16
Trace Minerals, Immune Function, and Viral Evolution
Melinda A. Beck1
Introduction
A number of trace elements have been shown to be important for adequate functioning of the immune system, including copper, zinc, and selenium. Both deficiencies and luxus levels of trace elements can influence various parameters of the immune system, such as antibody responses, cell-mediated immunity, and natural killer (NK) cell activity.
A functional immune system is required for the ability of the host to prevent or limit infections. This is particularly important for soldiers in the field, where exposure to novel infectious agents, as well as working in less-than-optimal hygienic conditions, is a real possibility. Clearly, the optimum level of trace elements and other nutrients for immune function needs to be included in any military diet.
In addition to the effect of trace elements on immune function, recent studies from Beck et al. (1995) have demonstrated that selenium (Se) levels can influence the genetics of a viral pathogen. Thus, trace element nutrition influences not only the host response to a pathogen but also the pathogen itself.
This paper reviews the effect of the trace minerals zinc (Zn), copper (Cu), and Se on immune function, as well as the effect of Se on a viral pathogen. Implications for soldiers in the field will also be discussed.
Zinc
Zn is perhaps one of the most studied trace elements with respect to its effect on the host immune system. Deficiencies in Zn have been classified into three syndromes by Henkin and Aamodt (1983): acute, chronic, and subacute deficiency. It has been suggested that subacute deficiency is the most common, affecting an estimated 4 million people in the United States (Walsh et al., 1994). Zn is obtained in the diet primarily from meat (50%), cereals and legumes (30%), and dairy products (20%) (USDA, 1986).
Zn deficiency has been noted to result in increased susceptibility to infectious disease (Bogden et al., 1988). In a mouse model of Zn deficiency (Fernandes et al., 1979; Fraker et al., 1978, 1986), a 30-d feeding period of suboptimal levels of Zn led to reduced thymus size and depleted macrophages and lymphocytes in the spleen. Suboptimal Zn status has also been associated with decreased T-cell function and antibody responses (Kruse-Jarres, 1989). If the Zn deficiency is corrected, immune status is restored (Walsh et al., 1994).
Excess levels of Zn have also been reported to be immunosuppressive, including decreased activities of polymorphonuclear leukocytes, decreased T-cell proliferation to mitogen, and decreased antibody production (Schlesinger et al., 1993). Thus, Zn status, both excess and deficient, adversely affects immune function.
Singh et al. (1994) found that supplemental Zn given prior to strenuous exercise reduced the amount of reactive oxygen species that occur post exercise. This antioxidant effect of Zn may be of importance to troops who are under chronic physical stress.
Driessen et al. (1995) found that, in vitro, lipopolysaccharide-stimulated peripheral blood mononuclear cell cultures exposed to 0.0125 mM Zn had elevated interleukin (IL)-1β levels that were 50 percent higher than cultures that were not supplemented with Zn. Secretion of interferon-gamma (INF-γ) increased 10-fold when cultures were supplemented with 0.1 mM Zn. However, monocyte stimulation by superantigens (staphylococcal enterotoxins A and E) was decreased in cultures supplemented with Zn. Thus, depending on the type of stimulus, supplemental Zn may exert different effects.
Zinc is a cofactor for a number of enzymes, including thymidine kinase, ribonuclease, and RNA and DNA polymerases. All of these enzymes are
important for cell division. In addition, Zn is an essential cofactor for thymulin, a peptide hormone that plays a key role in T-cell maturation.
Thus, Zn has been shown in a number of studies to have an effect on immune function. However, depending on the type of immune stimulus and/or concentration of Zn present, the immune system can either be stimulated or suppressed. Further studies with Zn are required to clearly delineate the role of zinc in immune system functioning.
Copper
Cu is an essential nutrient for humans, although Cu deficiency is rare. Cu-deficient animals are more susceptible to parasitic, bacterial, and viral infection (Newberne et al., 1968; Okmole and Onawunmi, 1979; Stabel et al. 1993). Children with Menkes syndrome, a genetic disease of Cu malabsorption, generally die from infectious bronchopneumonia (Prohaska and Failla, 1993).
Animal studies have demonstrated that Cu-deficient mice have impaired plaque formation to sheep blood red cells, demonstrating decreased B-cell activity, and decreased antibody responses to a number of antigens (Blakly and Hamilton, 1987; Failla et al., 1988; Koller, 1987; Prohaska and Lukasewycz, 1981, 1989, 1990; Vyas and Chandra, 1983).
Cell-mediated immunity has also been studied in Cu-deficient animals, with conflicting results. Jones (1984) reported delayed-type hypersensitivity (DTH) responses were enhanced in Cu-deficient mice, whereas Koller et al. (1987) reported normal DTH responses in Cu-deficient rats. However, T-cell proliferative responses to a variety of antigens are lower in Cu-deficient animals (Davis et al., 1987; Lukasewycz and Prohaska, 1983, 1985; Prohaska and Lukasewycz, 1981, 1989). NK cell activity is also decreased in Cu-deficient rats (Koller, 1987).
Lymphocyte subset populations are also altered in Cu-deficient animals. Higher numbers of B-cells and fewer T-helper (Th) cells are found in Cu-deficient animals as compared with animals with normal Cu nutriture.
In a human study, Kelley et al. (1995) fed healthy adult males a low copper diet for 66 days, followed by a period of normal Cu intake. Daily Cu intake was 0.66 mg/d for the first 24 days, 0.38 mg/d for the next 42 days, and 2.49 mg/d for the final 24 days of the study. A range of 1.5 to 3.0 mg/d of Cu is recommended for adults. Lymphoproliferative responses to mitogen were greatly reduced during the 0.38 mg/d feeding period, when compared with values at the start of the study, and were not restored within the 24 days of feeding the 2.49 mg/d Cu diet. Secretion of IL-2 receptor was also decreased. However, numbers of B-cells increased during the low Cu diet, although the numbers of total T-cells (CD3+) or T-cell subsets (CD4+, CD8+) did not change. Moreover, no changes were seen in neutrophil phagocytic function.
Although adequate Cu intake is important for immune function, the mechanism of action is not known. Cu has many biochemical activities, such as
a cofactor for ferroxidase, cytochome c oxidase, and Zn-Cu superoxide dismutase, an enzyme that is important in limiting oxidative stress. Further research is necessary to delineate the role of Cu in immune system activities.
Cu-Zn Interactions
Zn and Cu are antagonistic with one another: Zn deficiency leads to an increase in Cu levels in liver and bone (Burch et al., 1975; Moses and Parker, 1964; Petering et al., 1971; Prasad et al. 1969). Conversely, excess Zn leads to Cu deficiency. Under certain conditions, Zn and Cu can inhibit one another's absorption. For example, Zn absorption in Zn-adequate rats is decreased with excess Cu, but not in Zn-deficient rats (Evans et al., 1974; Walsh et al., 1994). Both Cu and Zn absorption are increased with a Zn deficiency. However, only Cu absorption is increased with a Cu deficiency.
Selenium
Selenium (Se) is an essential component of glutathione peroxidase, an antioxidant enzyme that plays an important role in removing hydrogen peroxide and organic hydroperoxides (Chaudiere et al., 1984). A deficiency in Se can induce a state of oxidative stress in the host. Oxidative stress is a term used to describe the overabundance of the production of free radicals and other oxidants in comparison with antioxidant defenses. Thus, oxidative stress describes a situation in which prooxidants are favored over antioxidants. Oxidative stress can affect host cells in a number of ways. Beckman and Ames (1997) have suggested that oxygen free radicals damage on the order of 10,000 DNA bases per cell per day, of which a small percentage are not repaired. Membrane integrity of cells becomes impaired due to oxygen free radical-mediated lipid peroxidation, leading to the decrease or loss of cellular function. Proteins are also susceptible to free-radical damage. For example, hydroxy radicals modify amino acid residues at metal-binding sites of proteins (Stadtman, 1992). Oxidized proteins are more rapidly degraded than unoxidized proteins (Davies and Goldberg, 1987; Farber and Levine, 1982; Rivett, 1985). Oxidant stress also alters ion movement across cellular membranes by interfering with Na+ pumps and Na+K+Cl− cotransporter activities (Elliott and Schilling, 1992).
Se deficiency has been associated with lower resistance to infection with Pasturella multocida and parainfluenza 3 virus (Chandra and Chandra, 1986; Dhur et al., 1990; Larsen, 1988; Larsen and Tollersrund, 1981; Reffett et al., 1988; Sheffy and Schultz, 1979, Stable and Spears, 1993). The increased susceptibility to infectious pathogens in Se deficiency may be due to decreased antibody production and impaired lymphoproliferative responses (Chandra and Chandra, 1986).
Se deficiency has also been associated with an endemic cardiomyopathy in China known as Keshan disease. Keshan disease (KD) is characterized by acute or chronic heart conditions affecting heart function such as cardiac insufficiency, enlargement of the heart, arrhythmia, atrial fibrillation, and tachycardia (Li et al., 1985). Histologically, KD is characterized by multiple focal necrosis and myocardial parenchymatous degeneration (Gu, 1983). Epidemiologists in China found that the disease occurred only in areas with low Se soil content. Subsequently, it was found that individuals residing in KD endemic areas were of low Se status. Supplementation with Se to normal nutritional levels has prevented the widespread occurrence of KD in endemic areas of China. However, the Se deficiency alone did not explain all aspects of the disease. The seasonal and annual incidence of KD suggested that an infectious cofactor may play an etiological role in the development of the disease. Indeed, virologists in China have isolated several enterovirus strains from blood and tissue specimens of KD victims (Su et al., 1979). One of the enteroviruses, Coxsackievirus B4 (CVB4), recovered from a blood sample of a KD patient, caused a higher incidence and more severe myocarditis in neonatal mice born to Se-deficient dams than in neonates born to dams that were fed a diet containing adequate levels of Se (Bai et al., 1980; Ge et al., 1987).
Coxsackieviruses, and particularly CVB viruses, are etiological agents of viral-induced myocarditis and are suspected agents of dilated cardiomyopathy (DCM) (Fuster et al., 1981; Leslie, 1989). DCM is the second leading indication for heart transplantation in this country (O'Connell and Robinson, 1985), which suggests that infections with CVB viruses are responsible for a great deal of morbidity and mortality. Coxsackieviruses are nonenveloped RNA viruses in the Picornaviridae family, subgroup enterovirus (of which the most commonly known enterovirus is poliovirus). The genome consists of approximately 7,500 base pairs in an open reading frame, flanked by both 3' and 5' nontranslated regions.
With over 3 decades of research, the mouse model of CVB-induced myocarditis is widely accepted as an appropriate animal model for human myocarditis (Woodruff, 1980). Inoculation with Coxsackievirus B3 (CVB3) induces a myocardial inflammatory infiltrate 10 to 14 days later (Leslie, 1989), consisting primarily of both CD4+ and CD8+ T-cells, a pattern similar to that seen in human cases of myocarditis (Woodruff, 1980; Woodruff and Woodruff, 1974). Although the response is initiated by the viral infection, the heart pathology is widely believed to be due to an immunopathological process. Evidence for an immunopathological basis for enterovirus-induced cardiomyopathy is provided by the lack of or diminished disease in CVB3-infected athymic (nude) mice and lack of disease in gamma-irradiated adult mice that were not reconstituted with T-cells (Woodruff and Woodruff, 1974). In addition, at the peak period of cardiac inflammation, virus is not generally detectable in mouse heart tissue (Woodruff, 1980).
Although the immune system clearly contributes to the pathology, it also performs a protective function. Strains of mice that can clear virus from the heart more rapidly and rapidly produce neutralizing antibody develop only a mild myocarditis. However, strains of mice that have delayed viral clearance and delayed production of neutralizing antibody develop severe myocarditis (Herskovitz et al., 1985). In addition, hearts from severe combined-immunodeficient (SCID) mice inoculated with CVB3 develop severe cardiac necrosis (Chow et al., 1992). These effects have been attributed to direct viral lysis of cardiac myocytes due to the absence of a functioning immune system to clear the virus.
To investigate the role Coxsackieviruses may play in the development of KD, this laboratory utilized its well-characterized murine model of CVB3-induced myocarditis to study, in collaboration with Orville Levander at the U.S. Department of Agriculture, how a specific nutritional deficiency affects the host's response to a virus (Beck et al., 1994a,c, 1995).
C3H/HeJ male mice immediately postweaning were fed either a diet adequate or deficient in Se. Following 4 weeks of feeding the diets, mice were bled and serum glutathione peroxidase levels were analyzed as a biomarker of Se status. Mice fed the Se-deficient diet had serum glutathione peroxidase levels significantly depressed when compared with mice fed the Se-adequate diet (4.7 +/- 0.2 milliunits/mg protein vs. 50.8 +/- milliunits/mg protein). Mice were then inoculated with either CVB3/20, a myocarditic strain of CVB3, or CVB3/0, an amyocarditic strain.
As shown in Figure 16-1, hearts from CVB3/20 (myocarditic strain)-infected mice that were fed a Se-deficient diet had increased pathology at an earlier time point than infected mice that were fed a Se-adequate diet (Beck et al., 1994c). In the deficient mice, myocarditic lesions were larger, were more necrotic, and had a greater number of calcium deposits. Of particular interest, as shown in Figure 16-2, CVB3/0 (amyocarditic strain)-infected mice that were fed a Se-deficient diet developed a moderate level of myocarditis, in contrast to CVB3/0-infected mice that were fed a Se-adequate diet, which did not develop any heart inflammation (Beck et al., 1994a). Thus, a dietary deficiency in Se permitted a viral phenotype change: CVB3/20 developed enhanced virulence, and CVB3/0 changed from an avirulent virus to a virulent one.
Because some of the individuals living in KD endemic areas were also of marginal vitamin E status, and because Se and vitamin E can act synergistically and spare one another's nutritional requirements, additional studies were performed in this laboratory. To mimic this situation, mice were fed diets deficient in vitamin E and adequate in Se prior to infection with CVB3. Hearts from mice fed vitamin E-deficient diets, but diets adequate in Se, had increased pathology when infected with CVB3/20. CVB3/0 infection, which is normally benign, could now cause disease in mice fed a Se-adequate, vitamin E-deficient diet (Beck et al., 1994b).
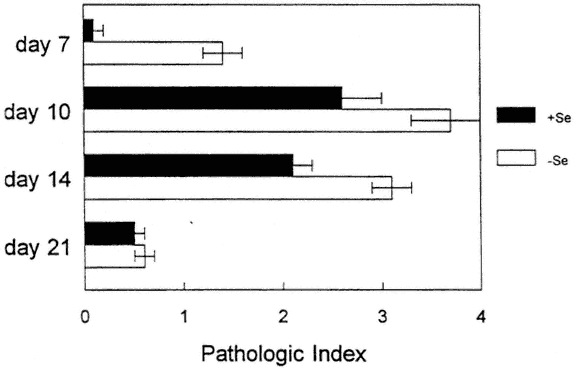
FIGURE 16-1
Histopathologic scores of CVB3/20 (myocarditic strain)-inoculated, selenium-adequate (+Se) or Se-deficient (-Se) mice at various times postinoculation. Scores: 0, no lesions; 1+, foci of mononuclear cell inflammation associated with myocardial cell reactive changes without myocardial cell necrosis; 2+, inflammatory foci clearly associated with myocardial cell reactive changes; 3+ - 4+, inflammatory foci clearly associated with myocardial cell necrosis and dystrophic calcification. Each bar represents the mean +/-SD of 10 mice.
FIGURE 16-2
Histopathologic scores of CVB3/0 (amyocarditic strain)-inoculated, selenium-adequate (+Se) or Se-deficient (-Se) mice at various times postinoculation. Scores as for Figure 16-1. Each bar represents the mean +/-SD of 10 mice.
Vitamin E also acts as an antioxidant, although by a very different mechanism from the antioxidant properties of Se. Thus, because either Se or vitamin E deficiency enhanced CVB3/20-induced myocarditis and allowed a normally benign CVB3/0 infection to become virulent, this author and colleagues proposed a common mechanism of oxidative stress.
Viral titers in various organs were examined to determine if viral replication patterns were altered as a result of replication in a nutritionally deficient animal. Altered patterns of replication may have been responsible for the increase in pathology seen in the deficient animals. As shown in Figure 16-3, cardiac virus titers were higher in the Se-deficient, CVB3/20-infected mice as compared with infected Se-adequate mice, although the kinetics of the response were identical. Thus, although the Se deficiency enhanced viral replication, reflected in higher titers, the replication time did not change, as virus was detected at identical time points in both deficient and adequate mice. As shown in Figure 16-4, for mice infected with CVB3/0, higher virus cardiac titers were also detected in deficient animals. However, in contrast to infection with CVB3/20, virus persisted for approximately 1 week longer in the deficient mice. Similar results were obtained with infected vitamin E-deficient mice (data not shown).
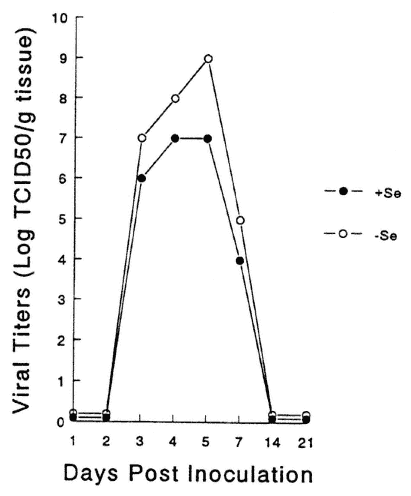
FIGURE 16-3
Heart virus titers from Se-adequate (+Se) or Se-deficient (-Se) mice infected with CVB3/20. Symbols represent the mean of 10 animals.
FIGURE 16-4
Heart virus titers from Se-adequate (+Se) or Se-deficient (-Se) mice infected with CVB3/0. Symbols represent the mean of 10 animals.
How does the increased oxidative stress affect either the host and/or the virus so that virulence is enhanced? Se and/or vitamin E deficiencies have a number of effects on immune function, including decreased proliferative responses to mitogen and reduced antibody production. For studies in this laboratory, several immune functions that are important in viral clearance mechanisms were examined: (1) B-cell function, measured by neutralizing antibody production, (2) T-cell proliferation against both antigen and mitogen, and (3) NK cell activity. In addition, mRNA was examined for IL-1, IL-6, and tumor necrosis factor-beta (TNF-β) in the hearts of deficient or adequate animals. All of these cytokines play critical roles in inflammatory processes.
No differences were found in neutralizing antibody titers between deficient and adequate mice (data not shown). However, spleen cell proliferative responses to both mitogen (Figure 16-5) and antigen (Figure 16-6) were decreased in Se-deficient mice when compared with Se-adequate mice. Mitogen responses were found to be much more depressed than antigen-specific responses. NK responses (data not shown) were also examined, as NK has been shown to be important in CVB3 clearance from normal animals (Gauntt et al., 1988). NK levels were only slightly depressed in Se-deficient mice, and this difference was not statistically significant.
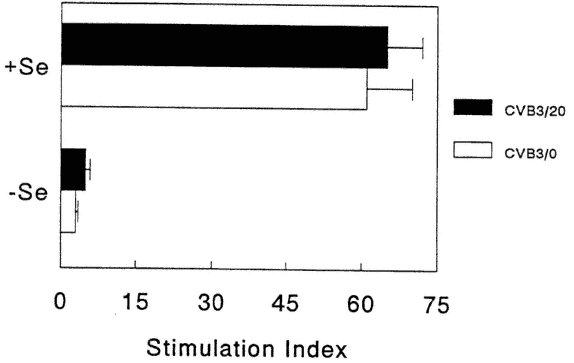
FIGURE 16-5
Mitogen-stimulated, spleen cell proliferative responses from Se-adequate (+Se) or Se-deficient (-Se) mice. Data expressed as stimulation indices calculated as ratio of counts per minute (cpm) in presence of mitogen over cpm in presence of medium (background). Each bar represents the mean +/-SD of 15 animals.
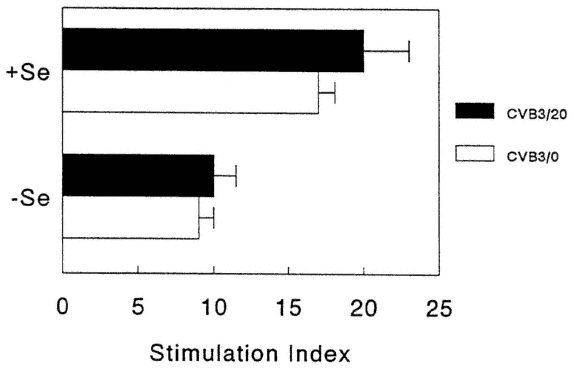
FIGURE 16-6
CVB3-antigen-specific-stimulated, spleen cell proliferative responses from Se-adequate (+Se) or Se-deficient (-Se) mice. Data expressed as stimulation indices calculated as ratio of counts per minute (cpm) in presence of antigen over cpm in presence of HeLa cell membranes (background). Each bar represents the mean +/-SD of 15 animals.
To examine cytokine production, heart tissue was isolated from CVB3/20-infected mice that were fed either Se-adequate or Se-deficient diets at various times post inoculation. Using reverse transcriptase-polymerase chain reaction (RT-PCR), PCR fragments were identified for IL-1, IL-6 and TNF-β. The fragments were normalized with α-tubulin and scanned using a laser densitometer. Figure 16-7 demonstrates the levels of mRNA for each sample isolated by measuring the area under the curve (AU) for each PCR fragment. As shown in Figure 16-7, mRNA levels for TNF-β and IL-6 are decreased in infected mice fed the Se-deficient diet as compared with mice fed the adequate diets. However, IL-1 levels were fairly similar between groups.
Levels of mRNA were also looked at for INF-γ in cultured spleen cells from infected mice with and without mitogen stimulation (10 days post-CVB3/20 inoculation, 48 hours in culture). As shown in Figure 16-8, INF-γ was produced by stimulated cells in both Se-deficient and Se-adequate mice, although no mRNA was found in the unstimulated cultures. Although exposure to mitogen could induce stimulation of INF-γ mRNA in cells from Se-deficient mice, it is not known if antigen stimulation of cells from CVB3-infected, Se-deficient mice can also stimulate mRNA for INF-γ. Thus, although neutralizing antibody and NK levels are unaffected, or slightly affected, under these conditions of nutriture, proliferation levels and mRNA for IL-6 and TNF-β are depressed in Se-deficient mice, which indicates that some immune dysfunction has occurred.
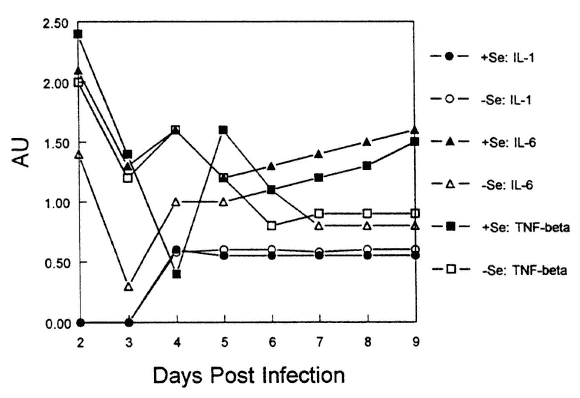
FIGURE 16-7
mRNA levels of cytokines in the hearts of Se-adequate or Se-deficient mice following infection with CVB3/20.
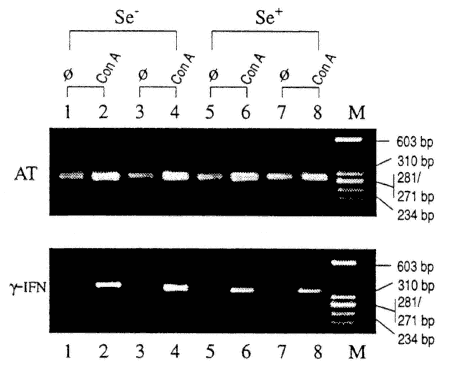
FIGURE 16-8
Agarose gel demonstrating cDNA fragments from RT-PCR of cultured spleen cells from four individual mice (two Se-adequate and two Se-deficient) with and without mitogen (Con A) stimulation. ϕ, control cultures with no added stimulation; Con A, concanavalin A; M, molecular weight markers (sizes noted on figure); AT, α-tubulin; γ-IFN, gamma-interferon.
These results may indicate a suppression of Th1 cells preferentially to Th2 cells. Th2 cells are necessary for providing B-cell help, secreting cytokines such as IL-4, IL-5, and IL-10. Th1 cells produce IL-2 and INF-γ. Because antibody responses were intact (suggesting that Th2 cells were functioning) and proliferation levels were decreased (suggesting a lack of IL-2 protein), the nutritional deficit may have affected Th1 activity.
The increased pathology seen in nutritionally deficient mice may have been due to changes in the host that allowed for the virus to cause increased damage, such as a decreased immune response or changes in heart cell physiology. A second possibility is that the virus itself changed as a consequence of replication in a Se-deficient host. To determine if this second possibility had occurred, virus obtained from Se- and vitamin E-deficient donors was passaged back into Se- or vitamin E-adequate recipients. The experiment was performed as follows: at 10 days postinoculation with either CVB3/20 or CVB3/0, hearts from mice that were fed either a Se-deficient or Se-adequate diet or a vitamin E-deficient or vitamin E-adequate diet were processed for virus isolation. The viruses recovered from the hearts were passaged onto HeLa cell monolayers, isolated, and titered. These viruses were then renamed to reflect the host from which they had been isolated. For example, CVB3/20 virus, which was isolated from a Se-adequate host, was designated CVB3/20Se+. Similarly, virus isolated from a Se-deficient host was designated CVB3/20Se-. The HeLa-passaged virus was then
inoculated intraperitoneally at 1 × 105 TCID50 in 100 μl medium into 7-weekold, male, C3H/HeJ mice fed a vitamin E- and Se-adequate diet. At 7 and 10 days postinoculation, mice were killed and their hearts examined for pathology and heart viral titers.
This laboratory was able to demonstrate that viruses from either Se- or vitamin E-deficient mice underwent a phenotypic change in the deficient animal, such that passage into a Se- and vitamin E-supplemented recipient also caused increased disease (Beck et al., 1994a, b, c). Not only did the Se deficiency enhance the virulence of the CVB3/20 myocarditic virus, the Se deficiency was also able to change the phenotype of the amyocarditic CVB3/0 virus from benign to virulent so that virus now caused damage even in a nutritionally adequate host (Beck et al., 1994c).
Was the phenotype change due to a change in virus genotype? To answer this question, four separate virus isolates obtained from CVB3/0-infected, Se-deficient mice and four separate isolates from CVB3/0-infected, Se-adequate mice were sequenced. The input virus was also sequenced for comparison (CVB3/0 is a cloned and sequenced virus). As shown in Table 16-1, it was found that six nucleotides in the viral genome had mutated in the virus recovered from the Se-deficient host. No virus mutations were found in the
TABLE 16-1 Nucleotide and Corresponding Amino Acid Differences between the Avirulent CVB3/0 and the Virulent CVB3/0Se– and Comparison with CVB3/20 (Virulent) Strain
|
|
CVB3 Strain |
|
||
|
Nucleotide Number (5'-3') |
CVB3/20* |
CVB3/0* |
CVB3/0Se–† |
Amino Acid Change |
|
234 |
T |
C |
T |
Nontranslated region |
|
788 |
A |
G |
A |
Arg |
|
2271 |
T |
A |
T |
Phe |
|
2438 |
C |
G |
C |
Gln |
|
2690 |
A |
G |
G |
None |
|
3324 |
T |
C |
T |
Val |
|
7334 |
T |
C |
T |
Nontranslated region |
|
* CVB3/20 and CVB3/0 are cloned and sequenced strains of CVB3. The CVB3/0 preparation used to inoculate both Se-deficient and Se-adequate mice was sequenced. † CVB3/0Se– virus was isolated from the heart of a Se-deficient mouse inoculated with CVB3/0. The same pattern of nucleotide changes was identified in three other virus isolates. These viruses are myocarditic when inoculated into Se-adequate mice. |
||||
isolates from the Se-adequate mice. Notably, all six nucleotides were identical to nucleotides found in the virulent CVB3/20 virus. Interestingly, one nucleotide, nt 2690, in the avirulent strain common to the cardiovirulent strain did not mutate. Thus, the CVB3/0Se-viruses were identical to each other and represented a hybrid between a known virulent strain and the avirulent input strain. To this author's knowledge, this is the first description of a specific host nutritional deficiency permitting an avirulent virus to develop virulence due to changes in the viral genotype (Beck et al., 1995).
Recently, this laboratory isolated virus from CVB3/0-infected, vitamin E-deficient mice. Identical nucleotide changes occurred in these viruses as well, suggesting that a common mechanism of oxidative stress leads to predictable nucleotide changes in the CVB3/0 genome, changing an avirulent virus to a virulent one (Beck et al., 1996).
Thus, the change in genotype in virus isolated from either Se- or vitamin E-deficient mice may be related to the virus' enhanced ability to replicate in deficient hosts. The most common causes of mutation in proliferating viruses are thought to be errors in polymerase transcription and endogenous damage to nucleic acid bases. Coxsackievirus is an RNA virus and therefore lacks the proofreading capability that is a feature of DNA replication. Because of the lack of RNA replicases with proofreading ability, RNA viruses are susceptible to high mutation rates. The high mutation rate of RNA viruses results in the continuous generation of virus mutants, producing a dynamic and changing viral population. Thus, any increase in viral replication may be expected to enhance the evolution of new viral mutants.
Two possibilities may account for the increased viral titers in the deficient mice. Virus may have escaped from normal immune clearance mechanisms, thus allowing a higher and more persistent degree of replication. A second alternative (although not mutually exclusive) is that cardiac cell membranes may have been compromised such that virus was able to replicate to higher titers in oxidatively stressed cardiac cells versus normal cardiac cells.
Another possible explanation for the change in viral genotype may be that the oxidative stress status of the host directly affected the virus. The reactive oxygen species (ROS) generated due to the nutritional deficiency may have damaged the viral RNA, which is similar to what occurs when ROS damages DNA. This damage may have resulted in the mutations. This possibility is currently being explored in this laboratory.
Thus, the work with Coxsackievirus and host Se deficiency demonstrates that not only can the nutritional deficiency affect the host, but effects on the pathogen must be considered as well. This work demonstrates that once the benign virus has mutated, as a result of replication in a nutritionally deficient host, it can now infect and cause disease even in mice of normal nutriture. The work has implications for troops deployed in the field. It may be possible that if a few soldiers are oxidatively stressed, as a result of a nutritional deficiency or severe physical activity, a viral pathogen could mutate into a potentially more
virulent pathogen and infect other soldiers who may or may not be oxidatively stressed. It would seem prudent to ensure that any food products provided for the troops contain a balance of nutrients to limit oxidative stress.
Author's Summary and Conclusions
Clearly, trace elements have been shown to be important for immune system functioning. A deficiency in Cu, Zn, or Se has immunosuppressive effects on the host, which can lead to increased susceptibility to infectious disease. The usual model for understanding the effect of nutrition on infectious disease is diagrammed as follows:
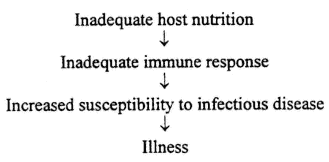
However, this author's work with Se and CVB3 demonstrates that host nutrition can affect not only the host but the pathogen as well. Therefore, the following model is proposed:

This model takes into account not only the effect that host nutriture has on the immune response but also the effect of host nutriture on the viral pathogen. This model more accurately represents the relationship between nutrition and infectious disease.
A number of studies, many of which have been cited in this paper, have examined the effect of trace elements on immune function. These studies are important, in that they indicate which immune parameters are sensitive to these nutrients. However, these studies are limited in that most do not correlate immune dysfunction with an actual increase in illness. For example, if a mild deficiency in Zn decreases T-cell proliferative responses by 20 percent, is this enough to increase susceptibility to infectious disease? It is entirely possible that some of the immune changes induced by nutritional status will not result in increased incidence or duration of illness. The immune system has many redundant pathways, and measurements of one particular aspect of this system
may not provide a true picture of the host's ability to respond to infection. The immune parameters that are examined need to be correlated with incidence and/or severity of infectious disease.
This author recommends that any food supplement tested in military personnel for its effect on immune function should also be ranked for protection against illness. Effects can be self-reported (although this is the least reliable measure) or medical personnel can record and monitor symptoms. Additionally, sampling of throat or nasal washes for common respiratory viruses could be obtained. Thus, a more ''real world'' indication of immune function—the ability to resist infection—can be determined.
References
Bai, J., S. Wu, K. Ge, X. Deng, and C. Su. 1980. The combined effect of selenium deficiency and viral infection on the myocardium of mice. Acta Acad. Med. Sci. Sin. 2:29-31.
Beck, M.A., B.R. Blakly, and D.L. Hamilton. 1987. The effect of copper deficiency on the immune response in mice. Drug Nutr. Interact. 5:103-111.
Beck, M.A., P.C. Kolbeck, L.H. Rohr, Q. Shi, V.C. Morris, and O.A. Levander. 1994a. Benign human enterovirus becomes virulent in selenium-deficient mice. J. Med. Virol. 43:166-170.
Beck, M.A., P.C. Kolbeck, L.H. Rohr, Q. Shi, V.C. Morris, and O.A. Levander. 1994b. Vitamin E deficiency intensifies the myocardial injury of Coxsackievirus B3 infection of mice. J. Nutr. 124:345-358.
Beck, M.A., P.C. Kolbeck, Q. Shi, L.H. Rohr, V.C. Morris, and O.A. Levander. 1994c. Increased virulence of a human enterovirus (Coxsackievirus B3) in selenium-deficient mice. J. Infect. Dis. 170:351-357.
Beck, M.A., Q. Shi, V.C. Morris, and O.A. Levander. 1995. Rapid genomic evolution of a non-virulent Coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat. Med. 1:433-436.
Beck, M.A., Q. Shi, V.C. Morris, and O.A. Levander. 1996. From avirulent to virulent: Vitamin E deficiency in mice drives rapid genomic evolution of a CVB3 virus [abstract]. FASEB J. 10(3):A191.
Beckman, K.B., and B.N. Ames. 1997. Oxidative decay of DNA. J. Biol. Chem. 272:19633-19636.
Bogden, J.D., J.M. Oleske, M.A. Lavenhar, E.M. Munves, P.W. Kemp, K.S. Bruening, K.J. Holding, T.N. Denny, M.A. Guarino, L.M. Krieger, and B.K. Holland. 1988. Zinc and immunocompetence in elderly people: Effects of zinc supplementation for 3 months. Am. J. Clin. Nutr. 48:655-663.
Burch, R.E., R.V. Williams, H.K.J. Hahn, M.M. Jetton, and J.F. Sullivan. 1975. Serum and tissue enzyme activity and trace element content in response to zinc deficiency in the pig. Clin. Chem. 21:568-577.
Chandra, S., and R.K. Chandra. 1986. Nutrition, immune response, and outcome. Prog. Food Nutr. Sci. 10:1-7.
Chaudiere, J., E.C. Wilhelmsen, and A.L. Tappel. 1984. Mechanism of selenium-glutathione peroxidase and its inhibition by mercaptocaroxylic acids and other mercaptans. J. Biol. Chem. 259:1043-1050.
Chow, L.H., K.W. Beisel, and B.M. McManus. 1992. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab. Invest. 66:24-31.
Davies, K.J., and A.L. Goldberg. 1987. Proteins damaged by oxygen radicals are rapidly degraded in extracts of red blood cells. J. Biol. Chem. 262:8227-8231.
Davis, M.A., W.T. Johnson, M. Briske-Anderson, and T.R. Kramer. 1987. Lymphoid cell functions during copper deficiency. Nutr. Res. 7:211-222.
Dhur, A., P. Galan, and S. Hercberg. 1990. Relationship between selenium, immunity, and resistance against infection . Comp. Biochem. Physiol. 96C:271-280.
Driessen, C., K. Hirv, H. Kirchner, and L. Rink. 1995. Zinc regulates cytokine induction by superantigens and lipopolysaccharide. Immunology 84:272-277.
Elliott, S.J., and W.P. Schilling. 1992. Oxidant stress alters Na+ pump and Na+K+Cl-cotransporter activities in vascular endothelial cells. Am. J. Physiol. 263:H96-H102.
Evans, G.W., C.I. Grace, and C. Han. 1974. The effect of copper and cadmium on 65Zn absorption in zinc-deficient and zinc-supplemented rats. Bioinorg. Chem. 3:115-120.
Failla, M.L., U. Babu, and K.E. Seidel. 1988. Use of immunoresponsiveness to demonstrate that the dietary requirement for copper in young rats is greater with dietary fructose than dietary starch. J. Nutr. 118:487-496.
Farber, J.M., and R.L. Levine. 1982. Oxidative modification of the glutamine synthetase of E. coli enhances its susceptibility to proteolysis [abstract 3482]. Fed. Proc. 41:865.
Fernandes, G., M. Nair, K. Onoe, T. Tanaka, R. Gloyd, and R.A. Good. 1979. Impairment of cell-mediated functions by dietary zinc deficiency in mice. Proc. Natl. Acad. Sci. USA 76:457-461.
Fraker, P.J., P. DePasqual-Jardieu, C.M. Zwick, and R.W. Luecke. 1978. Regeneration of T-cell helper function in zinc-deficient adult mice. Proc. Natl. Acad. Sci. USA 75:5660-5664.
Fraker, P.J., M.E. Gershwin, R.A. Good, and A. Prasad. 1986. Interrelationships between zinc and immune function. Fed. Proc. 45:1474-1479.
Fuster, V., B.J. Gersh, E.R. Giuliani, A.J. Tajik, R.O. Brandenburg, and R.L. Frye. 1981. The natural history of idiopathic dilated cardiomyopathy. Am. J. Cardiol. 47:525-531.
Gauntt, C.J., E.K. Godeny, and C.W. Lutton. 1988. Host factors regulating viral clearance. Path. Immunol. Res. 7:251-265.
Ge, K.Y., J. Bai, X.J. Deng, S.Q. Wu, S.Q. Wang, A.N. Xue, and C.Q. Su. 1987. The protective effect of selenium against viral myocarditis in mice. Pp. 761-768 in Selenium in Biology and Medicine Part B, G.F. Combs, O.A. Levander, J.J. Spallholz, and J.E. Oldfield, eds. New York: Van Nostrand/Reinhold.
Gu, B.Q. 1983. Pathology of Keshan disease: A comprehensive review. Chinese Med. J. 96:251-261.
Henkin, R.I., and R.L. Aamodt. 1983. A redefinition of zinc deficiency. Pp. 83-105 in Nutritional Bioavailability of Zinc, G.E. Inglett, ed. Washington, D.C.: American Chemical Society.
Herskovitz, A., K. W. Beisel, L.J. Wolfgram, and N.R. Rose. 1985. Coxsackievirus B3 murine myocarditis: Wide pathologic spectrum in genetically defined inbred strains. Human Pathol. 16:671-673.
Jones, D.G. 1984. Effects of dietary copper depletion on acute and delayed inflammatory responses in mice. Res. Vet. Sci. 37:205-210.
Kelley, D.S., P.A. Daudu, P.C. Taylor, B.E. Mackeuy, and J.R. Turnland, 1995. Effects of low-copper diets on human immune response. Am. J. Clin. Nutr. 62:412-416.
Koller, L.D., S.A. Mulhern, N.C. Frankel, M.G. Steven, and J.R. Williams. 1987. Immune dysfunction in rats fed a diet deficient in copper. Am. J. Clin. Nutr. 45:997-1006.
Kruse-Jarres, J.D. 1989. The significance of zinc for humoral and cellular immunity. J. Trace Elem. Electrolytes Health Dis. 3:1-8.
Larsen, H.S. 1988. Effects of selenium on sheep lymphocyte responses to mitogens. Res. Vet. Sci. 45:11-18.
Larsen, J.H., and S. Tollersrund. 1981. Effects of dietary vitamin E and selenium on phytohemogglutinea response and pig lymphocytes. Res. Vet. Sci. 31:301-305.
Leslie, K. 1989. Clinical and experimental aspects of viral myocarditis. Clin. Microbiol. Rev. 2:191-197.
Li, G., F. Wang, D. Kang, and C. Li. 1985. Keshan disease: An endemic cardiomyopathy in China. Hum. Pathol. 16:602-609.
Lukasewycz, O.A., and J.R. Prohaska. 1983. Lymphocytes from copper-deficient mice exhibit decreased mitogen reactivity. Nutr. Res. 3:335-341.
Lukasewycz, O.A., and J.R. Prohaska. 1985. Alterations in lymphocyte subpopulations in copper-deficient mice. Infect. Immun. 48:644-647.
Moses, H.A., and H.E. Parker. 1964. Influence of dietary zinc and age on the mineral content of rat tissues. Fed. Proc. 23:333-343.
Newberne, P.M., C.E. Hunt, and V.R. Young. 1968. The role of diet and reticuloendothelial system in the response of rats to Salmonella typhimurium infection. Br. J. Exp. Pathol. 49:448-457.
O'Connell, J., and J. Robinson. 1985. Coxsackie viral myocarditis. Postgrad. Med. J. 61:1127-1131.
Okmole, T.A., and O.A. Onawunmi. 1979. Effect of copper on growth and serum constituents of immunized and nonimmunized rabbits infected with Trypanosoma brucei. Ann. Parasitol. 54:495-506.
Petering, H.G., M.A. Johnson, and J.P. Horwitz. 1971. Studies of zinc metabolism in the rat. Arch. Environ. Health 23:93-101.
Prasad, A.S., D. Oberleas, P. Wolf, J.P. Horwitz, E.R. Miller, and R.W. Luecke. 1969. Changes in trace elements and enzyme activities in tissues of zinc-deficient pigs. Am. J. Clin. Nutr. 22:628-637.
Prohaska, J.R., and M.L. Failla. 1993. Copper and immunity. Pp. 309-332 in Nutrition and Immunology, D.M. Klurfeld, ed. New York: Plenum Press.
Prohaska, J.R., and O.A. Lukasewycz. 1981. Copper deficiency suppresses the immune response of mice. Science 213:559-561.
Prohaska, J.R., and O.A. Lukasewycz. 1989. Biochemical and immunological changes in mice following postweaning copper deficiency. Biol. Trace Elem. Res. 22:101-112.
Prohaska, J.R., and O.A. Lukasewycz. 1990. Effects of copper deficiency on the immune system. Adv. Exp. Med. Biol. 262:123-143.
Reffett, J.K., J.W. Spears, and T.T. Brown Jr. 1988. Effect of dietary selenium and vitamin E on the primary and secondary immune response in lambs challenged with parainfluenza 3 virus. J. Anim. Sci. 66:1520-1528.
Rivett, A.J. 1985. The effect of mixed-function oxidation of enzymes on their susceptibility to degradation by a nonlysosomal cysteine proteinase. Arch. Biochem. Biophys. 243:624-632.
Schlesinger, L., M. Arevalo, S. Arredondo, B. Lönnerdal, and A. Stekel. 1993. Zinc supplementation impairs monocyte function. Acta Paediatr. 82:734-738.
Sheffy, B.E., and R.D. Schultz. 1979. Influence of vitamin E and selenium on immune response mechanisms. Fed. Proc. 38:2139-2143.
Singh, A., M.L. Failla, and P.A. Deuster. 1994. Exercise-induced changes in immune function: Effects of zinc supplementation. J. Appl. Physiol. 76:2298-2303.
Stabel, J.R., and J.W. Spears. 1993. Role of selenium in immune responsiveness and disease resistance. Pp. 331-356 in Human Nutrition: A Comprehensive Treatise , D.M. Klureld, ed. New York: Plenum Press.
Stabel, J.R., J.W. Spears, and T.T. Brown Jr. 1993. Effect of copper deficiency on tissue, blood characteristics, and immune functions of calves challenged with infectious bovine Rhinotracheitis virus and Pasteruella hemolytica. J. Anim. Sci. 71:1247-1255.
Stadtman, E.R. 1992. Protein oxidation and aging. Science 257:1220-1224.
Su, C., C. Gong, J. Li, L. Chen, D. Zhou, and Q. Jin. 1979. Preliminary results of viral etiology of Keshan disease. Chin. J. Med. 59:466-472.
USDA (U.S. Department of Agriculture Nutrition Monitoring in the United States). 1986. A progress report from the Joint Nutrition Monitoring Evaluation Committee. DHHS publ. no. 1255. Hyattsville, Md.: USDA.
Vyas, D., and R.K. Chandra. 1983. Thymic factor activity, lymphocyte stimulation response and antibody producing cells in copper deficiency. Nutr. Res. 3:343-349.
Walsh, C.T., H.H. Sandstead, A.S. Prasad, P.M. Newberne, and P.J. Fraker. 1994. Zinc: Health effects and research priorities for the 1990s. Environ. Health Perspect. 102:5-46.
Woodruff, J.F. 1980. Viral myocarditis: A review. Am. J. Pathol. 101:427-482.
Woodruff, J.F., and J.J. Woodruff. 1974. Involvement of T-lymphocytes in the pathogenesis of Coxsackievirus B3 heart disease. J. Immunol. 113:17-26.
Discussion
RONALD SHIPPEE: Maybe you mentioned this, and I missed it. When you are depleting selenium in mice, is there a food intake problem?
MELINDA BECK: No, but we did weigh the mice because we were worried about that.
RONALD SHIPPEE: You don't have to worry about feeding or anything like that?
MELINDA BECK: No.
GERALD KEUSCH: In the selenium-deficient model, in your initial experiments, did you refeed selenium-deficient animals at some time during the course of the infection and attenuate it?
MELINDA BECK: No, we haven't looked at that yet. That is one of those things that we are interested in doing.
GERALD KEUSCH: The second part of your presentation was instructive, and you had four separate isolates that you sequenced, and is it my understanding that all of them had undergone all of those changes?
MELINDA BECK: Right.
GERALD KEUSCH: So, you got multiple point mutations occurring?
MELINDA BECK: Right. What we think is happening is that the RNA viruses have a high mutation rate because they don't have repair mechanisms. So, when they replicate, if they make a mistake, they cannot fix it, and what I think is happening is we are driving the evolution of the virus by causing the immune deficiency.
So, the viruses replicate fast and they replicate to higher titers, which increases the chances for the mutations to occur, and because of the way we are doing the experiment, we are looking at the time of peak pathology and then taking those viruses out and sequencing them.
So, we are seeing the winners of the race, and, I think if we looked earlier, we might see fewer point mutations. If we look at day 3, for example, you might see three changes and then maybe at day 6 you see four changes, and by the time when we are looking, it is like, boom, here is the maximum number of changes that the virus can tolerate.
GERALD KEUSCH: Are you certain that the inoculum itself was not mixed inoculum, and you selected rather than . . .
MELINDA BECK: Right, well, I mean with RNA viruses, you have a cloned virus, and a clone is never a clone when you are working with RNA viruses, because once I replicate it in cell culture, you are already introducing mutations.
What we are seeing is the consensus sequence of the dominant population. Nobody could ever say that what goes in is all the same sequence. So, you cannot do that.
GERALD KEUSCH: It is conceivable that it could be a selection of preexisting . . .
MELINDA BECK: Right.
LEONARD KAPCALA: Would you speculate on how this may have any application or relevance to HIV?
MELINDA BECK: Yes, I think it has a lot of relevance to HIV because you know, you have a lot of quasi-species in a single individual with HIV, and I
think that nutrition can be one mechanism for driving those changes. So, I think that is a strong possibility, and clearly people—I mean I am not an HIV expert, and you probably know better than I do—but I mean towards the end you see a lot of malnutrition and wasting-type syndromes in those people with HIV, and I think that can contribute to what we are seeing with the Coxsackievirus.
DAVID NIEMAN: I know this is an aside from what you are doing, but I think it is of importance to the military, especially the first couple of slides you showed, which is when animals are infected with the Coxsackievirus, depending on the strain, it can lead to infection of the heart and to mortality.
I know that there are some case reports in humans. In fact, there is one paper published on Air Force recruits in basic training where there were a number of deaths, and there was speculation in the paper that some of these—I think this was by Dr. Philips—may have been from infected recruits who exercised too heavily when they were infected.
Now, it is difficult for an individual to know if they are infected with the Coxsackievirus versus some other type of virus, but nonetheless, there are some cardiologists who are quite conservative on this and do recommend that there be no exercise at all during various respiratory types of infections, and I thought maybe you could give your opinion on this and maybe some of the military men in this room can talk to this because I doubt that when a ranger gets infected that they are allowed to rest. I think that that should be policy.
MELINDA BECK: I think you are right. I think that is a concern, and usually the types of instances where you see sudden death sorts of things other than anatomic problems with the heart is often when somebody has been exercising.
So, they are infected. They go out and they do strenuous exercising and they die, and like you said, it is difficult to know when do you have a rhinovirus and when is it a Coxsackievirus, and you shouldn't be out running around, and I mean I think it is a difficult problem.
I think the impression I get with the people in the Ranger course is if they get a sniffle or something they are certainly not going to go and complain about it. They are going to keep going.
DAVID NIEMAN: May I add to that, again, because I am sure you can answer this as well? I have a colleague at Ball State University who is the only researcher I know in the world who does this. They have rhinovirus number 16. As you know, there are more than 100 of them, but 16 is a moderate rhinovirus, and they spray this into the noses of the subjects whom they pay to do this, and then they exercise them to maximal exertion and look at whether or not the symptoms get worse, number 1, and number 2, whether or not their performance is affected, and they have found that there is no effect of a rhinovirus.
Now, my understanding though is Coxsackieviruses often feel like a bad cold, and so, rhinoviruses cause 40 percent of the common cold, and then there is a host of other viruses that cause similar symptoms.
So, the problem is going to be whether it is a benign virus like the rhinovirus or something more potent.
MELINDA BECK: I think even with Coxsackievirus—not every Coxsackievirus obviously is going to go on to infect the heart because there are many infections that people get and it doesn't go to the heart. So, I mean part of our research emphasis is to try to understand, you know, is it a difference in the virus or a difference in the person? Why person A when he gets a Coxsackievirus infection ends up with myocarditis and person B just gets a cold and it is fine?
RONALD SHIPPEE: As a nutritionist, this is fascinating. As someone who is working with the guys I am working with, it is scary, but you know, it even goes further because you cannot be sitting in a Ranger school in camp. You can have in 65 days, 72 hours in the clinic and that clock starts at day 1. So, if you go in for 4 or 5 hours of sick call, that is against your 72 hours. So, you are right, and these guys, there are notorious stories of guys walking around and finishing the course with broken toes, broken fingers, and they do. They suppress a lot, and this is fascinating.
DAVID NIEMAN: Look at Reggie Lewis(?).
RONALD SHIPPEE: Absolutely.
DAVID NIEMAN: It appears that he died from myocarditis, and he probably exercised too hard when he was infected.
RONALD SHIPPEE: Right, and you know, when Pål [Wiik] presented last night and this issue came up with his guys—they are going to finish this probably—but our guys, this is a career school, and if you are going to become a career officer in combat these days you are going to have a Ranger tab or Special Forces. So, when we go in to work with these folks and we do supplementation, we are messing around with their careers. So, that is the motivation there. This is fascinating.
G. RICHARD JANSEN: Is the converse true or have you tried the converse where the virulent strain could be transformed into a nonvirulent strain by passing through a selenium-adequate host?
MELINDA BECK: We have done the experiment where we pass it into a deficient host and made it like a super virus, and we haven't analyzed those sequences yet.
If you do multiple passages of . . . We have done multiple passages, well, I haven't but the lab I came from has done multiple passages of both the 20 and the 0, and we have never seen any changes in the nucleotide.
So, just passing through adequate animals either doesn't attenuate or they don't acquire virulence either way. So, just normal passage doesn't seem to do it.
G. RICHARD JANSEN: I am talking about the virulent strain.
MELINDA BECK: Right, but if you do multiple passages of the 20, the virulent strain into normal animals, you don't see any attenuation.























