Tennessee: An occupational window into a regional problem. Am. J. Public Health 81:587-591.
Trinchieri, A., A. Mandressi, P. Luongo, G. Longo, and E. Pisani. 1991. The influence of diet on urinary risk factors for stones in healthy subjects and idiopathic renal calcium stone formers. Br. J. Urol. 67:230-236.
U.S. Navy. 1988. Nuclear Powered Submarine Atmosphere Control Manual. S9510-AB-ATM-010/(U). Department of the Navy, Washington, D.C.
van Aswegen, C.H., P. Hurter, C.A. van der Merwe, and D.J. du Plessis. 1989. The relationship between total urinary testosterone and renal calculi. Urol. Res. 17:181-183.
Vandemark, N.L., B.D. Schanbacher, and W.R. Gomes. 1972. Alterations in testes of rats exposed to elevated atmospheric carbon dioxide. J. Reprod. Fertil. 28:457-459.
Vogel, J.M. 1975. Effect of spaceflight on bone mineral. Acta Astronautica 2:129-140.
Wamsley, J.R., E.W. Youngling, and W.F. Behm. 1969. High fidelity simulations in the evaluation of environmental stress: Acute CO2 exposure. Aerospace Med. 40:1336-1340.
Wang, T.C. 1975. A study of bioeffluents in a college classroom. ASHRAE Transact. 81:32-33.
Wasserstein, A.G., P.D. Stolley, K.A. Soper, S. Goldfarb, G. Maislin, and Z. Agus. 1987. Case-control study of risk factors for idiopathic calcium nephrolithiasis. Miner. Electrolyte Metab. 13:85-95.
Weitzman, D.O., and J.A.S. Kinney. 1969. Effect on Vision of Repeated Exposure to Carbon Dioxide. Rep. No. 566. Naval Submarine Medical Center, Naval Submarine Base, Groton, Conn.
West, J.B. 1979. Pp. 23, 74 in Respiratory Physiology: The Essentials. Baltimore: Williams & Wilkins.
Whedon, G.D., L. Lutwak, P.C. Rambaut, M.W. Whittle, M.C. Smith, J. Reid, C. Leach, C.R. Stadler, and D.D. Sanford. 1977. Mineral and nitrogen metabolic studies, Experiment M071. Pp. 164-174 in Biomedical Results from Skylab, R.S. Johnston and L.F. Dietlein, eds. National Aeronautics and Space Administration, Washington, D.C.
Whedon, G.D. 1984. Disuse osteoporosis: Physiological aspects. Calcif. Tissue Int. 36:S146-S150.
White, C.S., J.H. Humm, E.D. Armstrong, and N.P.V. Lundgren. 1952. Human tolerance to acute exposure to carbon dioxide. Pp. 439-455 in Rep. No. 1: Six Per Cent Carbon Dioxide in Air and in Oxygen. Aviation Med. (Oct.).
Wilson, A.J., and K.E. Schaefer. 1979. Effect of prolonged exposure to elevated carbon monoxide and carbon dioxide levels on red blood cell parameters during submarine patrols. Undersea Biomed. Res. 6(Suppl.):S49-S56.
Wright, J.R., and J.A. Clements. 1987. Metabolism and turnover of lung surfactant. Am. Rev. Resp. Dis. 135:426-444.
Wooley, W.D., S.A. Ames, and P.J. Fardell. 1979. Chemical aspects of combustion toxicology of fires. Fire Materials 3:110-120.
Yonezawa, A. 1968. [Influence of carbon dioxide inhalation on renal circulation and electrolyte metabolism.] Jpn. Cir. J. 32:1119-1120.
Zharov, S.G., Y.A. Il'in, Y.A. Kovalenko, I.R. Kalinichenko, L.I.
Karpova, N.S. Mikerova, M.M. Osipova, and Y.Y. Simonov. 1963. Effect on man of prolonged exposure to atmosphere with a high CO2 content. Pp. 155-158 in Proceedings of the International Congress of Aviation and Space Medicine. International Congress of Aviation and Space Medicine, Rome.
Zink, P., and G. Reinhardt. 1975. Carbon dioxide poisoning after prolonged exposure. Beitr. Gerichtl. Med. 33:211-213.