2
Three "Futures" for Glass
Since as early as 1970, there has been an consensus in the international scientific and technical communities that deep geological disposal with a system of multiple barriers to radionuclide release is the primary option for the disposal of high-level radioactive waste. Ideally, the system of multiple barriers should provide a redundant and independent means of preventing radionuclide release to the biosphere. The performance assessment methodology focuses on the evaluation of these separate barriers and potential interactions between barriers (e.g., waste form interactions with ground water in the near field). In the United States, probabilistic performance assessments are used to evaluate compliance with regulatory release limits. Within this strategy, each barrier—the waste form, canister, backfill, and repository—may be viewed as having primary importance, or as having no importance at all in the confinement of the radionuclides. The waste form assumes greater importance as other barriers are judged to have lower probabilities of successful performance. For the purpose of discussing the status of the science and technology of glass as a waste form for containment of radioactive materials, it is convenient to consider that glass can serve three very different "futures" or containment roles.
-
Glass as the only barrier to long-term release of radionuclides.
-
Glass as an effective but not necessarily the primary barrier to long-term release.
-
Glass as a totally ineffective barrier to long-term release, used only for handling, transportation, and short-term storage.
Discussion of glass in these three containment roles makes what has been accomplished in previous decades more readily apparent. It also allows more explicit definition of what remains to be accomplished in developing the appropriate scientific basis for understanding glass behavior and technology needs for the full-scale incorporation of nuclear waste into glass. Discussion of three possible futures for glass avoids the necessity of selecting a specific role at this time. There will be a variety of applications (e.g., transportation, interim storage, final disposal), each of which fails into the above "futures". Glass is commonly judged adequate in the context of short-term handling or as part of a multiple barrier system (Futures 2 and 3), but there has been no analysis of the value of having waste forms perform in the only barrier scenario (Future 1).
With developments of more robust glasses, glass as a waste form may move from the less demanding containment requirements of a medium for transportation toward the more demanding requirements of being a barrier to the release of radionuclides in the repository. Indeed, as part of the performance assessment analysis, the glass waste form in each of these three roles may be evaluated and the value of additional work to improve its properties determined. Total system performance analysis, as often practiced, can obscure the value of the waste form because of the large uncertainties associated with the geological systems (e.g., tectonics, climate change, flow in the unsaturated zone, sorption). The presentations and discussions at the NRC workshop led the steering committee to conclude that sensitivity analyses used to evaluate waste form performance should emphasize the materials properties of the waste form, not the total system performance.
In the following sections the science and technology needs for glass in each of these three "futures" are discussed.
FUTURE 1: THE ONLY BARRIER TO LONG-TERM RELEASE
If the canister, the engineered barriers, or the geology of the repository do not provide for containment of the radionuclides, glass emerges as the only barrier to the long-term release of radioactive materials. Although this is an extreme and highly unlikely assumption, it does provide a limiting condition and can give valuable guidance to further research and development on glasses. There are a number of compelling reasons to emphasize the waste form in any disposal strategy:
-
The radionuclides are located in the waste form. Initially, the only part of the repository that is radioactive is the waste form. The successful performance of the waste form results in near-field containment, rather than relying on the geological repository, long travel times, dispersal or dilution, and sorption. These geological processes implicitly presume release and movement of radionuclides over time.
-
It is easier to model the chemistry and physics of the corrosion and alteration of glass, with the subsequent release or retention of radionuclides over some range of conditions, than it is to develop coupled hydrological, geochemical, and geophysical models of the movement of radionuclides through the far-field of a geological repository. Also, extrapolation of the corrosion behavior of glass over long periods rests on a firmer scientific foundation than the extrapolated behavior of, as an example, hydrological systems (which are site specific and highly dependent on idealized boundary conditions, e.g., climate and recharge).
-
Natural glasses provide an approach to "confirming" the hypothesized long-term behavior of the nuclear waste glass in specific geochemical environments. Information from natural analog studies can be an important component of performance assessments.
Whatever the probability that glass waste forms will actually be the "principal barrier" to radionuclide release, it can be argued that scientists must conduct their research as if this were the case . An evaluation of the state of knowledge with respect to the long-term behavior of glass in geological environments is therefore a critical issue.
Science
The scientific understanding of glasses for the encapsulation of radioactive waste is extensive. This understanding was reflected in the presentations and discussions at the NRC workshop. Indeed, the committee noted that there is broad agreement on a phenomenological model to describe glass behavior in a repository environment.
The reaction of glass with water involves a number of different processes, including exchange of alkali metal ions in the glass with hydronium ions from water, matrix dissolution of the glass, formation of altered surface layers on the glass (including adsorption, structural changes, and mineral precipitation onto the surface), and changes in solution composition. Colloidal particles can break away from the glass surface into solution; colloids can absorb and carry glass constituents, especially highly charged ions such as actinides (see abstract by J. K. Bates in Appendix E). The critical result of glass corrosion is the release of radionuclides. Thus, the form of the nuclides in the solution is important; the nuclides can be molecularly dissolved or
suspended as colloids. The reaction of glass with water vapor also can occur under repository conditions. This reaction involves ion exchange, formation of surface alteration layers, and precipitation of crystalline phases.
During the long-term reaction of glass with water, three stages have been distinguished, which are schematically shown in Figure 1 (Bates et al., 1996; Grambow, 1991; Lutze, 1988). Stage I is the initial stage of the corrosion process in which the release rate (i.e., the forward rate of reaction) of elements is congruent and linear because the elemental concentrations in solution are lower than the solubility limits of most phases. Stage II occurs after appreciable loss of silica from the glass, when the dissolution rate decreases as the silica concentration in solution reaches a higher level. Glass reaction during Stages I and II commonly results in the formation of a reacted layer that may or may not be partially crystalline (Figure 2). The layer may become a chemical sink for released radionuclides due to the precipitation of phases, or it may be a physical barrier to diffusive loss of radionuclides. During Stage III, formation of surface hydration layers, adsorption of chemical species, and surface precipitation of crystalline phases (Figure 3) occur as concentrations in solution reach the solubility limits of principal phases. During this stage, the rate of dissolution of the glass can change discontinuously because of spalling of surface layers and loss of material. The rate of glass dissolution may finally reach very low levels, but it is never zero. This observation is often referred to as the "long-term rate" of corrosion. Further, as reaction continues, the precipitation of new crystalline phases may accelerate the Stage III glass dissolution process. The precipitation of aluminosilicates (e.g., zeolites) may lead to an acceleration of the glass corrosion rate due to removal of silicon from solution. The phases that form depend not only on glass composition but also on the composition of the water in contact with the glass. Figures 2 and 3 depict surface features of a typically corroded waste glass.
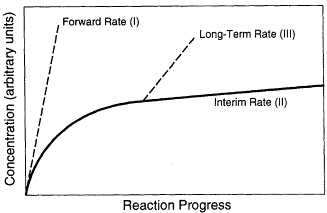
FIGURE 1 Schematic representation of reaction progress for glass. The Reaction Stages I, II, and III are discussed in the text. The curves represent the concentrations in solution of the most soluble element in the glass, usually boron (after Bates et al., 1996, and the discussion by Grambow, 1991).
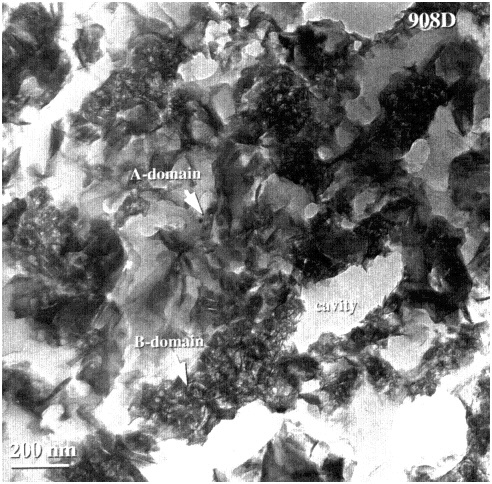
FIGURE 2 Transmission electron micrograph showing a cross-section of a surface layer. This poorly crystalline gel-like layer consists of two distinct domains: A-domains consist of coarsely crystalline fibrous clay (smectite) aggregates along void and cavity linings, and B-domains consist of needle-like crystallites (also smectite) in an amorphous matrix. The compositions of the domains differ. B-domains have high amounts of zirconium and rare earth elements and lower amounts of transition metal elements. The spherical ''holes'' (lower right corner) are the holey carbon substrate on which the sliced sample rests (from workshop poster by Gong et al., abstract included in Appendix E).
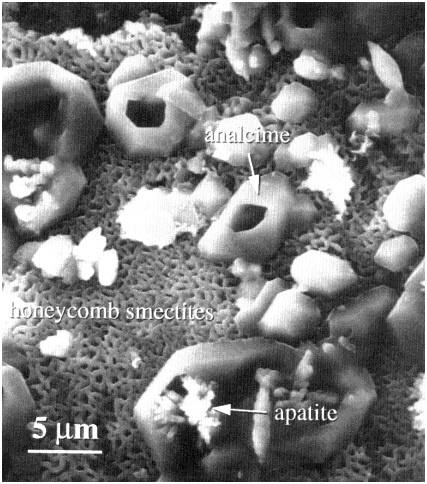
FIGURE 3 Scanning electron microscopy image showing typical surface morphology of a corroded nuclear waste glass surface. Crystals of aluminosilicates (analcime) and phosphates (apatite) form on the surface of a "honeycomb-like" layer of clay (smectite). This is a reference French glass (SON68), not containing radionuclides, subjected to vapor phase alteration for nearly 1,000 days (from workshop poster by Gong et al, abstract included in Appendix E).
Based on the presentations and discussions during the workshop, the steering committee made the following general observations:
-
There is consensus among scientists in the field of nuclear waste glass corrosion that much progress has been realized during the past decade in understanding nuclear waste glass properties. Experts are in general agreement in identifying the important factors that affect the long-term behavior of glass. Alteration and dissolution by ground water are considered the most critical factors. The intrinsic properties of glass (e.g., composition, proportion of crystalline phases, extent of fracture, and the radiation field that results from the type and concentration of radionuclides) and the extrinsic properties of the repository (e.g., ground water composition, flow rate, thermal history, compositions of canister and backfill) all have important effects on the chemical durability of glass. The interaction of these parameters during alteration and corrosion can have a significant impact on the long-term durability of glass. Thus, it is not possible to determine the chemical durability of a glass without considering the context of relevant repository conditions.
-
The description of the phenomenology of glass alteration by aqueous solutions is now quite satisfactory despite uncertainties in the understanding of basic processes and mechanisms. The uncertainties are most important when glass behavior is extrapolated to long times (e.g., thousands of years). The present understanding of glass corrosion is the result of extensive analysis of both solutions and solids, using an array of modem techniques. However, the basic mechanisms of glass alteration need further investigation. At present, kinetic models are based on simple first-order reactions in which silica is the only reactive species. Although these models have contributed to the present level of understanding, they are not consistent with an increasing body of experimental evidence for complex glasses because species other than silica are important in the alteration and dissolution process. More refined models should be developed to properly describe experimental results. Although refinements in the models may be forthcoming, major changes in the understanding of glass corrosion are not expected.
-
The glass alteration models used in performance assessments must be generally applicable to a wide range of glass compositions under a variety of laboratory conditions, as well as diverse natural geochemical environments. As already mentioned, the models developed to describe glass corrosion also should be applicable to natural glasses that are used as analogs in modeling the long-term behavior of nuclear glasses. The role of other species possibly present in solution (e.g., inorganic complexing ions and humic or fulvic acids) also must be assessed.
-
The relationship between molecular structure and reactivity of glasses during interaction with aqueous solutions should be more intensively investigated with appropriate techniques (e.g., nuclear magnetic resonance, X-ray absorption spectroscopy, neutron diffraction) for varied chemical compositions. Glass structure at surfaces in contact with solutions is of particular relevance but is difficult to study because there are a limited number of appropriate techniques. Such studies should identify not only the role of the major network formers and modifiers, but also the structural roles and atomic-scale environments of radionuclides of interest in long-term safety assessments (e.g., technetium-99 and the actinides). The atomic-scale description should be linked to macroscopic properties such as changes in density, dissolution rates, and fracture propagation.
-
Most scientists agree that the hydrated gel layer that forms at the surface of glass during aqueous alteration plays a critical role in both the kinetics of glass alteration and in the fate of most long-lived radionuclides. The processes of formation and the chemical and textural evolution of the hydrated gel layer is a key issue. The compositional, structural, and thermodynamic description of this poorly organized material may be extremely important,
-
particularly because it may be where long-lived nuclides, such as the actinides, become concentrated. This conclusion also is true for secondary crystalline phases such as oxides, oxyhydroxides, silicates, and carbonates that may incorporate radionuclides into their structures. Various methods of analysis are useful for following the course of the reaction of glass with water, including solution analysis and a wide variety of techniques to measure surface composition, phases, and elemental profiles of glass constituents. Both solution and surface analyses are needed to understand the reactions. More use of surface analysis techniques, such as optical and Raman spectroscopy, X-ray absorption spectroscopy, and X-ray photoelectron spectroscopy, should lead to an increased understanding of the surface structure and behavior of waste glasses. Future work should focus on the detailed characterization and evolution of the surface layer, particularly in long-term experiments that can then be compared to natural glasses.
-
Empirical studies based on actual glass compositions and fundamental studies with systematic variations in compositional and other parameters are needed in order to elucidate corrosion mechanisms over time. The complex interplay between kinetic parameters and thermodynamic driving forces must be considered more explicitly in improved alteration and corrosion models.
-
Widely used standard experimental tests have been designed for the measurement of alteration kinetics, identification of basic mechanisms, and comparison of glass performance as a function of chemical composition. However, there has been only a limited effort to develop tests to determine the long-term durability of glass. Standard leach tests are not sufficient for this purpose. The description of long-term behavior presently relies heavily on studies of natural glasses. Previous efforts to relate what is known about corrosion of natural glasses to the long-term behavior of nuclear waste glasses is quite limited. Surprisingly, there are only a limited number of reported results of leaching experiments on radioactive glasses.
-
Notable advances in modeling of glass alteration also have been made during the past decade. Both thermodynamic and kinetic approaches provide a much sounder scientific basis for the interpolation or extrapolation of glass alteration over a range of geochemical environments. Present models suggest that under appropriate conditions the expected lifetimes of glasses may be extended. In particular, the French computer model "LIXIVER" shows that, in silica-saturated solutions or for low silicon diffusion in gel layers, nuclear waste glasses may be orders of magnitude more durable than was previously expected. If the conclusions of such models can be confirmed by further research (e.g., long-term experiments on waste glasses or studies of natural glasses), the glass waste form might be considered not only as the initial but also as a principal barrier against release of radionuclides. An important conclusion is that any model used to describe waste glass corrosion also should be generally applicable to all glasses over a range of experimental conditions, including natural glasses in diverse geochemical environments.
-
The mechanical stability and the thermal stability of waste form glasses are now of secondary relevance or concern. Indeed, knowledge of the thermal stability (or the tendency towards devitrification) of nuclear waste glasses is more than adequate, and there appears to be no anticipated deleterious effect on their performance as a waste form. Similarly, knowledge of the mechanical properties is quite satisfactory. There were no serious issues identified concerning the physical properties of glass. The only issues identified for possible further study were the possible effects of phase separation and the formation of helium and/or oxygen bubbles on the mechanical properties of waste form glasses.
-
Radiation-glass interaction remains a controversial issue because some experimental simulations (e.g., doping with short-lived radionuclides) have failed to demonstrate a marked effect. Electron-beam irradiation studies suggest radiolytic decomposition of the glass and
-
bubble formation. The data are too sparse to come to any definitive conclusion in the absence of systematic studies. The effects of radiation are complicated and may include ionization, elastic interactions, solid-state radiolytic decomposition, bubble formation, phase separation, and transmutation. Often, previous studies have not used the most appropriate techniques for the observation of radiation effects in glass, an aperiodic solid. Differential etching does reveal selected removal of radionuclides along damage tracks formed during alpha decay of actinides in glass. Furthermore, radiation effects for appropriate dose levels have not been investigated for certain applications (e.g., development of glasses for the disposition of weapons plutonium). Workshop participants noted that little is known about the basic mechanisms of radiation-matter interactions in nuclear waste glasses. For instance, it is not possible at this time to predict even the sign of the volume change (±1.5 percent) for glasses of different compositions with increasing radiation dose. The issue of radiation effects on nuclear waste glasses was the subject of a workshop sponsored by the Council on Materials Science under the auspices of the U.S. Department of Energy in February 1996. A summary report of this workshop is available in Weber et al. (submitted for publication).
-
Because there is no repository yet selected in the United States, future repository conditions are difficult to define. Therefore, research to develop waste forms, notably glasses, whose behavior is as independent as possible of environmental fluctuations (e.g., flow rate, pH) should be encouraged. Conversely, glass compositions that appear to be chemically durable over rather narrow ranges of compositions should be avoided because it is difficult to guarantee constant conditions over geological times. The development of innovative glass compositions whose chemical durability is independent of critical parameters (e.g., temperature or flow rate) could lead to improved performance and would certainly simplify the assumptions required in complex performance assessments.
-
Glass alteration should be assessed in a geochemical system where other barriers such as canister, backfill, and near-field materials are considered to interact with the glass. Other factors, such as bacterial action and radiation effects, which have been considered as of minor importance in recent years, must not be completely neglected. For instance, radiation effects in the gel layer and their possible role on the release of radionuclides are still poorly known and must not be dismissed a priori as negligible. Few experiments have been specifically designed to investigate this issue. Such factors could become important in the future if glasses of increased durability (with notably different chemical compositions) are developed.
-
Physical and chemical properties of glass compositions must be reinvestigated and reassessed if new glass compositions (such as for weapons plutonium disposition) or hybrid materials (vitroceramics) are developed. As an example, if phosphate glasses are used, new research programs would have to be initiated to investigate relevant properties and phenomena, such as mechanical and thermal properties or radiation effects. Additional research will be required to understand the properties of glasses developed for new applications, such as plutonium disposition, to match the present level of understanding of borosilicate glasses. The range of conditions for which there is a good understanding of borosilicate glass behavior may not be appropriate to the new glass compositions (e.g., radiation dose).
-
The speciation of actinides in glass, in leached layers, and in solution still requires considerable research. There is a lack of fundamental thermodynamic data for realistic compounds (e.g., hydroxy-carbonates, clays, zeolites, zircon) expected to form in the direct near-field environment of glasses. This lack of data is particularly true for radionuclides that can have a major impact on the safety assessment, such as transuranic elements (e.g., neptunium). The role of colloids in the trapping and transport of certain radionuclides may be significant. This issue is linked with glass alteration because at least a fraction of inorganic colloids is likely to be formed
-
by the disaggregation of the hydrated gel layer. There are surprisingly few thermodynamic data for phases in proposed crystalline waste forms, and these data may be obtained in parallel with thermodynamic data on crystalline phases that form as corrosion products on glass.
Technology
If the glass waste form is the only barrier to the release of radionuclides, great care is needed to ensure that the glass lies within the established ranges of required composition and properties. This condition imposes stringent constraints on feed composition, vitrifier operating conditions, and canister-filling conditions such as cooling rate. Further, the condition requires a statistically significant sampling scheme to verify the properties of the glass product. At present, the final assurance of glass product consistency is obtained generally from careful sampling and adjustment of feed composition, control of vitrifier operating conditions, and glass canister-filling conditions.
If the requirements of glass as the only barrier are to be reached or even approached, it is likely that their attainment will be the result of a series of phased improvements in vitrification technology during development and demonstration. Even seemingly minor changes in melter design can sometimes have important effects on the vitrification process, perhaps allowing for a better sampling protocol to ensure product consistency. In fact, a phased approach to improved vitrification technology is occurring in several countries (e.g., France) that have substantial experience with vitrification. France is investigating the use of a cold wall melter in vitrification plants associated with the UP-1 and UP-2 reprocessing plants at La Hague, and China is building on the German experience that is reflected, in part, at Mol in Belgium. Russia also is developing a cold wall melter.
There are a number of essentially scientific issues that have a direct bearing on the development and application of vitrification technology. These require further work and include:
-
Determination of solubility limits of specific elements (e.g., actinides) in borosilicate glass.
-
Determination of the conditions and compositions for which phase separation may occur, particularly the formation of noble metal aggregates. In actinide-bearing glasses, the possibility of glass-glass immiscibility and/or crystallization requires careful attention, particularly for fissile nuclides.
-
Examination of samples of the actual waste glass for quality control, with archival samples retained for future examination. Because the glass logs may be in interim storage for a number of years before a repository is selected, built, and ready to accept the waste canisters, it is prudent to archive samples that can be used to study phenomena related to the aging of the glass.
FUTURE 2: AN EFFECTIVE, BUT NOT NECESSARILY THE PRIMARY BARRIER TO LONG-TERM RELEASE
This "future" for glass as a waste form represents the role presently played by glass in most nuclear waste disposal strategies. Glass is considered to provide an important barrier to release for an intermediate period of time (thousands of years). Such a role may be most appropriate for waste in which the dominant activity is due to short-lived radionuclides (e.g., high-level defense waste). Such a role may be very effectively used in handling low-level waste
or contaminated soils. Additionally, the glass serves as a solid for the immobilization of liquid waste, which facilitates transportation and interim storage.
Science
In establishing the scientific needs for this role, one may refer to the previous needs and issues listed above for waste glass performance in Future 1. The types of information and understanding required are similar, but the level of knowledge required may be distinctly different. Essential differences include:
-
The chemical durability requirements involved in this scenario are for shorter periods of time. This means, for example, that differences in extrapolated or interpolated results between different models of glass corrosion would be of less or even minor importance.
-
During the shorter periods of time, variations in the geological environment may be expected to be smaller; thus, the glass stability need only be confirmed for a restricted range of geochemical conditions.
-
Shorter time periods for glass durability will necessarily require more quantitative models of corrosion kinetics. Thermodynamic models may be of lesser importance. The kinetics of the corrosion process will have a greater impact on release of radionuclides than the final thermodynamic stability of the phase assemblage of corrosion products.
-
The decreased level of chemical durability means that the possibility of glass interactions with materials in the near field is much increased. The effect of canister-glass and rock-glass interactions should be thoroughly investigated by the appropriate matrix of experimental conditions and materials. For such applications in situ tests may assume greater prominence.
-
Some phenomena, for example, ionizing doses in defense waste glass, reach near-maximum values in short periods of time and would thus be of greater importance under this "future".
Technology
If the glass waste form assumes such an intermediate role in radionuclide containment, there is greater flexibility in using a number of technological options. As an example, pretreatment or processing of the waste may be used to change the proportions of high-level waste volume to low-level waste volume. This has an important impact on cost. The waste glass formulations may be adjusted to produce the optimal balance between high- and low-level waste volumes, without the more rigorous requirement of the performance of high-level waste glass. Additionally, pretreatment may be used to separate long-lived nuclides, such as the actinides, for incorporation into waste forms having higher durability. This approach was described in the Russian program in which highly durable ceramics will be used for the immobilization of actinides, while borosilicate glass will be used for the remaining higher-volume waste streams.
Quality control requirements for the glass product can be relaxed, and the need to have samples of the actual radioactive glass may be much reduced. This has an important impact on plant operation and decreases the measures needed to ensure worker safety.
Vitrification of radioactive waste has been an evolutionary process, and the technologies are of several different types, with quite different glass compositions. Both waste slurries and calcined wastes are being used as feed to the vitrifiers. Joule heated and "cold wall" crucibles have been used. Flat bottom and tapered bottom crucibles have been built and operated, and both
types are under construction, although there appear to be potential problems with flat bottom crucibles because of the possibility of sludge buildup. However, there is no compelling reason to believe that this wide variation in approaches to vitrification is bad or that it is likely to lead to a glass waste form that is unacceptable for the multiple barriers application. In fact, the Russian experience with phosphate-based glasses suggests that this role may still require several different glass formulations.
FUTURE 3: A TOTALLY INEFFECTIVE BARRIER TO LONG-TERM RELEASE
The glass waste form may be seen as a means for immobilization of liquid waste for short-term storage and transportation to a repository. Under this future, solid-state immobilization enhances handling and transportation but is not of consequence for disposal. This places a minimum requirement on waste form performance in the repository.
Such an assumption may be made in safety assessments, particularly for longer periods of time, but in this case one must rely on other technological barriers emplaced in the near field and, above all, on the geological barrier (i.e., the far field) to play the dominant role in isolating nuclear waste. However, it is almost impossible to improve by engineering means the far-field capability of a repository's geological barrier to prevent transport and dispersion of long-lived radionuclides. Therefore, however low the probability that the nuclear waste glass will actually play a role as a barrier to long-term release of radionuclides, some would argue that the disposal concept (with its system of multiple barriers) must be developed as if the glass will actually play no role as a barrier to the long-term release of radionuclides.
Science
The present level of knowledge of glass properties and performance appears adequate to meet this role.
Technology
If no credit is to be taken for the waste form for long-term containment, the primary constraints on vitrification of the high-level waste are those related to the costs and operation of the vitrification plant. Waste glass formulations may be chosen to simplify plant operation, reduce capital costs of construction and operation, and ensure ease and cost effectiveness for the dismantling and disposal of the vitrification plant. However, research may have important impacts on the technology, such as in the development of longer-lived melters and improved operating conditions.











