3
Overview of Programs of Research on Ethnic Minority and Medically Underserved Populations at the National Institutes of Health
The National Institutes of Health (NIH) is responsible for a broad range of basic, biomedical, behavioral, epidemiologic, and clinical research that addresses America's health needs. Research on the prevention, detection, treatment, and control of cancer is the primary responsibility of the National Cancer Institute (NCI). The National Cancer Act of 1971 (P.L. 75-244) directs NCI to plan and develop a coordinated research program that encompasses all institutes, centers, and divisions (ICDs) of NIH, as well as other federal and nonfederal research organizations, and to develop a cancer control program that demonstrates effective practices in cancer prevention and management. NCI interprets its mission as follows:
The National Cancer Institute coordinates the National Cancer Program, which conducts and supports research, training, health information dissemination, and other programs with respect to the cause, diagnosis, prevention, and treatment of cancer, rehabilitation from cancer, and the continuing care of cancer patients and the families of cancer patients. Specifically, the Institute:
- Supports and coordinates research projects conducted by universities, hospitals, research foundations, and businesses throughout this country and abroad through research grants and cooperative agreements.
- Conducts research in its own laboratories and clinics.
- Supports education and training in fundamental sciences and clinical disciplines for participation in basic and clinical research programs and treatment programs relating to cancer through career awards, training grants, and fellowships.
- Supports research projects in cancer control.
- Supports a national network of cancer centers.
- Collaborates with voluntary organizations and other national and foreign institutions engaged in cancer research and training activities.
- Encourages and coordinates cancer research by industrial concerns where such concerns evidence a particular capability for programmatic research.
- Collects and disseminates information on cancer.
- Supports construction of laboratories, clinics, and related facilities necessary for cancer research through the award of construction grants (National Cancer Institute, 1998d).
Although NCI directs a large and comprehensive program of cancer research within its portfolio and collaborates with other groups on research or cosponsors other cancer research at other ICDs, the committee finds that there is little evidence of a strategic plan for cancer research relevant to ethnic minority and medically underserved populations at NIH coordinated through NCI or any other central mechanism, as noted below.
This section describes the range of ongoing cancer-related research and programs at NCI and other ICDs, summarizes cancer-related research programs at NCI and other ICDs that are relevant to ethnic minority and medically underserved populations, and reviews the funding for these programs. Particular emphasis is placed on the programs and functions of NCI, given its stated role in coordinating cancer-related research at NIH.
Overview of NIH Appropriations and Funding for Cancer Research
Over the past decade, NIH and NCI have enjoyed significant increases in congressional appropriations, from periods of little to no growth in the early 1980s to steady increases in the mid-1990s (Figure 3-1). NCI experienced a slight decline in its budget from 1980 ($1 billion) to 1983 ($987.6 million), but by 1986 the Institute's budget reached $1.26 billion, and it had reached nearly $1.6 billion by the end of the decade (National Cancer Institute, 1998e). Annually, nearly 80 percent of the institute's budget is dedicated to research, whereas approximately 10 percent of the budget is allocated toward both resource development and cancer prevention and control activities. In fiscal year (FY) 1997, $1.411 billion was allocated for research grants, including $577 million for investigator-initiated grants (R01 grants) and $132 million for cancer center grants. More than $412
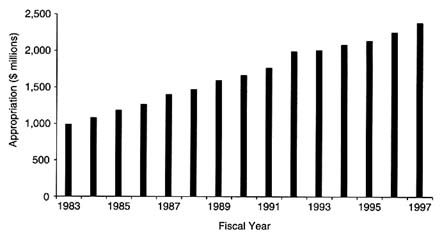
FIGURE 3-1 National Cancer Institute appropriations, 1983–1997.
SOURCE: National Cancer Institute.
million was reserved for intramural research and $231 million was allocated for cancer prevention and control.
NCI has appeared to prioritize extramural spending on traditional R01 grants (increasing allocations for R01 grants from $356 million in FY 1986 to $377 million in FY 1989, even though the number of such grants declined from 2,508 to 2,239), outstanding investigator grants (increasing spending from $23.2 million in FY 1986 to nearly $53 million in FY 1989), cooperative agreements (doubling spending of $10 million in FY 1986 to $20 million by FY 1989), and intramural research (increasing spending from nearly $214 million in FY 1986 to $294 million by FY 1989; National Cancer Institute, 1998e). (See Box 3-1 for a description of common NIH research grant mechanisms.)
By FY 1993, NCI's budget topped $2 billion for the first time, allowing the Institute to increase funding for R01 grants to $430 million (although the number of grants awarded decreased again to 1,955), increasing MERIT awards from $32 million in FY 1989 to $51.6 million in FY 1993, increasing spending on cancer control contracts from $33 million in FY 1989 to more than $52 million in FY 1993, and nearly doubling the cancer career grant program over FY 1989 levels to $14 million (National Cancer Institute, 1998e).
Congressional appropriations for NCI topped $2.38 billion in FY 1997 and fueled increases in both funding and the number of R01 grants made by the institute (more than $577 million was applied to 2,194 awardees). The number and amount allocated for First Independent Research Support
|
Box 3-1
|
and Transition awards also increased ($47 million in awards to 446 grantees, an increase of more than $18 million from FY 1993 levels), as was the case for U01 cooperative agreements (more than $81 million in awards, up from $56 million in FY 1993) and the Clinical Cooperative Program (more than $86 million in awards, an increase of more than $12 million from FY 1993). Spending on cancer control grants and contracts more than doubled to more than $70 million and $110 million each, respectively (National Cancer Institute, 1998e).
Cancer Research at Other ICDs
Although the increases outlined above do not represent the sum of spending on all cancer-related research at NIH, NCI has traditionally allocated the largest amount of any of NIH's ICDs on cancer research. Over the past several years, NCI's share of this budget has hovered at slightly greater than 85 percent of the total amount of NIH money spent on cancer-related research (National Institutes of Health, 1998). Many cancer-related grant programs sponsored by ICDs other than NCI enjoy joint sponsorship with NCI. In FY 1997, NIH spent approximately $2.76 billion on cancer research, more than 86 percent of which was funded directly by NCI. It is expected that by FY 1999 overall spending by NIH on cancer-related
research will exceed $3.23 billion, fueled in large part by the Clinton Administration's request for NCI appropriations of $2.77 billion.
The National Institute of Environmental Health Sciences (NIEHS) holds the second largest portfolio of cancer-related research among the institutes at NIH, with allocations of $84.44 million in FY 1997, a figure that approached nearly $90 million in FY 1998. The National Heart, Lung, and Blood Institute (NHLBI) is third in cancer-related funding with funding, of $57.6 million in FY 1997, followed by the National Institute of Allergy and Infectious Diseases (NIAID) with funding of $43 million and the National Institute on Diabetes and Digestive and Kidney Diseases (NIDDKD) with funding of $33.4 million (see Table 3-1).
Examined by spending on specific cancer sites, cancer types, diseases related to cancer, and types of research mechanisms, NIH reports spending the greatest amount of money on cancer clinical trials (more than $400 million in FY 1997, an increase of more than $150 million from FY 1990), followed by funding for breast cancer (more than $330 million in FY 1997, a fourfold increase over FY 1990 levels of $81 million), cancer prevention and control activities (nearly $240 million in FY 1997, up from $80.5 million in FY 1990), AIDS-related cancers ($224 million spend in FY 1997, up from $149 million in FY 1990), and lung cancer ($132 million in FY 1997, up from $65 million in FY 1990). Among cancers that disproportionately affect ethnic minority and medically underserved communities (in addition to the cancer types described above), NIH spent $74 million across ICDs on prostate cancer-related research in FY 1997 (up from $13.2 million in FY 1990), $54 million on cervical cancer (an increase of $30 million from FY 1990 levels), approximately $100 million on colorectal cancer (nearly doubling spending from $51.2 million in FY 1990), and $39 million on ovarian cancer (nearly four times the $10.5 million spent in FY 1990). In contrast, other cancers that disproportionately affect minority and medically underserved groups, such as liver cancer ($33 million in FY 1997) and uterine cancer ($8.6 million in FY 1997), have not received substantial increases in funding, with increases of only about $5 million and $2 million, respectively, since FY 1990. It must be noted, however, that funding for these disease areas can and often does overlap. Basic research and clinical research that benefits more than one type of cancer site are included in estimates of total funding for each cancer (see Table 3-2; National Institutes of Health, 1998).
Office of Research on Minority Health
The NIH Office of Research on Minority Health (ORMH) was established in 1990 by then-director of NIH William Raub and was authorized by the U.S. Congress in the 1993 National Institutes of Health Revitalization
TABLE 3-1 National Institutes of Health Cancer Research Initiative
|
|
Dollars (in thousands) |
|||
|
Participating ICDS |
FY 1997 Actual |
FY 1998 Estimate |
FY 1999 Estimate |
% Change 1999/1998 |
|
NCI |
$2,389,041 |
$2,547,314 |
$2,776,267 |
$9.0 |
|
Cancer % to Total |
86.50% |
86.6% |
85.9% |
|
|
NHLBI* |
57,620 |
59,815 |
67,539 |
12.9 |
|
NIDR |
16,448 |
17,313 |
19,811 |
14.4 |
|
NIDDK |
33,430 |
36,450 |
39,350 |
8.0 |
|
NINDS |
17,929 |
18,734 |
24,468 |
30.6 |
|
NIAID |
43,085 |
44,377 |
47,927 |
8.0 |
|
NIGMS |
22,574 |
24,421 |
30,421 |
24.6 |
|
NICHD |
10,311 |
11,000 |
11,900 |
8.2 |
|
NEI |
8,616 |
9,242 |
9,508 |
2.9 |
|
NIEHS |
84,368 |
89,430 |
94,796 |
6.0 |
|
NIA |
12,183 |
12,730 |
14,990 |
17.8 |
|
NIAMS |
5,303 |
5,690 |
6,220 |
9.3 |
|
NIDCD |
2,910 |
3,087 |
4,296 |
39.2 |
|
NIMH |
4,287 |
3,823 |
4,116 |
7.7 |
|
NIAAA |
2,700 |
2,000 |
2,500 |
25.0 |
|
NINR |
3,570 |
4,250 |
4,570 |
7.5 |
|
NHGRI |
17,084 |
22,158 |
30,151 |
36.1 |
|
NCRR |
25,926 |
28,754 |
37,874 |
31.7 |
|
FIC |
195 |
575 |
600 |
4.3 |
|
NLM |
0 |
0 |
4,500 |
0.0 |
|
NIH |
2,760,698 |
2,941,163 |
3,231,804 |
9.9 |
|
* All years adjusted to reflect Women's Health Initiative. SOURCE: National Institutes of Health. |
||||
Act (P.L. 103–43). Its mission, as established by Congress, is to coordinate the development of NIH policies, goals, and objectives related to minority health research and research training programs and to expand the level of participation of minorities in all aspects of biomedical research (including training of minority scientists and participation of ethnic minority individuals in NIH-sponsored clinical trials). ORMH seeks to accomplish these goals largely by working in partnership with other NIH ICDs, as well as other governmental agencies.
ORMH holds no independent grant-making authority; its primary function in addressing minority health research needs is to leverage research support by creating partnerships with other NIH institutes. In effect, ORMH collaborates with NIH institutes and centers (ICs) to support research and training projects. Administrative and professional support
TABLE 3-2 Research Dollars (in millions) by Various Cancers
|
|
1990 Actual |
1991 Actual |
1992 Actual |
1993 Actual |
1994 Actual |
1995 Actual |
1996 Actual |
1997 Actual |
President's Budget |
|
Total NCI* |
$1,644.3 |
$1,712.7 |
$1,947.6 |
$1,978.3 |
$2,076.2 |
$2,130.3 |
$2,254.9 |
$2,381.1 |
$2,441.7 |
|
AIDS |
$149.2 |
$160.9 |
$165.7 |
$173.0 |
$213.0 |
$217.4 |
$225.4 |
$224.7 |
$224.3 |
|
Brain and central nervous system |
29.8 |
31.5 |
32.5 |
40.5 |
41.7 |
43.0 |
41.6 |
44.2 |
46.3 |
|
Breast cancer |
81.0 |
92.7 |
145.0 |
211.5 |
267.6 |
308.7 |
317.5 |
332.9 |
338.9 |
|
Cancer prevention and control |
80.5 |
90.8 |
114.9 |
112.6 |
153.9 |
205.0 |
22.0 |
248.7 |
251.0 |
|
Cervical cancer |
21.9 |
22.3 |
30.7 |
42.2 |
42.3 |
45.5 |
51.6 |
54.0 |
56.0 |
|
Clinical trials |
246.0 |
254.4 |
314.5 |
326.8 |
339.0 |
384.8 |
393.8 |
403.9 |
412.6 |
|
Colorectal cancer |
51.2 |
56.5 |
69.2 |
74.2 |
83.1 |
96.5 |
98.0 |
99.0 |
100.0 |
|
Hodgkin's disease |
7.5 |
7.8 |
6.7 |
6.8 |
6.7 |
7.8 |
8.0 |
8.4 |
8.8 |
|
Leukemia |
50.4 |
60.1 |
64.6 |
74.2 |
77.7 |
77.5 |
79.3 |
83.0 |
86.0 |
|
Liver cancer |
28.3 |
29.8 |
30.7 |
37.5 |
37.9 |
38.0 |
31.4 |
33.2 |
34.7 |
|
Lung cancer |
65.1 |
68.7 |
76.3 |
92.9 |
106.4 |
113.9 |
119.4 |
123.3 |
128.2 |
|
Melanoma |
21.2 |
26.2 |
24.8 |
29.8 |
33.4 |
31.8 |
36.0 |
37.3 |
38.3 |
|
Non Hodgkin's lymphoma* |
|
|
33.4 |
40.1 |
38.7 |
39.7 |
49.9 |
51.5 |
52.7 |
|
Ovarian cancer |
10.5 |
13.6 |
20.7 |
32.5 |
33.5 |
33.9 |
36.5 |
39.0 |
40.6 |
|
Prostate cancer |
13.2 |
13.8 |
31.4 |
51.1 |
56.1 |
64.3 |
71.1 |
74.0 |
77.5 |
|
Uterine cancer |
6.5 |
7.0 |
7.8 |
6.3 |
7.2 |
7.7 |
8.1 |
8.6 |
9.0 |
|
* Includes AIDS funding. SOURCE: National Institutes of Health. |
|||||||||
for these collaborations is conducted by IC staff following an interagency transfer of funds from ORMH to ICs.
The ORMH priority-setting and funding processes appear to be driven by the professional judgment and research priorities of an ad hoc panel, as well as those of other ICs. In response to an inquiry from the study committee, ORMH writes:
ORMH begins its funding process by asking the ICs, ''What is it that we should be doing that we are not doing?" In practice the ORMH sends out two communications to the ICs annually. The first call is for the confirmation of projects for which ORMH has committed out-year support. The second call is for the submission of new projects or programs that the ICs consider meritorious and which fill a gap in minority health research and/or research training. Because the level of support requested by the ICs usually exceed the budget for the Minority Health Initiative, an ad hoc review panel is convened to assist ORMH in prioritizing the projects to support (National Institutes of Health, Office of Research on Minority Health, 1998a).
Research initiatives proposed by other ICs for ORMH co-funding are evaluated by the Center for Scientific Review and individual IC advisory councils for appropriateness. Proposals are then forwarded to ORMH for evaluation and prioritization.
Although the ORMH proposal review process has been conducted by an ad hoc panel since the office's inception, as of recently the newly appointed Advisory Committee on Research on Minority Health will advise the ORMH director on prioritizing the projects that ORMH will support. This committee, which held its first meeting in April 1998, is composed of 12 individuals with expertise in minority health research or research training, or both. The committee will advise the ORMH director regarding appropriate research priorities and activities for the enhancement of minority health for the inclusion of minority groups as subjects in clinical research and for the enhancement of minority participation in research and training programs. The committee is expected to meet twice a year and to produce a biennial report summarizing its advice and recommendations regarding NIH programs. The establishment of the Advisory Committee appears to represent the first step toward a "formalized" process of internal review of ORMH activities (see Chapter 4 for a more detailed discussion of the ORMH priority-setting process).
Specifically, at the first meeting of the committee, ORMH Director John Ruffin and NIH Director Harold Varmus asked for the committee's assistance in several areas, including reviewing the current portfolio of research co-funded by ORMH to identify potential gaps, assessing whether critical minority health research issues are being appropriately addressed through the Minority Health Initiative, and advising NIH regarding optimal
approaches for recruiting and training minorities for health research. Ruffin specifically asked for assistance in responding to new challenges, including developments in human genome research and changes to federal affirmative action policies that may affect minority scientist recruitment and training.
ORMH Research Funding
Funding for ORMH has increased significantly since the office was created in 1991, but its overall funding remains minuscule in comparison to the $14 billion overall budget of NIH. In FY 1991 the office initiated activities with a budget of $1.5 million. In FY 1993 ORMH's budget allocation increased to $48.4 million, coinciding with passage of the Minority Health Improvement Act of 1993. In FY 1994 and FY 1995 ORMH funding increased to $62.7 million and $67.8 million, respectively, but it saw its first budget decline in FY 1997, when the ORMH allocation dropped to $70.1 million from a high of $71.1 million in FY 1996.
In research relevant to the study of cancer among ethnic minority populations, ORMH reports that from 1992 to 1997, it provided nearly $20 million in funding to assist NCI minority initiatives. The bulk of this funding has been to support the Minority Adolescent HIV Prevention and Treatment Project (approximately $10 million from FY 1994 to FY 1997). Other significant expenditures include funding for grants to improve ethnic minority recruitment and retention in clinical trials, funds for training of minority investigators, and small research supplements. In FY 1997 ORMH allocated slightly less than $6 million to NCI (see Table 3-3), including $1.75 million to support the Minority Adolescent HIV Prevention and Treatment Project, $1 million to cancer centers to support minority recruitment to NCI-sponsored clinical trials, and nearly $750,000 to support other efforts to increase minority participation in clinical trials. These expenditures for cancer-related projects were approximately 9 percent of ORMH's total budget in FY 1997. ORMH reports that NCI did not provide additional funds beyond initial funding (e.g., for overall cancer center or clinical trial operations) to support these projects.
Estimated ORMH expenditures on cancer in FY 1998 reflect its two-fold mission. The office allocated $6.22 million to assist NCI projects on cancer among minorities in FY 1998. The three largest NCI projects supported by ORMH are the Minority Adolescent HIV Prevention and Treatment Project ($1.75 million), funds to encourage minority participation in NCI-sponsored trials ($1 million), and training supplements for under-represented minorities ($75,000; see Training of Minority Scientists below). Other expenditures include grants for regional workshops for minority
TABLE 3-3 National Cancer Institute Minority Initiatives Supported by the NIH Office of Research on Minority Health (FY 1997)
|
Projects |
ORMH |
Institute |
|
Minority Adolescent HIV and Treatment Project |
1,750,000 |
0 |
|
Enrollment of Minorities in NCI Clinical Trials |
59,535 |
0 |
|
Overcoming Impediments to Participation of Minorities and Special Populations in Clinical Trials |
500,000 |
0 |
|
Minority Participation in NCI-Sponsored Clinical Trials |
1,000,000 |
0 |
|
Barriers to Latino-American Participation in Cancer Clinical Trials |
21,011 |
0 |
|
Procurement of Prostate Tumor Tissues from African-American Patients |
52,326 |
0 |
|
Determination of Correlation Between Androgen Receptor CAG Trinucleotide Repeat Length and Prostate Cancer Risk |
291,000 |
0 |
|
Small Grant for Women and Minority Recruitment |
68,120 |
0 |
|
Cohort Study of African-American Men with Prostate Cancer |
77,225 |
0 |
|
Caucus on Prostate Cancer and Minorities |
45,528 |
0 |
|
Preventing Cancer in Hispanic Communities |
249,954 |
0 |
|
Collaborative Clinical and Molecular Correlative Studies |
350,000 |
0 |
|
Institute of Medicine Minority Cancer Study |
600,000 |
0 |
|
Baylor College of Medicine—Biennial Symposium on Cancer and Minorities |
30,000 |
0 |
|
NCI International Program Middle East Conference |
250,000 |
0 |
|
Regional Grantsmanship Workshops for Minority Investigators |
100,000 |
0 |
|
Minority Research Supplements |
750,000 |
0 |
|
TOTAL |
6,179,171 |
0 |
|
SOURCE: NIH Office of Research on Minority Health. |
||
investigators, for grants conferences to stimulate ethnic minority participation in clinical trials, supplemental funding for basic research related to prostate cancer and ethnic minorities, and small supplements for minority cancer control and prevention programs.
ORMH's estimates of its expenditures on cancer-related research may overstate the amount of funding that directly addresses the cancer research needs of ethnic minority and medically underserved populations. As noted above, funding for the Minority Adolescent HIV and Treatment project represents a large proportion of ORMH's allocation for cancer research. The committee questions, however, the relevance of this project for cancer research. Although neoplasms are a significant health concern among patients suffering from AIDS and HIV-related complications, an overview of the Minority Adolescent HIV and Treatment Project supplied
to the committee by ORMH does not mention the words "cancer," "neoplasms," or other related terms. Rather, this project's main focus appears to be the establishment of a community-based, comprehensive, and multidisciplinary health care center to monitor, treat, and enroll HIV-infected ethnic minority children and adolescents in HIV and AIDS Malignancy Branch clinical trials. It is unclear how many, if any, of the population enrolled in this program were treated for AIDS-related carcinomas. In addition, results of this research project may be limited; ORMH reports that the project was terminated in late FY 1998 "due to insurmountable contractual and legal issues" (National Institutes of Health, Office of Research on Minority Health, 1998a).
Assessment of ORMH Activities
ORMH serves as a focal point for the coordination of research on ethnic minority health at NIH. One of the office's major functions is to stimulate research on minority populations at relevant ICs of NIH by providing research supplements (including Minority Health Initiative funds) to "leverage" IC resources. ORMH has only recently, however, established a standing advisory panel to help guide the establishment of research priorities (this function had previously been assumed by an ad hoc panel) and does not participate in the Research Enhancement Awards Program with other specialty offices at NIH to coordinate funding proposals and priorities. Its criteria for program funding and research priorities have therefore been less open to public scrutiny. In addition, OMRH program funding appears to have supplanted, rather than leveraged, NCI resources for important research and program activities in many instances. The committee offers the following recommendation to strengthen ORMH's stated functions:
Recommendation 3-1: The Office of Research on Minority Health should more actively serve a coordinating, planning, and facilitative function regarding research relevant to cancer among ethnic minority and medically underserved populations across relevant institutes and centers of NIH. To further this goal, the Office of Research on Minority Health should:
- make criteria for Minority Health Initiative project support explicit;
- coordinate with other specialty offices (e.g., the Office of Research on Women's Health) by participating in NIH-wide coordination efforts such as the Research Enhancement Awards Program; and
- ensure that Minority Health Initiative funding does not supplant funding from institutes and centers for research and programs relevant to ethnic minority and medically underserved populations.
Overview of Scientific Infrastructure at NCI
Since 1996, NCI has undertaken several significant changes in its internal structure that affect both intramural and extramural scientific programs. Many of the these changes were initiated in response to a series of internal program reviews, including the 1995 Bishop-Calabresi report (Ad Hoc Working Group of the National Cancer Advisory Board, 1995), which recommended a complete organizational separation of intramural and extramural programs. Two new extramural divisions, the Division of Cancer Prevention (DCP) and the Division of Cancer Control and Population Sciences (DCCPS), were created. DCP was created to add "visibility, prominence, and strength to the NCI's prevention programs" (National Cancer Institute, 1998d, p. 11), whereas DCCPS was created from programs of the Division of Cancer Prevention and Control, which has been eliminated, as well as extramural portions of the Division of Cancer Epidemiology and Genetics.
Perhaps most significantly for ethnic minority and medically underserved groups, three new offices were created to develop partnerships with community-based groups that focus on cancer. The Office of Special Populations Research (OSPR) was formed to provide a focal point and coordinating center for research related to "special populations," defined by NCI as economically disadvantaged people, elderly people, and certain ethnic minority groups. OSPR works with other NCI offices to assist in defining scientific questions relating to special populations, as well as in evaluating the effectiveness of outreach efforts aimed at these populations. The Office of Liaison Activities links to national cancer advocacy organizations to facilitate communication between NCI and community-based groups. Similarly, the Office of Cancer Survivorship develops and coordinates research on cancer survivorship to address "the unique physical, social, psychological, and economic issues faced by these individuals" (National Cancer Institute, 1998d).
Intramural Research
Intramural research at NCI is conducted principally in the Divisions of Basic Sciences, Clinical Sciences, and Epidemiology and Genetics. This research encompasses basic, clinical, and population-based research. In addition, NCI intramural laboratories and clinics train cancer research
specialists. The intramural research relevant to ethnic minority and medically underserved populations is described below.
Extramural Research
NCI's principal activity involves the funding of extramural research. Extramural research accounts for more than 75 percent of the institute's budget and includes both laboratory and clinical investigations (including individual or program project grants and cooperative agreements), epidemiologic studies and surveys, cancer control projects, cancer centers, construction and general medical infrastructure, and education. These extramural programs are categorized under cancer research activities, cancer control, and cancer resource development (National Cancer Institute, 1998f).
NCI's extramural research program is driven largely by investigator-initiated proposals for funding, consistent with the philosophy of its director, Richard Klausner. Proposals are evaluated on the basis of several criteria, principally, whether the research affords the greatest scientific opportunities to increase the level of knowledge about cancer. A second factor that guides funding is the degree of burden posed by specific cancers. All investigator-initiated research proposals are evaluated by any of more than 100 study sections or peer-review groups, which evaluate the importance of the research topic, the rigor of the proposed methodologies or techniques, and the investigator's ability to meet the aims of the study. NCI anticipates awarding more than $1.19 billion in extramural research awards in FY 1998 to support 3,700 research grants, more than 1,000 of which are new or competing renewal projects (National Cancer Institute, 1998d).
Much of the extramural research infrastructure is supported by other peer-reviewed mechanisms, including the network of cancer centers, community clinical oncology programs, clinical cooperative groups, tissue banks, some surveillance activities, and training programs. Other components are funded by contract with NCI, including major cancer registries (see the description of the Surveillance, Epidemiology, and End Result [SEER] program below).
Cancer Centers
NCI currently supports 57 cancer centers for the purposes of conducting interdisciplinary research, training, and education. The Cancer Centers Program is designed to create a flexible infrastructure for innovative research and clinical and community applications to promote collaboration between basic, clinical, and population research scientists. A key element
of the cancer centers is to bring "the benefits of research more directly to local communities and regions of the country" (National Cancer Institute, 1998d, p. 34) by linking research and clinical application activities. Cancer center activities include the development of linkages with industry, state and local health agencies, and community organizations. However, no funding is provided for the development of such linkages.
Clinical Trials Infrastructure
Many of the NCI-supported cancer centers are involved in clinical trials. The largest source of support for clinical trials, however, is the NCI community Clinical Oncology Program (COP) and Clinical Trials Cooperative Group program. NCI supports hundreds of clinical trials via these mechanisms. Fifty-two CCOPs are currently funded in 30 states, with an additional eight minority-based CCOPs (MBCCOPs) funded to increase the numbers of ethnic minority patients in clinical trials research. The Clinical Trials Cooperative Group program includes 12 groups that annually place approximately 20,000 new patients into cancer treatment protocols.
Training and Education
NCI's Cancer Training Program supports individual fellowship and career awards and education grants to support the cancer research infrastructure. The institute pursues four strategies to achieve this goal: (1) maintaining the numbers of basic scientists studying underlying genetic and biological mechanisms of disease; (2) encouraging a greater proportion of basic scientists to develop interests in model systems of human disease; (3) attracting more young physicians, public health specialists, and other health care professionals into cancer research, especially in biostatistical, epidemiologic, behavioral, and other prevention and control sciences; and (4) using education grants to improve the curricula for health care and public health students.
Cancer Control
NCI-supported cancer control activities attempt to disseminate and apply new medical knowledge to routine practice. This includes research on the behavioral, psychosocial, health services, communication, and cancer surveillance aspects of cancer control.
Dissemination
NCI conducts several dissemination activities through the Office of Cancer Information, Communication, and Education (OCICE). The Cancer Information Service (CIS) provides information to cancer patients and their families through a toll-free telephone information service and through community outreach efforts and educational campaigns. The International Cancer Information Center (ICIC) provides cancer information to scientists, health care professionals, and the public through PDQ, the NCI's cancer information database, and the bibliographic CANCER-LIT database. NCI also disseminates information via its site on the World Wide Web.
NCI FY 1997 Programs and Resources Allocated to Addressing Ethnic Minority and Medically Underserved Populations
NCI categorizes research and training programs relevant to special populations (including ethnic minority and medically underserved populations) into two subgroups. Category I programs are defined as "research or training targeted to, or for, a specific special population or populations," whereas Category II programs are "research on a problem affecting all populations (thus, not targeted to any specific group). This research is, however, of special significance to a specific special population or populations" (National Cancer Institute, 1998b, p. 4). Both program subtypes are reported here.
Cancer Surveillance Activities
NCI's cancer surveillance effort is aimed at identifying and reporting on the disease frequencies in the U.S. population that may be useful in identifying trends and generating causal hypotheses. At the core of this effort is the SEER program, which is described in greater detail in Chapter 2. SEER program data emanate from 11 population-based registries including registries in the states of Connecticut, Iowa, New Mexico, Utah, and Hawaii and the metropolitan areas of Detroit, San Francisco-Oakland and the San Jose area south of San Francisco, Los Angeles, Seattle-Puget Sound, and Atlanta and the 10 counties in Georgia surrounding Atlanta. According to NCI, the population in geographic areas in the SEER program represent approximately 14 percent of the U.S. population, including 25 percent of the Hispanic American population, 41 percent of the Asian/Pacific Islander population (including 43 percent of all Chinese Americans and 60 percent of all Japanese Americans), 27 percent of American
Indian and Alaska Native populations, and 12 percent of the African-American population. More than half of the African-American population in SEER program coverage areas resides in either Los Angeles or Detroit, more than two-thirds of the SEER program Chinese-American population resides in either Los Angeles or San Francisco-Oakland, and 60 percent of the SEER program Hispanic population resides in Los Angeles.
The SEER program recently expanded its coverage explicitly to improve the coverage of minority populations (see Chapter 2). NCI allocated approximately $2.3 million in FY 1996 to expand the SEER program database to increase the coverage of the Hispanic population and $250,000 to increase the coverage of the Native American population.
To enhance SEER program data with regard to American Indian and Alaska Native populations, NCI is supporting and planning several Category I initiatives. The New Mexico SEER program registry receives NCI support to collect and report on data for American Indians in Arizona. NCI is also planning an operational system for the establishment of a cancer registry among the Cherokee population in Oklahoma. In addition, NCI has worked in collaboration with the Indian Health Service to support the Alaska Native Tumor Registry for cancer surveillance among Alaska Natives and previously supported a project to describe cancer incidence, mortality, and patterns of care, risk factors, and cultural obstacles to early detection and treatment of cancer among American Indians and Alaska Natives.
Among the products of the SEER program relevant to the study of ethnic minority and medically underserved populations is the program monograph entitled Racial/Ethnic Patterns of Cancer in the United States 1988–1992 (Miller et al., 1996). This publication provides incidence and mortality data for 13 U.S. "racial" and ethnic groups (mortality data are compiled from National Center for Health Statistics [NCHS] data).
A summary of other cancer surveillance activities based on information provided to the study committee by request is provided below (National Cancer Institute, 1998b).
Data Resources
To address questions regarding the effects of cancer, the use of cancer-related services, costs of the disease, and patterns of care among special populations, NCI has supported a number of special initiatives, some in collaboration with other organizations and federal agencies.
Staff of the Division of Cancer Epidemiology and Genetics (DCEG) and the DCCPS recently published an atlas of cancer mortality maps for U.S. non-white populations, a Category I and II project that illuminates rates of mortality from cancer at a range of anatomic sites by geographic
region. State economic areas experiencing variations in cancer risk among minority populations are also highlighted. Data from this study may provide leads about etiology and cancer risk that may be pursued by further epidemiologic research. The study has revealed noteworthy patterns, such as a higher rate of prostate cancer among African-American men in the south Atlantic states, increasing rates of stomach cancer among American Indians in the Southwest, and "limited declines" in cervical cancer among African-American women in the Southeast.
In addition, DCEG staff, in collaboration with the National Institute for Occupational Safety and Health and NCHS, have jointly sponsored a study of occupation and industry codes on death certificates for purposes of understanding cancer prevalence by occupational risk. This study now encompasses 24 states and includes more than 5 million records. NCI reports that data from this Category I and II study are available for whites, African Americans, and all minority populations combined.
NCI is also planning several case-control studies of specific cancers and cohort studies of noncancerous conditions that are disproportionately prevalent among African-American men using data from the U.S. Department of Veterans Affairs (VA) inpatient and outpatient medical records. These data are available for more than 1 million African-American male veterans, as well as 4 million white male veterans, and can be used to examine the risk of various cancers associated with serious medical conditions and procedures.
NCI is also collaborating with the Health Care Financing Administration (HCFA) to link SEER program data with Medicare data to assess costs and the use of selected screening procedures, diagnostic procedures, and treatment patterns for older patients (ages 65 years and older). Health claims data will be examined by race, income, education, and related variables to understand how screening and treatment patterns may differ for subpopulations. In addition, the data will be analyzed by using census tract information to detect differences by the socioeconomic status of geographic areas for this Category I and II study.
In 1994, NCI established the Breast Cancer Surveillance Consortium to study mammography access and utilization among women in community-based settings and the impact of access and utilization on cancer outcomes. This Category II study is expected to yield data on mammography screening practices and mammography performance. It will also provide data on screening among ethnic minority women, as well as other factors that influence mammography access and utilization, such as education, income, and urban-rural location.
In addition, NCI has funded five grants specifically related to special populations to assess the utility of health claims data for cancer surveillance. These Category I and II projects will explore the completeness and
accuracy of information reported in claims-based data systems to determine the utility of reimbursement claims for tracking cancer incidence and stage, the use of screening and diagnostic tests, long-term treatment, and cancer-related health care utilization.
Surveys
To address questions regarding health risk behaviors (e.g., diet, health habits, and use of cancer screening), NCI conducts or uses a number of surveys that may identify ethnic group differences related to cancer risk. As indicated above, many of these efforts are conducted in collaboration with other federal agencies or organizations.
NCI has collaborated with the U.S. Bureau of the Census to generate supplemental questions for the Current Population Survey, a monthly survey of approximately 50,000 households used to obtain information about the labor force. NCI questions provide surveillance information on tobacco use and tobacco control attitudes. These data have been used to provide estimates of tobacco use among minority and medically underserved populations and were published in the Journal of the National Cancer Institute in 1996.
NCI also periodically provides supplemental questions to the Centers for Disease Control and Prevention's (CDC's) annual National Health Interview Survey (NHIS), a nationally representative survey administered in person. NCI supplements to NHIS in 1987 and 1992 pertained to cancer risk factors, including tobacco use, diet and nutrition, knowledge and attitudes about cancer, use of cancer screening, and cancer survivorship. The survey has been translated into Spanish and has included a measure of ''acculturation to the Hispanic population" (National Cancer Institute, 1998b, p. 12). NCI's supplemental questions to NHIS in the year 2000 will expand the acculturation section to include questions on health beliefs and health service use and will expand acculturation questions to Asian Americans, as well as Hispanics. NCI has also collaborated with CDC in the prospective National Health and Nutrition Examination Survey (NHANES), which investigated the health and nutrition status of the U.S. population with a particular focus on high-risk populations. Several cohorts have been followed prospectively since the 1970s to obtain data on diet and health. Both NHANES and NHIS are classified by NCI as Category II studies.
As part of the effort to understand dietary patterns and cancer risk, DCCPS staff have also studied data from the U.S. Department of Agriculture's Continuing Surveys of Food Intakes by Individuals to explore ethnic group differences in dietary intake and compliance with recommended daily nutritional guidelines.
Differences in breast cancer screening practices among elderly women in various ethnic groups have been examined in an NCI-supported study to measure the effect of legislation allowing Medicare reimbursement for breast cancer screening. Analyses comparing African-American and white women's usage patterns are ongoing. In addition, NCI has sponsored a national survey of mammography facilities to understand the characteristics of mammography services and providers and participation in price-subsidized programs for low-income women. NCI classifies these studies as Category I and II studies.
NCI is also supporting a Category I study to develop and validate needs assessment instruments to measure the effectiveness of cancer control methods among American Samoans. This project will develop a culturally sensitive survey instrument to assess knowledge and attitudes regarding cancer among a sample of American Samoans in Los Angeles, Hawaii, and American Samoa.
Studies That Use Databases
DCCPS sponsors several population-based studies relevant to ethnic minority populations. The Black/White Cancer Survival Study, begun in 1983, investigates the role of "social, behavioral, lifestyle, biological, treatment, and health care factors as contributors to the observed differences in survival" among African-American and white cancer patients (National Cancer Institute, 1998b, p. 18). NCI notes that several publications have developed from this study, which followed 3,400 individuals with breast, colon, corpus uteri, or urinary bladder cancers. In addition, SEER program and Medicare data have been used by DCCPS staff to examine patterns of care and costs of cancer treatment, in some studies according to clinical and sociodemographic factors. These studies are described by NCI as Category I and II studies.
Several SEER program special studies are ongoing. They report on data on patterns of care and treatment outcomes among white and non-white populations collected as part of the SEER program. One project reports on differences in treatment outcomes for African-American and white men with non-metastatic prostate cancer and has found differences related to race and socioeconomic status. Another study examines quality-of-life issues for Asian-American and Pacific Islander cancer survivors. Another series of studies examines trends in treatment for early-stage breast cancer by age, race, geographic region, and socioeconomic characteristics. Similarly, the Prostate Cancer Outcomes Study provides information about diagnostic and treatment practice patterns for prostate cancer and describes health-related quality of life according to geographic region, racial or ethnic subgroup, income, education, and health insurance status of
patients. Of the more than 3,300 men participating in the study, approximately 500 African-American and 430 Hispanic men participated in the initial survey. Finally, feasibility studies are being conducted to examine patterns of care from several data sources, including the Indian Health Service, to provide more information on American Indian cancer patients, particularly those suffering from colon, lung, breast, prostate, and cervical cancers.
Epidemiologic and Etiologic Research
Nutrition Studies
To address questions about links between dietary patterns and cancer incidence and mortality, particularly among ethnic minority and medically underserved populations, NCI supports a number of nutrition studies.
The Women's Health Trial: Feasibility Study in Minority Populations was initiated to assess whether ethnic minority and low-income women could be recruited into a trial in sufficient numbers to evaluate a dietary intervention and test the intervention's effects on lowering fat consumption. More than 2,000 minority and low-income women were recruited for this randomized trial. Similarly, DCCPS supports a study assessing diet and breast cancer risk among a sample of 400 black women. This Category I study seeks to "yield statistical methods for enhancing the ability to assess diet-related breast cancer risks in Black women as well as provide relevant pilot data to support future studies" (National Cancer Institute, 1998b, p. 24).
DCEG staff are investigating the relationship between fatty acids and prostate cancer risk among African-American and white males. The levels of a variety of fatty acids are being measured in plasma collected from both African-American and white men in a large, multicenter, population-based case-control study to search for relationships between fatty acid profiles and prostate cancer risk. Ethnic-group differences in these profiles and their relationship to prostate cancer will be assessed, as will the relationship between diet and fatty acid profiles. NCI has classified this research as a Category I and II study.
The Multiethnic/Minority Cohort Study of Diet and Cancer prospectively examines the relationship of dietary and other lifestyle risk factors to cancer. Investigators at the University of Hawaii at Manoa are studying a total of 215,000 African-American, Japanese-American, Hispanic, and white subjects in the western United States to assess dietary patterns and group differences. Slightly more than $1 million was allocated to this Category I activity in FY 1997. In addition, NCI staff are collaborating on analyses of the contributions of dietary and nutritional patterns to the high incidence
of esophageal, pancreatic, and prostate cancer and multiple myeloma in African Americans.
NCI lists three other ongoing, prospective studies (the Nurses' Health Study, the FELS Early Nutrition and Growth Study, and the Framingham Heart Study) that examine the relationship between nutritional and other risk factors and cancer in special populations. By NCI's own admission, however, these studies include very few ethnic minority participants and unknown numbers of lower-income or medically underserved participants. It is therefore unclear how research questions specific to these populations (e.g., "How is diet affected by acculturation?" or "Is poor childhood nutrition among African-American women linked to premenopausal breast cancer?") may be answered.
Environmental Risk Factor Research
Many ethnic minority populations are at greater risk for a range of environmental exposures (e.g., some forms of radiation or chemicals and pesticides) and infectious diseases (e.g., human immunodeficiency virus [HIV] and human papillomavirus [HPV] infections) that are known carcinogens or that may be linked with cancer. NCI supports a number of studies that investigate the physical, chemical, and viral causes of cancer and their disproportionate burdens on ethnic minority and medically underserved populations.
DCEG is supporting a study of breast cancer, benign breast disease, and pesticide exposure among a predominantly African-American population in Triana, Alabama, that has been exposed to high levels of the insecticide dichlorodiphenyltrichloroethane (DDT) in a tributary of the Tennessee River. Mammographic screenings, clinical examinations, and blood chemistries will be provided to the study participants. Other health and health education needs of participants will be identified and provided. The Category I study will evaluate the relationship between serum DDT levels and the risk for breast cancer and breast disease.
NCI, in collaboration with the Indian Health Service, CDC, and the Alaska Area Native Health Service, has funded pilot research exploring associations between breast cancer and elevated levels of organochlorines among Alaska Native women. These women are at increased risk due to diets high in protein and fat from marine sources established as having high concentrations of organochlorines. Components of this Category I study will involve the collection of serum, urine, and adipose tissue samples from Alaska Native women undergoing breast surgery and analysis of samples for organochlorines.
HIV infection now disproportionately affects ethnic minority individuals in the United States. NCI is investigating techniques that can be used to
identify HIV-infected individuals who are at risk for rapid disease progression and who may benefit from early therapeutic intervention, thereby reducing associated cancer risks. In addition, NCI and the National Institute of Child Health and Human Development are sponsoring research to reduce the rate of mother-to-infant transmission of HIV.
Several studies are under way to understand adult T-cell leukemia (ATL) and human T-cell lymphotropic virus type I (HTLV-I) and type II (HTLV-II) infection. ATL and infection with its causal agent, HTLV-I, are more common among African Americans than among whites. NCI staff seek to define host susceptibility to infection and modes of transmission of HTLV-I and improve surveillance of ATL patients. Epidemiologic studies are also conducted to better understand the modes of transmission of HTLV-II. Similarly, Category I and II studies are being conducted to assess the roles of Epstein-Barr virus in Hodgkin's disease among Hispanic patients, Burkitt's lymphoma among Ghanaians, and gastric cancers among Japanese Americans. DCEG and DCP staff are also studying the relationship of HPV and the etiology of lymphoma, hepatocellular cancer, and cervical cancer in American Indians.
DCEG staff are also engaged in studies of occupational exposure to hazardous agents and cancer risk. These Category I studies examine links between exposure to chemical and other environmental agents across a range of occupations, racial and ethnic groups, and socioeconomic backgrounds, given that lower-income and ethnic minority workers are often exposed to carcinogens at higher levels. A number of studies assess cancer risks for farmers or individuals living in rural areas and have found excess incidence rates for several cancers. Another project assessed the feasibility of conducting studies on cancer risks among migrant workers of African, Hispanic, and Asian backgrounds. In addition, intramural staff are working in collaboration with investigators at the University of Minnesota to assess the linkages between occupational and environmental risk factors among women in Shanghai, China.
Access to Care and Cancer
NCI has attempted to stimulate research on patterns of health care, cancer, and variations by socioeconomic differences and racial and ethnic groups. This research also attempts to identify barriers to state-of-the-art diagnosis and care for patients in rural areas.
NCI sponsored two workshops, one in 1989 and another in 1992, on patterns of care and the economic and social burdens of cancer on families. In addition, NCI issued a program announcement to improve the understanding of the economics of cancer care. "Grants funded under this Program Announcement," according to an NCI report, "include studies
of the cost-effectiveness of increasing breast cancer screening and effective follow-up among African American women, the effects of tobacco taxation on tobacco use, the cost effectiveness of alternative strategies of managing Pap smear results, patterns of care for breast cancer in [health maintenance organization] HMO and fee-for-service settings, and the application of econometric techniques to cost and outcomes studies using Medicare data" (National Cancer Institute, 1998b, p. 34). NCI has collaborated with other federal agencies, including the Agency for Health Care Policy and Research, HCFA, and the National Institute on Aging, in sponsoring this research. Finally, DCP recently released a request for applications (RFAs) to assess ways of improving cancer diagnosis and treatment in rural areas. The aim of the RFA is to "strengthen the application of state-of-the-art cancer diagnosis and management practices in rural areas by enhancing links between rural health care providers and regional cancer specialists" (National Cancer Institute, 1998b, p. 34).
Cancer Etiology
NCI intramural staff are investigating a range of possible and confirmed etiologic factors, including genetic susceptibility, environmental carcinogens, diet, behavior and lifestyle, and other risk factors, and their relationship with race and ethnicity in conferring a risk for cancer. For example, NCI scientists examined the relationship of rare variable nucleotide tandem repeat alleles of Ha-ras-1 in African Americans and whites as a possible predisposing factor in lung cancer and determined that differences in lung cancer rates between the two groups were due to differences in smoking patterns and not polymorphic gene variance.
Other studies on cancer etiology are summarized below by cancer site.
Prostate Cancer
NCI scientists are studying the relationship between a variety of genetic, biochemical, behavioral, and environmental factors and prostate cancer in two large case-control investigations of African-American and white men in the United States and a sample of men in China at low risk for the disease. In that study vasectomy at a young age and family history are among the risk factors associated with prostate cancer, whereas researchers continue to examine the role of androgen metabolism and other biochemical markers in prostate cancer, DCEG and DCP staff are also studying these relationships in the NCI-sponsored Prostate, Lung, Colon, and Ovarian (PLCO) Cancer Screening Trial. These research efforts have been classified as Category I and II studies.
Breast Cancer
DCEG supports a wide range of Category I and II research aimed at understanding the causes of breast cancer and whether etiology
varies by racial or ethnic group. A population-based, case-control study in North Carolina focuses on the causes of breast cancer among African-American and white women who live in suburban and rural areas of eastern and central North Carolina. The study integrates epidemiology and molecular biology to explore risk factors and possible gene-environment interactions as causes of cancer. Other studies are aimed at understanding differences in breast cancer incidence among younger (under age 40) African-American and white women, diet and risk of breast cancer among Asian-American women, and whether racial or ethnic variations in breast cancer incidence and prognosis are attributable to various exogenous mutagens.
Cervical Cancer
The incidence of cervical cancer is disproportionately high among African-American, Hispanic, and some Asian-American women. NCI supports case-control studies in Jamaica to understand the etiologic risks for cervical cancer associated with HPV, HIV, and HTLV, as well as a large study in Costa Rica that examines genetic susceptibility markers and nutrition to assess why common HPV infections sometimes persist and progress to cervical cancer. Both are Category I studies.
Nasopharyngeal Cancer
NCI-supported scientists are studying the role of a range of environmental, lifestyle, and genetic factors in the development of nasopharyngeal cancer (NPC), the incidence of which is particularly high in Southeast Asia and among individuals of Chinese descent. A case-control study in the Philippines has revealed a strong link between occupational exposures to chemicals (e.g., formaldehyde), smoking, and other environmental risk factors and NPC. Scientists are also examining the role of oncogenes and tumor suppressor genes in the pathogenesis of NPC. The interplay of genetic factors and environmental exposures is also being assessed in a family-based study recently initiated in Taiwan. Finally, NCI is also supporting a study of 60,000 Chinese men in Singapore to investigate the relationship between diet, particularly ethnic foods such as salted fish, and NPC. These studies have been classified as Category I and II studies.
Oral and Pharyngeal Cancers
DCEG staff are investigating the relationship between smoking and alcohol consumption and oral and pharyngeal cancers, the rates of which are 30 to 100 percent higher among African Americans than whites. When the rates for African-American and white nonsmokers and nondrinkers are compared, they are nearly equivalent. These relationships are being studied further in a case-control study in Puerto Rico, an area with high rates of oral and pharyngeal cancers. This study has revealed a greater risk for oral cancer with increasing alcohol
consumption among persons with the ADH 31-1 genotype. These studies have been classified as Category I and II studies.
Esophageal Cancer
African Americans and Chinese Americans suffer from higher rates of esophageal cancer than whites. NCI-supported scientists are studying tumors that occur in excess among African Americans in a series of case-control studies. In addition, studies are in progress to collect DNA from samples of populations at high and low risk for esophageal cancer in Shanghai, China. In Linxian, China, NCI researchers are studying the impact of a nutritional intervention on late-stage progression of esophageal cancer among individuals in a high-risk population. These studies have been classified as Category I studies.
Stomach Cancer
Asian Americans, African Americans, and farmers all suffer from higher rates of stomach cancers than other Americans. NCI is studying the effect of a nutritional intervention on the progression of precancerous gastric lesions among subjects in Shandong, China. A screening program in China is also sponsored by NCI to evaluate the role of diet on precancerous lesions of the stomach. Similarly, DCEG staff are evaluating the risk posed by agricultural hazards such as pesticides, fertilizers, and dust on stomach cancers in a case-control study in Nebraska. In addition, DCEG staff, working with the U.S. Environmental Protection Agency and NIEHS, are evaluating stomach cancer and agricultural exposures among African-American and white farmers in North Carolina and Iowa in the Agricultural Health Study. To encourage further research in this area, NCI, along with NIDDKD, NIAID, the NIH Office of Research on Minority Health, and the American Digestive Health Foundation, recently issued an RFA on Helicobacter pylori and its relationship to digestive diseases and cancer, with an emphasis on research related to minority populations. This research has been classified as Category I research by NCI.
Colorectal Cancer
NCI supports several studies that are investigating a range of risk factors associated with colorectal cancer. In a case-control study being conducted in China and the United States, researchers found that Chinese-American men and white men have colorectal cancer rates seven times higher than those of men in China. High-fat diets and low levels of physical activity were among the identified risk factors. In addition, a new multicenter study is assessing the independent and combined effects of dietary factors, physical activity, body size, reproductive factors, and family history on the risk of colon cancer among African Americans and whites. Finally, staff of the Cancer Prevention Studies Branch of NCI are collaborating with VA to collect blood and tissue specimens from patients
in VA medical centers to create a large specimen bank for a prospective study of nutritional and genetic hypotheses of colorectal neoplasia. Specimens will be obtained from patients with large adenomas, patients with small polyps, and asymptomatic individuals to assess their relationship with serum micronutrients and molecular genetic markers. These studies are classified as Category I and II research by NCI.
Pancreatic Cancer
NCI-supported research on the etiology of pancreatic cancer includes a series of case-control studies among African Americans that examine the roles of smoking, diet, various medical conditions, and genetic factors. The role of hepatitis viruses in conjunction with other environmental and lifestyle risk factors in the development of pancreatic cancer is also being investigated in a case-control study in Senegal, West Africa. These studies are classified as Category I studies.
Multiple Myeloma
NCI-supported case-control studies are comparing risk factors for multiple myeloma among African-American and white populations. Findings indicate that occupational exposures (especially for those residing on farms and reporting exposure to pesticides) increase the risk for this cancer. NCI also supported a workshop on the epidemiology of multiple myeloma, with special attention to factors that may contribute to the excess incidence among African Americans.
Cancer Prevention and Control
An NCI document notes that behavioral and environmental influences are responsible for the majority of cancers in the United States. Reducing the cancer burden therefore requires "a balanced partnership between the biomedical and behavioral/public health sectors" (National Cancer Institute, 1998b, p. 46). NCI's definition of cancer control research attempts to reflect this view: "cancer control research is now defined as basic and applied research in the behavioral, social, and population sciences to create or enhance interventions that, independently or in combination with biomedical approaches, reduce cancer risk, incidence, morbidity, and mortality" (National Cancer Institute, 1998b, p. 46). Much of this research is funded through the newly established DCCPS.
Primary Prevention and Intervention Studies
Tobacco Use and African Americans
NCI has funded Category I research examining the effectiveness of culturally appropriate behavioral interventions to decrease the level of tobacco use among African Americans. In
one study, the incremental benefits of "culturally sensitive" adjuvant behavioral therapy and use of the transdermal nicotine patch are assessed among a population of urban African Americans. In another study, gender and racial or ethnic variations in perceptions of cancer risk are assessed by using population-sensitive measures of risk perception. Improved cancer-risk communication in this study is expected to lead to reduced smoking rates and increased rates of use of screening mammography among African Americans. Finally, the Enhancing Cancer Control in a Community Health Center project assessed the effectiveness of patient-, physician-, and system-directed interventions aimed at promoting the early detection of breast and cervical cancers and smoking cessation in a predominantly African-American population. Ethnically appropriate patient education materials and telephone counseling were combined with physician education and other interventions in a community health center setting.
Tobacco Use and Hispanic Americans
An NCI-funded Category I study is examining the effectiveness of a social influence model on cancer-risk behavior among migrant Hispanic adolescents. The intervention includes social skills development and enhancement of parental skills to reduce the rates of tobacco consumption and other cancer-risk behaviors. A total of 700 adolescents will be randomly assigned to intervention and control conditions, with 12- and 24-month follow-ups.
Tobacco Use and Native Americans
Several Category I studies supported by NCI examine the use of culturally sensitive interventions to reduce the rates of tobacco use and improve diet. A study in California adapted a Quit for Life smoking cessation program for the needs of Indian health clinics and health care providers, followed by home visits to patients by Indian Community Health representatives. Another study examined the effects of culturally relevant community interventions with the family to augment a school-based health curriculum on health knowledge among southwestern Indian children. In the Northeast, NCI-supported investigators studied the effects of an integrated overall health curriculum on decision making among Native American youth related to diet and tobacco use. In the Northwest, a study examined the effects of a consultative process and the use of materials to assist tribal councils in developing and implementing more stringent tobacco-use policies.
Tobacco and Disadvantaged Youth
NCI is funding a Category I study to develop and test community-based cancer prevention strategies among high-risk youth in New York whose families' incomes fall below the federal
poverty line. Skills intervention techniques for youth and parental skills intervention techniques will be used and their effects will be assessed. In addition, NCI has reissued an RFA for studies relating to the control of tobacco use among youth, including the ''identification and evaluation of factors influencing the decline of tobacco use among particular groups, for example, African American youth" (National Cancer Institute, 1998b, p. 51).
The American Stop Smoking Intervention Study
The American Stop Smoking Intervention Study (ASSIST) is a community-based intervention directed by local voluntary coalitions that plan and support tobacco control activities with the support of NCI, the American Cancer Society (ACS), and state and local health departments. More than 6,000 community organizations are involved in the initiative in 17 states. The ASSIST intervention model is based on smoking prevention and control methods established by research supported by NCI, as well as other research. NCI reports that although this Category II initiative is aimed at all populations in the targeted states, those groups with elevated smoking rates relative to that for the majority population, as well as those groups "that have displayed slower rates of decline (e.g., women, youth, the medically underserved, the less educated, and several ethnic minority populations)," will receive special focus (National Cancer Institute, 1998b, p. 52).
Noticeably absent from this portfolio of research on smoking interventions among specific ethnic groups and disadvantaged populations are smoking cessation research programs targeted to Asian-American and Pacific Islander populations (especially Southeast Asian populations, among whom tobacco use is among the highest of all U.S. ethnic groups) and medically underserved individuals (who also suffer from a high incidence of tobacco use).
Reducing Dietary Risk Behavior in Adolescents
The NCI-supported Category I and II study Reducing Cancer-Related Dietary Risk Behaviors in Adolescents targets a multiethnic population of lower-income students from two inner-city school districts in Minnesota to increase students' levels of consumption of fruits and vegetables and reduce their levels of intake of calories from total fat. Intervention components include a school curriculum addressing eating cues and the influence of advertising on food choices, a home intervention program to facilitate student-parent discussions of dietary choices, and a school environment component targeting food availability and incentives. Interventions will be implemented over a 2-year period, and evaluations will assess culturally appropriate strategies.
Risk Factor Prevention for Hispanic Youth
NCI is supporting a Category I study evaluating a comprehensive cancer-risk prevention intervention targeting preadolescents in schools serving predominantly low-income Hispanic families. School-based and parent interventions are coupled with a school food service intervention in 14 schools in San Jose, California, to increase healthful eating practices and levels of physical activity among youth and provide instruction in weight regulation skills. A primary objective is to reduce the level of prevalence of obesity at the end of the 2-year intervention, whereas secondary objectives include increasing the level of consumption of low-fat foods (including fruits and vegetables and dietary fiber), increasing the level of physical activity, and decreasing the level of consumption of dietary fat.
5 A Day Behavioral Research and Evaluation
NCI's 5 A Day program seeks to increase awareness of healthy dietary patterns and to increase levels of consumption of fruits and vegetables. These programs have been adapted to serve the needs of ethnic minority consumers. In North Carolina, an NCI-funded project mobilized community and religious organizations to tailor a 5 A Day program to the needs of an African-American community. Local businesses, churches, and media worked collaboratively with local health officials and cooperative extension staff to help implement the program. In Minnesota, an NCI-funded 5 A Day program targeted a multiethnic (45 percent minority) cohort of schoolchildren, including Asian-American, African-American, Hispanic, and American-Indian children, with a school-based intervention involving school curriculum, food service menu changes, and industry and media support. In Arizona, the 5 A Day-Healthier Eating for the Overlooked Worker project targeted a predominantly Hispanic population to compare the impacts of peer educational programs at work sites to those of traditional work-site wellness programs. The Treatwell 5 A Day Work-site Nutrition Intervention in Massachusetts serves a population that is approximately 33 percent African American and 33 percent Hispanic with a work-site intervention and family involvement component to assess their synergistic effects. The program is sponsored collaboratively by an NCI-supported comprehensive cancer center, the state health agency, cooperative extension, and industry. In Maryland, the effects of a program combining nutrition education, lay counseling, print materials, and community-based family involvement on levels of fruit and vegetable consumption among a low-income, primarily African-American population was assessed in the 5 A Day WIC Promotion Program (WIC is the Special Supplemental Food Program for Women, Infants, and Children). Finally, NCI has supported communications research, including focus groups with African-American men and women, to
explore perceptions about food and to develop strategies to improve 5 A Day messages tailored to the African-American community. Results of this research are found in messages in radio segments, media newsletters, and other outreach activities.
Chemoprevention Trials
NCI is sponsoring more than 60 chemoprevention trials to test compounds that may block, suppress, or retard cancer. Although none appear to be focused on issues of chemoprevention among ethnic minority or medically underserved populations, NCI provided information on two such trials that are "of extreme importance to several special population groups" and are therefore classified by NCI as Category II studies (National Cancer Institute, 1998b, p. 61). The Breast Cancer Prevention Trial, initiated in April 1992, tested the effects of tamoxifen in the prevention of breast cancer among high-risk subjects. Similarly, the Prostate Cancer Prevention Trial is designed to test the effectiveness of finasteride in the prevention of prostate cancer. Both studies, however, suffer from disproportionately low ethnic minority enrollment (see Chapter 4).
Secondary Prevention (Early Detection)
Breast Cancer Screening
The Breast Cancer Screening Consortium, funded through an NCI interactive grant mechanism, is a five-site study focused on identifying means of increasing the utilization of screening programs by women over age 50 who have not adhered to recommended screening guidelines. Telephone counseling and other interventions are examined in this study. NCI provided no information regarding the enrollment of ethnic minority or medically undeserved women in this trial. A smaller study, the Increasing Breast Screening Among Nonadherent Women study, evaluated the effectiveness and cost effectiveness of tailored telephone counseling and other intervention strategies in five regions of the United States in increasing the rates of breast cancer screening among nonadherent women, including elderly ethnic minority women. NCI classifies this study as a Category I and II study.
PLCO Cancer Screening Trial
The PLCO Cancer Screening Trial is a large-scale randomized study to determine whether screening tests will reduce the number of deaths related to prostate, lung, colorectal, and ovarian cancers. As of 1997, 89 percent of the participants in this Category II trial were white, 4.4 percent were African American, 1.4 percent were Hispanic, 4.3 percent were Asian American, and less than 0.5 percent were
Pacific Islanders, American Indians, or of other racial or ethnic backgrounds. To increase the levels of ethnic minority participation in the PLCO Cancer Screening Trial, NCI plans to cosponsor (with CDC) a new screening center to focus on the recruitment of African Americans and initiate another new center to focus on recruitment of Hispanics. A special study sponsored by CDC will assess psychosocial factors that influence older African Americans' decisions to undergo cancer screening. In addition, NCI is sponsoring a study to test literacy and develop culturally appropriate educational materials to encourage cancer screening among low-income African-American women.
Cervical Cancer Screening
The ASCUS/LSIL Triage Study is a 6-year clinical trial designed to determine the proper means of evaluating and managing minor Pap smear abnormalities. Four clinical centers are funded by NCI to enroll approximately 7,200 women with a recent diagnosis of abnormal Pap smears, of which nearly 40 percent are African American or Hispanic. Participants will be randomly assigned to one of three management groups and monitored for 3 years to help determine which patients are likely to experience progression to cancerous conditions.
Colorectal Cancer Screening
The South Carolina Colorectal Cancer Screening Study is an NCI-funded Category I study designed to develop new methods of recruiting low-income African-American women into colorectal cancer screening trials and to test literacy and develop culturally appropriate educational materials.
Other Screening Studies with Multiethnic Populations
NCI funded a large grant for a multicenter project administered by the Northern California Cancer Center, Pathways to Screening in Four Ethnic Groups. This project developed and evaluated culturally targeted cancer control interventions on the basis of the Pathways to Screening framework, a model of early cancer detection that focuses on the continuum from basic knowledge and attitudes to the procedural and organizational aspects of the delivery process. Pathways models were developed and evaluated for the Hispanic, Vietnamese, African-American, and Chinese-American communities in the San Francisco Bay area. Evaluation will assist in the development of culturally tailored interventions appropriate for racial and ethnic groups in other regions of the United States.
Clinical Trials
NCI Intramural Clinical Research Trials
NCI's Division of Clinical Sciences (DCS) supports ongoing intramural clinical trials on the NIH campus in Bethesda, Maryland. These trials include studies of clinical treatment protocols for lung, breast, ovarian, prostate, and bladder cancers and lymphoma. DCS maintains a central referral office that prospective patients can contact and a toll-free telephone number that prospective patients can call to obtain information or help in finding appropriate studies. All care at the clinical center is free of charge. In addition, NCI contributes to the Special Ambulatory Care Program to supplement travel, housing, and guardian expenses for patients while they are at the clinical center and for transportation to and from NIH. In addition, NCI has contributed to the Patient Emergency Fund, which is available to provide financial assistance to patients participating in clinical trials.
NCI reports that "statistical and financial evaluations of the populations recruited to intramural trials at NIH indicate that the majority of [NCI's] patients, and particularly pediatric patients, represent indigent populations from rural or inner-city areas" (National Cancer Institute, 1998b, p. 10). In FY 1996, 2,798 patients were enrolled at the NCI clinical center. Of these, 83 percent were white, 10.8 percent were African American, 2.5 percent were Hispanic, 1.4 percent were Asian or Pacific Islander, 0.1 percent were Native American, and 2.1 percent were of unknown racial or ethnic backgrounds.
Extramural Clinical Trials
CCOP
CCOP links patients and community-based physicians with researchers at clinical cooperative groups and cancer centers, thereby providing cancer patients, their physicians, and researchers with access to NCI-approved clinical trials and state-of-the-art care. NCI has funded nine clinical cooperative groups and three cancer centers to develop and manage clinical trials through CCOP. In addition, 51 community-based programs in 30 states were funded through CCOP and involved the participation of more than 300 hospitals and 3,300 physicians. NCI reports that nearly 20 percent of the more than 20,000 patients entering clinical treatment trials each year are ethnic minorities.
To increase the numbers of ethnic minority participants in CCOP, NCI initiated MBCCOPs in 1990. MBCCOPs are funded in seven states and Puerto Rico and have accounted for more than 50 percent of the
accrual of new ethnic minority cancer patients. NCI reports that minority accrual in CCOPs is proportionate to their representation in the United States (see Chapter 4 for more detailed information on minority enrollment) but adds that recruitment of minority and underserved populations remains a "special focus" of recruitment efforts (National Cancer Institute, 1998b). Approximately $2.5 million was allocated to this activity in FY 1997.
In addition, NCI's Cancer Therapy Evaluation Program has provided $1.1 million in funding in FY 1997 to 5 of the 11 cooperative groups to enhance the accrual of minorities in trials. Funds have been used to support community outreach in institutions with large minority patient populations, focus groups and educational opportunities for minority professionals, and advertising to increase minority awareness of clinical trials. Within the past 2 years, NCI has also reached agreement with VA and the Department of Defense to include eligible veterans and active-duty military personnel in clinical trial programs, potentially increasing the pool of minority participants. Finally, as noted above, ORMH of NIH has supported specific minority accrual projects at the NCI cancer centers, including the development of outreach literature for people with low levels of literacy, hiring of bilingual patient liaisons and a minority recruitment data manager, and other projects.
Conferences to Increase Accrual of Minorities
NCI has sponsored a series of conferences "to share current information and strategies that would aid cancer clinical investigators in recruiting and retaining minority participants in cancer clinical research" (National Cancer Institute, 1998b, p. 10). An RFA was issued to provide support for regional conferences; the RFA was preceded by a National Conference on the Recruitment and Retention of Minority Participants in Clinical Cancer Research. The national conference was cosponsored by NCI, ACS, the Oncology Nursing Society, NIH, and NIH's ORMH, and NIH's Office of Research on Women's Health. A monograph was published as a result of the national conference, and publication of the proceedings of each of the regional conferences is under way.
Basic Research
Basic research is the foundation of cancer research at NCI. This emphasis is reflected in the NCI FY 1999 budget request, which outlines four areas of "exceptional promise" for advancing knowledge of cancer: cancer genetics, including efforts to identify every human gene predisposing an individual to cancer; preclinical models of research, including the development of appropriate animal models of human cancers; imaging techniques
to improve diagnosis; and defining the signature of cancer cells to improve detection and diagnosis (National Cancer Institute, 1998d).
New developments in basic research have led to groundbreaking cancer treatments and prevention methods that have provided benefits to all populations. In some cases, significant gains have been made in technologies for the detection and treatment of cancers that disproportionately affect ethnic minority and medically underserved populations. Few examples of basic research at NCI dedicated to examinations of potential differences in underlying biological or genetic mechanisms among population groups exist, however. In a document provided to the study committee, NCI reports that "where there are biological differences among populations, these can only be found through basic research," yet it goes on to state that its "basic science projects are rarely categorized as 'targeted' research" (National Cancer Institute, 1998b, p. 80).
NCI provided to the study committee examples of ongoing basic research that may provide benefits for the detection, prevention, and treatment of several cancers that disproportionately affect ethnic minority and elderly populations. Only in the area of breast cancer was it apparent that research questions were directed toward understanding population-group differences in cancer etiologies, disease courses, and treatment responses. For example, NCI has initiated a study to investigate the reasons for the possible increased aggressiveness of breast cancer in African-American women. Analyses of breast tissue samples from African-American and white patients matched by age, stage of disease at diagnosis, and other critical factors are under way to determine if differences in molecular characteristics may account for the poorer prognosis of breast cancer in African-American women. In addition, NCI has supported research on Mapping by Admixture Linkage Disequilibrium (MALD) to assist in gene mapping among patients with sporadic cases of breast cancer. Such patients have no family history of the disease, precluding traditional genetic linkage analysis. Tissue specimens from African-American patients are being analyzed by MALD analysis to explore possible genetic links behind sporadic cases of breast cancer.
NCI is also supporting two pilot studies involving basic research on breast cancer among diverse population groups. One examines the kinetics as the well as the phenotypic and genotypic properties of breast cancer as they relate to tumor aggressiveness among African-American, Hispanic, and white women in south Florida. Another plans a comparative molecular analysis of the primary DNA sequence of the estrogen receptor gene in breast cancer tissue removed from African-American, Hispanic, and white women to look for potential gene rearrangements and deletions.
Information for the Public and Outreach Activities
NCI staff are involved in a wide range of outreach and public information efforts, some of which are tailored specifically to the needs of ethnic minority communities.
Information and Education
International Cancer Information Center
OCICE supports ICIC, which maintains the NCI web site CancerNet, as well as two comprehensive cancer databases, PD. and CANCERLIT. CancerNet (located at http://cancernet.nci.nih.gov) provides information to the general public and physicians and features a special section entitled "Information for Racial/Ethnic Groups." ICIC staff are working with advocacy groups on an initiative to provide layperson-oriented information about cancer clinical trials on CancerNet. PDQ is a database that describes the latest advances in cancer treatment, care, screening, and prevention and features a technical version for health professionals and a nontechnical version for patients and the general public. Users can obtain information on clinical trials in directories of more than 23,000 physicians and more than 11,000 organizations active in cancer care. PDQ is available in both English and Spanish. CANCERLIT is a bibliographic database that contains citations from the cancer literature published from 1963 to the present and contains more than 1.2 million citations.
ICIC has also sought to provide tailored outreach to the Hispanic community by using market research conducted by the Office of Cancer Communications (OCC). Direct mail communication with 37,000 Hispanic-serving health professionals, including information about NCI resources and how to access them, a Spanish-language brochure for patients, public service announcements in major Hispanic markets, and outreach to the National Council of Catholic Bishops are among the strategies used to target this population. ICIC is also sponsoring a pilot project in seven Maryland public libraries to increase the awareness and use of CancerNet in these settings, especially among individuals who are less likely to have access to computer resources at home or work.
Cancer Information Service Branch
The Cancer Information Service Branch oversees CIS, a national information and education network and toll-free telephone service that provides information to individuals and communities about recent developments in cancer research, prevention, and treatment. The CIS toll-free telephone service operates in both English and Spanish. To improve outreach to minority communities, CIS has engaged in collaborations with minority-based and minority-serving organizations.
For example, CIS is or has been working with the National Black Leadership Initiative on Cancer (NBLIC), the Appalachian Leadership Initiative on Cancer (ALIC), and the National Hispanic Leadership Initiative on Cancer (NHLIC) to support the development of community-based coalitions focused on cancer prevention, to promote nutritional awareness, and to air bilingual public service announcements as part of cancer prevention efforts, respectively. CIS staff have also worked directly with community groups to develop culturally sensitive cancer screening messages for Hawaii Natives and American Indians.
Patient Education Branch
The Patient Education Branch (PEB) of OCICE is involved in the development, implementation, and promotion of educational programs for persons living with cancer and their families, including culturally tailored education and outreach services for minority and medically underserved populations. PEB assists extramural scientists with outreach efforts to increase patient accrual to clinical trials, including promotional efforts targeted to minority health professional organizations and minority-serving media outlets. These efforts include the Cancer Clinical Trials Education Program, an information and education program geared to health professionals regarding clinical trials, including culturally tailored information for professionals serving ethnic minority patients and individuals with low levels of literary. A cancer survivorship training program has also been developed for health professionals addressing the common concerns of cancer survivors, including components tailored to minority audiences. Similarly, an information booklet was developed for health professionals to address patients' concerns regarding genetic testing. PEB has also supported demonstration projects to develop new and innovative ways of disseminating the Patient Information File for the PDQ database, including one demonstration specifically targeted to African-American and Hispanic consumers. Finally, PEB also supports the Cancer Patient Education database, which provides information on hundreds of cancer patient education resources and publications, including non-English publications and publications directed toward individuals with low levels of literacy, and disseminates educational resources to cancer patients and their families.
Health Promotion Branch
The NCI Health Promotion Branch (HPB) conducts research on health communication and develops materials for distribution through the CIS outreach program. For example, HPB staff have conducted focus group research to learn more about Hispanic consumers' attitudes, beliefs, and behaviors related to cancer prevention, early detection, and treatment and to learn more about African-American,
Hispanic, Native American, Asian-American, and white women's attitudes and knowledge regarding breast cancer and mammography. HPB has also conducted consumer research to determine how to better frame 5 A Day health messages to the African-American community. In addition, NCI staff have developed a geodemographic marketing database that links demographic, marketing, and health information to better tailor messages to the needs of specific communities.
HPB staff have also developed a number of publications and public service announcements for ethnic minority populations (see Appendix C for examples of titles). In addition, HPB supports media campaigns, such as the recent national media campaign on cervical cancer screening, that feature efforts to reach out to ethnic minority populations. The cervical cancer screening campaign included information on the incidence and rates of mortality from cervical cancer among older minority women in a media kit distributed to majority and minority media outlets.
Overall, OCC reports spending $7.4 million in FY 1997 targeting cancer information to minority and underserved populations, although this figure is derived by using "percent relevancy" calculations for monies allocated on the basis of the percentage of minority group individuals in the overall target pool. Among those programs in FY 1996 listed as "100 percent" relevant to minority populations, Hawaii's CIS received $393,000 for outreach activities targeted to Native Hawaiians, $250,000 each was allocated to the development of nutrition education materials targeted to African Americans with low levels of literacy and to community-based education projects directed at urban African-American residents, and $150,000 was allocated to the development and dissemination of culturally appropriate messages and materials for American Indians, Native Alaskans, and Native Hawaiians.
Outreach Activities and Community Education
NCI has placed a great deal of emphasis on three leadership initiatives to support minority outreach, research, and cancer control efforts.
NBLIC seeks to promote the participation of African-American community leaders in the mobilization and stimulation of community cancer prevention and control activities. NBLIC's objectives are to reduce cancer incidence and mortality rates and improve cancer survival rates among African Americans and to address barriers to cancer control services among other objectives. More than 60 community coalitions have been established through the work of NBLIC's four regional offices. NCI allocated approximately $1.7 million to this activity in FY 1997.
Through NHLIC, an initiative implementing community demonstration and health communications research projects, NCI is addressing the
cancer prevention and control needs of the Hispanic community. NCI has established two cooperative agreements for NHLIC, one with the Baylor College of Medicine and another with the National Coalition of Hispanic Health and Human Services Organizations (COSSMHO). COSSMHO has established nine local project sites across the United States and in Puerto Rico to address cancer research questions with community-based organizations. NHLIC's: En Acción, an NCI cooperative agreement with the Baylor College of Medicine, is a theory-based, research-oriented program combining national and regional health expertise with grassroots community leadership to reach the major Hispanic populations in six cities across the country. The project initiated the first comprehensive epidemiologic assessment of cancer risk factors among these Hispanic and Latino population groups and has developed state-of-the-art cancer prevention and control strategies tailored to those diverse Hispanic populations. NCI allocated approximately $1.2 million for these activities in FY 1997.
ALIC serves to facilitate the development of a strong cancer network in Appalachia. As a research program, ALIC is testing the effectiveness of community coalitions and partnerships as an intervention strategy for social and organizational change in achieving cancer control objectives. The program involves four individual projects, with four research universities as the lead agencies, working with a variety of partners in 10 different states in the Appalachian region. All four projects have initially focused their coalition development activities on the following specific outcomes: an increase in early-stage diagnosis of breast and cervical cancer with a concomitant decrease in late-stage diagnosis of these diseases, an increase in cancer survivorship, and a decrease in breast and cervix cancer mortality over time. A great deal of information about the society and about the health—and cancer problems—in Appalachia, and about the individual ALIC projects, can be found in a publication, Sowing Seeds in the Mountains, an ALIC monograph published by NCI in 1994. Preliminary results suggest that the ALIC intervention has enhanced capacity (defined as a greater degree of interconnectedness among partnering organizations involved in planted action) in the Appalachian cancer control system and that this additional capacity will increase screening activities for breast and cervical cancer in the region. NCI allocated approximately $1.5 million for these activities in FY 1997.
Cancer Research Networks
NCI supports two networks to coordinate meetings and enhance the efforts of researchers working to address the needs of ethnic minority and underserved populations. The Network for Cancer Control Research Among American Indian and Alaska Native Populations (NCCR-AIANP)
was established in 1990 to disseminate information regarding cancer control and prevention among American Indian populations, to increase collaboration with the Indian Health Service and CDC, to expand cancer surveillance among American Indian populations, and to promote new studies on patterns of care and cancer survivorship. NCCR-AIANP has convened three national conferences to discuss research and training issues relevant to American Indian populations and developed a National Strategic Plan for Cancer Prevention and Control Research in 1992. The Network also established in 1997 the Cancer Information Resource Center and Learning Exchange (CIRCLE) at the Mayo Comprehensive Cancer Center. This learning and resource center serves to link young American Indian cancer prevention and control researchers with experienced mentors. The Native Hawaiian and American Samoan Cancer Control Research Network focuses on the cancer control and prevention needs of Pacific Islander populations. The Network collaborates with a variety of public and private organizations, including Papa Ola Lokahi (the Native Hawaiian Board of Health), the Office of Hawaiian Affairs, the Cancer Center of Hawaii, Native Hawaiian Health organizations, traditional healers, scientific and lay community leaders, and others. In 1995 the two networks collaborated to sponsor a Native American Cancer Conference III in Seattle, Washington, to discuss their mutual problems.
It is not clear from the information provided by NCI how these networks differ from the leadership initiatives in purpose or mission. Similarly, it is unclear why no such network or leadership initiative exists for cancer control activities among Asian-American populations.
Training of Minority Scientists
Many ethnic minority groups are disproportionately underrepresented among cancer researchers in all relevant fields of study (e.g., oncology, psychology, epidemiology, molecular genetics). Increasing the pool of well-trained ethnic minority researchers can help to increase the quality and quantity of research on ethnic minority and medically underserved populations. For example, the small percentage of ethnic minority health care professionals and researchers has been identified as a significant barrier to the participation of ethnic minorities in clinical trials (Swanson and Ward, 1995). Further, such researchers are often able to address cultural and linguistic considerations in the conceptualization of research on ethnic minority populations and the interpretation of research findings.
NCI offers a number of training and career development programs designed to increase the number of minority scientists in biomedical fields, as well as to enhance the careers of those already in the field. NCI's Comprehensive Minority Biomedical Section (CMBS) in the Division of Extramural
Activities (DEA) and OSPR have funded the majority of these graduate, postgraduate, and collegiate fellowships and scholarships to encourage minority students to pursue careers in oncology research. In addition, funds are allocated to increase mentoring and training opportunities at minority-serving institutions.
Eligibility for minority training funds is typically restricted to U.S. citizens or resident aliens and to members of ethnic or racial groups determined by the granting institution to be underrepresented in biomedical or behavioral research. For minority research supplements, this definition includes African Americans, Hispanic Americans, American Indians, Alaska Natives, and Pacific Islanders but excludes many Asian-American groups. Furthermore, NIH gives priority to projects involving African Americans, Hispanic Americans, American Indians, Alaska Natives, and Pacific Islanders. The exclusion of Asian-American groups from these programs appears to reflect NCI's belief that Asian-American scientists are well represented within the cancer research field. Asian-American researchers, however, are underrepresented in behavioral and population sciences, highlighting the dangers of assessing the representativeness of populations in research on the basis of aggregated data across all cancer fields. NCI did not cite an empirical basis for the policy of exclusion of Asian Americans from training programs.
Dissemination of information regarding training programs appears to occur largely through government publications, such as the NIH Guide. Announcements about the minority research supplement grants, for example, are provided to principal investigators via the guide. Dissemination also occurs through the efforts of program staff attending professional conferences and meetings (e.g., the annual meeting of the American Association for Cancer Research [AACR]). Beyond these efforts, NCI did not provide evidence of any formal or systematic information dissemination or outreach plan regarding training opportunities for minorities.
NCI Programs for Underrepresented Minorities
Minority training and enrichment programs include the following:
- Science Enrichment Program. The Science Enrichment Program (SEP) is a 4- to 6-week summer residential science education program to encourage promising high school students to pursue professional careers in scientific research. SEP is open to youth from underrepresented minority and underserved groups, as well as those from areas where science education opportunities are limited. Three universities (the University of Kentucky at Lexington, the University of Massachusetts at Amherst, and the University of Southern California) now participate in the program. Approximately 150 youth participate in the program, which received funding of slightly more than $1 million in FY 1997.
- Minority Access to Research Careers Summer Research Training Program . NCI, in collaboration with the Minority Access to Research Careers (MARC) program of the National Institute for General Medical Sciences (NIGMS), provides support to students from MARC institutions to participate in a summer research traineeship program at NCI's intramural laboratories. The program is designed to increase research training opportunities for minority MARC scholars interested in cancer-related research careers. Approximately $25,000 was allocated in FY 1997 to fund six students.
- Travel Awards. CMBS provides travel fellowships for minority students to attend annual meetings of AACR. The program is intended to increase the participation of minority pre- and postdoctoral scientists in the annual meeting. Thirty-three awardees were funded in 1997; $73,000 was allocated for this activity in 1998. Similarly, funds were made available for the first time to support the travel of faculty at historically black colleges and universities (HBCUs) to attend the AACR conference.
- Mentored Career Development Award. NCI has invited individuals who have been recipients of an NIH Research Supplement for Underrepresented Minority Individuals in Postdoctoral Training or a Minority Investigator Supplement to apply for the Mentored Career Development Award, which is intended to provide a means of gaining scientific expertise while bridging the transition from a mentored research environment to an independent research or academic career. Approximately $950,000 was allocated for this K01 award, initially announced in an RFA in 1997, with approximately $1.25 million allocated in FY 1998. Ten awards were made in 1997.
More than $400,000 was allocated in FY 1997 for the following four Minority Health Professional Training Initiative awards:
- Minority Oncology Leadership Academic Award. This award, available to faculty members at minority health professional schools, is offered to promote independent cancer research.
- Clinical Investigator Award for Research on Special Populations. This K08 award is targeted to newly trained clinicians interested in designing research protocols aimed at improving the health of groups with disproportionately high cancer morbidity and mortality rates. The award enables clinicians to pursue 3 to 5 years of special study and supervised research experience and covers the transition between postdoctoral research and an independent research career.
- Mentored Research Scientist Development Award. This award supports the development of faculty members at minority-serving institutions in areas relevant to cancer. The awardee is provided with a mentored relationship with outstanding cancer researchers at major research institutions. The objective of the award is to ''broaden the experience of faculty members at minority schools, to increase the pool of biomedical and behavioral investigators in cancer research, and have graduate and undergraduate students, most of whom will be minority individuals, become more cognizant of research opportunities in cancer research" (National Cancer Institute, 1998b, p. 23). Two such awards were made in 1994 and 1995.
- Minorities in Medical Oncology. This award, first announced in an RFA in 1996, was reissued as a program announcement in 1997 and provides funds to encourage recently trained minority clinicians to gain research experience in medical oncology. Three awards were made in 1996.
Other Training and Career Development Programs
In addition to the awards described above, a number of NIH-wide funding mechanisms related to career development and training are offered by NCI, the majority of which are open to all cancer researchers.
Research Supplements for Underrepresented Minorities
Nearly $2.2 million was allocated for both the Minority Investigator Supplement to NCI's cancer centers for minority research and NCI grantees to support minority researchers involved in research projects. Minority Investigator Supplements were also available for promising high school students, undergraduate students, and graduate research assistants (nearly $1.2 million in FY 1997). In 1997, 128 awards were made; these grants were overwhelmingly made to African-American (61 awards) and Hispanic (60 awards) researchers, with 4 awards made to American Indian researchers and 1 award made to a Pacific Islander. In 1996, 112 awards were made; these were made exclusively to African-American and Hispanic scientists.
National Research Service Awards
Three National Research Service Awards (NRSA) are offered. A predoctoral fellowship for oncology nurses, minority students, and students with disabilities (F31 award) was established to increase the numbers of each group in the cancer research enterprise, whereas a postdoctoral fellowship (F32 award) is open to all cancer researchers holding the Ph.D. degree or its equivalent. Both fellowships require the awardee to work with an identified mentor at a sponsoring institution to supervise research and training. The NRSA Senior Fellowship (F33 award) was established for experienced scientists who seek to
acquire new research capabilities or change the direction of their research careers.
Other Awards
Other individual research training and career awards include the Howard Temin Award (K01 award), to enhance the research careers of the most promising doctoral-level scientists while they consolidate and focus their research programs and obtain independent funding; the Preventive Oncology Academic Award (K07 award), to promote the career development of researchers in the fields of cancer prevention, cancer control, and cancer etiology; the Mentored Clinical Scientist Development Award (K08 award), for clinically trained physicians who are committed to careers in cancer research to undertake 3 to 5 years of specialized study; and the Mentored Clinical Scientist Development Program Award (K12 award), an award to educational institutions to support career development experiences for clinicians that will lead to research independence in areas such as translation of basic research results into new clinical procedures and AIDS-related malignancies.
Overall, NCI reports that it allocated $80.4 million to training and education programs in FY 1997, including $43.7 million to the NRSA program, $20.9 million to the Research Career Program, and $12.2 million to the Cancer Education Program. DEA reports that it allocated approximately $15 million to minority training, including the programs cited above.
Other Extramural Research Support
NCI reports that several programs, in addition to the ones reported above, have been designed specifically to expand opportunities for minority researchers or to address research questions critical to minority and medically underserved communities. These are described in the following sections.
Minority Biomedical Research Support
More than $2.5 million was allocated for the Minority Biomedical Research Support program, which is offered in conjunction with NIGMS to expand opportunities for ethnic minority faculty and students by supporting specific cancer-related projects at minority-serving institutions. Nineteen such awards were provided in FY 1997.
Small Research Grants for HBCU Faculty
To increase the research base at historically black institutions, NCI
offers funding to new faculty members at HBCUs to conduct basic science research relevant to the goals of NCI.
Small Business Innovative Research Grants and Technology Transfer Research Grants
The Small Business Innovation Research Grant and the Small Business Technology Transfer Research Grants provide funds to small businesses that develop technological, innovative, and cost-effective solutions to translating basic scientific knowledge into clinical and preventive cancer services. NCI reports that it made 10 such awards for ethnic minority projects in FY 1997, including several projects that develop targeted cancer prevention and education messages for African-American, Hispanic, or Native American populations, and 2 awards for the development of cancer information materials for populations with low levels of literacy.
Small Grant Program
NCI has established an R03 award mechanism to fund projects solicited by RFAs or program announcements that are preliminary short-term research projects limited in time (typically 2 to 3 years) and amount of support ($50,000 annually). NCI provided examples of five projects related to special populations funded through this mechanism, which typically allows investigators to test new ideas or conduct pilot studies. In addition, NCI funded eight awards pertaining to underserved minority women in FY 1997. These awards were made following the release of an RFA pertaining to the recruitment and retention of ethnic minority women in clinical and prevention trials.
Funding for Extramural Research Relevant to Ethnic Minority and Medically Underserved Populations
NCI reports that 127 extramural awards were made in FY 1997 to address the needs of specific special populations, including some of the research projects summarized above. "Special populations" include ethnic minorities, medically underserved groups, elderly people, blue-collar workers, and people living in rural areas (see Chapter 2). Awards are included in this tabulation only if the grant primarily targets one or more special populations. Nearly $44 million in funding was allocated to special populations research in FY 1997, an increase of more than $30 million since FY 1990 (see Table 3-4 and Figure 3-2).
TABLE 3-4 Minority/Special Populations Awards (CRISP 1997; last update January 20, 1998)
|
Activity Code |
No. of Awards |
Award Amount |
|
F31 |
2 |
$30,814 |
|
F32 |
1 |
28,600 |
|
K07 |
3 |
161,095 |
|
N01 |
9 |
14,408,361 |
|
P01 |
4 |
704,346 |
|
P30 |
8 |
1,307,744 |
|
P50 |
2 |
367,519 |
|
R01 |
59 |
17,514,524 |
|
R03 |
12 |
1,297,779 |
|
R13 |
1 |
50,000 |
|
R25 |
12 |
1,445,109 |
|
R29 |
1 |
132,937 |
|
R43 |
2 |
199,493 |
|
R44 |
3 |
1,022,700 |
|
U01 |
4 |
3,967,931 |
|
U10 |
5 |
1,175,872 |
|
Total |
128 |
43,903,168 |
|
SOURCE: National Cancer Institute. |
||
Among these awards, NCI reports that 59 R01 awards—the principal NIH research mechanism for investigator-initiated awards—totaling more than $17.5 million were provided. Nine N01 awards totaling $14.4 million were granted. The awards principally support the PLCO Cancer Screening Trial and the recruitment of minority individuals into these screening trials. Twelve R03 and R25 awards supported small grants for time- and scope-limited projects (these were primarily projects to stimulate recruitment and retention of minorities in clinical trials) (nearly $1.3 million) and educational projects to develop programs in areas of education, information, technical assistance, or evaluation ($1.44 million), respectively. Eight P30 center core grant awards totaling $1.3 million were made to consortiums, including the Charles R. Drew University of Medicine and Science in Los Angeles. Nearly $4 million in funding was provided to support four U01 cooperative agreements, including the minority-serving leadership initiatives. A total of $704,000 in four P01 awards was awarded to support broad-based, multidisciplinary research. Many other awards were provided in other categories (see Table 3-4 for details).
FIGURE 3-2 National Cancer Institute awards targeting special populations
(total funding in millions of dollars). SOURCE: National Cancer Institute.
Allocation of NCI Resources for Research on Ethnic Minority and Medically Underserved Groups
The summaries of programs of research at NCI relevant to minority and underserved populations presented above include information regarding specific funding amounts, where available. Attempts to estimate the overall percentage of the NCI budget that has been allocated to the study of minority and medically underserved populations is difficult, in part because many basic and applied research programs that are geared to improving cancer prevention, treatment, and control among general populations may reap benefits for minority and medically underserved groups.
Another difficulty in accounting for funding allocated to the study of specific populations is posed by the accounting methods that NCI uses to estimate resource allocation. NCI, along with other agencies of the U.S. Department of Health and Human Services, is required to produce annual reports on the allocation of research funds for Minority Health and Assistance Programs (MHAPs). These include programs that are fully targeted to minority populations (100 percent relevant), in addition to those that are not specifically targeted to these populations but that have some bearing to minority populations by virtue of having minority groups among study samples, clinical trial groups, or the like. For the latter programs, NCI calculates the "percent relevancy" of the program on the basis of the
percentage of minority individuals targeted in a research grant or contract. This percentage is then used to estimate the amount of funding from the project that can be added to the total funding for minority health programs. For example, if a clinical trial research grant is funded at a total of $1 million and if 30 percent of the patients in the trial are ethnic minorities, $300,000 is counted toward total institute expenditures on minority health. No such calculations or figures are reported for medically underserved populations.
Such accounting methods, however, obscure and perhaps overstate the benefits of research for minority populations. If research questions are not geared toward illuminating unique issues in cancer treatment, control, and prevention for minority populations, then the findings may be of limited value in addressing disparities in health outcomes between minority and nonminority populations. It is unclear from percent relevancy accounting methods whether such studies address unique issues for minority populations or whether subpopulation analyses are overlooked entirely. In addition, studies with very small percentages of minority populations may not provide the statistical power necessary to examine within- or between-group differences that are relevant for minority groups, yet these percentages are used in calculations of total resources allocated to minority programs.
Using "percent relevancy" accounting methods, NCI reports that $124,399,000 was allocated to MHAPs in FY 1997. Many of these research programs were summarized earlier in this chapter and include both intramural and extramural research, contract, and training programs. This figure represented approximately 5.25 percent of NCI's total budget in 1997. According to the committee's own calculations, however, inclusion of only those research programs listed as 100 percent relevant to minority populations would result in a total figure for FY 1997 of $24,234,000, or approximately 1 percent of the total NCI budget.
These projects almost certainly focus research hypotheses on issues for ethnic minority populations (i.e., that are specific to these groups), whereas NCI's report of $124.4 million may reflect both research targeted specifically to ethnic minority groups, as well as research that indirectly offers benefits to these populations (i.e., that are relevant to ethnic minority groups). Further, NCI's report of allocating $43.9 million in FY 1997 to research projects specific to "special populations" includes funding for projects targeted to elderly and blue-collar populations, groups that were not included in the committee's analysis.
The committee offers the following recommendations regarding resource allocation and accounting methods for programs of research and
training relevant to ethnic minority and medically underserved populations:
Recommendation 3-2: Research and research funding relevant to cancer among ethnic minority and medically (as per Recommendation 3-3) underserved populations should be more adequately assessed and should be increased.
NCI reports that it allocated more than $124 million for research and training programs relevant to cancer among ethnic minority and medically underserved populations. This figure represents approximately 5.25 percent of the total NCI budget. The committee believes, however, that this is an overrepresentation of the amount of resources allocated to addressing the needs of ethnic minority and medically underserved groups (see Recommendation 3-3 below). When allocations are summed for projects exclusively directed to these populations, the figure is slightly more than $24 million, or approximately 1 percent of the total NCI budget. Other NIH ICs report on their allocations of money to minority health research and training programs, but these figures are small relative to the overall budgets of the respective ICs. The committee finds that these resources are insufficient relative to three criteria:
- the burden of disease in ethnic minority and medically underserved communities;
- the changing U.S. demographic picture (which indicates that the growth of many ethnic minority groups, such as Hispanics and Asians and Pacific Islanders, will significantly outpace that of other groups and that no ethnic group will constitute a "majority" by the year 2050); and
- scientific opportunities inherent in the study of diverse populations.
Recommendation 3-3: NIH should improve the accuracy of its assessment of research that is relevant to ethnic minority and medically underserved groups by replacing the current "percent relevancy" accounting method with one that identifies studies whose purpose is to address a priori research questions uniquely affecting ethnic minority and medically underserved groups.
NIH calculates the amount of money allocated to minority health research on the basis of the percentage of ethnic minority individuals in NIH study populations. Such percent relevancy accounting methods are inappropriate as an indicator of overall expenditures for studies that address minority health needs because they overstate the relevance of research for addressing ethnic minority health issues. Estimates of expenditures on minority-related health research should be determined by
assessing whether research questions for specific programs are focused on illuminating the particular needs of ethnic minority and medically underserved communities.
The committee found no evidence that NIH calculates total expenditures for research on medically underserved groups, apart from calculations derived for ethnic minority populations. A consistent definition of medically underserved individuals is needed (as discussed above), and calculations of research expenditures for these groups should be based on whether research questions specifically address the unique needs of these populations.
Research on Cancer among Ethnic Minority and Medically Underserved Groups at other ICDS
As noted above, several ICDs are conducting research, either in collaboration with NCI or independently, on cancer among minority and medically underserved populations.
National Institute of Environmental Health Sciences
As noted above, NIEHS supports the second-largest portfolio of cancer-related research at NIH, with overall funding of more than $84 million in FY 1997. Of this amount, NIEHS reports that it allocated $26.7 million to fund 138 extramural projects, including investigator-initiated research, co-funded projects, and Superfund and center grants (described below). Of these, NIEHS reports that 71 awards are relevant to minority and medically underserved populations, in that the awards specifically target minority or low-income communities or the research topic disproportionately affects minority and medically underserved populations. Total expenditures on these awards fell slightly under $9.5 million; eight awards were cosponsored with NCI, with a total of $672,389 expended by NIEHS. Several relevant programs are described below.
Community Outreach and Education Program
NIEHS supports community outreach efforts through the Environmental Health Sciences Center Grants Program, a program of core center support that seeks to address specific regional or community needs through outreach and educational activities. Centers serve to define environmental health issues of greatest concern to communities, with a focus on populations that may be at greatest risk as a result of environmental insults, including children, elderly people, and low-income communities. Centers are encouraged to sponsor local efforts through community organizations
and to collaborate with other existing outreach programs, especially those supported by other federal agencies (e.g., other NIH institutes, CDC, and the National Institute for Occupational Safety and Health).
Developmental centers, in particular, are encouraged to foster collaborations and develop outreach programs for ethnic minority and low-income communities. NIEHS currently supports three such centers. Columbia University's Harlem Center for Health Promotion and Disease Prevention, for example, has formed partnerships with community groups in Harlem and Washington Heights, New York, to promote public education, outreach, and environmental monitoring. Interdisciplinary and inter-institutional programs with Harlem Hospital, for example, have also provided a forum for sharing environmental health research findings across institutions. Community outreach forums have addressed a wide range of community concerns about environmental pollutants and public health problems. Similarly, the Tulane/Xavier Center for Bioenvironmental Research provides comprehensive environmental research and education for the City of New Orleans and the Gulf South region. This center combines the resources of a major research institution and historically black college to foster collaboration between local organizations; state, local and federal governments; industry; and students.
Community-Based Prevention/Intervention Research in Environmental Health Science
This initiative aims to implement culturally relevant prevention and intervention activities among economically disadvantaged and underserved populations that are adversely affected by an environmental contaminant. It is intended to foster refinement of scientifically valid intervention methods, whereas it also strengthens the participation of the affected communities in this effort. The long-range goal of this program is to reduce the incidence and mortality from environment-associated diseases among these populations. Five grants that concentrate on Native American, African-American, and Hispanic populations have been awarded. This program also serves as a model for other federal, state, and local agencies in designing their own environmental justice contaminant prevention programs.
Environmental Justice: Partnerships for Communication
The Partnerships for Communication program provides multiyear grants that foster collaboration between health providers and community residents to examine and assess environmental contamination specifically located in communities. The primary goal of the grants is to investigate the relationship between economic factors, social factors, and the health
status of the community members exposed to environmental hazards. Currently, 12 grants have been awarded across the United States. The majority of these grantees are located in lower-income communities confronting the redevelopment of brownfields.
Minority Worker Training Program
NIEHS, working in collaboration with the Environmental Protection Agency, has developed the Minority Worker Training Program as a series of national pilot programs designed to test strategies for the recruitment and training of young people for future environmental careers. The people selected live near hazardous waste sites or in communities at risk of exposure to contaminated properties. This program seeks to provide sustainable job development and training for young adults who live in areas with hazardous exposure potential, such as brownfields sites in urban communities. Community groups and HBCUs are involved in this partnership to teach math, science, and life skills to assist in reinforcing worker knowledge and positive behavior on the job.
Environmental Health Science Centers: Agricultural Chemicals and Farm Workers
The NIEHS Environmental Health Science Centers are academic institutions throughout the country that bring together individuals from many scientific disciplines to focus on particular environmental health problems. Three of these centers (those at the University of Iowa, University of California at Davis, and Oregon State University) focus on the health concerns of agricultural workers, many of whom are migrant workers or disadvantaged minorities. The results of this research will help define the true risks to this occupational group so that better prevention and intervention strategies can be developed to protect their health.
Agricultural Health Study
NIEHS is assisting NCI in a prospective study of cancer risk in a cohort of 75,000 pesticide applicators and their spouses. NIEHS manager and evaluates the part of the study devoted to noncancer endpoints and has assisted in recruiting African Americans into the study population. This study examines, in part, the unique risks to rural African Americans and other minority populations exposed to pesticides. Minority populations may have increased susceptibility to the effects of pesticides. For example, there is increasing evidence of racial differences in the prevalence of gene polymorphisms that affect the metabolism of chemicals including pesticides
and the organic solvents in which they are mixed. In addition, such groups may have enhanced vulnerability to pesticides due to underlying nutritional deficiencies or concomitant health problems often associated with poverty.
Brownfields National Partnership Action Agenda
NIEHS is participating to help support the Brownfields National Partnership Action Agenda, a public, private, and community initiative to redevelop brownfields into safer living areas. Brownfields are abandoned, idled, or underused industrial and commercial facilities where expansion or redevelopment is complicated by environmental contamination. These areas are not as toxic as Superfund sites but still face immense barriers to their redevelopment. Although brownfields are typically found in urban areas, they are prominent in rural areas as well. A large proportion of medically underserved and economically disadvantaged citizens reside near brownfields.
Mississippi Delta Project
The Mississippi Delta Project is a collaborative effort of government, academia, grassroots organizations in communities, and local and state health agencies to address environmental contamination in the Mississippi Delta Region, one of the poorest regions in the country and a region that is greatly affected by environmental pollution. NIEHS works with other federal agencies to identify key environmental hazards, promote environmental quality, and reduce and, where possible, prevent these hazards from affecting the health and environment of residents.
Working in collaboration with ORMH, NIEHS has initiated the Columbia Project, a community outreach program of the Mississippi Delta Project. This is a community-based effort to augment community participation and involvement in decisions concerning the environmental health of residents in the Delta Region. A Needs Assessment Workshop to identify demonstration projects in the Delta Region was held in February 1998 and included representatives from federal agencies, state and local health departments, HBCUs, grassroots organizations in communities, and health care professionals.
Institutional Capacity Building
NIEHS has supported several programs designed to enhance environmental health research and training capacity in underserved populations. NIEHS has initiated a series of planning activities organized through HBCUs
and majority academic institutions to assess the needs and opportunities for building environmental health research and training capacity for underserved populations. In addition, a state-of-the-art Molecular Research and Training Center has been established at a local (Durham, North Carolina) high school that is attended by a large number of African-American students.
Training and Education Workshop
NIEHS sponsors several programs designed to increase the number of minority scientists involved in biomedical research, increase the levels of awareness and participation of minorities in NIEHS and NIH intramural and extramural activities, and facilitate cooperative research between minority and majority scientists on the health problems that may disproportionately affect minorities and lower-income populations. For example, the Minority Faculty Development program is designed to strengthen the ability of minority institutions to provide instruction of the environmental health discipline.
National Institute of Allergy and Infectious Diseases
NIAID supports a number of research projects relevant to cancer among minority and medically underserved populations. This research focuses largely on cancer associated with AIDS and neoplastic complications of HIV infection, notably Kaposi's sarcoma, but also includes basic studies of the abnormal proliferation of immune cells and immune responses to cell proliferation. Given that HIV infection disproportionately affects ethnic minority populations, particularly African-American and Hispanic communities, NIAID's portfolio of research in this area is highly relevant to the overall NIH effort to address cancer among minority and medically underserved populations.
As noted above, NIAID supports the fourth-largest portfolio of cancer research among NIH ICDs, with $43 million in funding for 177 cancer-related research projects in FY 1997. Of these, NIAID reports that 63 projects, with funding totaling $4.49 million, are relevant to minority and medically underserved populations.
Clinical Trials
Through a range of intramural and extramural programs, NIAID directs a large national clinical trials network that tests and provides therapies for the treatment and prevention of HIV infection and its complications. These programs have enjoyed, on the whole, significant success in
the recruitment of minority candidates into clinical trials. NIAID works with these networks to identify and help develop culturally sensitive educational materials and to identify and overcome barriers to recruitment and retention of minority patients. Among the patients enrolled in the Adult AIDS Clinical Trials Group in 1996, for example, 35 percent were African American and 25 percent were Hispanic. Three institutions serving predominantly minority communities were originally funded in 1993 to assist in minority accrual to these trials. Similarly, the Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) enrolled 31 percent African-American patients and 12 percent Hispanic patients among new enrollees in 1996. CPCRA is a community-based trials network located in community settings such as health care clinics and centers, making these trials much more accessible to women and ethnic minorities.
NIAID supports epidemiologic research, clinical research, and research on the natural history and transmission of HIV infection in a variety of populations. Urban women and children are the focus of the Women and Infants Transmission Study, which evaluates issues of perinatal HIV transmission and disease progression in women and children. In 1996, 86 percent of the women enrolled in this program were ethnic minorities. Similarly, the Women's Interagency HIV Study examines the spectrum and clinical course of HIV infection in women and has enrolled 56 percent African-American and 24 percent Hispanic women. In contrast, NIAID's Multicenter AIDS Cohort Study, which examines the mechanisms by which HIV damages the immune system and how the immune system combats HIV infection among homosexual and bisexual men, has enrolled only 15 percent ethnic minority participants.
Training and Infrastructure Development
NIAID has provided support for a number of programs designed to increase the numbers of minority biomedical researchers and support the development of the biomedical research infrastructure at minority-serving institutions.
The Research Supplements for Underrepresented Minorities program served to increase the number of minorities in biomedical research by providing supplemental training funds for research grants currently being funded by NIH. In 1996, NIAID funded 40 such supplements at $3.6 million for minority investigators at the junior faculty, postdoctoral, predoctoral, undergraduate, and high school levels. Similarly, the Research Centers in Minority Institutions (RCMI) program assists predominantly minority institutions that offer doctoral degrees via grant support for laboratory and infrastructure development, faculty expansion, and other areas to assist these institutions in becoming more competitive in seeking research
funding. NIAID co-funds the AIDS Infrastructure Initiative of RCMI and supported 15 RCMI projects at eight institutions in FY 1996. To further enhance these efforts, NIAID offered its own AIDS Infrastructure for Minority Institutions RFA in FY 1990 and FY 1993 to provide funding for RCMI projects at three additional institutions.
NIAID has also collaborated with the National Institute of Mental Health to support an initiative pairing NIAID's 12 Centers for AIDS Research investigators with RCMI investigators to increase collaborations between ethnic minority and majority institutions. Eleven projects at seven schools were ultimately funded. In 1995, NIAID established its own Enhancement Awards for Underrepresented Minority Researchers to enable these individuals to establish clinical or basic AIDS research programs. Nine individuals were funded. Furthermore, NIAID's Office of Research on Minority and Women's Health has developed a number of outreach programs, including one for Washington, D.C., area high school science students and teachers, and other collaborations with the Interamerican College of Physicians and Scientists to increase awareness of NIAID programs and resources among Hispanic investigators.
Ethnic Minority Issues at NIAID
In 1994, the NIAID director established the Minority Scientists Advisory Committee (MINSAC) as a standing committee to advise the director on issues and concerns of the minority scientific community and provide suggestions on ways that the Institute can attract highly qualified minority scientists to its intramural and extramural programs. In particular, MINSAC has conveyed concerns regarding the underrepresentation of minorities in tenure, tenure-track, and postdoctoral positions within NIAID's intramural research program, resulting in the creation of additional lines to attract minority scientists. In addition, MINSAC has initiated the development of a minority constituency catalog, including minority scientists, institutions, and organizations, to assist in the identification of qualified minorities to participate in the NIH peer-review system.
National Institute of Diabetes and Digestive and Kidney Diseases
NIDDKD directs approximately 70 percent of its budget to investigator-initiated grants. These awards are largely focused on basic research to understand underlying mechanisms of disease and pathogenesis. Like other ICDs, NIDDKD uses RFAs or program announcements sparingly and largely in response to congressional directives or the need to stimulate research in small program areas.
Priority setting at NIDDKD is determined by a planning process that establishes a framework for program activities. This program plan is submitted to the NIDDKD Advisory Council for discussion. Of the six sections in the current program plan, one references ''minority activities" and highlights minority initiatives, research projects, center activities, and training and career development programs.
NIDDKD's cancer-related research is coordinated with NCI via a formalized memorandum of understanding that establishes NCI's primary responsibility for research on carcinoma of the prostate and NIDDKD's primary responsibility for research on noncancerous conditions, including research on the growth, development, and maturation of the prostate.
NIDDKD reports that it allocated a total of $33.4 million for research related to cancer among minority and medically underserved groups. This funding supports 142 programs or research grants. Nearly $1.5 million of this funding was allocated to the minority emphasis within the NIDDKD clinical trial on Medical Treatment of Prostatic Symptoms (MTOPS); this money represents 20 percent of the total funding for MTOPS. NIDDKD supported a workshop to identify barriers to the successful recruitment and retention of minority participants in this study and provided funding for 11 of 17 participating clinical centers to develop minority recruitment plans specific to their locales.
In collaboration with NCI and NIAID, NIDDKD has sought to stimulate research on Helicobacter pylori and its relationship to digestive diseases and cancer, particularly in minority populations, by establishing an RFA in January 1997. The RFA calls for studies on the epidemiology of Helicobacter in minority populations, genetic susceptibility to Helicobacter infection, and the clinical course of infection. The RFA notes that 10 to 12 awards are anticipated, with $2.5 million in funding available for awards. Before FY 1997, NIDDKD offered RFAs for studies of the regulation of prostate growth, the molecular epidemiology of prostate carcinogenesis, clinical trials of medical therapy in benign prostatis hyperplasia, hormonal regulation of breast-specific growth factors, and other studies of cancers that disproportionately affect ethnic minority populations.
National Heart, Lung, and Blood Institute
NHLBI reported a dramatic increase in cancer-related research expenditures from FY 1985 to FY 1997. In 1985, NHLBI allocated $3.87 million to cancer-related research; this figure increased to $50.7 million in FY 1993 and $57.6 million in FY 1997, spurred in large part by the cancer portion of the Women's Health Initiative (WHI; see below), of which $16.7 million is included in the FY 1993 total and $19.5 million is included in
the FY 1997 total. The bulk of NHLBI's cancer-related research funding is for basic scientific projects that are not targeted to specific populations.
NHLBI reports that before FY 1997 it did not support any cancer research relevant to minority and medically underserved populations. According to NHLBI Director Claude Lenfant, "The NHLBI does not have any processes for establishing priorities for cancer-related research among minority and medically underserved populations. Research on cancer and research on cancer in minorities are not part of the mission of the NHLBI, except for the WHI which NHLBI administers as part of a NIH consortium with the National Cancer Institute (NCI), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and the National Institute on Aging (NIA)" (Lenfant, 1998, p. 1).
In FY 1997, NHLBI supported aspects of WHI that included research on cancer (approximately 40 percent of the total WHI contract). NHLBI estimates that the minority population of these study trials is approximately 20 percent. WHI is a cross-institute collaboration that examines the effects of promising interventions, such as hormone replacement therapy and dietary modification, in preventing a number of common diseases among women, including coronary heart disease, osteoporosis, and cancer (specifically, breast and colorectal cancers). In addition, WHI attempts to identify risk factors for these diseases and assess the feasibility of various community-based programs of preventive care.
National Human Genome Research Institute
The National Human Genome Research Institute (NHGRI) was established in 1989 first as a center, then as an Institute in 1997 to lead NIH's efforts in the Human Genome Project, including chromosome mapping, DNA sequencing, DNA-based diagnostics and gene therapy development, database development, technology development for genome research, and studies of the ethical, legal, and social implications of genetics research. Cancer-related research represents a significant proportion of NHGRI's portfolio, including work conducted in the Laboratory of Cancer Genetics, where researchers are seeking to understand genetic changes in somatic cells that lead to cancer, inherited mutations that predispose individuals to cancer, and genes involved in the development of malignant characteristics in cancer cells, such as drug resistance and metastasis.
Of note in NHGRI's cancer research portfolio is a collaborative effort between NHGRI and NIH's ORMH to establish an intramural research project, the Center for Collaborative Research on Genomic Analyses of Diseases that Disproportionately Affect African Americans, at Howard University in Washington, D.C., an historically black university. NHGRI and ORMH have identified four principal objectives of this collaboration:
- to increase the competitiveness of minority researchers and minority institutions by developing a center that will become a self-sustaining entity through competitive grants and contracts;
- to promote collaborative research between majority and minority institutions (NHGRI and Howard University scientists will be involved in an exchange between the two campuses to foster cross-fertilization and training);
- to increase the inclusion of minorities in study populations; and
- to promote the study of diseases that disproportionately affect African Americans, such as diabetes and hereditary prostate cancer, which will be a focus of the Center.
In a summary of this initiative, NHGRI notes that there is a paucity of standardized, population-based data on the genetic and epidemiologic factors underlying the diseases that disproportionately affect African Americans. To address this need, the help of African-American physicians, research scientists, and institutions is needed to increase the participation of African Americans in research. The Center's initial objectives include the recruitment of African-American families for genomic research; the development of standardized protocols for the collection of genetic, clinical, and epidemiologic data; and the establishment of core facilities for the isolation, characterization, and storage of cells and DNA for study. Specific projects emerging from this collaboration include a study of sibling pairs with non-insulin-dependent diabetes mellitus in West Africa, genetic linkage studies of hereditary prostate cancer among African Americans (which has also received research support from NCI), and studies of the ethical, social, and legal aspects of genetic research and testing, such as the informed-consent process and the psychosocial impact of a genetic diagnosis on African-American patients and their families.
The training and education of young African-American scientists is also a major goal of the collaboration with Howard University. With funding from ORMH, several African Americans at the doctorate level have been recruited for these projects, and additional training for pre- and postdoctoral scientists will occur at NHGRI's laboratories in the Division of Intramural Research.
NHGRI proposes that the Center be supported for 5 years, with assistance from ORMH.
Summary and Recommendations
NIH, and particularly NCI, has funded an impressive array of research projects and training initiatives that may have a demonstrable impact in
addressing the disproportionate burden of cancer incidence and mortality among minority and medically underserved populations. The committee finds, however, that no "blueprint" or strategic plan exists to direct or coordinate this research activity (see discussion of priority setting in Chapter 4). As a result, model programs in one or more institutes are not replicated by other ICDs where indicated, some areas of research emphasis receive greater attention than others, and overall funding to address the needs of minority and medically underserved populations is inadequate. Several recommendations are therefore indicated:
Recommendation 3-1: The Office of Research on Minority Health should more actively serve a coordinating, planning, and facilitative function regarding research relevant to cancer among ethnic minority and medically underserved populations across relevant institutes and centers of NIH. To further this goal, the Office of Research on Minority Health should:
- make criteria for Minority Health Initiative project support explicit;
- coordinate with other specialty offices (e.g., the Office of Research on Women's Health) by participating in NIH-wide coordination efforts such as the Research Enhancement Awards Program; and
- ensure that Minority Health Initiative funding does not supplant funding from institutes and centers for research and programs relevant to ethnic minority and medically underserved populations.
Recommendation 3-2: Research and research funding relevant to cancer among ethnic minority and medically underserved populations should be more adequately assessed (as per Recommendation 3-3) and should be increased.
Recommendation 3-3: NIH should improve the accuracy of its assessment of research that is relevant to ethnic minority and medically underserved groups by replacing the current "percent relevancy" accounting method with one that identifies studies whose purpose is to address a priori research questions uniquely affecting ethnic minority and medically underserved groups.
Although the committee found evidence that NCI sponsors significant behavioral and social science research aimed at examining the range of behavioral, psychosocial, dietary, and other factors that enhance the risk for cancer or poor cancer outcomes among ethnic minority and medically
underserved groups, behavioral and social science research should be expanded, particularly with respect to prevention and outreach efforts. Lerman et al., (1997) note that approximately 65 percent of cancer deaths are attributable to behaviors such as smoking, sexual risk behavior, and to factors such as diet. For example, over the past half-century, more than 300,000 women have died from cervical cancer, including a disproportionate number of women from ethnic minority and medically underserved backgrounds, even though the technological tools have been available since the 1940s to vastly decrease mortality due to cervical cancer. Similarly, greater research is needed to illuminate barriers to cancer care (Chapter 5) and strategies to overcome them.
Although the committee does not seek to imply that NCI's research resources should be allocated entirely on the basis of likely etiologic factors, it is noteworthy that the NCI division with primary responsibility for funding behavioral and population-based research, DCCPS, allocated approximately $21 million to "minority health and assistance programs" in FY 1997 (as noted above, this figure is based on a percent relevancy calculation, which suggests that many projects were not specifically targeted to ethnic minority and medically underserved populations). This figure represents approximately 17 percent of the total NCI funding for MHAPs and is likely an inflated estimate of funding for behavioral and social science research, given that a large percentage of DCCPS's resources are allocated to important programs such as the SEER program and other nonbehavioral research.
The agenda for such research should be based on an analysis of the prevalence of particular cancers in these population and their preventability (Chen, 1994). Particularly for ethnic minority populations, this research should include investigations of ethnically appropriate interventions, including culturally competent and linguistically appropriate approaches. In addition, more research is needed on barriers to cancer care among these populations, and strategies to overcome them.
Recommendation 3-4: The newly established program of behavioral and social science research at NCI addresses an area of research that has been neglected in the past. The committee urges that this program of research identify as one of its highest priorities a focus on the cancer prevention, control, and treatment needs of ethnic minority and medically underserved groups.
Such focused research will require collaboration and coordination between DCCPS and the NCI Divisions of Cancer Prevention and Cancer Treatment. As will be discussed in Chapter 4, coordination of this research
activity is appropriately led by OSPR, provided that sufficient resources and authority are granted to OSPR to carry out this agenda.
The NHGRI-Howard University collaboration stands as an outstanding, but unfortunately rare, example of a partnership between the federal scientific research infrastructure and an historically black institution that meets multiple goals. Not only will this partnership result in improved recruitment of ethnic minority patients as research subjects but it will also improve opportunities for the training of minority scientists and allow focused investigation of genetic risk as it applies to some segments of the African-American population.
Recommendation 3-5: Collaborations between NIH and research and medical institutions that serve ethnic minority and medically underserved populations should be increased to improve the study of cancers that affect these groups and to increase the involvement of such entities and populations in scientific research.
Although the committee found evidence that NCI sponsors a significant portfolio of training programs designed to increase the numbers of ethnic minority investigators in cancer-related research fields, there is little evidence that NCI or NIH has undertaken a thorough assessment of training programs to determine whether these programs are producing adequate numbers of ethnic minority researchers in all appropriate cancer research fields (e.g., behavioral and social sciences, epidemiology, genetics, and cell biology) and to determine whether training programs have resulted in the increased representation of ethnic minorities in cancer research fields. Furthermore, there is little evidence that guidelines or other training criteria have been established by NCI or NIH to ensure that all trainees can receive high-quality instruction and mentoring. Such efforts would improve the planning and implementation of future training programs.
Recommendation 3-6: NIH should increase its efforts to expand the number of ethnic minority investigators in the broad spectrum of cancer research to improve minority health research. These efforts should (1) assess relevant areas of research needs and ensure that trainees are representative of these disciplines and areas of inquiry, (2) determine guidelines for the quality and expected outcomes of training experiences, and (3) maintain funding for a sufficient period of time to assess the impact of training programs on the goal of increasing minority representation in cancer research fields.





























































