3
Models and Risk Projections
INTRODUCTION
This chapter presents the committee's risk models relating lung-cancer to radon exposure and applies the models to exposures of the general population to estimate the burden of lung-cancer due to exposure to indoor radon. We discuss both the committee's models describing lung-cancer risk in miners and the application of the models in projecting lung-cancer risks in the general population. We also describe prior risk models and the basis for our approach to developing new risk models. The committee decided to use primarily miner-based data for risk estimation and to use models in which risk is linearly related to dose at low doses. Those two decisions follow those of the BEIR IV committee. However, the rationale for our model is supported more strongly than was that of the BEIR IV committee, being grounded in the biologic considerations developed in chapter 2 and in the stronger body of observational evidence provided by the pooled data from the studies of underground miners, as well as a meta-analysis of the reported 8 case-control studies of residential radon exposure and lung-cancer.
In this chapter, we provide risk projections that describe both the increment in lifetime risk of lung-cancer mortality for various exposure scenarios and the population burden of lung-cancer attributable to exposure to indoor radon. This chapter also addresses uncertainties associated with the models and with risk projections based on the models. Appendix A describes the modelling and uncertainty analysis procedures in detail.
RISK-ESTIMATION APPROACHES
This section briefly reviews alternative approaches to estimating lung-cancer risk associated at radon exposure levels typically found in homes and provides the rationale for the committee's selected approach. Figure 1-3 showed the alternative approaches considered and the related data sources.
Dosimetric Approach
The dosimetric approach applies the well-characterized radiation data from human exposures to γ rays, in particular data from the atomic-bomb survivors, to derive estimates of the risk associated with exposure to radon (ICRP 1990). This approach has the following steps:
-
Use physical dosimetric models of the lung to estimate alpha-particle dose to lung-airway epithelium for indoor radon exposure.
-
Convert the alpha-particle dose to an equivalent low-linear-energy-transfer (low-LET) dose for low-LET radiations, using an appropriate weighting factor for radon-progeny alpha particles in the bronchial epithelium.
-
Convert the equivalent dose to an effective dose, using the appropriate tissue-weighting factor for lung (ICRP 1990). (It is possible to omit step 3 and use lung-specific γ-ray-based risk estimates in step 4).
-
Use risk coefficients per unit of effective dose, based primarily on atomic-bomb survivor data, to estimate the risk per unit of cumulative exposure to radon.
One strength of this dosimetric approach is its use of the wealth of data from the continuing epidemiologic study of the atomic-bomb survivors in Hiroshima and Nagasaki. Lung-cancer risk has been well characterized in that cohort in relation to dose. However, the approach is weakened by the need for scaling factors to convert from the acute, whole-body, primarily γ-ray exposure to the chronic, localized, alpha-particle exposure of the lung from indoor radon. In addition, the data from Hiroshima and Nagasaki are subject to uncertainty owing to limitations of the dosimetry and the need to extrapolate from an exposed population in Japan to other population groups with differing background cancer rates.
Biologically Motivated Approach
Biologically motivated models are intended to provide realistic representation of the steps in radon carcinogenesis from energy deposition to the appearance of cancer. In this context, the parameters of the model have a direct biologic interpretation. One such model is the Moolgavkar-Venzon-Knudson 2-stage clonal expansion model, which incorporates both tissue growth and cell kinetics (Moolgavkar and Luebeck 1990). Such approaches to cancer risk estimation
have been proposed and reviewed by various authors (for example, Little and others 1992, 1994; Moolgavkar and others 1993; Crump 1994a,b; Moolgavkar 1994; Goddard and Krewski 1995; Little 1995).
This committee did not pursue biologically motivated cancer-risk models for several reasons. First, the mechanisms of radon-induced carcinogenesis must be known with sufficient certainty before an appropriate biologically motivated model can be constructed. Despite the considerable amount of information summarized in chapter 2, the committee recognized that current knowledge of radiation cancer mechanisms remains incomplete and any postulated model would necessarily be an oversimplification of a complex process. Second, application of a fully biologically motivated model requires information on fundamental biologic events, such as mutation rates and cell kinetics, that is not readily available in the present application. Third, a comprehensive biologically motivated model involving many parameters, such as the 2-stage clonal-expansion model used by Moolgavkar and others (1993) to describe the Colorado miner data, cannot be fruitfully applied without comprehensive longitudinal data on personal exposures to both radon progeny and tobacco. When the various steps in radon-induced carcinogenesis are more fully understood, the biologically motivated approach might become the preferred approach. However, the committee considered an empirical approach to be preferable at present.
Empirical Approach
Statistical methods for the analysis of epidemiologic data, particularly cohort data, have evolved rapidly since the 1970s. These statistical methods can be used to estimate lung-cancer risks directly from epidemiologic data, as done by the BEIR IV committee. To implement the now-common empirical approach, it is assumed that disease rates in narrow time intervals are constant, or at least can be accurately approximated by mean disease rates in the time intervals. Epidemiologic cohort data are summarized in a multidimensional table, in which each cell contains information on person-years at risk, number of events (lung-cancer deaths) occurring within the cell, and variables that identify the cell, such as age, cumulative exposure, and exposure rate. For each cell, the observed number of events is assumed to follow a Poisson distribution, with a mean equal to the underlying disease rate for the cell multiplied by the person-years at risk. Poisson events are assumed to be infrequent and have a distribution in which the variance equals the mean.
In the development of an empirical risk model to describe rates of radon-induced lung-cancer in miners, several a priori assumptions are needed about either the shape of the exposure-response function or the factors that influence risk. In its most general implementation, empirical modeling is sufficiently flexible to offer some degree of biological plausibility with only minimal assumptions needed about the structure of the model. That generality, as well as the
ability to model without assuming any underlying biologic mechanism of disease, leads to the characterization of the modeling approach as empirical or descriptive. The empirical modeling approach also allows for evaluation of diverse factors that modify risk, such as attained age and exposure rate, through formal statistical testing. Given the limitations of the available data and the resulting difficulty in discriminating among plausible alternative models, the empirical approach undoubtedly results in models that are relatively crude and at best yield rough approximations of actual patterns of risk. While the committee relied on data on lung-cancer mortality in underground miners to construct its proposed risk models, a series of assumptions is needed to extend the miner-based model to the general population. For example, the committee used models in which the exposure-risk relation is linear at low exposures, based on the mechanistic considerations discussed in chapter 2. Other assumptions made in projecting population risks are described later in this chapter.
RATIONALE FOR THE COMMITTEE'S CHOSEN METHOD FOR RADON RISK ESTIMATION
The committee critically assessed the principal approaches (see Figure 1-3) that could be used to estimate the risk associated with exposure to indoor radon, with respect both to sources of data for developing risk models and to techniques for modeling. The combinations of data resources and risk estimation approaches of present interest are as follows:
-
Biologically motivated analysis of miner data.
-
Dosimetric approach using low-LET data (for example, atomic-bomb survivor data).
-
Empirical analysis of miner data.
-
Empirical analysis of data from residential case-control studies.
The strengths and limitations of the three different data sources are summarized in Table 3-1.
With regard to the first approach, the committee recognized that use of biologically motivated risk models is a highly desirable goal, but it felt that such models have not reached a stage at which they can be used for radon risk assessment. Specifically, the complexity and multiplicity of the processes involved in radiation carcinogenesis were noted, as were the gaps in knowledge of the most-basic relevant processes. The paradigms describing carcinogenesis in general and radiation carcinogenesis in particular are changing rapidly. For example, the potential importance of delayed genomic instability (Chang and Little 1992; Kadhim and others 1992; Morgan and others 1996), not incorporated in currently formalized biologically motivated models, was not apparent until within the last few years.
The second approach, the dosimetric approach based on the atomic-bomb
TABLE 3-1 Relative strengths of data for alternative approaches for estimating the risks posed by indoor radon
survivors, has both strengths and weaknesses. Its strengths include the availability of estimates from a large cohort of men, women, and children exposed to a wide range of doses; the extensively characterized dose estimates for the survivors; and the 45-year period follow-up. For the present application to radon progeny, weaknesses include the very different types of radiation and exposure patterns to which the bomb survivors were exposed—acute whole-body doses of gamma rays and, to a lesser extent, neutrons. In particular, the radiation weighting factor needed to relate gamma-ray risks to alpha-particle risks is probably not known to better than within a factor of about 5 (Burchall and James 1994; Brooks and others 1994; Brenner and others 1995). The risk estimates from the study of atomic-bomb survivors are also subject to uncertainty (NRC 1990). The committee reasoned that the uncertainties in extrapolating risks from acute whole-body γ-ray exposure to prolonged, localized alpha-particle exposure were too great to justify use of this approach.
Over the last decade, considerable resources have been devoted to case-control studies of residential radon exposure. A number of studies have been completed, and some are still in progress. These studies are reviewed in appendix G. In principle, residential studies yield the most relevant risk estimates, because they relate directly to the population of interest. However, because of the very low risk associated with exposures at residential levels, risk estimates obtained from these studies, even estimates based on meta-analysis of several studies, are very imprecise. Furthermore, the residential studies offer little opportunity for evaluating with the modifying effects of such factors as smoking and time since exposure. For those reasons, the committee rejected a model based on the residential-radon studies (the fourth approach) as the primary source of risk estimation. However, the committee did compare risk estimates based on the residential data with the low-exposure risk estimates that it generated from the miner data.
Having thus considered the various alternative approaches, the committee chose to follow the general approach of the BEIR IV committee and of Lubin and others (1994a) to the analysis of pooled miner data and to base risk estimates on an empirically derived model (the third approach). This approach provided the committee with well-established databases and methods as a starting point.
Empirical or descriptive modeling of risk allows a unified approach for testing the validity of the form of the model and of the significance of model parameters. For example, the committee used a relative risk model rather than an absolute risk model to describe lung-cancer risk to radon exposure. It had been observed that a relative risk model, with time-varying covariates, provided a more parsimonious description of the miner data than an absolute excess-risk model (Lubin and others 1994a).
The flexibility of the modeling approach allowed the incorporation of specific biologically based patterns of risk. Two important choices in the committee's analysis are the incorporation of an inverse exposure-rate effect and the assumption of linearity of the exposure-response relationship at low cumulative exposure. For radon-induced lung-cancer, those choices have a plausible biologic rationale, as well as some experimental justification (see chapter 2).
PREVIOUS MODELS
A number of models have been previously developed for estimating lung-cancer risk posed by exposure to radon and its progeny. Models developed through the middle 1980s were described in the BEIR IV report (NRC 1988). These and other models are discussed in detail in appendix A to this report. With the exception of preliminary reports from 2 studies which later changed, these models have all assumed linearity of the exposure-response relationship. All models used risk estimates derived from the studies of miners.
The earliest risk models specified effects of exposure in terms of the absolute excess risk of lung-cancer from radon-progeny exposure. The absolute (excess) risk model represents lung-cancer mortality as r (x, z, w) = r0 (x) + g (z, w), where r0 (x) is the background lung-cancer rate and g (z, w) is an effect of exposure. (Here, w denotes cumulative exposure, x represents covariates that determine the background risk, and z denotes covariates that modify the exposure-response relationship.) The model proposed by the BEIR III committee allowed the absolute excess risk to vary by categories of attained age with allowance for different minimal latent periods for each category. A descriptive model for the absolute excess lung-cancer risk, proposed by Harley and Pasternack (1981), served as the basis of risk estimates in Reports 77 and 78 of the National Council of Radiation Protection and Measurements (NCRP 1984a,b). That model assumed that exposure has no effect on risk before age 40 years and that, after a latent period, the absolute excess risk declines exponentially with time since exposure. The model was proposed specifically to address risk associated with radon-progeny expo-
sure, and, although miner data were not used to define its form, published results of analyses of miner data were used to specify latent periods and parameter values thought to be reasonable and appropriate (NRC 1988).
A meta-analysis of miner-study results by Thomas and McNeil (1982), the BEIR IV committee analysis of pooled data from 4 miner cohort studies (NRC 1988), and the findings from a number of individual cohort studies of miners suggested that models of the relative risk (RR) were preferable to models of the absolute excess risk.
Recent descriptive models for lung-cancer risk associated with radon-progeny exposure have also modeled the relative risk rather than the absolute risk. Under the general relative risk model, the lung-cancer rate r (x, z, w) can be written as r(x, z, w) = r0 (x)RR(z, w), where r0 (x) is the lung-cancer rate among nonexposed, and RR (z, w) is the exposure-response function. Of particular interest is the linear relative risk model:
RR = 1 + ßw, (1)
where ßw estimates the excess relative risk (ERR), w is exposure, and ß estimates the increment in ERR for unit change in exposure w.
The simplest of the relative-risk models was proposed in Report 50 of the International Commission on Radiological Protection (ICRP 1987). The ICRP model for extrapolation to indoor exposures was a linear model for ERR in relation to cumulative exposure. The value of ß was derived by reducing a value thought to be representative of the miner studies to reflect differences in conditions between mines and homes. On the basis of findings in the atomic-bomb survivors and dosimetric considerations, ß was increased by a factor of 3 for exposures occurring before age 20 years. The assumption of a constant relative risk and the higher risks assigned to exposures in childhood can be questioned. Detailed analyses of miner data, however, have indicated that the exposure-response relationship is not constant but varies with other factors (NRC 1988; Thomas 1981). In addition, there is little evidence of enhanced effects of exposure at young ages in the miner data (Lubin and others 1994a).
The BEIR IV committee analyzed pooled data from 4 cohort studies of radon-exposed miners (NRC 1988). It found that the simple linear ERR model did not fit the data adequately and that the exposure-response parameter ß varied with time since exposure and attained age. Since its publication in 1988, the BEIR IV model has served as the primary basis for assessing risks for underground miners and the general population. Using the BEIR IV model as a starting point, Jacobi and others (1992) proposed a related "smoothed" model for the relative risk of lung-cancer from radon-progeny exposure, which served as the basis of risk estimation in ICRP Report 65 (ICRP 1993). Expanding the analytic approach in the BEIR IV report, Lubin and others (1994a, 1995b) pooled data from 11 cohort studies of miners, including the 4 studies used in the BEIR IV analysis, and fitted similar types of models for the ERR. Lubin and colleagues
(1994a) again found that the exposure-response relation varied with time since exposure and attained age, but they also found variation with exposure rate. Lower exposure rates were associated with increased risk. The BEIR VI committee used the work of Lubin and colleagues (1994a) as a starting point for the analyses described in this chapter.
BEIR VI RISK MODEL FOR LUNG CANCER IN MINERS
Introduction
This section considers the sources of data, methods of combining data from diverse populations, and assumptions that underlie the lung-cancer risk model developed by the committee in its analysis of miner data. The committee used a relative-risk model that relates lung-cancer rate in miners to their occupational exposure to radon.
In the analysis, exposure refers to occupational exposure to radon progeny during employment in underground mines, and relative risks refer to the additional risks associated with occupational exposure to radon progeny beyond the background risk from lung-cancer, which reflects other exposures, including indoor radon. Residential radon-progeny exposures of the miners are not considered in the analysis data and are implicitly assumed to be the same, on average, at all levels of occupational exposure. Any bias in the modeling due to ignoring nonmine exposures is likely to be small, because residential radon concentrations are generally much lower than mine concentrations.
The committee's model is based on a linear relationship between exposure and the relative risk of lung-cancer. This linear relationship was based on an empirical evaluation of the 11 individual miner studies. In analyzing the miner data, Lubin and others (1994a) explored various models for describing the form of the relative risk in relation to radon exposure. Within the range of exposures in miners, linear models provided an adequate characterization of each cohort except the Colorado Plateau uranium miners. In the Colorado data, the authors found a relative-risk pattern that was concave at high cumulative exposures. Accordingly, in the analysis of pooled data, data from the Colorado study were limited to exposures below 11.2 Jhm-3 (3,200 WLM), below which relative risks were consistent with linearity.
Sources of Data
Pooled data from 11 cohort studies of radon-exposed underground miners were used to develop the committee's risk models; these data were derived from all the major studies with estimates of exposure for individual miners (Table 3-2). Data were available from 7 studies in addition to those considered by the BEIR IV committee. These data are described in detail in appendixes D and E.
TABLE 3-2 Epidemiologic studies of underground miners used in the BEIR VI analysisa
Since the 1994 publication of the original pooled analysis by Lubin and colleagues (1994a), data from 4 studies (Chinese tin miners and the Czechoslovakia,1 Colorado and French uranium miners) have been updated or modified (Lubin and others 1997). In assembling the original data for the China study, the original investigators (Xuan and others 1993) assumed that all miners worked 285 d/yr until the early 1980s, which corresponded to the end of the follow-up less the
5-yr lag period. Recent information has indicated, however, that miners worked 313 d/yr before 1953, 285 d/yr in 1953–1984, and 259 d/yr after 1984. Estimates of exposures have been updated accordingly. An extensive reevaluation of exposure histories and of follow-up and vital status has been carried out for the Czech cohort (Tomásek and others 1994a). There were 705 lung-cancer cases in the updated data, compared with 661 in the previous data set, and the cohort was enlarged from 4,284 to 4,320 miners. For the Colorado study, follow-up has been extended from December 31, 1987, through December 31, 1990 (Hornung and others 1995). In the updated data used by the committee, there were 336 lung-cancer deaths at exposures under 11.2 Jhm-3 (under 3,200 WLM) in a total of 377 cases, compared with 294 lung-cancer deaths at exposures under 11.2 Jhm-3 in a total of 329 total cases in the prior pooled analysis. For the French miner data, the investigators made small corrections in exposure estimates and in health outcomes other than lung-cancer.
In addition to the data changes for those cohorts, there has been a reassessment of estimates of exposure of a nested case-control sample within the Beaverlodge cohort of uranium miners, including all lung-cancer cases and their matched control subjects (Howe and Stager 1996). For these Beaverlodge miners, exposure estimates were about 60% higher than the original values. Because of the computational difficulties of merging case-control data with cohort data, only the data from the Beaverlodge cohort study with the original exposure estimates were used in the committee's analysis.
Analysis of Pooled Data from Different Studies
In the development of risk models, it is important to take account of the totality of evidence from all relevant studies. When data from many different sources are available, this is most effectively accomplished by analyzing combined or pooled data. The models developed by Lubin and others (1994a) were based on analyses of data from 11 miner cohorts. Other examples of analyses of pooled data are those by Cardis and others (1995) on cohorts of externally irradiated nuclear workers in the United States, the United Kingdom, and Canada and Lubin and others (1994b) on data from 3 case-control studies of indoor radon exposure and lung-cancer.
Analyses of pooled data can provide more precise estimates of parameters than those based on individual studies—an advantage that is especially important for investigating modifying factors, which requires comparing risks among subsets of the data. They can also test whether differences in findings among studies represent true inconsistency or simply result by chance. The application of similar methods to data from all studies and the presentation of results in a comparable format facilitate comparisons of results from different studies.
Analysis of pooled data from diverse sources must, however, be done with care because the data might not be fully comparable. In the present context, the
cohorts differ with respect to the methods used to estimate radon-progeny exposure, the completeness of mortality follow-up, and the accuracy of disease diagnosis. The cohorts also differ with respect to demographic characteristics, other exposures encountered in the mines, and smoking patterns. Such differences can lead to heterogeneity in risk estimates. Heterogeneity can be partially addressed by adjusting for modifying factors, such as exposure rate, on which data are available. However, lack of adequate data on all covariates that affect risks and biases in the data can result in residual heterogeneity even with extensive adjustment for covariates. It is important to take account of heterogeneity in analyzing the data, particularly in expressing the uncertainty in the risk estimates obtained.
Statistical methods for analyzing data sets derived from different sources, taking into account heterogeneity among sources, are described in appendix A. Random-effects models (Davidian and Giltinan 1995) provide a natural statistical approach for combining data from different sources in the presence of heterogeneity. Specifically, heterogeneity is accommodated by allowing for random perturbations in parameter values from cohort to cohort, and this results in a random-effects distribution of parameter values across cohorts. The mean of the distribution constitutes an overall summary of the parameter value across cohorts and its variance describes the component of uncertainty due to unaccounted for differences between cohort studies. Two-stage statistical methods have also been used in analysis of pooled data from different studies. With the 2-stage approach, estimates of the model parameters specific for each cohort are derived, and an overall estimate is then obtained by an appropriately weighted linear combination of the cohort-specific estimates, taking into account variation within and between cohorts. The 2-stage approach was used in recent analyses by Lubin and others (1994a) and also by Burnett and others (1995) in combining data on air pollution and respiratory health in 16 Canadian cities.
Both the random-effects and 2-stage approaches were used to combine data from the 11 miner cohorts (see appendix A), but the results presented in this chapter are based on the 2-stage method. In simple modeling situations, the 2-stage and random-effects models were generally found to be in good agreement. In the more-complex modeling conducted by the committee, however, the random-effects approach proved to be computationally more burdensome, and convergence was not always obtained with the iterative numerical methods required in model fitting. Consequently, the committee relied primarily on the 2-stage method in conducting its combined analyses. The committee's 2-stage approach can be viewed as a simplification of, and an approximation to, the full random-effects approach.
The committee recognized that each of the 11 miner studies has certain unique characteristics that contribute to the observed differences in risk among cohorts. In the presence of such differences, the desirability of pooling data from heterogeneous populations can be questioned. Pooling makes maximal use of all relevant data in an objective manner and provides an overall summary measure of
risk. Provided that cohort heterogeneity is acknowledged in the pooling process, the standard error of this overall risk estimate will be an appropriate measure of its statistical uncertainty. The committee considers that, if done carefully, analyses of pooled data can be informative. The committee also believes that, in the absence of clear reasons to exclude particular cohorts from the analysis, it is preferable to make use of all the available data for risk-assessment purposes.
Model Based on Full Data Set
Selection of the committee's risk models was guided by the extensive analysis by Lubin and others (1994a) of the 11 miner cohorts. That analysis indicated that a linear model was sufficient to describe the miner data. Because of the present focus on residential exposures, which are generally at or below the low end of the range of exposures experienced by miners, a linear exposure-response model was considered appropriate for purposes of this report. This choice was supported by the committee's review of the biologic basis of radon carcinogenesis, set out in chapter 2.
Lubin and others (1994a) examined models that took into account factors that modify cancer risk, including time since exposure, attained age, duration of exposure, and intensity of exposure. Those models are described in detail in appendix A. Briefly, they found that models that took into account time since exposure, attained age, and either duration of exposure or concentration of radon progeny as an indicator of exposure rate provided equally good fits to the miner data. Models with fewer than 3 of the modifying factors did not provide comparable fits to the available data. For a given total exposure, duration of exposure is inversely related to the concentration at which exposure was received (the exposure rate), and either duration of exposure or average concentration provides an indication of average exposure rate.
Models with both categorical, that is, discrete, and continuous covariates were considered. The categorical models were preferred by the committee for purposes of risk projection. Both types of models led to comparable predictions of risk within the range of exposure experienced by the miners, but projections of risk to lower exposures based on categorical models depended less on observations at high doses than those from continuous parametric models.
On the basis of previous experience in modeling the miner data, the committee concluded that 2 categorical models (referred to as the exposure-age-duration and exposure-age-concentration models) provided the most-appropriate basis for risk assessment. The concentration model includes categories for exposure in three windows of time since exposure, for age, and for concentration; in the duration model, duration is the measure of exposure rate. Although the committee did not repeat the comprehensive model-selection exercise conducted by Lubin and others (1994a), it analyzed in detail the updated data from the miner studies with these and related models (see appendix A).
The committee's preferred models for predicting relative risk were of the form
where β is the exposure-response parameter; total exposure, w, is partitioned into temporal exposure windows with w5–14, w15–24, and w25+ defining the exposures incurred 5–14 yr, 15–24 yr, and 25 yr or more before the current age; and θ15–24 and θ25+ represent the relative contributions to risk from exposures 15–24 yr and 25+ yr or more before the attained age. The factor 1 in equation (2) represents the background RR for lung-cancer without occupational exposure but with outdoor and indoor exposures. Note that θ5–14 = 1 by definition for w5–14. The parameters ![]() age and γz define effect-modification factors and represent, respectively, multiple categories of attained age (
age and γz define effect-modification factors and represent, respectively, multiple categories of attained age (![]() age) and of either exposure rate or exposure duration (γz). Details of the model fitting are given in appendix A.
age) and of either exposure rate or exposure duration (γz). Details of the model fitting are given in appendix A.
Preliminary analyses indicated that the effects of time since exposure, attained age, and exposure duration or concentration were similar in most cohorts, so that these parameters were constrained to be the same in all 11 cohorts when the models were fitted to the pooled data. However, the parameter β varied considerably across cohorts. Consequently, the overall estimate of β was obtained by using the 2-stage method, so that the associated standard error reflects variation both within and between cohorts.
Estimates of the parameters in the committee's 2 models, based on the 2-stage approach, are given in Table 3-3. (Similar results were obtained with the random-effects approach discussed in appendix A.) Although the parameter estimates changed slightly with the updated miner data, the general pattern of effects was comparable with that observed in the original analysis by Lubin and others (1994a). For a given level of exposure, ERR declined with increased time since exposure (θ5–14 > θ15–24 > θ25+), and with attained age. Lung-cancer risk increased with either lengthening duration of exposure or decreasing exposure rate.
Model Based on Exposure-Restricted Data
The mean exposure in the analysis of pooled data on the miners was 0.57 Jhm-3 (162 WLM), about 10 times the exposure from lifetime occupancy in an average U.S. home. The mean duration of exposure for the miners was about 6 yr, about one-tenth the duration of residential exposures. Thus, mean exposure rates of miners were about 100 times those of residents of typical houses. Those differences in exposure profiles between the entire group of miners and the general population are a source of uncertainty in the model based on the full data set.
To reduce that uncertainty, the BEIR VI committee limited the miner data to exposures that approach those in typical residences; even with this restriction of the
TABLE 3-3 Parameter estimates from BEIR VI models based on original (Lubin and others 1994) and updated pooled (Lubin and others 1997) miner data
|
|
Exposure-age-duration modela |
Exposure-age-concentration modela |
|||
|
|
Original data |
Updated data |
|
Original data |
Updated data |
|
βb × 100 |
0.39 |
0.55 |
β × 100 |
6.11 |
7.68 |
|
Time-since-exposure windows |
|||||
|
θ5–14 |
1.00 |
1.00 |
θ5–14 |
1.00 |
1.00 |
|
θ15–24 |
0.76 |
0.72 |
θ15–24 |
0.81 |
0.78 |
|
θ25+ |
0.31 |
0.44 |
θ25+ |
0.40 |
0.51 |
|
Attained age |
|||||
|
|
1.00 |
1.00 |
|
1.00 |
1.00 |
|
|
0.57 |
0.52 |
|
0.65 |
0.57 |
|
|
0.34 |
0.28 |
|
0.38 |
0.29 |
|
|
0.28 |
0.13 |
|
0.22 |
0.09 |
|
Duration of exposure |
Exposure rate (WL) |
||||
|
γ<5 |
1.00 |
1.00 |
γ<0.5 |
1.00 |
1.00 |
|
γ5–14 |
3.17 |
2.78 |
γ0.5–1.0 |
0.51 |
0.49 |
|
γ15–24 |
5.27 |
4.42 |
γ1.0–3.0 |
0.32 |
0.37 |
|
γ25–34 |
9.08 |
6.62 |
γ3.0–5.0 |
0.27 |
0.32 |
|
γ35+ |
13.6 |
10.2 |
γ5.0–15.0 |
0.13 |
0.17 |
|
|
|
|
γ15.0+ |
0.10 |
0.11 |
|
a Parameters estimated on the basis of the model b Units are WLM-1. |
|||||
data, the number of lung-cancers exceeded the total number analyzed by the BEIR IV committee. Models were developed on the basis of data on exposures under 0.175 Jhm-3 (50 WLM) and under 0.350 Jhm-3 (100 WLM) (Lubin and others 1997). The remaining data include sufficient lung-cancer cases for analysis and cover the range of cumulative exposures for most of the general population. For the exposures used, the inverse exposure-rate effect is not considered to be important (see chapter 2). Table 3-4 describes the unrestricted and restricted data. There were 274,161 person-years of observation among occupationally nonexposed workers, including 115 lung-cancer cases.2 For exposures under 0.350 Jhm-3 (100 WLM), there were 564,772 person-years (64% of total exposed person-years) and
TABLE 3-4 Numbers of lung-cancer cases and person-years and exposure information for cases in the pooled miner data and in data with restrictions of exposure
|
|
0.175 Jhm-3 (<50 WLM) |
0.35 Jhm-3 (<100 WLM) |
No restrictions |
|
Cohort |
|||
|
China |
77 |
116 |
980 |
|
Czech |
15 |
77 |
705 |
|
Colorado |
15 |
22 |
336 |
|
Ontario |
180 |
231 |
291 |
|
Newfoundland |
21 |
24 |
118 |
|
Sweden |
17 |
36 |
79 |
|
New Mexico |
8 |
11 |
69 |
|
Beaverlodge |
42 |
49 |
65 |
|
Port Radium |
20 |
25 |
57 |
|
Radium Hill |
52 |
53 |
54 |
|
France |
22 |
33 |
45 |
|
|
All data combineda |
||
|
Lung-cancer deaths |
|||
|
Nonexposed |
115 |
115 |
113 |
|
Exposed |
353 |
562 |
2674 |
|
Person-years |
|||
|
Nonexposed |
274,161 |
274,161 |
271,457b |
|
Exposed |
454,159 |
564,772 |
883,996 |
|
Mean values for exposed lung-cancer cases |
|||
|
WLM |
19.7 |
40 |
493.6 |
|
WL |
0.9 |
1.2 |
4.1 |
|
Years since last exposure |
17 |
17.4 |
13.8 |
|
Duration of exposure, yr |
5.4 |
6.6 |
14.1 |
|
Attained age, yr |
50 |
58.6 |
58.5 |
|
a Totals exclude 115 workers and 12 lung-cancer cases that were in both the Colorado and New Mexico studies. The cases had exposures in excess of 0.35 Jhm-3 (100 WLM). b The numbers of "nonexposed" person-years and lung-cancer cases differ because of the factors that define the person-years. |
|||
562 lung-cancer deaths (21% of total exposed cases); for exposures under 0.175 Jhm-3 (50 WLM), there were 454,159 person-years (51% of total exposed person-years) and 353 lung-cancer deaths (13% of total exposed cases).
In addition to fitting of the full model (2), the constant linear RR model in equation (1) was fitted to the restricted data. For model (1), estimates of ß exposures restricted to less than 0.175 Jhm-3 (< 50 WLM) and < 0.350 Jhm-3 (< 100 WLM) were 3.343/Jhm-3 (95% CI, 0.571-7.143) and 2.286/Jhm-3 (95% CI, 0.857-4.000), respectively. For the unrestricted data, the estimate of ß was 1.257/Jhm-3 (95% CI, 0.571–2.857). For exposures under 0.350 Jhm-3, there was some suggestion of nonlinearity, but fitting a nonlinear model (RR = 1 + βωγ) did not significantly improve the model fit (p = 0.30). In accord with the biophysical
model for the inverse exposure-rate effect, the gradients of increasing effects with increasing duration of exposure and of decreasing effects with increasing exposure-rate suggest diminution of the inverse exposure rate effect at less than 0.350 Jhm-3, compared with the results with the unrestricted data. Other patterns of modification of the exposure-response coefficient (ß) in the restricted analyses were consistent with the patterns with the unrestricted data for all factors except attained age. The excess RR declined with time since exposure and exposure rate, and increased with exposure duration. Although the exposure-response relationship did not vary significantly with exposure rate and duration factors in the restricted data, it increased with attained age for exposures under 0.350 Jhm-3, whereas it decreased with attained age in the unrestricted data. Lubin and others (1997) fit the exposure-duration and exposure-rate models to the unrestricted data. The models with parameters fixed were then applied to data restricted to < 0.175 Jhm-3 (< 50 WLM). The deviances from these fits were similar to the deviances obtained using the simple linear excess RR model in WLM for restricted data. These results indicate that the exposure-duration model and the exposure-rate model obtained from the unrestricted data adequately described the low-exposure data.
COHERENCE OF EVIDENCE FROM MINERS AND FROM THE GENERAL POPULATION
The committee also assessed the comparability of the miner-based models with the evidence from the case-control studies in the general population, an additional source of information on risks posed by indoor radon. A meta-analysis of 8 case-control studies, each having 200 or more lung-cancer cases and estimates of exposure, has been conducted (Lubin and Boice 1997; see appendix G). The committee also assessed the comparability of risk estimates from these data resources: the full miner data set, the exposure-restricted miner data set, and the residential case-control studies.
The miner-based models and the results from the meta-analysis are not strictly parallel; the miner-based models are time-and age-dependent, and the meta-analysis is based on a loglinear RR model for the time-weighted average exposure rate in an exposure window selected by each of the investigators. A loglinear model for RR gives estimates of risk comparable with those from the linear model (equation 1) with relative risks near unity. To compare estimates made with the different models, the RR was calculated for 30 yr of exposure at 148 Bqm-3 (4 pCiL-1). The results are as follows.
-
Miner-based exposure-age-duration or exposure-age-concentration models, full data set: For a person 65–69 yr old, the estimated RR is 1.11 with the exposure-age-duration model and 1.26 with the exposure-age-concentration model.
-
Miner-based constant-ERR model, data restricted set to under 0.175 Jhm-3 (50 WLM): For a person in the same age category, the estimated RR is 1.17. This estimate is not age-dependent.
-
Model based on meta-analysis of residential case-control studies: The estimated RR for a person living in a home for 30 years at 148 Bqm-3 (4 pCiL-1) is 1.14 (95% CI, 1.01–1.30). This estimate is not age-dependent.
The models derived from the full and restricted miner data provide similar RR estimates for the scenario of indoor exposure. That comparability supports the assumption of linearity of the exposure-response relationship across the range of exposures encompassed by the miner data. There is also coherence between the miner-based risk estimates and the estimate based on the meta-analysis of case-control studies, in which the exposures of participants span the range of typical indoor exposures (see Figure 3-1 and appendix G). The coherence further supports the extrapolation of the exposure-response relationship from the miner studies to the general population. However, the meta-analysis could not describe the exposure-response relationship at the lower end of residential exposure precisely. The comparison of the 3 models does not explicitly take into account any differential effects of smoking on radon risks in the 3 data sets—the full and restricted miner data and the case-control data. However, differences in proportions of ever-smokers in the 3 data sets were likely to have been small and were not likely to have introduced major bias in the comparison of radon risks.
Results from the indoor case-control studies do not provide direct information on lifetime risks posed by radon exposure. The excess risk of 14% at 148 Bqm-3 corresponds to only 30 years of exposure in a house at a constant radon concentration and hence does not reflect the risk of lung-cancer associated with lifetime exposure, where the estimated excess lifetime relative risk at 148 Bqm-3 based on the miner models is 40–50% (Table 3-5). Estimated relative risks from indoor studies and from miner-based models reflect a 30-year exposure period at 148 Bqm-3 and not lifetime exposures at this level. Thus, if exposures outside this 30-year period influence lung-cancer risk, as suggested by the miner data, then the 14% excess relative risk at 148 Bqm-3 from indoor studies is a biased estimate of the lifetime relative risk at this concentration and therefore cannot be used to estimate attributable risks for a population.
BEIR VI RISK ASSESSMENT FOR LUNG CANCER IN GENERAL POPULATION
Introduction
To extrapolate the risk model from the BEIR VI analyses of miner risks to residential exposures, several assumptions must be made (Table 3-6). The assumed shape of the exposure-response relationship is critical. A linear relation-
TABLE 3-5 Estimated lifetime relative risk of lung-cancer risk associated with lifetime indoor exposure to radon
|
|
|
|
|
|
LRR |
|||||||
|
|
|
|
|
|
Exposure-age-concentration model |
|
Exposure-age-duration model |
|||||
|
|
|
|
|
|
Male |
|
Female |
|
Male |
|
Female |
|
|
Concentration |
|
|
Exposure |
|||||||||
|
Bqm-3 |
pCiL-1 |
WLa |
WLM/yb |
Jhm-3/y |
Ever-smokers |
Never-smokers |
Ever-smokers |
Never-smokers |
Ever-smokers |
Never-smokers |
Ever-smokers |
Never-smokers |
|
Multiplicative model: |
||||||||||||
|
25 |
0.7 |
0.003 |
0.10 |
0.00035 |
1.090 |
1.097 |
1.099 |
1.103 |
1.060 |
1.065 |
1.066 |
1.068 |
|
50 |
1.4 |
0.005 |
0.19 |
0.00067 |
1.179 |
1.194 |
1.197 |
1.206 |
1.120 |
1.130 |
1.131 |
1.137 |
|
100 |
2.7 |
0.011 |
0.39 |
0.00137 |
1.352 |
1.388 |
1.391 |
1.411 |
1.237 |
1.259 |
1.261 |
1.274 |
|
150 |
4.1 |
0.016 |
0.58 |
0.00203 |
1.521 |
1.582 |
1.582 |
1.616 |
1.352 |
1.389 |
1.390 |
1.410 |
|
200 |
5.4 |
0.022 |
0.78 |
0.00273 |
1.684 |
1.775 |
1.769 |
1.821 |
1.464 |
1.518 |
1.517 |
1.547 |
|
400 |
10.8 |
0.043 |
1.56 |
0.00546 |
2.290 |
2.542 |
2.490 |
2.637 |
1.892 |
2.033 |
2.012 |
2.091 |
|
800 |
21.6 |
0.086 |
3.12 |
0.01092 |
3.303 |
4.057 |
3.797 |
4.255 |
2.649 |
3.053 |
2.939 |
3.174 |
|
Submultiplicative modelc: |
||||||||||||
|
25 |
0.7 |
0.003 |
0.10 |
0.00035 |
1.081 |
1.194 |
1.089 |
1.206 |
1.054 |
1.130 |
1.059 |
1.137 |
|
50 |
1.4 |
0.005 |
0.19 |
0.00067 |
1.161 |
1.388 |
1.177 |
1.411 |
1.108 |
1.259 |
1.118 |
1.274 |
|
100 |
2.7 |
0.011 |
0.39 |
0.00137 |
1.318 |
1.775 |
1.352 |
1.821 |
1.214 |
1.518 |
1.235 |
1.547 |
|
150 |
4.1 |
0.016 |
0.58 |
0.00203 |
1.471 |
2.159 |
1.525 |
2.229 |
1.318 |
1.776 |
1.352 |
1.819 |
|
200 |
5.4 |
0.022 |
0.78 |
0.00273 |
1.619 |
2.542 |
1.694 |
2.637 |
1.420 |
2.033 |
1.466 |
2.091 |
|
400 |
10.8 |
0.043 |
1.56 |
0.00546 |
2.174 |
4.057 |
2.349 |
4.255 |
1.809 |
3.053 |
1.915 |
3.174 |
|
800 |
21.6 |
0.086 |
3.12 |
0.01092 |
3.120 |
7.008 |
3.549 |
7.440 |
2.507 |
5.058 |
2.760 |
5.317 |
|
a Based on radon gas at 40% equilibrium with its decay products. b Based on 70% occupancy of the home. c Based on the committee's preferred submultiplicative model for the joint effect of smoking and radon. |
||||||||||||
TABLE 3-6 Assumptions required for extrapolating lung-cancer risk estimates from miners to general population
|
Characteristic |
Assumption |
|
Shape of exposure-response function |
Linear |
|
Exposure rate |
Risks at residential levels comparable with those in miners exposed at less than 0.298 Jm-3 (0.5 WL) (exposure-rate model) or for durations longer than 35 yr (exposure-duration model) |
|
Sex |
Ratio of ERR to exposure is the same for males and females |
|
Age at exposure |
Ratio of ERR to exposure is the same for all ages at exposure |
|
Tobacco smoking |
Submultiplicative interaction of smoking and radon; on basis of analyses of ever- and never-smoking miners, the ratio of ERR to exposure for never-smokers is about twice that for ever-smokers |
|
Dosimetry of radon progeny in the lung |
No modification of risk required, because dosimetric K factor estimated to be 1 |
|
Other differences between miners and those exposed in homes |
Ratio of ERR to exposure not dependent on these differences |
ship was assumed in analysis of the miner data on the grounds that it is the simplest model that provides a satisfactory fit to the data in the range of exposures received by the miners. However, to estimate population risks at exposures outside the range of the miner data, a particular exposure-response relationship is assumed at exposures lower than those received by the miners. This assumption needs to be supported by underlying biologic mechanisms.
The committee chose to use a linear relationship between risk and low doses of radon progeny without a threshold. That choice is based primarily on the mechanistic considerations described in chapter 2. In brief, those considerations are related to the stochastic nature of the energy deposition by alpha particles; at low doses, a decrease in dose simply results in a decrease in the number of cells subjected to the same insult. That observation, combined with the evidence that a single alpha particle can cause substantial permanent damage to a cell and that most cancers are of monoclonal origin, provides the mechanistic basis of the use of a linear model at low doses. In addition, as discussed above, exposure-response relationships estimated from the observational data in miners with the lowest exposures, and from the case-control studies of indoor radon, are consistent with linearity (Figure 3-2).
Another critical issue in the extrapolation of risks to the general population is that exposure rates in homes are a thousand-fold to a hundred-fold less from those in most mines. At levels of exposure experienced by miners, biologic consider-
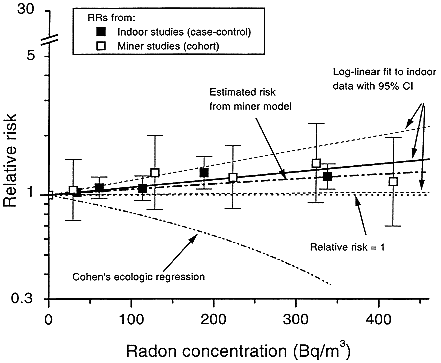
FIGURE 3-2 Summary relative risks (RR) from meta-analysis of indoor-radon studies and RRs from pooled analysis of underground-miner studies, restricted to exposures under 0.175 Jhm-3 (50 WLM). Included are RR of 1, fitted exposure-response and its 95% confidence interval from indoor-radon studies, and estimated linear RR based on ecologic analysis by Cohen (1995).
ations reviewed in chapter 2 suggest that, for a particular total exposure, longer duration of exposure is associated with increased risks. However, for extrapolating from the lowest category of exposures in mines to typical residential exposures (Figure 3-1), any modification of risk by exposure rate is thought to be negligible because of the low probability of multiple alpha-particle traversals of epithelial cells. Exposure-rate effects are observed in the miner data (Lubin and others 1995a) and have been incorporated into the committee's models. The consistency of the risk estimates based on data at exposures lower than 0.175 Jhm-3 (50 WLM) and on the meta-analysis offers some support for using the committee's models in adjusting for exposure-rate effects at residential doses.
In addition to differing in exposure levels and exposure rates, the general population differs from the miner cohorts in including females and persons exposed at all ages and in tobacco-smoking and other exposures. Assumptions
regarding the possible modifying effects of those factors are required to extrapolate the miner-based risk estimates. These assumptions are discussed below.
As in the BEIR IV report, we applied the same risk model to baseline rates for males and females, assuming a multiplicative joint association for exposure and sex; that is, the ratio of ERR to exposure is assumed to be the same for males and females, given specific ages, exposure rates and times since exposure. The background lung-cancer risk for females is lower than that for males, so this assumption results in a smaller lifetime absolute excess risk for females attributable to radon exposure. There are no directly relevant data on modification by sex of the risk posed by radon exposure. Two somewhat contradictory analogies can be made. After occupational risk factors are adjusted for, the effects of cigarette-smoking, for given durations and intensities, are at least as great in females as in males (Doll and others 1980; Lubin and others 1984; Risch and others 1993); this indicates consistency with a multiplicative interaction (USDHHS 1990). In contrast, among the Japanese atomic-bomb survivors, the ERR per Gy for lung-cancer mortality was about 4 times greater in females than in males, although the absolute excess risk was only about 50% greater in females (Shimizu and others 1988). That pattern of sex differences suggests that the proportional translation of radiation effects from males to females could be incorrect. The relevance of the observation in the Japanese atomic-bomb survivors, who received whole-body acute exposure to gamma radiation and some neutron radiation, to people exposed to localized lung doses of alpha radiation throughout their lives is unclear.
The committee also assumed that the ERR per unit exposure does not vary with age at exposure. Analyses of the underground-miner data provided little evidence of variation of the ratio of ERR to exposure with age at the start of mining, but data on those exposed under the age of 20 yr are limited. The atomic-bomb survivor studies, which include many subjects exposed under the age of 20, also give little indication that age at exposure modifies lung-cancer risk, although estimates of parameters describing age effects for lung-cancer are imprecise. In contrast, risks for several other radiation-induced cancers have shown strong age dependency in atomic-bomb survivors and other cohorts. There remains considerable uncertainty with regard to a difference in effects between radon exposure in childhood and in adulthood.
Analyses of the miner data indicate a combined effect of smoking and radon intermediate between additive and multiplicative (see appendix A). This type of combined effect indicates synergism between smoking and radon, but the degree of synergism could not be characterized with a high degree of precision. In estimating lifetime risk for ever-smokers and never-smokers, we used 2 approaches based on modeling the miner data. In the first approach, we assumed a multiplicative relationship (applying the risk model separately to lung-cancer rates in ever-smokers and in never-smokers), recognizing that the risks to ever-
smokers might be overestimated and the risks to never-smokers underestimated. In the second approach, based on analysis of data from known ever-smoking and never-smoking miners, we observed a submultiplicative relationship for the joint association, wherein the ratio of ERR to exposure is greater in never-smokers than in ever-smokers. Acknowledging the limitations of the available data on smoking, we considered the submultiplicative relationship to be preferable for predicting population risk. This preference was based on consideration of model fit and on the higher ratio of ERR to exposure in never-smokers.
For the adjustment of age-specific lung-cancer mortality rates to reflect rates in ever-smokers and never-smokers, we used assumptions based on 1993 data (CDC 1995): relative risks for ever-smokers of 14 and 12 compared with never-smokers and percentages of ever-smokers in the population of 58% and 42% for males and females, respectively. By definition, ever-smokers include both current and former smokers. These assumptions imply that 95% of cases of lung-cancer in men and 90% of cases in women are in ever-smokers; these percentages are consistent with recent data reviewed in appendix C. We assumed 18 yr as the age of starting to smoke regularly. Because of limitations of available data, risks were not estimated separately for former smokers.
Finally, extrapolation from mines to homes requires consideration of the factors that affect the relationship between exposure and dose to target cells in the lung, such as particle size, distribution, and bronchial structure (appendix B). We have incorporated the formalism proposed in BEIR IV (NRC 1988) and the later Panel on Dosimetric Assumptions (NRC 1991), in which the dose per unit exposure in homes is related to the dose per unit exposure in mines by a dimensionless factor K, described in detail in appendix B. Figure 1-2 gives the components of the extrapolation. The exposure-dose factors can be addressed by modeling the dose produced by a given exposure for relevant groups, including miners and the various groups of occupants of homes (men, women, children, and infants).
Because the estimated median K-values for males, females, and children are all close to 1, population risks have been projected with miner-based risk models under the assumption that the dosimetric K is equal to 1. However, uncertainty analyses of population-risk projections were conducted to estimate the significance of deviations from 1.
Measures of Risk
To assess the population lung-cancer risk posed by indoor radon, the model for the exposure-response relationship is applied to the observed distribution of residential radon exposures received by the U.S. population. This risk-characterization step yields the information needed by policy-makers and by stakeholders, primarily the general public in this instance. Previous estimates of the numbers of lung-cancer deaths per year from indoor radon are based on such calculations.
Several measures can be used for risk characterization: population-level indicators that describe the total risk to a population and individual-level indicators that describe the risks to individuals who have specific exposures and particular characteristics, such as, gender and smoking status. For describing the risk to the population, we have used the population attributable risk (AR), which describes the burden of lung-cancer deaths that, in theory, could be prevented if all exposures to radon were eliminated. AR estimates include cases in ever-smokers and never-smokers. The cases in ever-smokers include those resulting from the synergism between smoking and radon exposure; in principle these cases could have been prevented either by smoking prevention (the smokers remained never-smokers) or by reduction of indoor radon concentration to outdoor levels. Although the latter cannot be practically achieved, the committee provides AR estimates for several radon mitigation scenarios that might be feasible through programs to reduce exposures to indoor radon. At the individual level, the committee provides estimates of lifetime relative risk (LRR). Those estimates are based on a constant exposure rate over a lifetime for a cohort followed to extinction compared with a similar cohort exposed to no radon also followed to extinction. The risks have been corrected for competing causes of death by using standard life table methods as described in BEIR IV.
Relative-Risk Estimates
Lifetime Relative Risks
LRRs were computed with the committee's exposure-age-concentration and exposure-age-duration models (Table 3-5). The LRR describes the proportional increment in lung-cancer risk posed by radon exposure beyond the background level (exposures from outdoor air). In addition, LRR estimates were calculated with the BEIR IV model and a constant-relative-risk model based on exposure-restricted data for purposes of comparison. Estimates were computed for exposure scenarios that reflect indoor-radon exposure patterns of interest.
Table 3-5 shows the estimated LRRs for lifetime exposure at constant radon concentrations of 25, 50, 100, 150, 200, 400, and 800 Bqm-3 (0.7, 1.4, 2.7, 4.1, 5.4, 10.8, and 21.6 pCiL-1). Those concentrations were converted to annual exposures by assuming an equilibrium of 40% between radon and its progeny (appendix B) and assuming 70% of time spent at home. The LRRs are similar for the exposure-age-concentration model and the exposure-age-duration model. The LRRs estimated with the BEIR VI models and the BEIR IV model are also similar, even with the inclusion of exposure rate in the new model. As anticipated, the LRR increases with exposure. Women have a somewhat steeper increase in LRR with increasing exposure than men because of their lower background lung-cancer mortality. The higher LRRs in never-smokers than in ever-smokers also reflect differing background mortality rates.
Population-Risk Estimates
Estimation of Attributable Risk
Following Levin (1953) and Lubin and Boice (1989), the attributable risk (AR) of lung-cancer due to ionizing radiation can be defined as the proportion of lung-cancer deaths attributable to exposure to radon progeny. The AR estimates indicate the proportion of lung-cancer deaths that theoretically may be reduced by reduction of indoor radon concentrations to outdoor levels. The AR of lung-cancer from indoor exposure to radon can be estimated given the exposure-response relationship for radon and lung-cancer risk, the distribution of indoor radon concentrations, and mortality from lung-cancer and from all causes. Mortality can be adjusted, as done by the USEPA (1992a), to reflect the mortality pattern of a hypothetical population not exposed to radon. However, the effect of such an adjustment would be small and, because of the uncertainty inherent in AR estimates, was not carried out.
ARs of lung-cancer from indoor radon in the U.S. population were computed with the exposure-age-duration and exposure-age-concentration models with the parameter estimates from Table 3-3, the BEIR IV risk model, and the linear ERR model fitted to data at exposures under 0. 175 Jhm-3 (50 WLM). AR calculations were described in the BEIR IV report (NRC 1988), and are given in appendix A. The BEIR VI risk assessment used the results of the National Residential Radon Survey, which were based on a statistical sample of U.S. residences (Marcinowski and others 1994). The ARs based on the committee's risk models and on the constant linear ERR model were similar (Table 3-7) and consistent with those reported previously (USEPA 1992b; Lubin and Boice 1989; Lubin and others 1995a; Puskin and Nelson 1989).
When the model was applied separately to males and females and to ever-smokers and never-smokers, the ARs were similar. The ARs for the various risk models used 1985–1989 mortality data (for both lung-cancer and all causes of death) and the same distribution for domestic radon concentration as shown in Table 3-7. The computed ARs for the total population with the current models ranged from about 10 to 15%. The AR estimates based on the various models were similar both to each other and to the estimates based on the BEIR IV model.
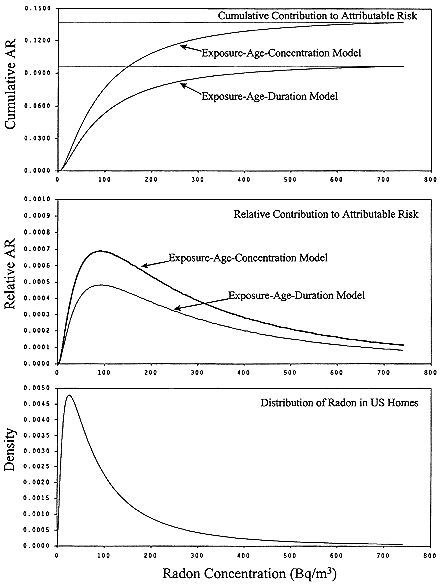
In this analysis, we assumed a lognormal distribution for residential radon concentrations, with a median of 24.8 Bqm-3 (0.67 pCiL-1) and a geometric standard deviation of 3.11 (Marcinowski and others 1994). In Table 3-8 and in Figure 3-2, we present the ARs from each portion of the distribution of radon concentrations in U.S. homes. For males (results for females are similar), the overall AR based on the exposure-age-duration model is estimated to be 0.099, as compared to 0.141 with the exposure-age-concentration model, about 30% less. However, the percentage contributions to the overall distribution from different percentiles of the exposure are very close for the two models. The
TABLE 3-7 Comparison of attributable risks for indoor radon progeny exposure in the U.S. for ever-smokers and never-smokers, using the risk models with and without adjustment for a submultiplicative relationship between radon progeny exposure and smoking
|
|
|
Multiplicative modelb |
Submultiplicative modelc |
||
|
Model |
Total populationa |
Ever-smokers |
Never-smokers |
Ever-smokers |
Never-smokers |
|
Males |
|||||
|
Committee preferred models |
|||||
|
Exposure-age-concentration |
0.141 |
0.136 |
0.149 |
0.125 |
0.258 |
|
Exposure-age-duration |
0.099 |
0.096 |
0.105 |
0.087 |
0.189 |
|
Other models |
|||||
|
CRRd (< 0.175 Jhm-3, < 50 WLM) |
0.109 |
0.105 |
0.117 |
0.096 |
0.209 |
|
BEIR IV |
0.082 |
0.079 |
0.086 |
0.071 |
0.158 |
|
Females |
|||||
|
Committee preferred models |
|||||
|
Exposure-age-concentration |
0.153 |
0.149 |
0.156 |
0.137 |
0.269 |
|
Exposure-age-duration |
0.108 |
0.105 |
0.110 |
0.096 |
0.197 |
|
Other models |
|||||
|
CRRd (< 0.175 Jhm-3, < 50 WLM) |
0.114 |
0.111 |
0.117 |
0.101 |
0.209 |
|
BEIR IV |
0.087 |
0.085 |
0.089 |
0.077 |
0.163 |
|
a No adjustment for smoking. b Unadjusted risk model applied to each group, implying a joint multiplicative relationship for radon progeny exposure and smoking. c Models adjusted by multiplying the baseline ERR/WLM by 0.9 for ever-smokers and by 2.0 for never-smokers. d CRR = constant relative risk. |
|||||
homes with higher radon concentrations contribute disproportionately to the AR; the 49.9% of homes with radon levels of 25 Bqm-3 (0.68 pCiL-1) or less account for only 12.8% (12.7% under the exposure-age-duration model) of the overall AR, whereas the 5.9% of homes with radon levels above 148 Bqm-3 (4 pCiL-1), the Environmental Protection Agency (EPA) action level, account for about 30% of the AR.
Attributable Risk Accounting for Smoking Status
The committee's two risk models were initially developed without explicitly incorporating the available data on smoking status. For estimation of the number of lung-cancers in the general population due to indoor exposure to radon progeny, the BEIR IV committee and other groups have recommended that the same
TABLE 3-8 Distribution of attributable risks for U.S. males from indoor residential radon exposure, based on BEIR VI models
|
Contribution to AR |
|||||
|
Exposure range, Bqm-3 |
Proportion of homes in Range, % |
Exposure-age-concentration model |
Exposure-age-duration model |
||
|
|
|
Actual |
% |
Actual |
% |
|
0–25 |
49.9 |
0.018 |
12.8 |
0.013 |
12.8 |
|
26–50 |
23.4 |
0.026 |
18.5 |
0.018 |
18.4 |
|
51–75 |
10.4 |
0.020 |
14.2 |
0.014 |
14.2 |
|
76–100 |
5.4 |
0.015 |
10.5 |
0.010 |
10.5 |
|
101–150 |
5.2 |
0.020 |
13.9 |
0.014 |
13.9 |
|
151–200 |
2.4 |
0.013 |
9.2 |
0.009 |
9.2 |
|
201–300 |
1.8 |
0.014 |
9.6 |
0.010 |
9.7 |
|
301–400 |
0.7 |
0.007 |
5.2 |
0.005 |
5.3 |
|
401–600 |
0.4 |
0.006 |
4.5 |
0.005 |
4.6 |
|
601 + |
0.4 |
0.002 |
1.5 |
0.002 |
1.6 |
|
Total |
100.0 |
0.141 |
100.0 |
0.099 |
100.0 |
risk model be applied to ever-smokers and to never-smokers, thereby assuming the joint relationship of radon-progeny exposure and smoking to be multiplicative (NRC 1988). However, the current analysis, like some previous analyses, indicates that, although the joint effect of radon-progeny exposure and smoking is consistent with a multiplicative model, the most likely relationship is intermediate between multiplicative and additive. This intermediate combined effect implies that estimates of the number of radon-associated lung-cancer deaths among ever-smokers and never-smokers predicted with a multiplicative assumption are too high for ever-smokers and too low for never-smokers.
Using the results of the analyses of the effect of radon-progeny exposure among never-smokers and ever-smokers, we can modify the exposure-age-concentration and exposure-age-duration models to account for smoking status. There were insufficient data to develop a risk model for never-smokers directly, but we can adjust the risk models on the basis of the relative difference in the exposure-response relationships for ever-smokers and never-smokers. When only data on miners for whom some smoking information was available were used, the overall ERR/0.0035 Jhm-3 was estimated to be 1.02% among never-smokers (95 % CI, 0.15–7.18%), and 0.48% among ever-smokers (95 % CI, 0.18–1.27%). Among these same miners, the overall ERR/0.0035 Jhm-3 ignoring smoking status was 0.53% (95% CI 0.20%, 1.38%). An influence analysis, involving omission of data from each of the cohorts with smoking data one at a time, did not identify any one cohort as having a dominant effect on these estimates.
The estimated ratios of ERR per unit exposure based on the miner data on ever-smokers and on never-smokers are directly comparable only insofar as mean
age, time since exposure, and exposure rate—factors known to modify the ratio—are similar for ever-smokers and never-smokers. Those modifiers were found to differ only slightly between the two groups: never-smokers were 1 yr older, their time since last exposure was 6 mo longer, and their exposure rate was 90% of that of ever-smokers. We therefore assumed that the estimated ratios approximate the relative effects of radon-progeny exposure in ever-smokers and never-smokers.
Among miners with smoking data, the proportional effect of exposure among ever-smokers relative to the overall effect without considering smoking status was 0.9 (0.48/0.53); among never-smokers, the relative effect was about 1.9 (1.02/0.53). To modify the risk models for smoking status, we adjusted the estimated baseline ERR/0.035 Jhm-3 without altering the parameter estimates to take into account the various modifying factors. Specifically, in the exposure-age-concentration model, the overall estimate for ß of 0.0768 for all miners combined was reduced to 0.069 for ever-smokers and increased to 0.153 for never-smokers. In the exposure-age-duration model, the estimate of 0.0055 was reduced to 0.0050 for ever-smokers and increased to 0.011 for never-smokers. ARs for indoor radon-progeny exposure in the US for ever-smokers and never-smokers, based on risk models with and without this adjustment, are given in Table 3-9.
In 1995, about 157,400 lung-cancer deaths—95,400 in men and 62,000 in women—occurred in the United States (Boring and others 1995). Most lung-
TABLE 3-9 Estimated attributable risk (AR) for domestic exposure to radon using 1985–89 U.S. population mortality rates based on selected risk models
|
Model |
Population |
Ever-smokersa |
Never-smokersa |
|
Males |
|||
|
Committee's preferred models |
|||
|
Exposure-age-concentration |
0.141 |
0.125 |
0.258 |
|
Exposure-age-duration |
0.099 |
0.087 |
0.189 |
|
Other models |
|||
|
CRRb (< 0.175 Jhm-3, < 50 WLM) |
0.109 |
0.096 |
0.209 |
|
BEIR IV |
0.082 |
0.071 |
0.158 |
|
Females |
|||
|
Committee's preferred models |
|||
|
Exposure-age-concentration |
0.153 |
0.137 |
0.269 |
|
Exposure-age-duration |
0.108 |
0.096 |
0.197 |
|
Other models |
|||
|
CRRb (< 0.175 Jhm-3, < 50 WLM) |
0.114 |
0.101 |
0.209 |
|
BEIR IV |
0.087 |
0.077 |
0.163 |
|
a Based on the committee's preferred submultiplicative model for the joint effect of smoking and radon. b CRR = constant relative risk. |
|||
cancer cases occur among ever-smokers: about 95% of cases in male ever-smokers and 90% in female ever-smokers (appendix C). This assumption places a higher proportion of cases in smokers than did the previous report of Lubin and colleagues (1994a), which assumed only 85% of cases to be in smokers. Table 3-10 shows the estimated lung-cancer deaths in the United States in 1995, including those attributable to indoor radon progeny exposure according to the preferred BEIR VI models.
Effect of Radon Mitigation on Attributable Risk
The overall AR describes the anticipated consequences of virtual elimination of indoor radon exposures under the committee's risk models. A more-realistic assessment of the reduction of attributable risk due to radon exposure mitigation focuses on exposure-reduction scenarios that might actually be achieved. Lubin and Boice (1989) considered three such scenarios: all homes above 148 Bqm-3 (4 pCiL-1) are reduced to zero, that is, the outdoor level, all homes above 148 Bqm-3 (4 pCiL-1) are distributed at a lower concentration below 148 Bqm-3
TABLE 3-10 Estimated number of lung-cancer deaths in the U.S. in 1995 attributable to indoor residential radon progeny exposure
|
|
|
Lung-cancer deaths attributable to radon progeny exposure (No.) |
|||
|
Smoking status |
Number of lung-cancer deaths |
Exposure-age-concentration model |
Exposure-age-duration model |
||
|
Malesa |
|||||
|
Total |
95,400 |
13,000b |
12,500c |
9,200b |
8,800c |
|
Ever-smokers |
90,600 |
12,300 |
11,300 |
8,700 |
7,900 |
|
Never-smokers |
4,800 |
700 |
1,200 |
500 |
900 |
|
Femalesa |
|||||
|
Total |
62,000 |
9,300 |
9,300 |
6,600 |
6,600 |
|
Ever-smokers |
55,800 |
8,300 |
7,600 |
5,900 |
5,400 |
|
Never-smokers |
6,200 |
1,000 |
1,700 |
700 |
1,200 |
|
Males and Females |
|||||
|
Total |
157,400 |
22,300 |
21,800 |
15,800 |
15,400 |
|
Ever-smokers |
146,400 |
20,600 |
18,900 |
14,600 |
13,300 |
|
Never-smokers |
11,000 |
1,700 |
2,900 |
1,200 |
2,100 |
|
a Assuming that 95% of all lung-cancers among males occur among ever-smokers, and that 90% of all lung-cancers among females occur among ever-smokers. Percentages of ever-smokers in the population were 58% and 42% for males and females respectively. b Estimates based on applying same risk model to ever-smokers and never-smokers, implying a joint multiplicative relationship for radon progeny exposure and smoking. c Estimates based on applying a smoking adjustment to the risk models, multiplying the baseline ERR/WLM by 0.9 for ever-smokers and by 2.0 for never-smokers, the committee's preferred approach. |
|||||
(using a truncated lognormal distribution), and all homes above 148 Bqm-3 (4 pCiL-1) are reduced to exactly 148 Bqm-3 (4 pCiL-1). These AR apply to a mixed population of male ever-smokers and never-smokers with exposures corresponding to the 1993 distribution of radon concentrations in U.S. homes. Because the distribution of radon concentration is approximately lognormal with the bulk of the population exposed to very low levels, the effective AR is substantially lower than the total AR. Effective ARs (EARs), which indicate the fraction of total lung-cancer deaths that would be eliminated by implementing one of these mitigation scenarios, are shown in Table 3-11. For example, under the exposure-age-concentration model, mitigating homes at or above 148 Bqm-3 (4 pCiL-1) would result in an estimated reduction in lung-cancer mortality of 4.2% if homes were mitigated to zero, 3.7% if homes were distributed across the range of levels below 148 Bqm-3 (4 pCiL-1) (on the basis of a truncated lognormal distribution), or 1.7% if homes were mitigated to exactly 148 Bqm-3 (4 pCiL-1). The second mitigation scenario is probably the most-appropriate characterization, and the first and third scenarios are limiting scenarios. Eliminating exposures at concentrations above 148 Bqm-3 (4 pCiL-1) results in an EAR of about 4%. Thus, 10–15% of all lung-cancers are estimated as attributable to indoor radon, and eliminating exposures in excess of 148 Bqm-3 (4 pCiL-1) would reduce the lung-cancer burden from radon to 7–11% of all lung-cancer cases.
TABLE 3-11 Effective attributable risks (EARa) for lung-cancer from residential radon exposure to radon using 1985–89 U.S. population mortality rates and the BEIR VI models
|
|
|
|
|
Modified radon distributionsb |
||
|
Model |
''Eliminate" all exposures |
Cut-off in Bqm-3 |
Cut-off in pCiL-1 |
"0" |
Truncated |
Exact cut-off |
|
Exposure-age-concentration |
0.141 |
|
|
|
|
|
|
|
|
37 |
1 |
0.110 |
0.092 |
0.068 |
|
|
|
74 |
2 |
0.078 |
0.065 |
0.040 |
|
|
|
148 |
4 |
0.042 |
0.037 |
0.017 |
|
Exposure-age- duration |
0.099 |
|
|
|
|
|
|
|
|
37 |
1 |
0.077 |
0.065 |
0.049 |
|
|
|
74 |
2 |
0.055 |
0.047 |
0.028 |
|
|
|
148 |
4 |
0.031 |
0.027 |
0.012 |
|
a EAR, the effective attributable risk, indicates the fraction of total lung-cancer deaths that would be eliminated by radon-exposure mitigation. b population distribution of radon concentrations in homes based on Marcinowski and others (1994) new residential radon survey. All homes over cut-off are assumed mitigated: to zero exposure (denoted "0"); to levels under cut-off based on a truncated lognormal distribution (denoted "truncated"); or to exactly cut-off (denoted "Exact cut-off"). |
||||||
The rationale for and the biologic justification of the committee's choice of a linear, nonthreshold exposure-response relationship for the calculation of risk, particularly at low total exposures, is given in chapter 2. Results of the miner studies and of studies of indoor radon exposure are consistent with a linear relationship throughout the entire range of residential radon exposures, and there is no evidence of a threshold exposure below which exposure carries no risk. However, data on the lowest residential exposures are sparse, and a threshold exposure below which exposure does not increase risk is theoretically possible.
Table 3-12 shows the effects on the AR and the EAR of assuming a linear model, but with various threshold exposures below which there is no increased risk and from which threshold risk increases with the slope used in the nonthreshold model. In this table, threshold exposures are specified in terms of concentrations: exposures at or below the indicated concentrations are assumed to not increase lung-cancer risk. With the assumed thresholds, the total estimated lung-cancer burden to the population is lower than with the committee's no-threshold model. For example, if the threshold were 74 Bqm-3 (2 pCiL-1), the total AR—the proportion of lung-cancer eliminated on removal of all risk from residential radon exposure—is reduced from 0.141 to 0.083 according to the committee's exposure-age-concentration model, or from 0.099 to 0.058 according to the exposure-age-duration model. However, mitigating homes over 148 Bqm-3 (4 pCiL-1)—distributing high-radon homes to levels below this—increases the effective AR from 0.037 to 0.044 (exposure-age-concentration model) or from 0.026 to 0.031 (exposure-age-duration model). The increased effectiveness of mitigation is the result of lowering levels in homes with concentrations over
TABLE 3-12 Estimated attributable risk (AR) and effective attributable risk (EAR) for lung-cancer deaths in the U.S. from residential exposure to radon and it progeny and the consequences of assuming a threshold for exposure, below which there is no risk of lung-cancer deatha
|
Thresholdb |
Exposure-age-concentration model |
Exposure-age-duration model |
|||
|
Bqm-3 |
pCiL-1 |
AR |
EARc |
AR |
EARc |
|
0 |
0 |
0.141 |
0.037 |
0.099 |
0.026 |
|
37 |
1 |
0.113 |
0.040 |
0.079 |
0.028 |
|
74 |
2 |
0.083 |
0.044 |
0.058 |
0.031 |
|
148 |
4 |
0.048 |
0.048 |
0.033 |
0.033 |
|
a Estimates are based on the committee's exposure-age-concentration and exposure-age-duration models and assume constant lifetime exposure. b Exposures at or below the indicated threshold level are assumed to carry no risk of lung-cancer. c Effective attributable risk estimates the proportion of radon-induced lung-cancer deaths which could be prevented if the distribution of radon concentration in houses was modified. In this example, EAR is based on reducing the radon concentration in all homes above 148 Bq/m3 to a level between the outdoor level and 148 Bq/m3 (4 pCiL-1), based on a truncated lognormal distribution. |
|||||
148 Bqm-3 (4 pCiL-1) to concentrations below 148 Bqm-3 (4 pCiL-1), which carry some risk assuming no threshold, to levels which carry no risk, assuming an exposure threshold. Thus, if there were a threshold exposure level, then the estimated total lung-cancer burden attributable to radon would decline, whereas the percentage reduction in the lung-cancer burden that could be achieved through any practical radon mitigation strategy would increase.
SOURCES OF UNCERTAINTY
Quantitative estimates of human cancer risk are subject to a number of uncertainties, which need to be considered in risk management decision making. We discuss below sources of uncertainty in estimates of lung-cancer mortality associated with exposure to indoor radon in the United States. The initial discussion is in qualitative terms; the committee's quantitative treatment of uncertainty is described fully in appendix A and summarized later in this chapter.
Table 3-13 lists sources of uncertainty and categorizes them according to the steps in the risk-assessment process as discussed in appendix A. The material that follows discusses only uncertainties in the model relating lung-cancer risk to exposure (Table 3-13, part I). The model for addressing differences in radon-progeny dosimetry in mines and in homes (Table 3-13, part II) is discussed
TABLE 3-13 Sources of uncertainty in estimates of lifetime risk of lung-cancer mortality resulting from exposure to radon in homes
|
I Sources of uncertainty arising from the lung-cancer risk model |
|
A Uncertainties in parameter estimates derived from miner data 1 Sampling variation in the underground miner data; 2 Errors and limitations in the underground miner data; a) Errors in health effects data including vital status and information on cause of death; b) Errors in data on exposure to radon and radon progeny including estimated cumulative exposures, exposure rates and durations; c) Limitations in data on other exposures including data on smoking and on other exposures such as arsenic. B Uncertainties in application of the model to the general 1 Shape of the exposure/exposure rate response function for estimates at low exposures and exposure rates; 2 Temporal expression of risks; 3 Dependence of risks on sex; 4 Dependence of risks on age at exposure; 5 Dependence risks on smoking status. |
|
II Sources of uncertainty arising from the exposure/dose model |
|
III Sources of uncertainty arising from the exposure distribution model |
|
1 Estimate of the average radon concentration; 2 Estimate of the average equilibrium fraction; 3 Estimate of the average occupancy factor. |
|
IV Sources of uncertainty in the demographic data used to calculate lifetime risk |
briefly in appendix A and more thoroughly in appendix B. Uncertainties in the exposure distribution for the U.S. population (Table 3-13, part III) apply to AR estimates (Tables 3-7 through 3-11), but do not apply to estimates of LRR associated with specified exposure scenarios (Table 3-5). The exposure distribution used in calculating AR was taken from Marcinowski and others (1994) and is discussed in appendix G. Uncertainties in the demographic data (Table 3-13) are briefly discussed in appendix A. Puskin (1992) discusses sources of uncertainty in BEIR IV risk estimates; much of this discussion is relevant, at least in general terms, to the BEIR VI risk estimates.
Uncertainties in Parameter Estimates Derived from Underground-Miner Data
Uncertainty Due to Sampling Variation
Uncertainty resulting from sampling variation differs from uncertainty of most other sources in that it can be quantified using statistical methods. However, because estimates of lifetime risks are a complex function of the parameters of the risk model, Monte Carlo simulations are needed to obtain confidence intervals for such estimates. The committee's simulations, which include sampling variation as well as other sources of uncertainty, are described later in this section.
Errors in the Underground Miner Data
Errors in the data from the 11 miner cohorts used to construct the committee's risk model contribute additional uncertainty in parameter estimates. Errors in determination of vital status and cause of death could also potentially result in bias. However, because the analyses of the miner cohorts were based on internal comparisons within each of the cohorts, the effect of any bias from errors in health outcome data is not likely to be large, unless the errors depended on the level of exposure, which seems unlikely.
Errors in estimates of miner exposures are more likely to have biased estimates of risk. These errors occurred because measurements of radon and radon progeny were limited for many of the mines, especially during the early periods of mine operation (see appendix F). These limitations could have led to both systematic and random errors in estimates of exposure of individual miners. With regard to systematic bias, historical accounts were not sufficient to determine the overall magnitude or even the overall direction of bias. It is possible that different types of systematic bias affecting the estimates might not negate each other. It is well known that random measurement error in the exposure estimates can lead to underestimation of risk (see appendix G). The complex nature of the error structure and the lack of adequate data on all sources of error make it difficult or impossible to quantify its impact on risk estimates.
For most of the miner cohorts, errors in exposure estimates were likely to have been largest in the earliest periods of operation, when exposure rates were highest and fewer measurements were made or, in some cases, simple estimates were made in the absence of measurements. For that reason, measurement errors might affect not only the estimates of the overall risk coefficient, but also estimates of parameters that describe the relationship of risk with time-related variables, such as exposure rate, time since exposure, and age at risk. However, analyses restricted to exposures below 0.175 Jhm-3 and below 0.350 Jhm-3 led to attributable risk estimates that were very similar to those obtained with the committee's recommended models based on the full miner data set. Miners exposed at those low levels were employed predominantly in later periods when exposure-assessment methods had improved substantially, and their exposure estimates were probably affected to a lesser degree by measurement error than those of the miners who worked in the earlier periods.
In addition to radon progeny, some underground miners were exposed to arsenic, silica, and diesel fumes (see appendix F). Because those exposures might be positively associated with radon exposures, there is a potential for confounding if no adjustment is made. The other exposures might also have enhanced the risk posed by radon for miners through synergism. Such potential synergism constitutes a further source of uncertainty in extending miner-based risk estimates to the general population, which is not exposed to those agents. Limitations in the available data on miners make it difficult to evaluate bias in risk estimates for radon progeny because these other agents are not adequately considered.
In spite of the differences among the cohorts, an influence analysis showed that the AR estimates did not reflect an undue influence by any particular cohort (Table 3-14). The AR estimate changed by a few percent at most as individual cohorts were removed from the analysis.
TABLE 3-14 Influence analysis for the population attributable risk, based on calculating the AR after omitting data from each of the 11 cohorts
|
|
Population attributable risk (AR) |
|
|
Cohort excluded |
Exposure-age-concentration model |
Exposure-age-duration model |
|
None |
0.141 |
0.099 |
|
China |
0.175 |
0.124 |
|
Czechoslovakia |
0.137 |
0.101 |
|
Colorado |
0.137 |
0.104 |
|
Ontario |
0.151 |
0.099 |
|
Newfoundland |
0.135 |
0.100 |
|
Malmberget |
0.144 |
0.099 |
|
New Mexico |
0.136 |
0.096 |
|
Beaverlodge |
0.128 |
0.083 |
|
Port Radium |
0.148 |
0.101 |
|
Radium Hill |
0.129 |
0.081 |
|
France |
0.150 |
0.106 |
Smoking is also of potential concern both as a confounder and as a modifier (appendix C). The high prevalence of smoking among underground miners is a potential source of uncertainty in extrapolating the BEIR VI models to the general population. Because the committee does not expect strong correlations between smoking and radon exposure, it is not greatly concerned about uncontrolled confounding of radon risk estimates by cigarette-smoking. Limitations of the available smoking data are of greater concern in evaluating the modifying effects of smoking on risk posed by radon-progeny exposure.
Uncertainties in Specification of the Lung Cancer Exposure-Response Model and Its Application to Residential Exposure of the General U.S. Population
Uncertainties in model specification and application are especially important for extrapolating risks from underground miners to persons exposed in homes.
Shape of the Exposure/Exposure-Rate Response Relations
Cumulative exposures and exposure rates were generally much higher in underground mines than in homes, consequently, it might be thought of that the most-critical aspect of the committee's model development process is the choice of method for extrapolating risks to residential exposures. However, the committee's recommended risk models included parameters that specifically estimated effects at average exposure rates less than 0.03 Jm-3 (0.5 WL) and average exposure durations exceeding 35 yr, the rates and durations of principal interest for residential exposures. Furthermore, restricted analyses based on miners with lower cumulative exposures (less than 0.175 or 0.350 Jhm-3) and the meta-analysis of data from case-control studies of indoor radon led to risk estimates that were very similar to those based on the committee's recommended models.
Temporal Expression of Risk
The committee's risk model provides for a decline in risk with time since exposure and with age at risk. Although those patterns were consistently identified in nearly all the underground-miner cohorts, estimates of the effects could have been biased by errors in exposure measurements and in classification of death as due to lung-cancer, as well as changes in smoking habits over time. We note that lifetime risk estimates based on miner data restricted to concentrations below 0.175 or 0.350 Jhm-3, and on the assumption that risks did not decline with time since exposure or with age at risk, were similar to estimates based on the committee's recommended models.
Dependence of Risks on Sex
The cohorts used to develop the committee's model included only men. The committee has chosen to assume that risks to males and females are comparable on a multiplicative scale, which leads to risks for females that are about one-third those for males and about one-third of those which would have been obtained had risks been assumed to be comparable in males and females on an absolute scale. The committee had no basis for further quantifying the uncertainty associated with the choice of the multiplicative rather than the additive scale.
Dependence of Risks on Age at Exposure
Using the limited data available, the committee did not find evidence that age at exposure modified lung-cancer risks in underground miners; the preferred models were therefore based on the assumption that the excess relative risk did not depend on age at exposure. Data from the miner cohorts on exposures in childhood are limited primarily to the Chinese tin miners; even in this cohort, data on miners exposed in early childhood are sparse. Clearly, the uncertainty in lung-cancer risks in adults resulting from exposure in childhood is much greater than in risks resulting from exposure in adulthood.
Dependence of Risks on Smoking Status
Evaluation of the modifying effect of smoking on lung-cancer risk is limited by the extent of the available data on smoking. Because of the need to extrapolate from miners to the general population, with a lower proportion of ever-smokers, uncertainties in the characterization of the combined effect of smoking and radon exposure can affect estimates of overall and average population risks. Uncertainty in the combined effect has particular implications for the attribution of cases into ever-smoking and never-smoking groups. In the United States, about half of persons who have ever smoked have now stopped (USDHHS 1990). However, we did not calculate risks posed by radon exposure of former smokers, because we lacked data on changes in lung-cancer mortality in miners in relation to age and time since stopping smoking.
UNCERTAINTY ANALYSIS
The previous section identified factors that can contribute to uncertainty in radon risk estimates. In addition to a qualitative discussion of sources of uncertainty, it is desirable to quantify the extent to which those sources contribute to uncertainty in lung-cancer risk estimates. Risk analysts recognize the importance of addressing uncertainty in risk assessment (NRC 1994b) and have developed methods for quantifying uncertainties (Bartlett and others 1996). Hoffman and
Hammonds (1994) have recently applied such methods in assessing the cancer risks associated with radionuclide exposure.
Methods for quantitative uncertainty analysis are discussed in detail in appendix A, including the general framework for the analysis of uncertainty developed by Rai and others (1996). That framework is applicable when the risk depends on a series of risk factors X1,..., Xp, each of which can be subject to uncertainty and might vary among individuals in the population at risk. Some risk factors, such as body weight, might be subject to little uncertainty but vary widely in the population of interest. Other risk factors, such as genetic susceptibility to particular types of cancer, might vary little in the population but be subject to appreciable uncertainty. If the uncertainty and the variability in each of the risk factors can be specified, the overall effect of uncertainty and variability in risk can be evaluated. It is also possible to identify which risk factors contribute most to overall uncertainty and variability.
Although not all potential sources of uncertainty were quantified, the committee conducted a number of limited analyses, which proved to be informative. A complete quantitative analysis of all sources of uncertainty in factors affecting radon lung-cancer risk is not feasible for two principal reasons. First, it is difficult to enumerate all factors that may influence the lung-cancer risk associated with environmental exposures to radon. Second, characterization of the extent of both interindividual variability and uncertainty of some of these factors may not be possible using existing information.
To address uncertainty in radon risk estimates, the committee focused on those factors included in the committee's two preferred models: the exposure-age-concentration model and the exposure-age-duration model. In addition to the four risk factors considered in each of these two models, the committee evaluated the impact of uncertainty in the K-factor on the uncertainty range of AR estimates.
The committee used the methods proposed by Rai and others (1996) to evaluate uncertainty in radon risk estimates. That approach is applicable whenever the risk can be expressed as a function H(X1,..., Xp) of p risk factors X1,..., Xp, and when both uncertainty and variability in each factor can be specified. The analysis is considerably simpler when the function H is multiplicative, with H = X1x...xXp.
For purposes of the present report, the committee focused its attention on a quantitative uncertainty analysis of the population attributable risk, this being of most interest from the public health point of view. Because the AR is a measure of population rather than individual risk, the interindividual variability in lung-cancer risk is effectively averaged out in this analysis. In other words, since the AR is calculated by jointly integrating over the distribution of radon exposures and the distribution of K among individuals in the population, the AR is subject to uncertainty, but not variability.
The population attributable risk depends on the risk model used to describe the exposure-response relationship between radon and lung-cancer, the distribut-
tion of radon concentration in U.S. homes, and the dosimetric K-factor. The methods adopted by the committee to evaluate uncertainty in the AR require specification of the prior uncertainty in each of the factors affecting risk. As detailed in appendix A, the uncertainty in the parameters in the BEIR VI risk models was described by lognormal distributions, with dispersion at least as great as the sampling error in the estimated parameter value. Variability among radon concentrations in U.S. homes was characterized by a lognormal distribution used to describe the results of the National Residential Radon Survey; uncertainty in individual radon measurements (measurement error) was not addressed in this analysis. Variability in the K-factor was also described by a lognormal distribution based on a sample of observations in U.S. homes; uncertainty in K-factors for specific homes was described by a log-uniform distribution. Although the committee exercised some judgment in specifying distributions, the result represents a best attempt to allow for some degree of uncertainty in a number of the critical factors affecting the AR.
Because the AR is not a simple multiplicative function, Monte Carlo methods were used to evaluate uncertainty under the committee's preferred risk models. As shown in Figure 3-3, this analysis leads to an uncertainty distribution reflecting the likelihood of different possible values for the AR, centered roughly at the best estimates of the AR given in Table 3-7. Because the uncertainties in the model parameters are largely statistical, the uncertainty distributions reflecting only uncertainty in the parameters of the committee's risk models (Figure 3-3, case I) can be used to obtain approximate confidence intervals for the AR. For males (Figure 3-3a), 95% of the mass of this distribution falls in the range 0.09–0.24 for the exposure-age-concentration model, and 0.07–0.16 for the exposure-age-duration model. For females (Figure 3-3b), the corresponding limits are similar: 0.10–0.26 for the exposure-age-concentration model and 0.08–0.18 for the exposure-age-duration model. In this analysis, a constant value of K = 1 was used.
A second uncertainty analysis was conducted in which variability in K was taken into account (Figure 3-3, case II). Allowing for variability in K does not increase the dispersion of the uncertainty distribution for the AR, but does shift the distribution to the right. For males, the 95% uncertainty intervals for the exposure-age-concentration and the exposure-age-duration models were 0.10–0.22 and 0.08–0.18, respectively. For females, the corresponding limits were 0.10–0.28 and 0.08–0.19, respectively.
The final analysis of uncertainty in the AR further acknowledged uncertainty in the observed radon concentrations in U.S. homes as well as uncertainty in K (Figure 3-3, case III). This increased the range of uncertainty in the AR under both risk models. For males, the 95% uncertainty intervals were 0.10–0.26 for the exposure-age-concentration model and 0.08–0.19 for the exposure-age-duration model. For females, the corresponding limits were 0.10–0.28 and 0.09–0.29, respectively.
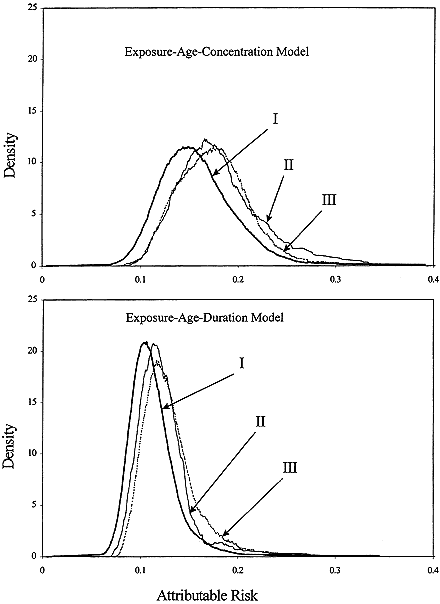
FIGURE 3-3a Uncertainty distributions for the population attributable risk (AR) for males. I: uncertainty in model parameters. II: uncertainty in model parameters; variability in K; variability in radon levels. III: uncertainty in model parameters; uncertainty/variability in K; variability in radon levels.
FIGURE 3-3b Uncertainty distributions for the population attributable risk (AR) for females. I: uncertainty in model parameters. II: uncertainty in model parameters; variability in K; variability in radon levels. III: uncertainty in model parameters; uncertainty/variability in K; variability in radon levels.
The uncertainty in the estimated values of the AR gives bounds to predictions of the number of lung-cancer cases attributable to residential radon exposure in the United States. For the exposure-age-duration model, the approximate 95% confidence limits on the AR imply a range of 11,400–26,200 lung-cancer cases for males and females combined. This range reflects statistical uncertainty in the central estimate of 15,400 cases given in Table 3-10. For the exposure-age-concentration model, the corresponding range is 14,800–38,600 cases, with a central estimate of 21,800 cases. The uncertainty ranges that take into account variability in K (case II in Figure 3-3) and uncertainty/variability in K (case III in Figure 3-3) are comparable in width but shifted slightly to the right because of the off-centering effect apparent in Figure 3-3 when variability in K is incorporated in the analysis. It is important to note that although these uncertainty limits encompass 95% of the mass of the uncertainty distributions in Figure 3-3, the uncertainty distributions place most of the mass nearer to the central values, indicating that values closer to the center of the distribution are most likely.
Because both the exposure-age-duration and exposure-age-concentration models fit the miner data equally well, the committee was unable to express a preference for either model. However, the committee noted that in as much as these 2 models are based on exposure levels in mines that generally exceed those in homes, model-based projections of the number of lung-cancer cases due to the presence of radon in U.S. homes are appropriate only if the models apply equally well at residential exposure levels.
To address that issue, the committee also calculated 95% uncertainty intervals for the projected number of lung-cancer cases attributable to residential radon exposure by using the constant-relative-risk (CRR) model restricted to exposures less than 0.175 Jhm-3 (50 WLM) (Table 3-9). Because this simple CRR model involves only a single unknown parameter ß (estimated to be 0.0117/WLM), 95% confidence limits on ß (0.002–0.225/WLM) can be used to obtain corresponding confidence limits on the AR. This simple uncertainty analysis, which focuses on the subgroup of miners with exposure levels closest to those in U.S. homes, provided 95% confidence limits of 3,300–32,600 lung-cancer cases about the central estimate of 17,500 cases based on the estimates of the AR given in Table 3-9. Although these confidence limits are wider than those based on the committee's 2 preferred models because of the smaller sample, the CRR model is based on observations closest to residential exposure levels.
As discussed previously and in appendix A, other factors might contribute to uncertainty beyond those included in this analysis. Nonetheless, this limited analysis does indicate that the population AR of lung-cancer due to radon in homes is subject to considerable uncertainty. The committee acknowledges that this analysis of uncertainty and variability depends on the specific assumptions made about uncertainty and variability in each of the factors affecting the AR. Because characterization of variability and especially uncertainty in the factors is
difficult, these particular assumptions reflect to a large extent the committee's best judgement.
COMPARISONS WITH BEIR IV
The BEIR VI committee's risk models are closely related to the model developed in the BEIR IV report. The BEIR IV committee combined data from 4 cohort studies of underground miners (Colorado Plateau, Ontario, Sweden, and Beaverlodge studies) and applied Poisson regression methods in model fitting. The starting point for the BEIR VI committee was the recent pooling of 11 studies by Lubin and others (1994a, 1995a), which included the same or updated data from the original 4 cohorts and data from seven additional cohorts. Closely comparable statistical methods were applied by both the BEIR IV and BEIR VI committees.
BEIR IV AND BEIR VI RISK MODELS
The committee's models are a direct extension of the BEIR IV model, which included parameters for time since exposure and attained age, but not exposure rate or exposure duration, as in the BEIR VI models. The form of the BEIR IV model is obtained from the BEIR VI models by setting ![]() and γz = 1, that is,
and γz = 1, that is,
Parameter values for the BEIR IV model and the BEIR VI models show the same general declining patterns for increasing time since exposure and attained age. The decline in the ratio of ERR per unit exposure with attained age, however, is more pronounced in the current models. Note that the values for β are not directly comparable, because the values reflect different baseline levels due to the inclusion of different modifying factors in the BEIR IV and BEIR VI models.
The overall ERR per unit of exposure in the absence of all modifying factors is not an adequate description of the relative risk from the miner studies and should not be used for formal comparisons. Nevertheless, the estimate of the overall ERR/Jhm-3 for the 4 cohorts used in the BEIR IV report was 3.8 Jhm-3 (0.0134/WLM), whereas the value was 1.4 Jhm-3 (0.005/WLM) for the pooled analysis of the 11 miner cohorts (Lubin and others 1994a). Those values afford a somewhat crude comparison, suggesting that the combined risk for miners in the 11 studies was less than the BEIR IV estimate. Figures 3-4 through 3-6 show more-direct comparisons of estimated LRR for selected exposure patterns in miners. Figure 3-4 shows LRRs by exposure rate from 0–5.95 Jm-3 (0–10 WL) for 5, 10, and 20 yr of exposure. BEIR IV estimates of LRRs for exposure rates of 0.60 Jm-3 (1.0 WL) and greater were higher than estimates from current models. Similar patterns are seen in Figure 3-5, which shows LRRs by duration
of exposure for exposures at constant rates of 0.30 Jm-3 (0.5 WL), 0.60 Jm-3 (1.0 WL), and 2.98 Jm-3 (5.0 WL).
Figure 3-6 provides comparisons of the various projections of LRR for lifetime exposure to radon at concentrations found in homes. A K-factor of 1.0, an equilibrium ratio of 0.4, and 70% home occupancy are assumed. Slightly higher LRRs are estimated with the exposure-age-concentration model than with the exposure-age-duration and BEIR IV models; however, estimates of LRRs are generally similar for concentrations of 1000 Bqm-3 (27.03 pCiL-1) and below, levels that include the large majority of dwellings.
SUMMARY AND CONCLUSIONS
Radon is one of the most extensively studied known human carcinogens. The series of cohort mortality studies of underground miners in countries throughout the
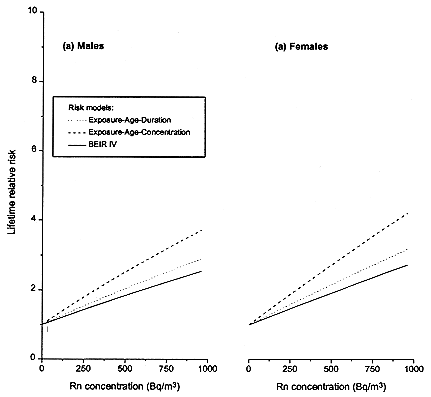
FIGURE 3-6 Predicted lifetime relative risk of lung-cancer for males and females by ''residential" radon concentration. Exposure occurs over a lifetime at a constant radon concentration.
world is highly informative with respect to the risk of lung-cancer associated with exposure to radon. In each of those studies, miners have been shown to be at excess risk for lung-cancer under past conditions of exposure. The quantitative estimates of exposures experienced by the miners, although subject to error, allow characterization of exposure-response relationships for radon and lung-cancer. These data formed the basis for the development of the committee's risk models.
Case-control studies of residential radon exposure and lung-cancer have also been conducted in various countries. Although also informative, the lower exposures of people in these studies and methodologic problems make it very difficult to identify the relationship between residential radon exposure and lung-cancer mortality in an individual study. However, the estimate of lung-cancer risk based on a recent meta-analysis of these 8 studies is in close agreement with the risk predicted on the basis of miner data.
The committee was fortunate to have available an update of the data on the 11 miner cohorts previously analyzed by Lubin and others (1994a). The most-recent data were used in developing the committee's risk models. The committee recognized that great care is needed in combining data from different cohorts of underground miners around the world. The levels of exposure to radon and other relevant covariates, such as arsenic and tobacco smoke, differed appreciably among groups of miners. The completeness and quality of the data available on relevant exposures also differed notably among the cohorts. Information on tobacco consumption was available for only 6 of the 11 cohorts; of these 6, only 3 had information on duration and intensity of exposure to tobacco smoke. Lifestyle and genetic factors that influence susceptibility to cancer might also account for heterogeneity among cohorts.
Despite those differences, the committee concluded that the best possible estimate of lung-cancer risk associated with radon exposure would be obtained by combining the available information from all 11 cohorts in a judicious manner. The committee used statistical methods for combining data that both allowed for heterogeneity among cohorts and provided an overall summary estimate of the lung-cancer risk. Confidence limits for the overall estimate of risk allow for such heterogeneity.
The committee's risk models described the ERR as a simple linear function of cumulative exposure to radon, allowing for differential effects of exposure during the periods 5–14 years, 15–24 years, and 25 years or more before lung-cancer death. The most weight was given to exposures occurring 5–14 years before death from lung-cancer. The committee entertained 2 categorical risk models in which the ERR was modified either by attained age and duration of exposure or by attained age and exposure rate. The ERR decreased with both attained age and exposure rate and increased with duration of exposure. For cumulative exposures below 0.175 Jhm-3 (50 WLM), a constant-relative-risk model without these modifying factors appeared to fit the data as well as the 2 models that allow for effect modification.
Lung-cancer risks associated with radon exposure were characterized in several ways. The LRR was used to describe the lifetime risk of lung-cancer among people continually exposed to radon throughout the course of their lifetime relative to the risk among unexposed individuals.
The percentage of lung-cancer cases that can be attributed to residential exposure to radon is of particular interest for risk management. The committee used data from the National Residential Radon Survey in combination with its 2 categorical risk models to estimate the AR posed by residential radon exposures. The ARs were estimated to be in the range 10–15%. These estimates are somewhat higher than the estimate of about 8% based on the data and methods of BEIR IV. About 30% of the AR was associated with homes having concentrations above 148 Bqm-3 (4 pCiL-1).
Although the AR percentages were comparable for ever-smokers and never-smokers under the multiplicative model, the number of radon-related lung-cancer cases was much higher among ever-smokers than for never-smokers under the multiplicative model. Of the approximately 157,000 lung-cancer deaths occurring annually, radon was estimated to play a role in about 15,000 to 22,000 cases. Of these, 13,000 to 19,000 were in ever-smokers and 1,000 to 3,000 in never-smokers, depending on the choice of the model. These computed values represent the best estimates of the lung-cancer risk attributable to radon that can be made at this time.
The committee recognized that these estimates are subject to uncertainty, including kinds of uncertainty that are not captured by statistical confidence limits on risk estimates. Consequently, the committee attempted a quantitative analysis of the uncertainty associated with estimates of the population AR. This analysis was itself limited, inasmuch as characterization of such sources of uncertainty as exposure measurement error in the miner data is difficult. Using data whenever possible and expert judgment otherwise, the committee attempted to describe the sources of uncertainty in its 2 categorical risk models.
The best estimates of the population AR were in the range 10 to 14% on the basis of the committee's preferred risk models. The quantitative analysis conducted by the committee provided limits within which the AR was considered to lie with 95% certainty. For the exposure-age-concentration model, the uncertainty interval ranged from about 9 to 25%, with central estimates of about 14%. This reflects a substantial degree of uncertainty in the AR, although the uncertainty distributions indicated that values near the central estimates were much more likely than values near the upper and lower limits. For the exposure-age-duration model, the AR ranged from 7 to 17%, and centered at about 10%. The committee also computed uncertainty limits for the simple constant-relative-risk model fitted to the miner data below 0.175 Jhm-3 (50 WLM), which is based on observations in miners closest to residential exposure levels. The latter analysis, which minimizes the degree of extrapolation outside the range of the miner data, led to uncertainty limits of 2–21%, with a central estimate of about 12%.
The committee also noted that its quantitative estimates of risks posed by residential radon exposure depend strongly on the assumption of a linear relationship, without a threshold, between low-dose exposure to radon and risk. As reviewed in chapter 2, that assumption is based on our current understanding of the mechanisms of radon-induced lung-cancer, although it is recognized that this understanding is incomplete. The committee did not attempt to quantitatively address uncertainty due to the linear, no-threshold assumption, because specific mechanistically plausible alternative dose-effect relationships were not identified in the committee's review.
Despite the uncertainty, the committee concluded that the weight of evidence of the available data supports a finding that residential-radon exposure increases lung-cancer risk. The best estimate of risk that can be obtained at this time is based on the committee's analysis of the combined updated data on the 11 cohorts of underground miners. The committee noted that this estimate is consistent with that derived from a recent meta-analysis of summary relative risks from the 8 residential case-control studies conducted to date.
The committee questioned whether further residential studies are likely to clarify the uncertainty surrounding residential-radon lung-cancer risks. The case-control studies conducted to date have been somewhat inconsistent. While most are compatible with the hypothesis of an elevated cancer risk, their results could also be interpreted as compatible with the hypothesis of no increase in risk in light of inherent uncertainties in data. Further, the residential studies offer only very limited information on never-smokers. Clear evidence of an increased lung-cancer risk in miners, many of whom were exposed to levels of radon only about two-fold higher than associated with residence in some homes, played an important role in supporting the committee's conclusions about the likelihood of an increased lung-cancer risk due to residential radon exposures. The committee agreed with the recommendations of workshops conducted by the Department of Energy and the Commission of European Communities: further studies should not be initiated until studies now in progress are completed and the data are pooled from these studies and studies already completed are pooled.
The committee examined the effect of reductions in radon levels in U.S. homes on lung-cancer risk, assuming different scenarios of the efficiency of reduction. On the basis of the committee's categorical risk models, reducing radon concentration in all homes that are above 148 Bqm-3 (4 pCiL-1) to below 148 Bqm-3 (4 pCiL-1) is estimated to result in the avoidance of about 3 to 4% of lung-cancers.