Executive Summary
Breast cancer is the most common malignancy among women and the second leading cause of cancer deaths in women in the United States. Despite the explosion of new knowledge from a variety of disciplines, women born in the United States have, on average, a 12.6% (or one in eight) chance of developing breast cancer. Estimates for 1996 predicted that more than 184,000 new cases of breast cancer would be diagnosed, and an estimated 44,300 women would die from breast cancer during this period (ACS, 1995). The incidence of breast cancer climbed at a rate of 1% to 2% per year during the past several decades until 1990 (Harris et al., 1992a; Miller et al., 1993). From 1990 to 1992, the incidence rate has been steady at approximately 110 cases per 100,000 women for all races. However, incidence and mortality rates vary by race. In 1992, age-adjusted incidences in Caucasian and African-American women were 113.1 versus 101.0 cases per 100,000 women, respectively. While mortality rates for Caucasian women have declined since 1990, mortality rates for African-American women have increased steadily since the 1970s. The 1992 age-adjusted mortality rates for Caucasian and African-American women were 26.0 and 31.2 deaths per 100,000 women, respectively, despite the lower incidence in African-American women (Kosary et al., 1996).
Breast cancer occurs when the epithelial cells of the breast begin to grow and divide uncontrollably, although there is some controversy as to what stage of this process is officially termed cancer. What causes the cascade of events that converts a normal breast cell into a malignant cell is unknown, but it is generally thought to involve a complex interaction of inherited genetic, hormonal, dietary, and environmental factors causing multiple new genetic changes in the involved cells.
The past decade has been a time of both great optimism and frustration in breast cancer research. The optimism stems in part from the emerging insights into the basic genetic and biochemical mechanisms of breast cancer; the frustration stems from the fact that while systemic treatment of breast cancer continues to make advances, the progress is relatively slow. This slow progress may be a reflection of the natural history of the disease, or a reflection of the lack of knowledge required to specifically target newer therapies and lower the toxicity of treatment. Scientists agree that until the causes of breast cancer are understood, its prevention or eradication is unlikely.
CHARGE TO THE COMMITTEE
In late 1995 the U.S. Army Medical Research and Materiel Command (USAMRMC) asked the Institute of Medicine (IOM) to review the implementation and progress of the Breast Cancer Research Program (BCRP). Specifically, the IOM was asked to: (1) review the portfolio of breast cancer research that has been funded by the Army's BCRP as well as breast cancer research supported by other public and private funding agencies; (2) provide an analysis of the BCRP as it has been implemented in response to the IOM (1993) recommendations, specifically assessing program management and program achievement; and (3) provide recommendations delineating important areas of research for which current funding and programs are not yet in place or in which additional emphasis is needed.
To undertake the stated task, the IOM appointed a multidisciplinary committee consisting of 13 individuals, including experts in basic, clinical, and public health research; surgical, radiation, and medical oncology; genetics; sociology; epidemiology; nursing; obstetrics and gynecology; health services research; health administration; and law. One member was also a breast cancer survivor with formal ties to a breast cancer advocacy group.
THE ARMY'S BREAST CANCER RESEARCH PROGRAM
For fiscal year (FY) 1992, Congress appropriated initial funding of $25 million for breast cancer research in the Army's Research, Development, Test, and Evaluation program for the purpose of pursuing interservice research on breast cancer screening and diagnosis for military women and dependents of military men (Public Law 102-172). This marked the beginning of the Army's BCRP. In FY 1993, Congress included $210 million to support a peer-reviewed competitive grants program in breast cancer research in the Defense Appropriations Act (Public Law 102-396). The Army subsequently assigned these funds to its Medical Research and Materiel Command, which continues to administer the BCRP. This appropriation was largely the result of the successful
lobbying efforts of the National Breast Cancer Coalition, which has continued to garner yearly support from Congress—$30 million in FY 1994 (Public Law 103-139), $150 million in FY 1995 (of which $35 million was earmarked for breast imaging technology and breast cancer centers) (Public Law 103-335), $75 million in FY 1996 (Public Law 104-61), and $112.5 million in FY 1997 (see Figure 1).
Because the FY 1993 appropriation represented a nearly tenfold increase in funds for the BCRP, and because Congress stipulated that the research funded must be externally peer-reviewed, the Army requested that the IOM provide recommendations regarding programmatic investment strategies and scientific peer review. The IOM issued the report Strategies for Managing the Breast Cancer Research Program: A Report to the U.S. Army Medical Research and Development Command (IOM, 1993). This report recommended a program designed to advance breast cancer research specifically by nurturing new avenues of investigation and attracting new investigators into the field. It recommended a three-pronged programmatic investment strategy: (1) scientist training and recruitment, (2) infrastructure enhancement, and (3) investigator-initiated research. The report also recommended implementation of a two-tiered system of peer review—the first tier to assess the scientific excellence of the research proposals and the second tier to award funding based on their programmatic relevance. The report emphasized the importance of ''channeling
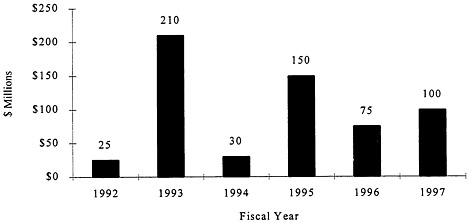
FIGURE 1. Appropriation history of the BCRP.
research funds in directions that stimulate innovative ideas, involve interdisciplinary research, enhance the use of existing research resources, and reward scientific excellence among all disciplines" (IOM, 1993).
METHODS
The current committee had access to a broad array of information concerning the Army's BCRP and its portfolio of funded research. It also benefited from discussions with the program director and staff, program contractors and scientific advisors, consumer participants, and others directly involved in the scientific peer review process. Written comments were received from almost 100 grantees of the program in response to a "Dear Colleague" letter sent to all grantees by the IOM. The committee also held discussions with representatives of other major breast cancer research funding organizations, both public and private, and with representatives of breast cancer advocacy groups. Extensive searches of published literature and of federally funded research in progress provided the committee with citations and abstracts of research specific to breast cancer. The committee used these information sources and called upon its collective expertise to assess the Army's BCRP and develop its recommendations.
FINDINGS
The Army's Breast Cancer Research Program Operation
The Army's BCRP has evolved over the last 5 years from a small research program pursuing interservice research on breast cancer screening and diagnosis into an organization pursuing a broad-based, competitively awarded research portfolio covering all areas of breast cancer research with approximately $500 million appropriated by Congress over the 4-year period. In its brief history as a peer-reviewed, competitive grants program, the BCRP has reviewed over 7,000 research proposals and developed a diversified $465 million research portfolio of approximately 800 projects distributed to public and private research institutions across the United States and internationally.
The BCRP is unique among breast cancer funding sources because it includes consumers (breast cancer survivors or other qualified persons) as voting members of both the scientific peer review panels and the programmatic review panel, and the management framework of the program allows relatively quick changes in direction. These positive aspects of the program provide linkage to highly interested constituents and great opportunity to respond to new research breakthroughs.
The current structure of the BCRP uses two outside contractors—one to support the activities of the scientific peer review process and the other to support the activities of programmatic review. This structure acknowledges the Army's limited expertise in managing scientific peer review of a competitive grants program in areas not directly relevant to the military mission. This structure appears to work well overall, and has kept annual overhead costs to under 10% of program dollars. The program management team and the Integration Panel that fulfills the role of the "advisory council" envisioned by the 1993 IOM report have instituted yearly improvements in the requests for proposals (Broad Agency Announcements) and in the scientific review process, have refined the programmatic vision and goals, and have streamlined the application process. For the 1993/1994 and the 1995 funding cycles, the program closely followed investment strategies and funding allocations recommended originally by the 1993 IOM committee; but significant changes were made for the FY 1996 funding cycle.
The Breast Cancer Research Program Portfolio
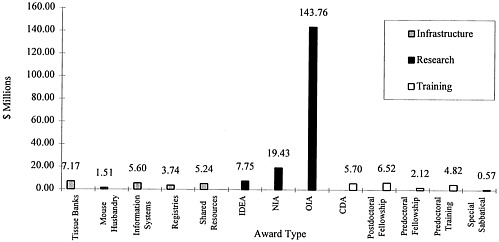
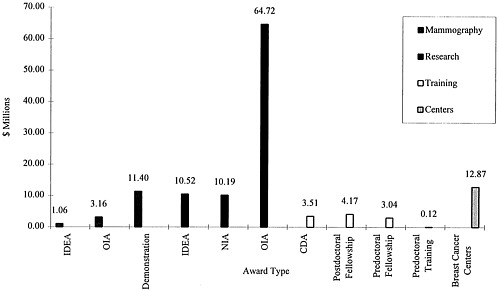
As recommended by the report Strategies for Managing the Breast Cancer Research Program: A Report to the U.S. Army Medical Research and Development Command (IOM, 1993), the Army has pursued an investment strategy that included research, training, and infrastructure enhancement in the FY 1993/1994 funding cycle (Figure 2). Of the total program expenditures of $218.8 million in FY 1993/1994, approximately 78% of the funds ($170.9 million) went to research projects and the remaining funds ($47.9 million) went for training and infrastructure enhancement. The funded research projects can be further subdivided into New Investigator Awards (NIAs) with 11.4% of the research funds, IDEA grants with 4.5% of the research funds, and more traditional Other Investigator-Initiated Awards (OIAs) garnering 84.1%. In FY 1995 (Figure 3), of the $86 million specified for funding research projects a greater proportion was directed toward IDEA grants (12%) while a proportionately smaller amount was directed to more traditional OIA grants (76%). NIAs stayed constant with approximately 12% of research funds.
The FY 1995 appropriation included $35 million designated by Congress for mammography and breast cancer centers. In FY 1996, the BCRP made a significant change in direction, targeting over 50% of funding for IDEAs, 20% for translation research, and 27% for training grants.
To date, the Army's investment in its research portfolio for breast cancer—across all types of awards—has, like the NCI, been heavily focused on cell and molecular biology and genetics (50%–60%), with 5%–9% of funded research on risk factors, 3%–5% on epidemiology, 8%–11% on detection, 6%–11% on
mammography, 3%–5% on psychosocial research, and 4%–7% on studies of health care delivery. In 1993/1994, only 3.8% of funded awards focused on minority or underserved populations; this increased to 9.6% of all awards in 1995 despite the smaller amount of funding available. However, from the 1993/1994 funding cycle to the 1995 funding cycle there was a slight decrease in the percentage of grants funded in basic research, and slight increases in the percentage of grants funded in the other categories.
Support for Breast Cancer Research Other Than the Army's Program
The Department of Defense (DOD) supports other breast-cancer-related research in addition to the Army's BCRP. Total DOD expenditures for breast cancer research, outside the Army's program, were $3.7 million in 1994 and $1.7 million in 1995. This included grants funded under the Defense Women's Health Program and the TriService Nursing Research program. A large percentage of the studies funded are in detection, imaging, and basic science.
Outside the DOD, a number of other federal agencies and private organizations fund breast cancer research (Table 1). Approximately $15 million was awarded by the Department of Energy (DOE), the National Science Foundation. (NSF), the U.S. Department of Agriculture (USDA), and the Department of Veterans Affairs (DVA) in 1994 (see Table 1, "Other federal government"), and about $13.3 million was awarded by these agencies in 1995. Of these agencies, DVA was the largest funder at $7.9 million in 1994. The vast majority of these funds went to VA medical centers for clinical trials of new chemotherapeutic agents, medical and surgical interventions, and prosthetic research. DVA also funded investigations in the behavioral sciences and patient education. DOE provided $5.7 million in research support, with an additional $1.2 million from the NSF and $420,000 from USDA. The research focus among these agencies is generalized: basic science, epidemiology, clinical trials, and technical advancement in diagnostics.
The National Institutes of Health (NIH) of the Department of Health and Human Services (DHHS), along with the Army's BCRP, is the major federal contributor to breast cancer research in the United States. The NIH consists of 21 institutes and centers but the majority of its cancer research is funded through the National Cancer Institute (NCI). Of the approximately 1,500 grants related to breast cancer research awarded by NIH in FY 1994, 1,200 were funded by the NCI. There are other institutes and centers within NIH that also fund breast cancer research, either directly or indirectly. The NCI dedicated $308.7 million to breast cancer research in FY 1995, approximately 16% of its total budget exclusive of funding for AIDS research.
In 1993 the California State Assembly established a breast cancer research and breast cancer control program to be funded with revenue from an increase
in the state tobacco tax and to be administered by the University of California. This program awarded approximately $20 million in grants as of FY 1995 (CBCRP, 1997).
The American Cancer Society (ACS) and the Susan G. Komen Foundation are the two largest private funding sources for breast cancer research. In 1996 the ACS funded over $14 million in research on breast cancer.
TABLE 1. Dedicated Breast Cancer Research Funding in the United States ($ thousands)
|
Federal Government |
|
|
Department of Defense |
|
|
USAMRMC—BCRP |
$75,000 (FY 1996) |
|
Other DOD expenditures |
3,869 (FY 1994) |
|
Department of Health and Human Services |
|
|
National Cancer Institute |
336,700 (FY 1996) |
|
Other National Institutes of Health Centers |
At least 30,000 (FY 1996) |
|
National Action Plan on Breast Cancer |
14,500 (FY 1995/1996) |
|
Other federal government |
13,300 (FY 1995) |
|
State Governments |
|
|
California Breast Cancer Research Program |
20,000 (as of FY 1995) |
|
Private Foundations |
|
|
American Cancer Society |
14,000 (FY 1996) |
|
Susan G. Komen Foundation |
6,700 (FY 1996) |
This is in addition to the $64.5 million provided by ACS in support of basic cancer biology research with its overlapping application to breast cancer (ACS, 1996a). The Susan G. Komen Foundation provided over $6 million in breast cancer research funding in 1996. One aspect of the Komen Foundation program is its focus on identifying and supporting opportunities involving education and health care delivery (Komen Foundation, 1996).
In addition, a 1995 survey by the Pharmaceutical Research and Manufacturers of America indicated that approximately 215 new medications are being tested in cancer therapy trials, including 48 drugs specifically for breast cancer (PhRMA, 1995).
Research Advances and Opportunities
Searches of the published literature on breast cancer research indicate that approximately 50% of the over 4,000 results for 1994 and 1995 address the basic genetic, cellular, and molecular factors relevant to the origin and
progression of breast cancer. Approximately 17% and 13% of published studies were relevant to epidemiology and the analysis of risk factors, respectively. Another 12% focused on breast imaging, including mammography, while studies examining psychological, social, and quality of life issues represented 5% of the reported studies. Health care delivery was the focus of only 3 of the more than 4,000 published reports, making up less than 0.1%.
Studies in genetics, cellular biology, and molecular biology are providing glimpses into the intricate mechanisms that determine when a cell is to grow, differentiate, or die. These studies are providing insights into how the genes involved in cancer disrupt this process. Several genes have been identified that are associated with breast cancer; however, many of the gentic changes identified occur during tumor progression and not in initiation of the malignant process. There are two recently discovered genes (BRCA1 and BRCA2) that appear to be responsible for a significant fraction of inherited breast cancer as well as some ovarian cancer. But extensive epidemiological studies, spanning decades, have demonstrated that the etiology of breast cancer is extremely complex, involving multiple endogenous and exogenous risk factors.
Progress has been slow in the areas of detection and treatment of breast cancer although a variety of new screening techniques are under investigation. The major focus of systemic treatment continues to involve conventional therapies such as chemotherapy and hormonal therapy. Current advances include the development of a new class of therapeutic agents, and the integration of laboratory advances in monoclonal antibody production into the clinical arena.
There is a need to incorporate newer therapies into clinical trials and to better understand the effectiveness of these, as well as standard approaches of systemic therapies, in women traditionally underrepresented in clinical studies— women who are older, less affluent, and ethnically diverse. For these women there are also differences in access to medical care.
The diagnosis of breast cancer and its treatment frequently results in a significant emotional, social, and financial toll on patients and their families. While the capability exists to measure these consequences, research has only begun to address them. A better understanding of psychological, social, and quality of life issues can contribute to the process of continuing care, thus supporting women and their families in their efforts to cope with issues of survivorship and recurrence. In addition, tests for mutations in the BRCA1 and BRCA2 genes are becoming clinically available. This capability has multiple ethical, legal, and psychosocial consequences that have as yet not been fully understood or addressed.
CONCLUSIONS
The committee concluded that the USAMRMC has succeeded in establishing a fair peer review system and a broad-based research portfolio by stimulating scientists from a wide range of disciplines to participate as applicants, reviewers, and advisers. The committee commends the Army for developing such a program under the serious time constraints and fluctuations in funding that have characterized the program to date. Moreover, the program fills a unique niche among public and private funding sources for cancer research. It is not duplicative of other programs and is a promising vehicle for forging new ideas and scientific breakthroughs in the nation's fight against breast cancer.
Among the most outstanding features of the program are the flexible approaches for setting priorities annually; the involvement of breast cancer advocates (consumers) in the grant peer review process; the level of commitment and diligence of the individuals who serve the program in various capacities; the commitment and support of the program director; the low administrative costs that allow the greatest share of funding resources to be awarded as grants; the use of outside experts for evaluation; and the unwavering respect and advocacy for this program among breast cancer advocacy organizations nationwide.
Based on abstracts of funded projects in the 1993/1994 and 1995 cycles, the committee determined that the portfolio covers science that is responsive to the range of six questions posed in the 1993 IOM report. As envisioned by that report the majority of funds support studies on the basic molecular and cellular biology of breast cancer. Since research results in the form of peer-reviewed publications were not yet available, the committee considered it premature to evaluate the quality of the portfolio of funded projects and, indirectly, the success of the BCRP investment.
The committee is concerned about the wide range of responsibilities currently given to the integration panel (IP). It recognizes a need for independent evaluation of the function of both tiers of review by an oversight group outside the Army, given the lack of scientific infrastructure within the Army.
RECOMMENDATIONS RELATED TO PROGRAM ACHIEVEMENT AND MANAGEMENT
-
Continue the Army's BCRP and make efforts to obtain multi-year authorization of and funding for it. Longer-term stability would allow longer-range programmatic planning, establishment of standing peer review panels, and implementation of more efficient and effective grants administration procedures (e.g., more timely release of the Broad Agency Announcement (BAA),
-
recruitment of appropriate reviewers, and optimization of review assignments). This could be achieved through either incorporation of the program into the annual DOD budget or multi-year authorization of funding by Congress.
-
Develop and implement a plan with benchmarks and appropriate tools to measure achievements and progress towards goals of the BCRP annually and over time. This would allow an evaluation of the effectiveness of the different funding mechanisms, with particular emphasis on IDEA grants (e.g., have the IDEAs generated new avenues of research or provided major breakthroughs) and recruitment and training grants. Elements of the process could include examination of records of publications and presentations, success in obtaining other grant support relevant to breast cancer, and identification and tracking of investigators who were recruited into breast cancer research by BCRP funding. Program evaluation should also measure achievements of the programmatic aims outlined in the 1993 IOM report.
-
Consider establishing a permanent non-Army oversight committee that is independent of both the IP and the contractors. Since responsibility for recommendations on policy and executive functions both rest with the IP, some members of the committee agreed that a separate mechanism for oversight and evaluation of the BCRP should be established. For other committee members, the fact that the IP has responsibilities in both areas was of lesser concern since no evidence was detected that the IP had failed to meet or had abused its responsibility. Despite differing views on the committee regarding the need for a group to oversee the work of the IP and the BCRP in general, the majority of this committee agreed to recommend the establishment of a relatively small permanent oversight group that would be responsible for quality assurance and program evaluation activities. This group would include scientists and clinicians experienced in directing research programs, widely respected leaders in cancer research, as well as a consumer representative. Members could come from academic, medical, and other relevant organizations. The group would report directly to the BCRP Director and would have access to all information needed to oversee and rigorously evaluate the program in an ongoing fashion.
The committee also recommends the following for program improvement (Box 1), with rationale for these recommendations provided in Chapter 7 of the report.
|
BOX 1. Other Recommendations
|
RECOMMENDATIONS FOR FUTURE RESEARCH DIRECTIONS
The 1993 IOM report identified six questions on the causation, prevention, screening, detection, diagnosis, and optimal treatment of and recovery from breast cancer that were to be used as a framework for breast cancer research. Noting that 50% of the funding to date has gone to address the first two questions, the committee reiterates the continuing importance of the other questions and finds that the six fundamental questions remain a useful framework for elaborating its recommendations for future research emphasis, as follows:
-
What genetic alterations are involved in the origin and progression of breast cancer?
-
What are the changes in cellular and molecular functions that account for the development and progression of breast cancer? The first two questions address a single fundamental issue, the identification of the cellular events involved in the pathogenesis of breast cancer. The identification and characterization of the genes involved in breast cancer initiation and progression, including invasion and metastasis, will facilitate study of the basic physiology and biochemistry of the normal breast, because it will become
-
possible to assess the role of these genes in normal breast development and function.
Studies to understand the mechanisms involved in tumor initiation and progression, the sequential steps from normalcy to malignancy in the breast, and the biochemical and biological functions of the relevant gene products present great opportunities for the development of new approaches to control this disease. Such studies may result in the development of diagnostic tools capable of identifying heritable and acquired changes that can be detected before the cells become invasive, or even in the premalignant phase, and also in knowledge of the likelihood of an in situ cancer's progressing to invasion. Furthermore, novel therapies capable of eliminating or terminally differentiating the breast cells carrying the genetic changes predisposing to malignancy could be developed. The development of such gene therapy requires a better understanding of the genetic and immunological basis of breast cancer, with the vaccine approach to prevention and treatment facilitated by knowledge of the new altered gene products and peptides expressed in cancer cells. Innovation and progress in any one of the areas noted here depends on progress in other diverse areas.
-
How can endogenous and exogenous risk factors for breast cancer be explained at the molecular level? The challenge to epidemiology is to move beyond examination of traditional risk factors to basic and applied investigations using genetic information to assess both risk and prognosis factors. Knowledge of the genes involved in the complex cascade of events leading to tumor development and progression will not, by itself, tell us how best to intervene in the process. The goal should be a complete understanding of the natural history of breast cancer through molecular epidemiological research. Studies of interactions of genetic and environmental or other nongenetic factors should be given high priority. This work will require close collaboration among clinical and basic scientists. The natural history of breast cancer and factors that influence prognosis need to be understood at both a histological and a molecular level. Epidemilogical studies should evaluate new and existing risk factors at the molecular level with emphasis on hormonal, geographic, and family history variables. Emphasis should be placed on identification of new factors whose molecular mechanisms explain cancer risks not explained by known risk factors. There is an ongoing need for methodological research—investigations into measurements of exposure, intermediate markers of carcinogenic processes, and sources of bias that can affect new types of studies.
-
How can investigators use what is known about the genetic and cellular changes in breast cancer patients to improve prevention, detection, diagnosis, treatment, and follow-up care? Knowledge of a woman's genetic makeup should allow determination of whether she would benefit from a particular treatment and of what her chances would be for good health and quality of life. Studies to determine the optimal way to counsel women with
-
genotypes that place them at risk will assist in developing informed consent procedures for testing and methods for effectively communicating test results. Implementation of preventive measures in high-risk women requires the full understanding of the natural history of breast cancer and the efficacy of various interventions, stratified by genotype information.
Multi-institutional, randomized, and controlled clinical trials should precede the widespread clinical application of promising clinical research. Long-term outcome studies based on established clinical trial principles and statistical methods should be continued to validate (or not) the final outcome—for example, mortality. The outcome studies should include quality of life and risk tolerance issues. Finally, there is a need to update periodically systematic reviews of these trials.
Furthermore, since 1993, women with breast cancer have had increasing influence in discussions relating to the direction and content of breast cancer research and they will continue to do so. For example, in testimony to this IOM committee, consumers have asked for additional research in the areas of prevention and treatment of lymphedema, long-term effects of axillary node dissection, living with metastatic disease and treatment for it, hormone replacement therapy for menopause, detection and prevention measures for women with inherited susceptibility to breast cancer, and weight management.
Complementary and alternative medicine interventions should be subjected to the same standards of testing as traditional interventions. About one-third of Americans are using complementary and alternative medicine, and breast cancer patients are particularly interested in these approaches, despite the widespread negative views held by physicians trained in the Western world.
-
What is the impact of risk, disease, treatment, and ongoing care on the psychosocial and clinical outcomes of breast cancer patients and their families? Behavioral, psychological, and social research has focused increasingly on racial, ethnic, and cultural differences, and the psychological effects of genetic testing for breast cancer susceptibility. Work in these areas should continue where gaps remain. There is increasing recognition of the importance of survivorship issues, especially because growing numbers of women are living longer with the disease. Survivorship issues are encompassed under the rubric of ''health-related quality of life" research. Studies are needed to better understand how breast cancer and its treatment influence women's evaluation of the quality of their lives and which variables are most influential in terms of diminishing or improving the health-related quality of life of breast cancer survivors and their families. Thus, there is continuing concern with improving knowledge of the range of disease and treatment consequences that occur such as body image, depression, early menopause, the psychological impact of long-term treatments, the impact of breast cancer on family and caregivers, economic hardship (e.g., loss of earnings, treatment costs), functional limitations (e.g., sexual and physical), and social role disability.
-
Studies of disability prevention are also essential for maximizing the breast cancer survivor's ability to participate in valued social roles and activities.
-
How can investigators define and identify techniques for delivering effective and cost-effective health care to all women to prevent, detect, diagnose, treat, and facilitate recovery from breast cancer? The IOM (1993) outlined a number of target topics for health services research including: barriers to state-of-the-art health care, health care seeking behavior, patient treatment preferences, and barriers and inducements to participation in clinical trials. These topics remain important. Other areas for investigation that have emerged include access to care, patterns of utilization of health services, patient—provider communication, provider education and behavior, economic and cost analyses, issues relating to policy setting and guidelines, and health care delivery systems.
Use of computer information systems is increasingly important in patient tracking, tissue bank administration, networking genetic information, and facilitating enrollment in clinical trials. These systems require additional investigation prior to widespread implementation because of confidentiality and acceptability issues.
Studies regarding ethnic, cultural, and personal differences in health beliefs and health care seeking behavior will yield important information for those providing care and setting policy. Also necessary is accurate, reliable, unbiased information on direct and indirect costs associated with genetic testing, prevention strategies, screening and diagnostic techniques, or a given treatment; such information is a critical component of realistic health care planning and delivery. An area of urgent importance is the effect of managed care on breast cancer screening, detection, treatment and follow-up. There is concern about the trade-off between quality and cost of health care.