5
Advancing State-of-the-Art Treatment and Prevention
In the previous chapters, the committee has reviewed in detail the National Institutes of Health's (NIH) portfolio of research on cancer among ethnic minority and medically underserved populations and, pursuant to the study charge, has commented on the adequacy and comprehensiveness of this portfolio in addressing the cancer research needs of these populations. The committee has reviewed the priority-setting processes at NIH that underlie decisions regarding resource allocation and the areas of scientific inquiry that are emphasized. Ultimately, however, the utility of this research in reducing cancer incidence and mortality and increasing rates of survivorship among ethnic minority and medically underserved populations is dependent upon NIH's ability to bring the fruits of such research to affected communities. This includes the application and testing of new knowledge in field-based clinical and prevention trials and the dissemination of research findings to community-based health care providers, to organizations engaged in cancer prevention, and to members of affected communities. Accordingly, this chapter addresses two aspects of the study charge:
- It conducts ''an examination of how well research results are communicated and applied to cancer prevention and treatment programs for minority and medically underserved communities"; and
- It assesses "the adequacy of NIH procedures for equitable recruitment and retention of minority and medically underserved populations in clinical trials."
This chapter begins with an assessment of NIH's efforts to include ethnic minority and medically underserved populations in NIH-sponsored cancer treatment and prevention trials. Particular attention is paid to the unique issues involved in recruiting these populations and retaining them in clinical trials, given the high quality of care generally afforded to patients enrolled in clinical trials and the importance of testing hypotheses with diverse populations to ensure the generalizability of findings. Next, the chapter reviews the strategies that NIH uses to disseminate information regarding cancer research to ethnic minority and medically underserved populations, their providers, and community-based health organizations.
Recruitment and Retention of Ethnic Minority and Medically Underserved Participants in Clinical Cancer Research
Clinical research forms the backbone of scientific advancements in medicine. New medications, preventive and rehabilitative interventions, and other innovations must be tested under the rigorous conditions of clinical trial research to understand whether these applications will work effectively, under what conditions they will work, and whether patients will be exposed to unintended harmful effects. Because patients are monitored closely under most clinical trial protocols, they often receive a higher quality of medical care and follow-up than patients who are not enrolled in clinical trials. This holds true even among patients in clinical trials assigned to "no-treatment" or "placebo" control groups in randomized trials.
Ethnic minority and medically underserved populations, however, have historically not participated in clinical trial research at rates proportional to participation rates among middle- and upper-income whites. Many factors may underlie this disparity. Examples of historical abuse of ethnic minorities in research abound; most researchers, and in particular, many in African-American communities, point to the Tuskegee syphilis experiment as a significant source of minority mistrust of the scientific establishment. In that study, federal researchers followed approximately 400 lower-income African-American men in rural Macon County, Alabama, who were infected with syphilis to study the natural history of the disease. Left untreated, syphilis can cause a host of life-threatening medical and cognitive complications. Yet, despite the availability of treatments such as penicillin, these men were denied treatment and were not informed of their infection. When news of the study became public in 1972, the study was abruptly halted, and the federal government and other public and private research
entities began developing a series of procedures designed to protect the rights of human subjects participating in research.
Efforts to obtain informed consent and protect participants in clinical trials, however, may have resulted in the exclusion of ethnic minority and medically underserved populations from some clinical trial settings. Many researchers found recruitment of these populations into clinical trials to be challenging; some researchers who were unaccustomed to working with ethnic minorities as potential research subjects encountered difficulties in obtaining their informed consent for participation in trials, whereas other researchers may have been too cautious in attempting to protect research subjects from unethical behaviors (Durso, 1997). Such attitudes may have furthered the gap of mistrust between the scientific community and ethnic minority communities. Some researchers dismissed the possibility of recruiting research subjects from ethnic minority communities altogether, citing difficulties in recruitment.
Mistrust of the scientific community among ethnic minority populations is also heightened by well-publicized claims concluding that African Americans and other minority groups are genetically inferior to whites, despite the repudiation of such work by large segments of the scientific community. Publications such as Richard Hernnstein and R.J. Murray's, The Bell Curve, which argues that African Americans are intellectually inferior to whites and Asians as a result of genetic differences between these groups, may reinforce the perception of many ethnic minorities that the "scientific establishment" views them as inferior and less deserving of high-quality medical care (Durso, 1997). Similarly, a large body of evidence indicates that African Americans and other minorities receive a lower intensity of medical and surgical care (Sullivan, 1991), reinforcing this view-point.
Structural issues within the health care research industry also pose challenges to the recruitment of ethnic minority and medically underserved individuals. Many urban, low-income, uninsured, underinsured, or ethnic minority individuals receive treatment in large public hospitals, as opposed to private hospitals or university-affiliated research hospitals. The latter often capture a larger share of federal research dollars. Increasingly, time and financial constraints prevent many physicians working in public hospital settings from participating in research projects and enrolling their patients as subjects.
Researchers working with lower-income and minority communities may also face greater costs in conducting research as a result of the need to address financial barriers to participation in clinical trials. Recruitment often requires more than placing ads in newspapers; researchers must expend resources to build relationships with community groups and hire outreach personnel. Clinical trial participants often must visit a doctor's
office or clinic regularly, which for some entails transportation and child-care costs, which typically are not covered by federal research grants. Some research programs have offered meals as a means of assisting low-income patients' participation.
Thus, the combination of historical experience and unequal access to health care has created a dynamic of mistrust on the part of ethnic minority and medically underserved communities and, in many quarters, resignation to low levels of minority participation in clinical research among investigators and health practitioners.
From a scientific perspective, however, it is critical to include diverse populations in clinical trials to ensure that research findings are generalizable to the entire population. (As discussed in Chapter 4, Zora Kramer Brown provides an example of the dangers involved when scientists and public health officials attempt to generalize research findings from relatively homogeneous study populations to broad, more diverse populations.) From a social justice perspective, it is important that research supported by taxpayer dollars be inclusive of and applicable to the diverse populations of the United States.
To address these needs, NIH and the U.S. Congress worked to develop standards in the late 1980s and early 1990s that mandated the participation of women and ethnic minorities in federally supported human subjects research. In 1993, as part of the NIH Revitalization Act (P.L. 103–43), Congress passed legislation that called for (1) the inclusion of women, ethnic minorities, and subpopulations into clinical trials; (2) the inclusion of adequate numbers of women and ethnic minorities for performance of valid analyses; (3) preventing cost from being an applicable reason for the exclusion of these groups; (4) a determination of the circumstances under which inclusion of these groups may be inappropriate; and (5) clinical trials and outreach programs to be designed and executed in ways that encourage recruitment and retention of women and ethnic minorities (Penn, 1996). This legislation was designed to ensure that biomedical and behavioral research results are applicable to all affected populations and include detailed information about the effects of gender, racial, and socioeconomic factors that might influence the development and outcomes of cancer.
In response to the law, guidelines for inclusion were developed and published (US. Department of Health and Human Services, 1994), and a 1-year comment period was provided. The policy states that women and ethnic minorities and their subpopulations must be included in all NIH-supported biomedical and behavioral research projects involving human subjects unless there is a clear and compelling justification that the inclusion of these groups is inappropriate with respect to the health of the subjects or the purpose of the research. NIH policy also demands that
studies be designed to allow valid analyses to be performed (i.e., to detect a significant difference that is of clinical or public health importance on the basis of scientific data). Investigators are required to report on actual accrual and inclusion of women and minorities in progress reports and for supplementary grant applications. In the future, reporting on intent to recruit will also be mandated. A computerized tracking system that enables NIH institutes to report on the actual number of ethnic minorities and women included in NIH-sponsored research studies has been developed.
The NIH Revitalization Act did not, however, address the many practical and ethical concerns that affect recruitment of ethnic minorities into clinical trials. As noted above, the cost of research may vary, with some populations being more costly to recruit into clinical trials. Balancing cost considerations with the need for fair recruitment into clinical trials can be challenging and can involve trade-offs that constrain researchers. Another concern is the applicability of results of studies with the general population to each of the relevant subpopulations. If certain groups of individuals are not included in clinical trials, then the principle of justice would support the need for a remedy to this situation. This leads to a separate concern: whether it is ethical to target the recruitment of ethnic minorities into clinical trials. Targeted studies are based on the conceptual framework that individuals differ on the basis of gender, "race" or ethnicity, culture, age, and other factors. However, these studies also raise the concern that the researchers who are involved in these studies believe that there are biological differences among the "races," a concept that is controversial (see Chapter 2). In certain cases, such assumptions that individuals differ are reasonable, such as in the evaluation of biological differences in the context of genetic conditions or in behavioral studies, especially if the behavioral studies address institutional racism that is associated with outcome differences between ethnic minorities and nonminorities. Finally, trust is an important consideration in recruitment efforts. Much existing evidence indicates that African Americans are less trusting of clinical research efforts than whites. No data are available indicating whether low-income whites share this mistrust. These concerns are not unreasonable, given the large degree of evidence indicating the lower intensities of medical and surgical care for African Americans.
NIH Efforts to Increase Participation of Minority and Medically Underserved Groups in Clinical Trials
The National Cancer Institute (NCI) has reported on several initiatives that have been used to increase the participation of ethnic minority populations and groups with low levels of literacy in clinical trials.
Outreach efforts to inform ethnic minority groups and populations with low literacy levels about clinical trials include versions of the NCI publication What Are Clinical Trials all About? in Spanish and in a version for people with low literacy levels, and a Spanish-language version of the videotape Patient to Patient: Cancer Clinical Trials and You. Fact sheets on cancer prevention and treatment studies have been developed in both English and Spanish. Similarly, a new training program for health professionals that addresses common patient concerns about the trial process, from initial decision making to trial participation and follow-up was developed in English and Spanish.
To address the increasing complexity of the informed-consent process, representatives from NCI, the Office of Protection from Research Risks, and the Food and Drug Administration have organized a working group charged with developing recommendations to make the informed-consent process more understandable. Informed-consent documents are lengthy, complex, and often difficult to understand, thereby hindering the process of providing accurate information to potential subjects. The working group developed recommendations for an informed-consent template and sample consent documents that are undergoing field testing.
NCI's Cancer Therapy Evaluation Program provided supplemental funding in fiscal year (FY) 1997 for 5 of the 11 Clinical Trials Cooperative Groups (see below for description) as part of an initiative to increase the accrual of ethnic minority populations. Funds were used to support focus groups and educational opportunities for ethnic minority health professionals, to advertise and support outreach efforts in ethnic minority communities, to hire translators, and to conduct other community-based education efforts.
To increase the number of ethnic minority patients enrolled in Community Clinical Oncology Programs (CCOPs), NCI developed the Minority-Based Community Clinical Oncology Programs (MBCCOPs) in 1990. The MBCCOP involve more than 300 physicians and eight program sites located in areas with large minority populations, such as San Juan, Puerto Rico; Mobile, Alabama; Honolulu, Hawaii; and San Antonio, Texas.
The NIH Office of Research on Minority Health (ORMH; see Chapter 3) has provided funding to the NCI Cancer Center Program to support programs and personnel to increase the accrual of ethnic minorities in cancer center trials. These funds have supported the hiring of personnel involved in minority recruitment efforts, such as translators, data managers, bilingual patient liaisons, and others and have supported activities such as the development of recruitment brochures specifically targeted to ethnic minority patients.
Finally, NCI has sponsored several conferences to promote strategies for the development and sharing of information among investigators to
increase ethnic minority accrual. NCI released a request for applications in 1996 to support regional conferences on the recruitment and retention of ethnic minorities in clinical trials. Eleven such conferences were funded to address particular issues for investigators and patient populations at each locality. These regional conferences followed a national conference entitled Recruitment and Retention of Minority Participants Clinical Cancer Research, held in Washington, D.C., that was cosponsored by the American Cancer Society (ACS), the Oncology Nursing Society, the NIH Office of Research on Women's Health, and ORMH, among others. A monograph of the conference proceedings outlining specific needs and strategies for recruitment and retention of ethnic minority and underserved populations was published by NCI (see Box 5-1).
Ethnic Minority Accrual in NIH-Sponsored Trials
As noted in Chapter 3, NCI sponsors approximately 500 clinical trials, including those of the Clinical Trials Cooperative Groups and CCOP, intramural clinical trials, and trials conducted at NCI-funded cancer centers. The Clinical Trials Cooperative Group Program performs more than half of the NCI-sponsored trials, conducting approximately 900 of the 1,500 trials. Thirteen cooperative groups that included participants from 194 universities and 1,839 hospitals and more than 23,700 physicians were funded in 1977. CCOP links community-based physicians with Clinical Trials Cooperative Groups and cancer centers for cancer prevention and treatment trials. More than 50 community-based programs in 30 states, nine cooperative groups, and three cancer centers were funded in 1997 and involved more than 300 hospitals and 3,300 physicians. Finally, NCI sponsors four large cancer prevention trials, described in greater detail below and in Chapter 3: the atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intra-epithelial lesions (LSIL) Triage Study (ALTS) of cervical cancer screening, evaluation, and management; the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Trial; the Prostate Cancer Prevention Trial (PCPT); and the Breast Cancer Prevention Trial (BCPT). The levels of ethnic minority accrual in these trial groups are summarized below. Except in a few instances as noted below, NCI and the trial groups did not report on accrual by socioeconomic status or other indicators of medically underserved populations.
Determinations of whether the level of accrual of ethnic minorities into trials is proportionate to cancer burden can typically be accomplished by comparing the percentage of cancer diagnoses among racial and ethnic groups in the U.S. population within a given time frame and the percentage of minority enrollment within trials for each cancer studied. Such
|
BOX 5-1 In January 1996, the National Cancer Institute (NCI), in conjunction with the National Cancer Advisory Board, the American Cancer Society, the Oncology Nursing Society, and the National Institutes of Health (NIH) Office of Research on Women's Health and Office of Research on Minority Health, organized a conference entitled "Recruitment and Retention of Minority Participants in Clinical Cancer Research." The 2-day conference brought together national experts on minority health and clinical trials research to share perspectives and strategies to improve the rate of inclusion of ethnic minorities in research. The proceedings of the conference were published by NCI (National Cancer Institute, 1996d). In the Executive Summary, conference participants concluded that the achievement of equity in clinical trials will require that four goals be met:
The committee supports the findings and recommendations of the five conference panels, some of which are summarized below with respect to specific issues and populations. ETHICAL ISSUES IN RECRUITMENT OF MINORITY PARTICIPANTS Three ethical principles should guide the behavior of individuals who conduct clinical research, particularly with respect to oversight by institutional review boards (IRBs), according to Nancy Kass of Johns Hopkins University. These ethical principles are respect for autonomy, beneficence, and justice. Occasionally, these principles conflict with each other, which requires priorities to be considered. CULTURAL ADAPTATIONS FOR OVERCOMING BARRIERS Native Americans Cultural factors significantly affect the recruitment of Native Americans into clinical trials, according to Linda Burhansstipanov, Director of |
|
the Native American Cancer Research Program in Denber, Colorado. Relevant considerations include insurance, poverty, racial identification, and distrust of science and research, especially by underserved and underrepresented population. IRB processes are very different for Native Americans, especially with respect to considerations of the protection of individuals and sovereignty. Although the Native American health care system provides excellent access to care, the rate of survival from cancer for this group is the poorest among all ethnic groups. Informed-consent forms and procedures may serve as a barrier to recruitment, as the language commonly used in such forms and procedures may be considered offensive by some Native Americans. In many cases, the informed-consent process is poorly understood by Native Americans. In addition, body language and styles of interaction between researchers and potential research subjects may affect recruitment. Other factors including intonation are important. Among this population, fear of research is real and telephone recruitment is generally unhelpful. Involvement of the community elders is important in studies among Native Americans. Face-to-face recruitment is likely to be the most successful strategy. Incentives, such as food for research subjects' children or grandchildren, and other rewards are being tested, but they have yet to show results. Although programs such as the Native Sisters (a social and emotional support system) have been implemented, their success is limited. Tribal beliefs are difficult to change, and protocols are often inflexible. The result is that it may take three times as long to recruit Native Americans into clinical trials. African Americans As described by Carolyn Harvey, cultural variations in communication styles also affect the recruitment of African Americans to clinical trials. Linear models of information are preferred among whites, with an emphasis on written messages. In contrast, among African Americans, a cyclical model of consent and information is important, with information being disseminated through real-life situations rather than statistics or written messages. African Americans are more likely to communicate via a church or an interactive educational session and often need the freedom to respond and interrupt during a teaching session. African Americans may also require flexibility and are responsive to nonverbal aspects of communication, accounting for the importance of tone, inflection, and body language. A model for this approach has been developed in East Texas (the Visible Messenger Model), where people who are known, trusted, and accountable in the community are recruited to provide cancer awareness messages. |
|
Asians/Pacific Islanders As outlined by Marjorie Kagawa-Singer, among Asians/Pacific Islanders many factors are barriers to recruitment, including structural concerns (such as a wide range of fluencies in English), cultural considerations (e.g., different conceptions of health and fear of preventive interventions), and cultural differences (decisions are made by family conference and often take a long time). Confidentiality is also a concern because these individuals often require the town elder to participate in the consent process, representing a breach of confidentiality. Written consent forms are off-putting to the patients. Successful strategies include the use of research team members familiar with Asian and Pacific Island cultures. Allocation of adequate lead times with focus groups is also required, because additional services are often needed. Hispanics As outlined by Edward Trapido, Hispanics consist of individuals of diverse races and ethnicities, with many subpopulations. Differences exist with subpopulations according to culture, language, religion, race, age, gender, family role, education, and length of time in the United States. Focus groups consisting of individuals from Miami revealed several important beliefs of the Hispanic participants: physicians did not communicate well with patients; the medical staff was insensitive to their culture and language; they had not been well informed about their cancer; many felt that physicians had financial interests in prescribing surgery or advising enrollment in surgical trials; chemotherapy would bring on certain death; physicians were concerned that patients would not follow up with care; communication difficulties were common when physicians preferred to speak English, even if the patient understood the language (even if the patient's native language was Spanish); patients were often not asked to participate in the trials; and the participants had a high level of concern over Medicare fraud, inconsistent public health policies, the commercialization of medicine, and the need to involve pharmacists and nurses in the process. Other cultural considerations include a sense of fatalism. Patients are often not told their diagnosis to spare them from pain. General recommendations of this panel included the following:
|
PUBLIC AND PROVIDER EDUCATION Approaching Community Physicians Worta McKaskill-Stevens points out that community physician involvement in cooperative trials is needed. The National Medical Association (22,000 minority physicians, two-thirds of whom are primary care providers) is working closely with the Eastern Cooperative Oncology Group to develop a protocol-specific patient brochure. The text includes sections on IRBs and who the participants are, and a layperson is on the IRB. It provides general information for clinical trials. Recruiting Asian Americans for Smoking Cessation Research Moon Chen, Jr., points out that researchers are challenged to recruit Asian Americans if they overlook the tremendous diversity within Asian-American populations. The majority of Asian Americans are foreign born (especially true with regard to the Southeast Asian populations in the United States), thereby posing linguistic barriers to recruitment. There is a relative dearth of research information on these populations, which is especially alarming given the high rate of health risk behaviors such as smoking (for example, 57 percent of Southeast Asian males in the San Francisco Bay area smoke). A research project in Ohio solicited community support and hired workers from Asian ethnic groups to find study samples via the telephone book and home visits, in part to establish rapport for a long-term relationship instead of simply collecting data. Similarly, educational outreach efforts in California proved successful when antismoking messages were tailored to particular groups and were presented by use of outdoor billboards and educational classes in settings where community members gather. Successful Recruitment of African-American Men: The DEED Program Isaac Powell describes the Detroit Education and Early Detection (DEED) program, which studied prostate-specific antigen changes among African-American men enrolled in a prostate cancer screening program. Researchers assessed these men's attitudes toward the health care system and found that fear of a positive diagnosis was a significant barrier to participation (many men held fatalistic attitudes toward a diagnosis |
|
of cancer). Concern about the loss of sexual function and other quality-of-life issues was also a significant barrier. Another significant impediment was distrust: many African-American men sampled believed that they might be abused as medical subjects in large health care institutions. After identifying potential barriers, the DEED program recruited subjects via African-American churches and related networks. Researchers found that when the church minister and other leaders were recruited for testing, participation among church members increased two- to threefold. In addition, a group of prostate cancer survivors assisted in recruitment by discussing their experiences. DEED program researchers reported high levels of success in recruiting and retaining African-American men in the in the longitudinal project. Working with Communities: A Key to Success Noel Chavez described several efforts that are needed for health education and research outreach in medically underserved communities. Chavez stressed the need for assessment of community norms and beliefs, to find out who in the community is entrusted with leadership positions, and how the community relates to the larger social environment. In addition, researchers need to find ways to help communities gain ''ownership" of research programs, including involvement in project planning and development, recruitment, and protocol design. Finally, researchers need to be prepared to assist communities in solving problems rather than assuming that work that can be done for or with a community. The development of trust and support within the community requires time, effort, flexibility, and resources, but the relationships built in this process will improve the likelihood of success. General recommendations of this panel included the following:
Implementation Strategies Alternative strategies for recruitment of ethnic minorities into clinical trials have not been evaluated in most settings. Roshan Bastani describes the experience of the University of California at Los Angeles (UCLA) in improving recruitment of ethnic minorities into clinical trials. An important consideration in this effort is the difficulty that some individuals have with the written informed-consent process. An appeal was made to the UCLA IRB to allow verbal consent in place of written consent, |
|
because in one study of low-income women (many of whom had low levels of literacy, were recent immigrants, or were illegal aliens), the written informed-consent process was too intimidating. The IRB allowed the use of verbal consent in conjunction with mailing of explanatory information on the study to all participants. Use of verbal consent dramatically increased the level of recruitment of members of this population. Tom Welty described logistical considerations related to the recruitment of American Indians into cancer research. The Indian Health Service (IHS) acts like a health maintenance organization and is faced with the rationing of health care services because of budget constraints. IRB concerns are very lengthy, involving the IHS or tribal facility, a second level of federal approval, and tribal approval. Publication of trial results requires approval of the tribe, the Area Publication Committee, and the IHS Publication Committee. Strategies to improve the IRB process in the IHS setting are needed. However, the safeguards have been adopted for good reasons, because much of the previous work provided little benefit to the Indian population and was intrusive and offensive to many. Recommendations from this panel included the following:
|
analyses, however, are sometimes complicated by several statistical and data limitations (J. Unger, Southwest Oncology Group, personal communications, 1998). For example, information regarding incidence is typically derived from Surveillance, Epidemiology, and End Result (SEER) program data, which are less reliable for some populations (e.g., American Indians)and nonexistent for others (e.g., rural medically underserved populations), as discussed in Chapter 2. Furthermore, as Tejeda et al., (1996) state:
[T]rue determination of proportional representation [of minorities] in trials is difficult because the United States does not have a national, population-based cancer registry from which the racial/ethnic composition of the population with cancer can be counted. Such a registry would make the determination of racial/ethnic composition of the newly diagnosed population with cancer a simple arithmetic exercise (p. 815).
Calculation of the percentages of members of ethnic groups with cancer
diagnoses is also dependent on the quality of the overall population data from the U.S. Bureau of the Census (i.e., the "denominator"), which are less reliable for some populations (especially many ethnic minority groups) within SEER program coverage areas. Finally, for many rare cancers and for cancers among smaller ethnic groups, incidence data are unreliable, therefore limiting the kinds of analyses that can be performed (Tejeda et al., 1996). Only a few published studies account for these limitations, and these are cited below. The committee therefore interprets the proportionality of ethnic minority participation in clinical trials with caution.
Sufficient data are not available from NCI to evaluate clinical trial accrual for medically underserved populations.
Cancer Cooperative Group Trials
Of the 13 cooperative groups funded in 1997, 4 are focused on pediatric cancers (the Children's Cancer Group [CCG], the National Wilms' Tumor Study [NWTS], the Intergroup Rhabdomyosarcoma Study Group [IRS], and the Pediatric Oncology Group [POG]), 5 are focused on specific cancers (the Brain Tumor Cancer Group, the Gynecologic Oncology Group [GOG], IRS, the National Surgical Adjuvant Breast and Bowel Project [NSABP], and NWTS), and 2 are small or regional groups (the M.D. Anderson Cancer Center [MDA] and the North Central Cancer Treatment Group [NCCTG]). These groups all face special circumstances that may affect the accrual of minorities in clinical trials. The groups focused on pediatric cancers, for example, enroll the majority of pediatric cancer patients in the United States (approximately 70 percent), making proportionate accrual of minority patients more likely (Bleyer, 1977; Tejeda et al., 1996). The remainder, however, are large nonspecialty trial groups that recruit subjects from broad adult patient populations.
NCI Clinical Trials Cooperative Group statistics indicate that the overall level of participation of U.S. cancer patients in Clinical Trials Cooperative Group is about 2.5 percent. In an examination of accrual patterns by ethnicity and age for all cooperative group trials during the period between January 1991 and June 1994, Tejeda et al., (1996) found that overall, ethnic minority representation is proportional in comparison to the incidence of cancer among ethnic minorities in the U.S. population. Specifically, the level of enrollment of African Americans was found to be 9.6 percent, that of Hispanics was 5.6 percent, and that of whites was 84.8 percent. These figures are very close to the estimated proportions of individuals with cancer in these groups in the United States (9.4 percent among African Americans, 3.4 percent among Hispanics, and 87.2 percent among whites).
Among the various age groups, Tejeda et al., (1996) found that overall, younger patients tend to be heavily represented in Clinical Trials Cooperative Groups, because slightly less than half of trial participants are under age 50 years, even though more than 85 percent of cancer diagnoses occur among people 50 years of age or older. Within age groups, the level of accrual of African-American and Hispanic patients appeared in most instances to be proportional to the incidence or slightly below the incidence of cancer in these populations. Among African-American and Hispanic patients ages 0 to 19 years, the level of enrollment (11.0 and 12.0 percent respectively) was very close to rates of incidence (12.4 and 11.9 percent, respectively); the same held true within the 20- to 49-year-old age group (10.6 and 4.8 percent enrollments for African Americans and Hispanics, respectively, compared to incidence rates of 11.4 and 6.0 percent, respectively) and the group consisting of those 50 years of age and older (8.5 and 2.5 percent enrollment for African Americans and Hispanics, respectively, compared to incidence rates of 9.1 and 2.9 percent, respectively).
When accrual was examined by specific cancer sites (leukemia and breast, colorectal, lung, and prostate cancer), Tejeda et al., (1996) found that, with a few exceptions, the level of ethnic minority enrollment in Clinical Trials Cooperative Groups generally was proportional to or greater than the expected incidences. Enrollment of African Americans in trials involving colorectal cancer (8.4 percent enrollment) and prostate cancer (9.8 percent enrollment) lagged slightly behind the incidence rate among this group (9.4 percent of cases of colorectal cancer and 11.1 percent of cases of prostate cancer among African Americans). Similarly, the level of enrollment of Hispanics in trials involving lung cancer (1.4 percent enrollment) also lagged slightly behind the incidence (2.2 percent). The greatest disparities in accrual were observed among African Americans ages 20 to 49 years with leukemia (9.4 percent enrollment compared with 12.4 percent incidence), colorectal cancer (10.2 percent enrollment compared with 15.7 percent incidence), and lung cancer (17.2 percent enrollment compared with 20.4 percent incidence) and among Hispanics ages 20 to 49 years with lung cancer (1.8 percent enrollment compared with 3.2 percent incidence).
The analysis of Tejeda et al., (1996), however, did not examine cancer incidence among Asian-American, Alaska Native; and American Indian populations in determining the proportionality of accrual patterns because of the small number of cases of cancer among these groups in the SEER program database. Similarly, the study does not report on the proportionality of accrual of low-income, low-literacy-level, rural, or otherwise medically underserved populations, presumably because these data are not reported in the SEER program registry (see Chapter 2).
NCI provided to the committee raw data and percentages of total enrollment
for the Clinical Trials Cooperative Groups in 1997. Although the proportions of cases of cancer among ethnic minority patients during the period of trial enrollment were not provided by NCI, enrollment data are presented here for purposes of comparison across trial groups. Where data on expected incidences are available, these are reported, but the committee urges caution in their interpretation. Percent accrual of ethnic minorities in each trial group in 1997 is depicted in Figures 5-1 and 5-2.
Among the pediatric clinical trial groups, both CCG and POG appear to have high levels of representation of ethnic minority patients on the basis of 1997 enrollment data. Among the 2,300 children enrolled in CCG,
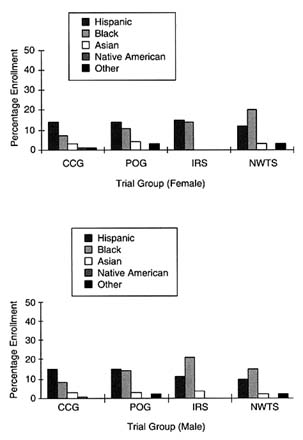
FIGURE 5-1 Percent minority accrual for 1997 cooperative pediatric groups. SOURCE: National Cancer Institute.
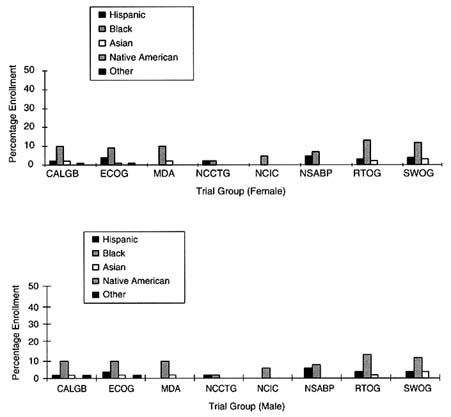
FIGURE 5-2 Percent minority accrual for 1997 cooperative groups.
SOURCE: National Cancer Institute.
30 percent are ethnic minority patients. Significantly, 14 percent are Hispanic, whereas 7 percent of the participants are African American. POG enrolls 31 percent minority females and 34 percent minority males, including 14 and 15 percent female and male Hispanics, respectively; 11 and 14 percent female and male African Americans, respectively; and 4 and 3 percent female and male Asian Americans, respectively (Figure 5-1). Bleyer et al., (1997) published a report supporting the conclusion that the accrual of ethnic minorities in POG and CCG is proportional to the incidence of cancer among ethnic minority group children, using enrollment data for nearly 30,000 children enrolled in these trials from January 1991 through June 1994. This study found that 11.6 percent of patients were Hispanic, 10.4 percent were African American, and 4.7 percent were from
other racial groups. On the basis of SEER program data (1989 to 1991 crude incidence data), the expected distribution of patients of the same age in the United States was 9.1 percent for Hispanic children, 10.7 percent for African-American children, and 4.3 percent for other ethnic groups. The authors found that the level of ethnic minority enrollment was equal to or greater than the rate of cancer for 24 to 27 subgroups.
Among the pediatric specialty clinical trial groups, NWTS and IRS appeared to have a high level of representation of ethnic minority patients, with 38 percent of females and 34 percent of males enrolled in NWTS identified as ethnic minorities and 30 percent of females and 36 percent of males enrolled in IRS identified as ethnic minorities. More than half of the ethnic minority females in NWTS were African American (20 percent overall), whereas less than 4 percent each were Asian American, American Indian, or other groups.
Among the adult specialty clinical trial groups, GOG enrolled 21 percent minority women. Of the 1,604 women accrued in 1997, 5.4 percent were Hispanic, 12.8 percent were African American, 2.3 percent were Asian American, and 0.2 percent were American Indian. NSABP reported data on enrollment to 5 treatment trials, including breast, colon, and rectal cancer treatment protocols. Among the colon and rectal cancer trials, 86 percent of the 267 males enrolled in this trial were identified as white, 5.2 percent were identified as Hispanic, 7.1 percent were identified as African American, and 0.3 percent were identified as Asian American. No American Indian men were accrued in these trials. Among the 240 women enrolled in these trials, 85 percent were identified as white, whereas 1.7, 10.4, and 1.2 percent were identified as Hispanic, African American, and Asian American, respectively. One American Indian woman was recruited into this trial. Similarly, of the 2,175 women recruited into NSABP's breast cancer treatment trials, 1,842 (85 percent) were white and 175 (9.0 percent) were African American. NSABP did not collect more detailed data on ethnicity in two of its breast cancer treatment protocols (instead, data were collected on the basis of a former categorization system), but reported that 5.4 percent of women in these trials were of "other" ethnic minority groups. Three newer breast cancer treatment trials did include detailed ethnicity data in accordance with NIH guidelines and revealed that 2.6 percent of the women enrolled were Hispanic, 1.0 percent were Asian American, 0.3 percent were American Indian, and 1.5 percent were of other ethnic minority backgrounds.
The small, regional clinical trial groups experienced low ethnic minority accrual overall. NCCTG was able to enroll only 11 percent female ethnic minority and 4 percent male ethnic minority patients, whereas MDA enrolled 12 percent ethnic minority patients overall. Among NCCTG's patient population, 3.1 percent of females and 1.5 percent of males were
Hispanic, 5.5 percent of females and 1.5 percent of males were African American, 1.5 percent of females and 0.4 percent of males were Asian American, and less than 0.3 percent of both the male and female populations were American Indian. MDA reported the accrual of no Hispanic individuals, whereas 9.2 percent (4 of 42) and 9.6 percent (5 of 52) of the enrolled patient population were African-American females and males, respectively. Only one Asian-American male was accrued to this trial group.
Among the large, nonspecialty clinical trial groups, the level of ethnic minority accrual appeared to be slightly less than those for the other clinical trial groups. The Cancer and Acute Leukemia Group B trial reported that in 1997, 16 percent of the enrolled patient population were ethnic minorities, whereas ECOG reported that 12 percent of females and 15 percent of males were ethnic minorities. RTOG and the Southwest Oncology Group (SWOG) fared similarly, as 16 percent of the females and 19 percent of the males enrolled in RTOG were ethnic minorities, whereas 17 percent of the females and 19 percent of the males enrolled in SWOG were ethnic minorities.
SWOG has conducted an analysis of its accrual to therapeutic trials between 1993 and 1996, comparing accrual rates to the percentage of cancer diagnoses in the U.S. among women, African Americans, and elderly people (ages 65 and older) at four cancer sites. As in the analysis of Tejeda et al., (1996), Unger and his colleagues (Unger et al., 1998) calculated expected values based on 1992 to 1994 SEER program incidence data and U.S. census data. Unger et al., (1998) found that the overall SWOG accrual rate for African Americans (10.2 percent) was almost identical to the estimated percentage of African Americans among U.S. cancer cases (10.1 percent). Similarly, the accrual rate for women (41 percent) was comparable to the estimated percentage of women among all cases of cancer in the U.S. (43 percent). The elderly, however, were substantially under-accrued, as 25 percent of SWOG's trial population was 65 or older, whereas 63 percent of cancer diagnoses occur among this population in the U.S. When examined by cancer site, Unger et al., (1998) found that the elderly were significantly under-accrued in breast, colon and rectal, and lung cancer trials, while African Americans were significantly under-accrued in lymphoma trials (7 percent accrual in SWOG, compared with 11 percent of African Americans among all lymphoma cases during the same period).
Similarly, Chamberlain et al., (1998) report on the sociodemographic characteristics of more than 4,000 patients enrolled in RTOG studies between January 1991 and June 1994, using SEER program and U.S. census data to determine the representativeness of the study sample to the overall U.S. population of cancer patients who have received radiation therapy. Using chi-square analyses, investigators determined that the educational
characteristics of older African Americans enrolled in RTOG were not significantly different from those of African Americans in all other age groups except the youngest age group (20 to 54 years), according to data from the U.S. census. African Americans in the youngest age group were two to three times less likely to have completed high school (45.3 percent of African-American males and 52.3 percent of African-American females who were in this age group and who were enrolled in the trial did not complete high school, whereas the expected rates are 18.9 and 17.5 percent, respectively). Chamberlain et al., (1998) also found that the proportion of African-American men (11.9 percent) and women (16.3 percent) enrolled in RTOG significantly exceeded the proportion of patients who were African American and received radiation therapy as a primary treatment (10.4 and 8.7 percent, respectively, on the basis of SEER program data). Similarly, for prostate and cervical cancers, enrollment of African Americans significantly exceeded the expected proportions of African Americans with cancer at those sites. The level of enrollment of African Americans diagnosed with cancers of the brain, head and neck (among females only), and lung (among females only) did not differ significantly from the expected incidence. Enrollment of African-American men with lung cancer, however, fell significantly below the percentage of African-American men with lung cancer who received radiation therapy (7.9 percent enrolled versus 13.2 percent expected). As is the case with Tejeda et al., (1996), Chamberlain et al., (1998) do not provide data on ethnic minority groups other than African Americans, because the stratification of the sample by age and education precluded an analysis of the small number of individuals of other ethnic backgrounds enrolled in RTOG. Similarly, the report provides no socioeconomic data for other populations. It is important that comparable studies be done for low-income whites as well as for other populations.
Overall, the Clinical Trials Cooperative Groups appear to accrue ethnic minority populations in clinical trials at rates proportional to the rates of cancer among those groups. When examined by specific trial groups and types of cancers, however, there appear gaps in accrual that serve to suppress overall ethnic minority accrual.
CCOPs and MBCCOPs
As noted above, 51 CCOPs were funded in FY 1997 to enroll patients in prevention and treatment trials. NCI provided raw data on patient accrual to these trials between June 1996 and February 1997 but did not provide information on accrual by specific ethnic minority groups. These data indicate that of the 4,363 patients enrolled in treatment protocols, 664 (15.2 percent of the total) were ethnic minorities. As is the case with
the Clinical Trials Cooperative Groups, accrual of ethnic minorities varied considerably with the site of the CCOP. Compared with the percentage of ethnic minorities residing in the state in which the CCOP is located, it was found that only 12 of the 51 CCOPs met or exceeded this percentage of accrual of ethnic minorities. (It should be noted that the percentage of ethnic minority residents in a state is only a crude standard for accrual, given that cancer incidence rates for various ethnic groups within a population may not be equivalent to their percentages within the total population. Data on the percentage of ethnic minority residents within a CCOP catchment area were not available for all CCOPs.) Of those CCOPs that reported high rates of accrual of ethnic minorities, those in the Oakland, California; Tampa, Florida; Miami Beach, Florida; and Manhassett, New York, reported accrual rates of 32 to 50 percent. Data on patient accrual in four of the CCOPs were unavailable.
In CCOP prevention trials, the rate of accrual of ethnic minorities was poor. Data provided by NCI revealed that of the 4,172 patients enrolled in prevention trials, 289 (6.9 percent of the total) were ethnic minorities. Only 5 of the 51 CCOPs were able to enroll ethnic minority patients at rates equivalent to the proportion of ethnic minorities living in the states in which CCOPs were located. Furthermore, only the Miami Beach and Tampa sites were able to enroll ethnic minority subjects at rates of one-third or more of the total subject population. Data on patient accrual from four CCOP sites were missing.
The eight MBCCOPs appear to have increased the numbers of ethnic minority patients in the overall CCOP pool, yet in some cases they have not performed better than the CCOPs accruing the highest numbers of ethnic minorities. One trial group (in Richmond, Virginia) did not recruit a greater proportion of ethnic minorities in treatment (31 percent) and prevention (24 percent) trials than the proportion of ethnic minorities in the catchment area (38 percent). Two other MBCCOPs reported less than 50 percent ethnic minority enrollment in treatment and prevention trials. In addition, the numbers of ethnic minorities brought into clinical trials via MBCCOPs between June 1996 and February 1997 are small: although data are missing for one of the eight MBCCOPs, the seven remaining groups brought only an additional 215 ethnic minority patients into treatment trials and 102 ethnic minority patients into prevention trials. Overall, 79 percent of the patients enrolled in MBCCOP treatment trials were ethnic minorities, whereas 58 percent of the subjects enrolled in prevention trials were ethnic minorities.
Cancer Prevention Trials
As noted above and in Chapter 3, NCI sponsors five large cancer prevention
trials to test the effectiveness of various chemoprevention strategies and cancer screening methodologies in reducing cancer incidence and mortality. The ALTS cervical cancer screening trial assesses the screening and management of minor Pap smear abnormalities. Four sites are involved in this trial, including the University of Pittsburgh, the University of Alabama at Birmingham, the University of Washington, and the University of Oklahoma. The PLCO screening trial is a large-scale, prospective randomized study to determine whether screening for these types of cancers will reduce the rate of associated morbidity (all four types of cancers account for nearly half of all cancer deaths in the United States). Ten centers around the United States are participating in this trial. To increase the rate of accrual of ethnic minorities, two screening centers focused on the recruitment of ethnic minorities were to be added in 1998, in addition to a new center cosponsored by the Centers for Disease Control and Prevention (CDC) to focus on the recruitment of African Americans. CDC is also working with the Henry Ford Health System in Michigan and the University of Pittsburgh PLCO screening trial center to identify effective methods for increasing the rate of recruitment of ethnic minorities and reducing barriers to participation. PCPT includes 222 sites across the United States and is coordinated by SWOG; its purpose is to test whether the drug finasteride will prevent prostate cancer in a randomized trial involving 18,000 men. Finally, BCPT is a randomized, double-blind trial that will assess whether the drug tamoxifen can prevent breast cancer in women at increased risk of developing the disease. More than 13,000 women were recruited from nearly 180 sites for this trial, managed by the National Surgical Adjuvant Breast and Bowel Project.
Data provided by NCI on the rates of accrual of subjects as of February 1998 for these trials indicate that only one trial, ALTS, experienced success in recruiting ethnic minorities. ALTS reports that of the 1,910 women enrolled, 1,102 (57.7 percent) are non-Hispanic whites, whereas 629 (32.9 percent) are African American. Recruitment of other minority groups into this trial, however, is inconsistent: of the women enrolled in the trial, only 65 individuals (3.4 percent) are identified as ''white Hispanic" (no data are provided for non-white Hispanic groups), an additional 3.7 percent are Asian, and 2.25 percent are identified as American Indian or Alaska Natives. Figure 5-3 depicts these accrual patterns.
The PLCO trial screens patients for three cancers simultaneously, including prostate, lung, and colorectal cancers for male patients and lung, colorectal, and ovarian cancers for female patients. Of the more than 80,000 participants in this trial, 72,000 (88.9 percent) are white, 3,546 (4.4 percent) are African American, 1,135 (1.4 percent) are Hispanic, 3,505 (4.3 percent) are Asian American, 439 (0.5 percent) are Pacific Islander, and 151 (0.2 percent) are American Indian or Alaska Natives. The rate of
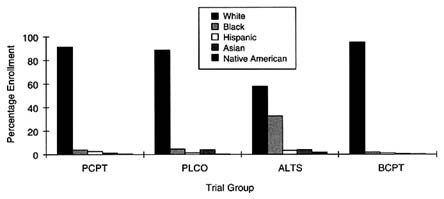
FIGURE 5-3 Enrollment in prevention trials by race/ethnicity.
SOURCE: National Cancer Institute.
accrual of all ethnic minority groups, with the exception of Pacific Islanders, lags behind the percentage of these individuals diagnosed with prostate, lung, colorectal, and ovarian cancers in the United States, according to figures from NCI. NCI reports that among Americans ages 55 to 74 years, the proportion of African Americans diagnosed with prostate, lung, colorectal, and ovarian cancer is 8.7 percent; similarly, the proportions for Hispanics, Asian Americans, and American Indians/Alaska Natives are 3.3, 8.6, and 0.5 percent, respectively. Thus, the rate of accrual of most ethnic minority groups in this trial is two- to threefold lower than the respective incidence of these cancers among these groups.
Similarly, PCPT suffers from a disproportionately low rate of accrual of ethnic minorities. Of the 18,861 patients randomized in this trial as of February 1998, 17,367 (92.1 percent) are white, 709 (3.8 percent) are African American, 500 (2.7 percent) are Hispanic, 157 (less than 0.1 percent) are Asian American, 59 (less than 0.1 percent) are Native American, and 21 (less than 0.01 percent) are Pacific Islander. According to SEER program data (Miller et al., 1996), 10.1 percent of cases of prostate cancer in the United States occur among African Americans. The rate of accrual of this population in PCPT is therefore 2.5 times lower than the national incidence of prostate cancer for this population.
PCPT did, however, provide data on the characteristics of other participants, such as levels of education and income, that may be relevant for targeting cancer control interventions. Of the total sample, 4 percent reported that they had some high school education or less, 15 percent reported only a high school degree, 30 percent reported vocational training or some college education, 16 percent reported a college (bachelor's) degree, and 35 percent reported postgraduate education. The median
annual income reported by the sample is $32,000, with 42 percent reporting incomes of $30,000 or less, 49 percent reporting incomes of between $30,000 and $50,000, and 9 percent reporting incomes of greater than $50,000.
BCPT recently yielded data suggesting that tamoxifen may be an effective chemopreventive agent for women at increased risk of breast cancer. This trial, however, which included more than 13,000 women, performed extremely poorly with respect to the accrual of ethnic minorities. Of the 13,266 women enrolled in the trial, 12,630 (95.2 percent) were white, 275 (2.1 percent) were African American, 163 (1.2 percent) were Hispanic, 112 (0.8 percent) were Asian or Pacific Islander, and 33 (0.2 percent) were Native American. According to SEER program data (Miller et al., 1996), African-American women make up 8.2 percent of the population with breast cancer, and Hispanic women make up 3.2 percent of the population with breast cancer. Therefore, for African Americans and Hispanics, the rates of accrual to BCPT were fourfold and almost threefold less, respectively, than the national breast cancer incidence. Asian Americans, Pacific Islanders, and Native Americans were slightly underrepresented in this study, according to national incidence data.
As is the case with the CCOP and MBCCOP prevention trials, the committee finds that the rate of accrual of ethnic minorities into large NIH-sponsored prevention trials in most cases lagged behind that into treatment trials. Especially for African-American and Hispanic populations, the rate of accrual of these groups appeared to be several fold less than the incidence of the various types of cancer in these groups.
How Can Low Ethnic Minority Accrual Be Addressed?
The data presented above indicate that NCI has done well in ensuring the proportional representation of ethnic minority individuals in clinical treatment trials. NCI has not enjoyed the same success, however, in accrual the of ethnic minorities in prevention trials. These data underscore the difficulties that researchers face in recruiting and retaining ethnic minority research participants. As noted above, issues of community mistrust of the medical and scientific establishments, researcher reluctance to extend extra effort to recruit ethnic minority and medically underserved populations, and greater costs associated with recruitment are all significant factors in the low rates of accrual. The same difficulties may apply to the recruitment of medically underserved populations, but data are lacking to draw appropriate conclusions. According to NCI's analyses and the experiences of other researchers, several additional factors may contribute to these disparities.
Comorbidity appears to be an important factor that potentially limits
enrollment of ethnic minorities in some trials. For example, NCI reports that between 1990 and 1994 there was an overall protocol availability of 20 to 30 percent for adults with newly diagnosed cancers. Eligibility varied by ethnic group, however, because only 33 percent of Hispanics and 40 percent of African Americans were eligible for participation, whereas more than 50 percent of whites were eligible. Ethnic group differences in eligibility were related to the rates of occurrence of comorbid diseases, such as higher rates of hypertension among African Americans. However, after taking into consideration the differences in the extent of comorbidities among the eligible patients, entrance rates did not differ among African Americans (60 percent), whites (62 percent), and Hispanics (70 percent).
Other important factors affecting the rates of recruitment into clinical trials include convenience, costs, conflicts with patient care, complexity, and effect on the patient-physician interaction, including the effects of third-party reimbursement. For example, studies with long-term survivors of pediatric cancers require patients to obtain third-party reimbursement for diagnostic tests such as cardiac echocardiography, which limits accrual of ethnic minorities and low-income populations into these long-term studies. In addition, Medicare and Medicaid health maintenance organization coverage of costs associated with enrollment in a clinical trial is poor and inconsistent, limiting the ability of populations covered by these plans to enter clinical trials.
For prevention trials, researchers face many ethical and practical concerns in recruiting ethnic minority and low-income individuals, especially those who lack insurance coverage or who have inadequate insurance coverage. A patient in whom an abnormality is detected in a screening trial, for example, should receive immediate and appropriate follow-up care. NIH funding for prevention protocols cannot be used for follow-up care of indigent patients. As noted earlier, NCI is attempting to address such downstream costs associated with prevention trials by coordinating with other federal agencies. The committee encourages these efforts and urges greater federal coordination to ensure that downstream costs do not limit the accrual of ethnic minority and low-income individuals.
Recommendation 5-1: NIH and other federal agencies (particularly the Health Care Financing Administration) should continue to coordinate to address funding for clinical trials, particularly to address the additional diagnostic and therapeutic costs associated with prevention trials and third-party payment barriers associated with clinical treatment trials.
Mistrust of biomedical research among many in ethnic minority communities may exacerbate the difficulties associated with their recruitment
into prevention trials. Minority individuals who believe themselves to be healthy may experience a greater reluctance to participate in prevention trials, especially if they have no immediate experience with cancer among their family and friends. This wariness may again be traced to the history of medical abuse and exploitation of ethnic minority group members. Despite the considerable patient protections present in human subjects research today, many ethnic minorities, especially older individuals, have firsthand experience with civil rights violations that occurred despite the existence of legal protections that supposedly applied to all Americans. Lessons from these experiences are not likely to be altered, despite the institution of lengthy informed consent procedures (indeed, such procedures may heighten suspicion), especially for individuals who are asymptomatic.
As noted in several of the presentations at the NCI conference on recruitment and Retention of Minority Participants in Clinical Cancer Research (Box 5-1), the informed-consent process should not be seen as a "barrier" to the recruitment of ethnic minority and underserved populations into clinical trials. The informed-consent process is the first step in developing a bond of trust between the researcher and the patient. Improvements in the informed-consent process, particularly alternative means of obtaining consent (e.g., oral consent methods), may help to clarify the research process for potential subjects and help define roles and expectations for both the patient and the researcher. Such alternate means of obtaining consent may also help improve the accrual of populations with low levels of literacy, which may disproportionately include ethnic minority and medically underserved individuals. The committee urges NCI to continue to encourage examination of the informed-consent process.
Recommendation 5-2: NCI should continue to work with other appropriate federal agencies and institutional review boards to explore creative approaches to improving patients' understanding of research and encouraging them to provide consent to participate in research. These approaches should address cultural bias, mistrust, literacy, and other issues that may pose barriers to the participation of ethnic minority and medically underserved groups.
Another potential reason for the low rate of accrual of ethnic minorities in prevention trials is that the physicians who recruit subjects into prevention trials are different from those who recruit subjects into treatment trials. For example, many oncologists are not involved in prevention and screening trials, whereas they are more likely to participate in treatment trials. In addition, investigators with some of the large NCI-supported
screening studies, such as the PLCO study, are constrained by the U.S. Office of Management and Budget mandate that a collaborating site must have adequate numbers of patients with all four diseases included as part of the PLCO study. This eliminates centers such as the Veterans Affairs Hospital system as a potential partner, despite the large numbers of people eligible for the prostate, lung, and colon cancer part of the study.
These barriers point to the need for NIH-supported researchers to form partnerships with community-based health service providers and community leaders. In an extensive review of the literature regarding recruitment of ethnic minorities into clinical trials, Swanson and Ward (1995) emphasize the need for researchers to develop community networks, provide community outreach services and programs, establish bonds with community leaders, and recruit as investigators ethnic minority physicians and other health service providers who serve primarily an ethnic minority clientele. Involving community members early in research design and implementation plans can also yield benefits for subject recruitment, as well as for ensuring the smooth operation of a study. Some researchers, for example, have enlisted the cooperation of churches and pastors in African American communities to overcome suspicion of research goals, generate referrals, train lay health workers, and generally promote awareness of healthy behavior. Swanson and Ward, however, note that "contrary to pervasive beliefs about this approach, it is not a panacea for achieving high participation rates of African Americans in clinical trials" (Swanson and Ward, 1995, p. 1753). Multiple approaches and strategies are necessary, they note, to ensure the "buy-in" of community members.
Community-based health care providers and community health clinics, such as the federally supported Community Health Centers (CHCs), offer great potential to researchers as sites to recruit patients and health care providers, to bridge cultural and linguistic gaps between researchers and target populations, and to train new researchers. CHCs serve as primary care providers for more than 9 million low-income patients, many of whom are ethnic minority. NCI support for training, research, and data collection in these settings should be explored not only as a means of increasing the accrual of ethnic minority and medically underserved populations in clinical trials, but also as a point of information dissemination regarding cancer prevention and treatment.
Finally, greater education of researchers is an important component of an overall strategy to improve the rate of recruitment of ethnic minority and medically underserved individuals into clinical trials. Some researchers may not be aware of the importance, from scientific and ethical perspectives, of demographically diverse subject pools; others may reject such goals as "political" or "inconvenient." However, anticipation of when interventions are expected to affect subpopulations differently is critically important.
Although it is not known what data are needed to suggest that treatments are likely to affect ethnic minority individuals differently from the ways in which they affect whites, attention to this consideration is needed. More research into the biological, genetic, and pathophysiological considerations of differences in certain cancers among ethnic groups would be helpful in this regard.
Data on the retention of ethnic minorities and medically underserved individuals in treatment and prevention trials have not been addressed in prior NCI reports. Retention in trials, however, is also likely to be problematic for many ethnic minority groups and medically underserved populations. The committee urges routine collection and analysis of data for patients who have dropped out of clinical trials, as well as for those who have been retained, to determine if there are patterns among these groups that may lead to better prediction of who is more likely to drop out and to potential intervention strategies. In addition, the committee urges that NCI require clinical trial investigators to report on the accrual of medically underserved patient populations, including rural residents, low-income individuals, individuals with low levels of literacy, and medically indigent populations. This information should be reported publicly by NCI as part of its effort to inform scientists, health advocates, policy makers, and the general public of progress in clinical trial accrual.
Recommendation 5-3: NCI should report on the accrual and retention of ethnic minority and medically underserved populations in clinical trials using a consistent definition for medically underserved populations, including such characteristics as rural versus urban population, insurance status, socioeconomic status, and level of literacy.
Dissemination of Research Findings to Ethnic Minority and Medically Underserved Communities
As noted above, the committee is charged with reporting on "how well research results are communicated and applied to cancer prevention and treatment for minorities." The term research results is very vague, ranging from poster sessions at a conference and publications available through a MEDLINE search to clinical practice guidelines. Similarly, the term dissemination covers both health care providers and consumers at large. Therefore, for the purposes of this report, the committee interpreted both terms very broadly as the dissemination of scientific information with regard to standards of care. The committee did not separate among cancer prevention, screening and diagnosis, or treatment and follow-up. The committee also looked at literature related to a variety of cancer sites and did not
attempt to distinguish between what may be more useful for addressing different types of cancer. For background, the committee reviewed documents provided by NIH, primarily from NCI (these documents are listed in Appendix E). The committee also directed specific questions to NCI and reviewed related research literature.
The remainder of this chapter comprises that report, which is divided into three sections. The first section reviews pertinent research literature. The second section describes examples of dissemination practices by NCI and racial and ethnic patterns of use of the Cancer Information Service (CIS). The third section concludes with recommendations by the committee.
Related Literature on Ethnic Minority and Medically Underserved Populations
The committee's review of the literature reveals that historically, federal, state, and local public health officials have failed to establish specific programs, guidelines, or initiatives to improve health service delivery for minority and medically underserved populations. Significantly, the literature also consistently reveals that ethnic minority and medically underserved individuals are more likely to receive less appropriate or less aggressive treatment for cancer once in the health care system, a fact that may contribute to disparities in cancer survival and mortality.
Studies of disparities in health service delivery indicate that ethnic minority patients, especially African Americans, are less likely to receive appropriate screening, diagnostic, and treatment services for cancer. Some evidence suggests that while "race" and income both predict differences in clinical treatment, "race" may serve as the "overriding determinant of disparities in care" (Geiger, 1996, p. 816). Many such studies have controlled for age, gender, insurance status, income, disease severity, concomitant morbid conditions, and underlying incidence and prevalence rates in the population groups under study. For example, Gornick et al., (1996), in a study of health service delivery to a multiethnic population of Medicare beneficiaries, found that African-American patients were less likely than whites to receive mammography. This difference was attenuated modestly when patient income was controlled, although it did not eliminate "racial" differences. While further study is needed to determine whether some of the differences in clinical care are due to other potential confounding factors, such as patient preferences and lack of information about the need for care, the presence of such systemic disparities suggests that racial discrimination by health care providers and institutions, perhaps operating at "unconscious'' levels (Gieger, 1996), may be the more likely explanation.
How Can Care Be Improved for Ethnic Minority and Underserved Groups?
The U.S. Preventive Services Task Force is an expert panel established by the federal government in 1984 to develop evidence-based practice guidelines on screening tests and other preventive services (Woolf et al., 1996). The second edition of the Guide to Clinical Preventive Services (U.S. Preventive Services Task Force, 1996) does not address multicultural or minority health issues. In the context of patient education, the physician is advised, "In considering a patient's belief system, the provider is challenged to facilitate the bridging of cross-culture gaps as well. Culturally sensitive education and counseling requires that clinicians assess their own cultural beliefs and be aware of local ethnic, regional, and religious beliefs and practices. Such knowledge aids the development of culturally specific health teaching" (U.S. Preventive Services Task Force, 1996, p. 77).
In one study, researchers examined 21 major health data systems of the U.S. Department of Health and Human Services and concluded that data on Hispanics are not included in several departmental national health data collection systems and that even when such data are collected, data on Hispanic subpopulations are found in few of the systems. When available, the databases often do not collect sample sizes adequate for analyzing any one of the four major Hispanic subpopulation. The researchers therefore support the call to collect data on Hispanics in all systems, to provide samples and subsamples large enough for statistical analysis, to support researchers and a research infrastructure for the Hispanic populations, and to support the broad dissemination of findings from the data that are presented in formats that are useful to Hispanic community-based organizations (Delgado and Estrada, 1993). Although the researchers do not address cancer per se, one researcher notes that hypertension is difficult to detect among groups such as Hispanics, for whom an array of impediments, including language and degree of acculturation, may retard the dissemination of information, making it difficult to detect and treat hypertension (Martinez-Maldonado, 1995).
In a survey of chronic disease directors in 50 states, only 16 states (32 percent) reported that they had sponsored or directly supported cancer prevention and cancer control services specifically targeted to American-Indian and Alaska-Native populations. Few state public health agencies had developed culturally relevant cancer education materials for American Indians and Alaska Natives. Although the respondents directed chronic disease or cancer prevention and control programs in their states, many were unfamiliar with the cancer patterns or general health problems among American Indians and Alaska Natives (Michalek and Mahoney, 1994).
The Problem with Guidelines
Legitimate differences of opinion exist, for example, regarding the effectiveness of clinical practice guidelines (e.g., frequency of prostate cancer screening, for which the benefits remain theoretical [Collins and Barry, 1996; Collins et al., 1997]). Several issues are related to efforts to make guideline material useful for patient-specific decision support: (1) incongruity of cancer screening guidelines with local standards, (2) insufficient specificity, (3) insufficient comprehensiveness, and (4) use and dissemination in today's health care environment (Zielstorff et al., 1996).
Despite encouragement from physicians and other health care providers, primary care physicians often do not comply with cancer screening guidelines (Burnett et al., 1995; Greenberg et al., 1996; Guerra et al., 1994; Hovell et al., 1996; Kripalani et al., 1996; Shapira and Levine, 1996). In private-practice settings, the use of preventive medicine services in general has not always been successful. Patient education materials may not be used after they have been ordered and made available to patients. However, several factors that seem to affect the successful implementation of preventive medical services in a clinical setting are often under the control of the physician, such as support from upper levels of administration, "stakeholding" by all of the staff, designation of an individual to manage implementation of a program offering such services, and provision of continuous auditing and feedback to participants (Dickey et al., 1994; Griffith and Rahman, 1994). Although systems for effective physician office-based preventive services can be implemented, they often are not, and strategies for their dissemination may require the involvement of professional medical organizations and managed care companies (Leininger et al., 1996; Simmons and Goforth, 1997).
In fact, it is not clear what factors are both necessary and sufficient to effect change in physician behavior. One 4-year study targeted health care providers in a community receiving an intervention and two control communities. The intervention included the formation of local physician planning groups, mailing of a series of informational packets, provision of medical office staff training sessions, and use of reminder system support. However, the study found no significant postintervention differences in the self-reported ordering of mammography's by physicians practicing in the intervention and control areas. Indeed, over the 4-year study period, routine ordering of mammographies across both groups increased by 36 percent, such that it was ordered for 80 percent of the respondents. The researchers attribute the increase to secular trends resulting from the diffusion of strategies that promoted the use of mammography in general (Taylor et al., 1996). In another study, researchers classified 2,357 Medicaid
outpatient barium enemas and 896 outpatient colonoscopic examinations as appropriate, inappropriate, equivocal, or miscoded on the basis of current guidelines in the medical literature. Significantly more colonoscopic examinations were rated as inappropriate, suggesting that more stringent criteria need to be used by physicians in ordering diagnostic examinations of the colon, particularly colonoscopy. The researchers encourage further investigation of the appropriateness, development, and dissemination of such guidelines (Levine et al., 1997).
Physicians are apparently more likely to comply with treatment guidelines than with guidelines related to prevention and screening (Katterhagen, 1996; Olivotto et al., 1997). For instance, negative publicity regarding fraudulent data from a large clinical trial of breast-conserving surgery (BCS) versus modified radical mastectomy apparently did not result in a decline in the rate of use of BCS (Polednak, 1996). However, the only region with an increase in the rate of BCS use from 1986 to 1990 was New England, and in general, use of the guidelines tended to increase only in hospitals that were large and in urban areas, that had higher patient volumes or a cancer center, or that were already using more BCS (Nattinger et al., 1996). In a different study, more disturbing findings indicated that the use of a lumpectomy versus a mastectomy was associated with younger age, private health insurance, and treatment in urban hospitals. Only 45.3 percent of women with Medicaid coverage who had a lumpectomy during the study period received the requisite follow-up radiation therapy, whereas 77.5 percent of private insurance subscribers and 88.1 percent of Medicare beneficiaries had the requisite follow-up radiation. The investigators concluded that research that uses data other than administrative claims data and practice patterns is necessary to determine the reasons for the lack of compliance with treatment protocols by women or physicians (Young et al., 1996).
In fact, significant differences in compliance with practice guidelines can be found between two large hospital providers of cancer services within the same medical community. In one study, a university hospital followed the guidelines more than a community hospital did both before and after publication of an NCI Clinical Alert regarding adjuvant therapy after primary treatment for node-negative breast cancer. The investigators concluded that physician referral patterns influenced the provision of adjuvant therapy, but they also concluded that the process of developing guidelines should include strategies for enhancing compliance with them (Studnicki et al., 1993).
Guidelines themselves must provide more than just a summary of scientific findings; they must make clear recommendations, and their diffusion into general medical practice must involve regional medical groups and their opinion leaders (Starreveld, 1997). The dissemination of standards
of treatment may be facilitated (or impeded) by physician and practice setting characteristics such as specialty, affiliation with a CCOP hospital, time in practice, professional centrality (level of participation in cancer information networks), solo practice, and number of colon cancer patients (McAfee et al., 1996).
Health behavior is complex, however, and any given behavior often exists among a constellation of other behaviors. One study looked at this relationship in two ways. The researchers compared women 50 to 70 years of age who had received three screening examinations—a clinical breast examination (CBE), mammography, and Pap smear—in the previous 2 years with those who had not received all three examinations. They also compared women who had a Pap smear and at least one breast screening examination (i.e., a mammogram or a CBE) in the previous 2 years with women who were underscreened. Black women were more likely to use screening services in the office setting (i.e., BCE and Pap smear), without a corresponding use of mammography. Nevertheless, more black women than white women received a routine Pap smear in combination with a CBE, a very positive trend with respect to the successful diffusion of at least two screening procedures among older black women. The data suggest that barriers to mammography screening remain even among women who are screened by a CBE and a Pap smear. The researchers point out the need to educate both primary care physicians and their patients that the various components of preventive health are equally necessary and inter-related (Pearlman et al., 1996).
What Works
Many different dissemination and implementation strategies are available; however, traditional passive dissemination methods (for example, publication in professional journals or mailing of practice recommendations to health care professionals) are generally ineffective. Greater attention needs to be given to coordinating active dissemination and implementation strategies to ensure effective professional practice (Grimshaw, 1996). A variety of methods, such as the use of letters of invitation, community mobilization, and mobile service delivery, can be used to increase levels of participation in screening programs (Bryant, 1996). Long-term, ongoing community participation and coalition building in all phases of the research are essential to generating community acceptance of innovative and effective interventions (Matsunaga et al., 1996; Suarez et al., 1993; Ward et al., 1993).
In an example not related to cancer per se (i.e., nursing education and the use of enteral feeding catheters), nurses' primary source of knowledge about the subject was clinical practice (56.9 percent) and consultation
with peers (21.7 percent); only 19 percent had received in-service training on the topic. Although written guidelines from the agency varied considerably, two factors significantly associated with use of the guidelines were (1) assistance from pertinent ancillary and support staff and (2) attendance at a relevant seminar or in-service training program. Among their conclusions, the researchers note that research-based guidelines and a more formal means of dissemination of information to clinical staff are needed (Belknap et al., 1997).
The diffusion of new technology is significantly affected by coverage and reimbursement decisions. Medical innovation may improve the quality of health care but may also increase the cost of health care delivery. The Medicare program has a significant influence on the coverage policies of public and private third-party payers. This influence is especially visible in coverage and reimbursement decisions for cancer-related diagnostic procedures and systems. Coverage for these procedures and systems is not widespread because payers have not been convinced of the clinical usefulness of the assays (Logue, 1993).
Clinical practice guideline dissemination or implementation processes have had mixed results. Variables that affect the adoption of guidelines include the quality of the guidelines, the characteristics of the targeted health care professionals, the characteristics of the practice setting, incentives, regulations, and patient-related factors. Specific strategies fall into two categories: primary strategies involving mailing or publication of the actual guidelines and secondary interventional strategies that reinforce the guidelines. Such intervention can be weak (e.g., didactic, traditional continuing medical education and mailings), moderately effective (e.g., audit and feedback, especially concurrent feedback, targeted to specific providers and delivered by peers or opinion leaders), and relatively strong (e.g., reminder systems, academic detailing, and multiple interventions). The evidence shows serious deficiencies in the adoption of clinical practice guidelines in practice. Future implementation strategies must overcome this failure by considering the forces and variables that influence practice and by using methods that are practice and community based rather than didactic (Davis and Taylor-Vaisey, 1997).
Following implementation of a legislative mandate requiring the dissemination of practice guidelines regarding deliveries by cesarean section to obstetric physicians, one study found that the guideline certification program did not accelerate the consistent but gradual downward trend in deliveries by cesarean section that had already been evident in the 3 prior years. The data did suggest a possible greater effect on repeat cesarean deliveries more than on primary cesarean deliveries. The date of guideline implementation reported by hospitals was not related to any systematic change in observed cesarean delivery rates. The researchers conclude that
the mere dissemination of practice guidelines by a state agency may not achieve either the magnitude or the specificity of the desired results without an explicit and thorough guideline implementation program. Simple legislative mandates may therefore be ineffective when multiple initiatives are already achieving the desired outcomes (Studnicki et al., 1997).
What is Needed
In the area of clinical trials, formal overviews or meta-analyses are now widely accepted as reliable means of evaluating the evidence from multiple trials that have assessed a particular form of therapy. Such analyses can have many benefits: the results are particularly clear and therefore have substantial public health impact, the areas of statistical and medical agreement on the evidence can be defined, and the areas of uncertainty (and hence future research priorities) can be clarified prior to the dissemination of the results (Sandercock, 1993).
It has been argued that cancer has lagged behind many other areas of medicine in pursuing outcomes research and management (Weeks and Pfister, 1996). For more than 20 years, studies have documented the substantial geographic variability in medical practice around the United States and have found that such variability is often driven by nonmedical factors. Outcomes management has several key components: the development and implementation of guidelines; measurement, pooling, and dissemination of clinical and outcomes data; and refinement of guidelines on the basis of an analysis of those data. In the case of the National Comprehensive Cancer Network (NCCN), the goal of outcomes research within NCCN member institutions is to pool and analyze selected patterns of care and outcomes data, ultimately creating a uniform outcomes measurement system to monitor guideline compliance (Weeks and Pfister, 1996). Further research, however, is required to determine optimal dissemination strategies and evaluate the impacts of these guidelines (Levine et al., 1996). In workplace environments, there is also a need for better assessment of the effectiveness of information dissemination, along with greater understanding of the barriers to implementation of the information by workers and management and improved hazard surveillance (Fine, 1995).
From the perspective of theory, several gaps regarding diffusion and dissemination should be considered: (1) research, in terms of better understanding of efficacious treatment and alternative treatments as well as prevention; (2) clinical practices, in that practitioners may not be up to date on diagnostic practices, which can be addressed through medical and continuing medical education; and (3) communication, both from the doctor's perspective and from that of the patient, such that health consumers need to be trained in how the medical system works and on how to
participate actively in their own care (Bourque, 1996). Arguably, the epidemiologic knowledge of health care workers, media, decision makers, and the general public has improved, and all groups are aware of the main cancer risk factors. Direct assessment of primary prevention activities has proved to be very difficult because many years may pass between the time that the level of exposure to a carcinogen is reduced and the time that the consequences in terms of public health are observed (Hill, 1996).
Although the development of treatment guidelines has evolved a somewhat systematic framework, further research is required to determine optimal dissemination strategies and to evaluate the impacts of these guidelines (Levine et al., 1996). Dissemination and diffusion are complex, long-term endeavors, even within a single institution with multiple sites. In one study that used the context of continuous quality improvement, the intervention arms of the study were training, consultation, networking, and reinforcement for internal multidisciplinary teams as they work through a structured process to understand and improve their clinic's process for providing preventive services. Training alone lasted up to 9 months (Solberg et al., 1996). Given the danger of overloading health care providers with new guidelines, there needs to be a timed strategy for their introduction. Dissemination of guidelines alone is not enough without an appropriate implementation strategy that may require marketing of new ideas to change practice (Forrest et al., 1996).
NCI Cancer Information Dissemination Practices
Of the information supplied to the committee, ethnic minority or medically underserved populations were rarely the targets of specific dissemination activities. The information in general reflect a varied picture of the dissemination process. For instance, consensus development panels are convened to expedite transfer of new knowledge and "medical technologies into the practice of medicine for the benefit of patients." However, one study related to follow-up of a consensus conference on prostate cancer treatment failed to note any consequent change in recommended therapies (Sherman et al., 1992). Clinical Alerts can have an immediate impact on the treatment of certain segments of patient populations (Johnson et al., 1994). Community Clinical Trials Networks can also be used to increase access to cancer care. However, success rates vary with organizational and management styles, the clarity of the goal statement, adequacy of funding, and so forth (Kaluzny et al., 1995b).
The Cancer Information Service as a Dissemination Tool
NCI's CIS is a successful mechanism for cancer information dissemination.
According to NCI, the service received 500,000 calls in 1996. In one survey, users tended to be generally very satisfied with the communications from their treating physicians, had strong information needs, and preferred to participate in their treatment plans (Manfredi et al., 1993). However, in that study, 80 percent of the respondents were patients; that is, it was not clear how well CIS disseminates cancer prevention, screening, and early detection guidelines before diagnosis. In addition, no data on the minority status of the users were available.
In addressing these questions, CIS provided the information presented in Tables 5-1 and 5-2. It should be noted that the committee did not formally analyze these data statistically. However, Table 5-1 indicates that less than 15 percent of calls to the CIS telephone service were from minorities,
TABLE 5-1 Race or Ethnicity of Callers to CIS Telephone Service (1997)
TABLE 5-2 Subjects of Inquiry to CIS Telephone Service
proportionately less than the proportions of calls from whites. Given the disproportionate incidence of cancer among minority populations, one would hope for appropriately increased numbers of calls from affected groups. For instance, prostate cancer incidence and mortality rates are higher among African-American men than among white men. Compared with white women, African-American women have a lower incidence of breast cancer but higher rates of mortality from breast cancer (American Cancer Society, 1997, 1998). Table 5-2 indicates that treatment topics are addressed much more than are prevention and screening (C. Thomsen, CIS, personal communication, 1998).
Scientific Journal Publications as a Tool for Dissemination
NIH provided a list of 888 scientific journal publications related to cancer among minorities and medically underserved populations that have resulted from awards of programs of NIH in 1985. Having neither the actual publications nor their abstracts in hand, it was difficult to tell how much the articles were in fact related to cancer among ethnic minority and medically underserved populations. The committee therefore analyzed only the titles of the articles listed to determine the distribution of publications across racial and ethnic groups and the number of publications related to dissemination. It should be noted that the categorizations may underestimate references to ethnic minority and medically underserved populations in the articles listed.
The committee examined whether the following specific terms actually appeared in the title:
- Multicultural: "race," "racial," "ethnic," "cultural,'' "multicultural"
- Not multicultural: no reference to any such terms at all or clearly indicating only a sample of white, Caucasian, or Anglo individuals.
- African American: "African American," "black."
- Hispanic: "Hispanic," "Latin/Latino/Latina," "Mexican," "Mexican American," or "Puerto Rican."
- Asian/Pacific: "Asian Islander," "Oriental," "Chinese," "Japanese," "Vietnamese," "Hawaiian."
- American Indian/Alaska Native: "Native American," "Alaska Native," "tribal."
- Related to dissemination: "screening," "education," "guidelines," "media," "disseminate/diffuse."
An article was listed in more than one column if its title included more than one appropriate term, for example, "Hispanic" and "American
Indian." As shown in Table 5-3, starting in 1995, there was a large increase in both the number of publications and the number of titles with some relation to dissemination compared with those in previous years. The reasons for these increases are not clear. For instance, more projects may have been funded, researchers may more frequently have published multiple articles that were based on the same numbers of projects, community and political activism may have increased pressure for both, or there may be other less obvious reasons worth investigating.
Other Sources of Cancer Information
NCI also made available approximately 600 publications and other informational resources. In its response to the Institute of Medicine, NCI listed several examples that are targeted to African-American, Hispanic, Native American, and multiethnic populations. However, no data on the proportion of all materials that are targeted to minority and medically underserved groups were available. The committee did not find any publication comparable to ACS's Cancer Facts & Figures—1997 (American Cancer Society, 1997), which provided a general comparison of the cancer-related needs of different racial and ethnic groups.
NCI's computerized information systems have been designed to help physicians cope with the information explosion by translating the medical literature into usable forms that are accessible by computer and fax. Systems developed by NCI's International Cancer Information Center provide access to a comprehensive source of bibliographic citations on cancer research (the CANCERLIT database) and to current, peer-reviewed syntheses of state-of-the-art clinical information on cancer (the PDQ database [Hubbard et al., 1995]). However, programs such as the NIH Consensus Development Program and Physician Data Query system have not been shown to have major impacts on treatment patterns (Kosecoff et al., 1987; Lomas et al., 1989). A method that assesses how usage patterns for these programs and systems may vary between minority and nonminority physicians does not seem to exist.
Admitting its lack of experience reaching high-risk populations, particularly those characterized by high proportions of socioeconomically disadvantaged individuals, ACS has begun to fund and provide technical assistance to community demonstration projects, followed by dissemination of model projects to selected ACS divisions with various resources and capabilities (e.g., outreach to high-risk populations, planning, program development, and evaluation) for replication (Corcoran and Robinson, 1994). Since January 1996, NCI has sponsored a series of regional conferences on the recruitment and retention of minorities in clinical trials, in part to promote interaction between community groups and researchers
TABLE 5-3 Articles from NIH-Sponsored Programs Relative to Cancer and Cancer Information Dissemination Among Minority and Medically Underserved Populations by Key Terms in Title
|
|
No. of Articles |
Key Term in Title |
|
|
Year |
Total |
Not Multicultural |
Multicultural |
|
1997 |
110 |
50 |
28 |
|
1996 |
129 |
42 |
20 |
|
1995 |
119 |
55 |
13 |
|
1994 |
83 |
22 |
12 |
|
1993 |
62 |
27 |
27 |
|
1992 |
66 |
40 |
10 |
|
1991 |
63 |
33 |
2 |
|
1990 |
50 |
29 |
3 |
|
1989 |
38 |
15 |
5 |
|
1988 |
32 |
24 |
2 |
|
1987 |
33 |
16 |
2 |
|
1986 |
39 |
19 |
3 |
|
1985 |
64 |
31 |
8 |
|
Total |
888 |
403 |
135 |
|
SOURCE: National Cancer Institute. |
|||
to share strategies. There are plans for a future publication about what has been learned through this process. However, it is not clear how NCI identifies successful intervention programs and models or supports their replication. Also, it is not clear whether or how NCI addresses the problem of studying subgroups of target populations.
Recommendations
In Chapter 1 of this report, the committee poses a fundamental question: Is there a strategic plan for reducing the numbers of deaths from cancer among minority and medically underserved population? If there is a plan, when can results be expected? On the basis of the documentation that the committee reviewed, the committee concludes that such a strategic plan and schedule do not exist. Although NIH has devoted resources to the needs of special populations, with regard to dissemination there is a difference between the delivery of information and the impact of information. The committee could find little to no dissemination research or activities
|
African American |
Hispanic |
Asian/Pacific |
Native American/ Alaska Native |
Dissemination |
|
22 |
7 |
10 |
5 |
26 |
|
25 |
9 |
11 |
16 |
38 |
|
17 |
20 |
10 |
4 |
35 |
|
17 |
9 |
6 |
6 |
2 |
|
31 |
24 |
12 |
10 |
4 |
|
22 |
3 |
7 |
2 |
6 |
|
3 |
5 |
11 |
1 |
|
|
6 |
4 |
6 |
|
6 |
|
5 |
3 |
6 |
5 |
3 |
|
1 |
2 |
3 |
|
|
|
4 |
3 |
3 |
|
2 |
|
3 |
6 |
7 |
2 |
|
|
3 |
6 |
14 |
4 |
1 |
|
159 |
101 |
106 |
57 |
123 |
specifically targeting health care providers who come from minority and medically undeserved groups or who, regardless of their own backgrounds, serve those groups. As might be expected, much published research simply describes the results of specific projects or studies. There is little to no documentation of the long-term impacts of these studies for either health care providers or consumers at large. As a result, in the research literature, some investigators have begun to call for the use in dissemination of studies outcomes measurement approaches like those used in clinical studies. This may evolve into something like "clinical guidelines" for dissemination and diffusion.
The overall recommendation of the committee is that NCI and NIH should develop a strategic plan and timetable to address the issues raised in this chapter. The strategic plan should use established cancer guidelines and recommendations in the areas of cancer prevention, screening and early detection, diagnosis, treatment, and follow-up. The strategic plan should provide a "gold standard" against which knowledge, attitudes, and behaviors can be benchmarked across various target populations of health
care consumers and providers, regardless of racial, ethnic, or socioeconomic status.
Recommendation 5-4: NCI should continue to assess its dissemination practices to identify effective cancer information delivery strategies among ethnic minority and medically underserved populations, revise and implement the strategic dissemination plan on the basis of the results of that research, and institute an ongoing system of monitoring to assess its effectiveness.
As part of the strategic planning process, NCI and NIH should:
- Establish a formal reporting mechanism, perhaps through the National Cancer Advisory Board or the President's Cancer Panel, on the formulation and achievement of the strategic plan.
- Establish baseline data regarding dissemination research for both researchers and their target populations. For instance, ethnic group and gender data should be recorded regarding (1) the principal investigators submitting research proposals, (2) the principal investigators eventually funded to do research, (3) the populations targeted in research, and (4) the practical impact of funded research on those populations. This process should use scientifically appropriate methods that would not bias the application review process.
- Establish a more structured framework for disseminating cancer guidelines and recommendations both to health care consumers in ethnic minority and medically underserved communities and to the health care professionals who serve them. Among health care professionals, dissemination should target the continuum from undergraduate through postgraduate training and continuing education for those already in practice.
- Establish a more structured framework for monitoring the knowledge and application of cancer guidelines and recommendations by health care consumers and providers, noting differences in minority, nonminority, and medically underserved populations.
- Establish a framework in which implementation of guidelines by both consumers and providers can be planned.
Summary
The committee finds that the inclusion of minority and medically underserved populations in clinical trials and that the dissemination of information to minority and medically underserved communities and their health care providers is a critical link connecting scientific innovation with improvements in health and health care delivery. Enhancing this link is
clearly within the purview of NCI and NIH. While many factors pose challenges to improvement (e.g., community mistrust), without a concerted effort to enhance this process, minority and medically underserved communities will continue to lag behind the American "majority" in benefiting from the tremendous recent scientific advancements and medical breakthroughs in cancer prevention, treatment, and control. The following recommendations were offered in this chapter:
Recommendation 5-1: NIH and other federal agencies (particularly the Health Care Financing Administration) should continue to coordinate to address funding for clinical trials, particularly to address the additional diagnostic and therapeutic costs associated with prevention trials and third-party payment barriers associated with clinical treatment trials.
Recommendation 5-2: NCI should continue to work with other appropriate federal agencies and institutional review boards to explore creative approaches to improving patients' understanding of research and encouraging them to provide consent to participate in research. These approaches should address cultural bias, mistrust, literacy, and other issues that may pose barriers to the participation of ethnic minority and medically underserved groups.
Recommendation 5-3: NCI should report on the accrual and retention of ethnic minority and medically underserved populations in clinical trials using a consistent definition for medically underserved populations, including such characteristics as rural versus urban population, insurance status, socioeconomic status, and level of literacy.
Recommendation 5-4: NCI should continue to assess its dissemination practices to identify effective cancer information delivery strategies among ethnic minority and medically underserved populations, revise and implement the strategic dissemination plan on this basis of the results of that research, and institute an ongoing system of monitoring to assess its effectiveness.











































