17
Exercise, Infection, and Immunity: Practical Applications
David C. Nieman1
Introduction
Although publications on the topic of exercise immunology date from early in the twentieth century, not until the 1980s did a large number of investigators, worldwide, begin to dedicate their effort to this research area. Modern-day interest in the immunology of exercise coincided with a brief review article published in the Journal of the American Medical Association in 1984 (Simon). In this report, Simon urged that ''there is no clear experimental or clinical evidence that exercise will alter the frequency or severity of human infections'' (p. 2737). This was the same opinion registered more than 50 years earlier by Baetjer (1932), who complained that comparatively little experimental work had been done to test the relationship between exercise and infection.
During the past decade, a plethora of worldwide research has greatly increased understanding of the relationship among exercise, the immune system, and host protection. Although much more investigation is needed, enough high-quality exercise immunology data exist to provide athletes, military recruits, and
the general population with preliminary practical guidelines in the areas of exercise prescription, respiratory infection, aging, and athletic endeavor.
Exercise Prescription and the Immune Response to Acute Exercise Bouts
From early in this century, it has been regularly reported that during recovery from high-intensity, cardiorespiratory exercise, subjects experience a sustained neutrophilia and lymphocytopenia (Garrey and Bryan, 1935). Of all immune cells, natural killer (NK) cells, neutrophils, and macrophages (of the innate immune system2) appear to be most responsive to the effects of acute exercise, both in terms of numbers and function (Gabriel et al., 1992; Nieman and Nehlsen-Cannarella, 1994; Pyne, 1994). The longer and more intense the exercise bout (e.g., marathon race competition), the greater and more prolonged the response, with moderate exercise bouts (>60% maximal aerobic power and >60 minutes duration) evoking little change from resting levels (Nieman et al., 1989, 1991, 1993b, 1994).
Mechanisms Behind the Acute Immune Response to Exercise
Many mechanisms appear to be involved in the acute immune response to exercise, including exercise-induced changes in stress hormone and cytokine concentrations, body temperature changes, increases in blood flow, and dehydration (Brenner et al., 1995; Cupps and Fauci, 1982; Pedersen and Ullum, 1994).
Following prolonged running at high intensity, serum cortisol concentrations are significantly elevated above control levels for several hours (Nieman et al., 1995a) (Figure 17-1). Cortisol has been related to many of the immunosuppressive changes experienced during recovery (Cupps and Fauci, 1982). Glucocorticoids administered in vivo have been reported to cause neutrophilia, eosinopenia, lymphocytopenia, and a suppression of both NK and T-cell function, all of which occur during recovery from prolonged, high-intensity, cardiorespiratory exercise. Figure 17-2 demonstrates that a significant correlation exists between the change in serum cortisol and the change in the neutrophil/lymphocyte ratio following 2.5 to 3 hours of running (Nieman et al., 1995d). The neutrophil/lymphocyte ratio, which rises strongly after heavy, prolonged exertion, has been proposed as an excellent index of the physiologic stress on the immune system (Linden et al., 1991).
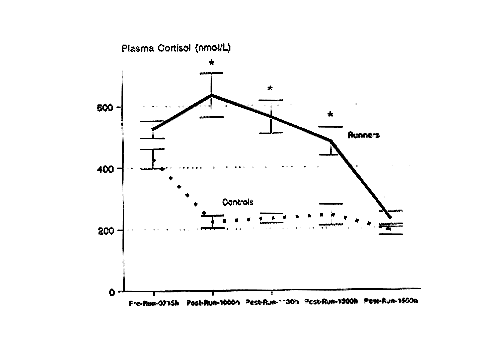
FIGURE 17-1
Serum cortisol response to 2.5 hours of running at approximately 75 percent ![]() O2bmax in marathon runners compared with values obtained from resting, sedentary controls. The pattern of change was significantly different between groups (F[4,27] = 9.39, P < 0.001). * P < 0.0125, between groups at given time point.
O2bmax in marathon runners compared with values obtained from resting, sedentary controls. The pattern of change was significantly different between groups (F[4,27] = 9.39, P < 0.001). * P < 0.0125, between groups at given time point.
SOURCE: Data from Nieman et al. (1995a).
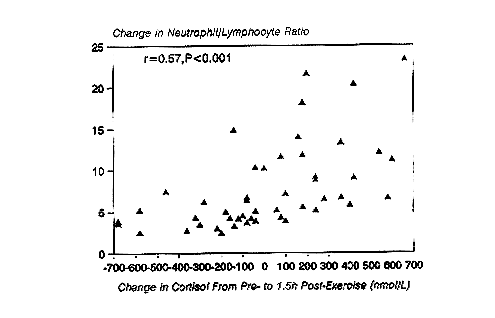
FIGURE 17-2
Correlation between change in cortisol and the neutrophil/lymphocyte ratio from pre- to 1.5-h postexercise in 50 marathon runners who ran 2.5 to 3 hours at 75.9 ± 0.9 percent ![]() O2max.
O2max.
SOURCE: Data taken from Nieman et al. (1995d) and unpublished data from author's laboratory.
The following have all been reported to be suppressed for at least several hours during recovery from prolonged, intense endurance exercise: NK cell activity (Mackinnon et al., 1988; Nieman et al., 1993b, 1995a; Shinkai et al., 1993) (Figure 17-3), mitogen-induced lymphocyte proliferation (Eskola et al., 1978; Nieman et al., 1995d), upper airway neutrophil phagocytosis and blood neutrophil oxidative burst (Macha et al., 1990; Müns, 1993), and salivary IgA concentration (Mackinnon and Hooper, 1994; Mackinnon et al., 1987; Tomasi et al., 1982).
During this "window of decreased host protection," viruses and bacteria may gain a foothold, increasing the risk of subclinical and clinical infection (Pedersen and Bruunsgaard, 1995). This may be especially apparent when the athlete goes through repeated cycles of heavy exertion (Pyne, 1994).
Taken together, these data suggest that the immune system is suppressed and stressed following prolonged endurance exercise, which decreases host protection. Hoffman-Goetz and Pedersen (1994) have proposed that the immunological responses to acute exercise can be viewed as a subset of stress immunology. Other physical and mental stressors such as space travel (Barger et al., 1995), thermal and traumatic injury, surgery, acute myocardial infarction, and hemorrhagic shock have all been associated with immunosuppression (Pedersen et al., 1994). Prolonged mental stress and anxiety have been associated with immunosuppression and increased risk of infection (Cohen et al., 1991). Thus, it makes sense that physical exercise when performed at stressful levels may be related to the same outcomes.
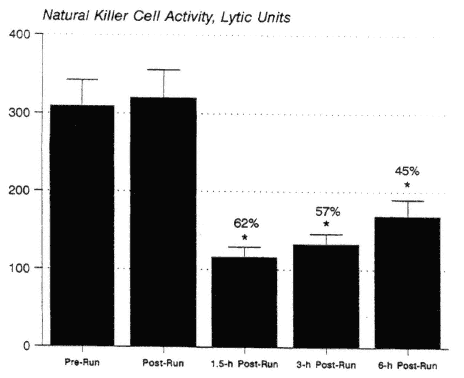
FIGURE 17-3
The pattern of change in natural killer cell activity over time in 50 marathon runners who ran 2.5 to 3 hours at 75.9 ± 0.9 percent ![]() O2max. * P < 0.001, comparison with pre-exercise.
O2max. * P < 0.001, comparison with pre-exercise.
SOURCE: Data taken from Nieman et al. (1995a) and unpublished data from author's laboratory.
There are few convincing data at this time, however, supporting the notion that exercise-induced changes in immune function explain the increased risk of upper respiratory tract infection (URTI) seen among some athletes. In a small study of elite squash and hockey athletes, Mackinnon and coworkers (1993) have demonstrated that low salivary IgA concentrations precede URTI. However, exercise training-induced changes in T-cell or neutrophil function in two other studies (one in U.S. Air Force Academy cadets during basic training) have not been significantly associated with URTI (Lee et al., 1992; Pyne et al., 1995). Further research with larger groups of individuals is needed.
Practical Applications for Exercise Prescription
Nonetheless, in light of available data, it is prudent to advise the general public that exercise bouts of low-to-moderate intensity (< 60% ![]() O2max) and duration (< 60 minutes/bout) exert less stress on the immune system than do prolonged sessions (> 90 minutes) of heavy exertion (> 75%
O2max) and duration (< 60 minutes/bout) exert less stress on the immune system than do prolonged sessions (> 90 minutes) of heavy exertion (> 75% ![]() O2max). Moderate- versus high-intensity exercise results in a reduced stress hormone response, which has been associated with a more favorable immune response.
O2max). Moderate- versus high-intensity exercise results in a reduced stress hormone response, which has been associated with a more favorable immune response.
Chronic Exercise and Immunity
Ideally, to test the effect of regular physical activity on immune function, a large group of individuals randomly assigned to exercise and sedentary control groups would be followed for at least 1 year with multiple immune measures taken before, during, and after the study. This study, which has not yet been conducted, will require strong financial support before it becomes feasible. At present, only a few small longitudinal and cross-sectional studies (most comparing athletes and nonathletes) are available.
Cross-sectional comparisons of human endurance athletes and nonathletes for NK cell activity, neutrophil function (phagocytosis and oxidative burst), and lymphocyte proliferative response (T-cell function) have provided interesting but somewhat inconsistent data.
NK Cell Activity
Cross-Sectional Studies
The majority of cross-sectional studies support the finding of enhanced NK cell activity in athletes when compared with nonathletes, in both younger and older groups (Nieman et al., 1993a, 1995c; Pedersen et al., 1989; Tvede et al., 1991). In one study, NK cell activity was 57 percent higher in experienced marathon runners compared with sedentary controls (Nieman et al., 1995c) (Figure 17-4). The data of Tvede and coworkers (1991) support a higher NK
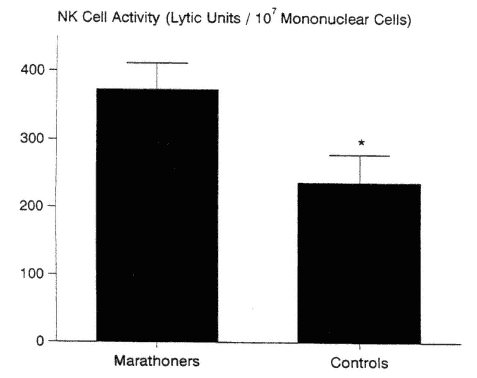
FIGURE 17-4
Natural killer (NK) cell cytotoxic activity was 57 percent higher in the marathon runners versus sedentary controls when expressed in lytic units per 107 mononuclear cells. * P < 0.05.
SOURCE: Data from Nieman et al. (1995c).
cell activity in elite cyclists during the summer months (intensive training period) when compared with the winter (low training period). Not all studies, however, support the finding of a higher NK cell activity in athletes versus nonathletes (Nieman et al., 1995b).
Prospective Studies
Several prospective studies utilizing moderate endurance training regimens over 8 to 15 weeks have reported no significant elevation in NK cell activity relative to sedentary controls (Baslund et al., 1993; Nieman et al., 1990b, 1993a). Together, these data imply that endurance exercise may have to be engaged in for a prolonged time period (i.e., years) before NK cell activity is chronically elevated.
Neutrophil Function
The cross-sectional data on neutrophil function are in contrast to those for NK cell activity (both components of the innate immune system). No researcher has reported an elevation in neutrophil function (phagocytic and/or oxidative burst) among endurance athletes when compared with nonathletes (Baj et al.,
1994; Hack et al., 1992, 1994; Pyne et al., 1995; Smith et al., 1990). Instead, during periods of high-intensity training, neutrophil function has been reported to be suppressed in athletes. This is especially apparent in the studies by Hack and coworkers (1994) and Baj coworkers (1994), where neutrophil function in athletes was similar to controls during periods of low training workloads but significantly suppressed during the summer months of intensive training. Pyne and coworkers (1994, 1995) reported that elite swimmers undertaking intensive training had a significantly lower neutrophil oxidative activity at rest than did age- and sex-matched sedentary individuals and that function was further suppressed during periods of strenuous training prior to national-level competition.
Because neutrophils are considered the body's best phagocyte, suppression of neutrophil function during periods of heavy training is probably a significant factor explaining the increased URTI risk among athletes. Müns (1993) has reported that neutrophils in the upper airway passages of athletes have a decreased phagocytic capacity when compared with those of nonathletes and that following heavy exertion, a further suppression is experienced for 1 to 3 days afterwards.
Other data from Müns and coworkers (1989) have also shown that IgA concentration in nasal secretions is decreased by nearly 70 percent for at least 18 hours after individuals have raced 31 km. Following a marathon race, subjects' nasal mucociliary clearance is significantly slower for nearly a week compared with that of control subjects (Müns et al., 1995) These data suggest that host protection in the upper airway passages is significantly suppressed for a prolonged time after endurance running races. These data may be the most important evidence to date linking risk of respiratory infection with athletic endeavor. Repeated cycles of heavy exertion may thus put the athletes at increased risk of URTI.
T-Cell Function
Data on the mitogen-induced lymphocyte proliferative response (generally a measure of T-cell function) to athletic endeavor are less clear than for NK cells and neutrophils, but the data usually support no significant difference between athletes and nonathletes (Nieman et al., 1995c, d; Tvede et al., 1991). Baj and coworkers (1994) reported no difference between elite cyclists and nonathletes during low training periods (March) but increased levels in the athletes for PHA (phytohemagglutinin) and anti-CD3 mAb (monoclonal antibody to clonaldeterminant 3+ containing cells) (but not Con A [Concanavalin A] or PWM [pokeweed mitogen]) during intensive training. Interleukin (IL)-2 generation, however, was suppressed in the athletes versus controls during intensive training. These data contrast with that of Tvede and coworkers (1991) who found no difference between athletes and nonathletes during both low or high training periods.
Among highly conditioned elderly women (average age 73 years), PHA-induced lymphocyte proliferative response was reported to be 56 percent higher than among sedentary controls (Nieman et al., 1993a). Data from Japan also support enhanced T-cell function among trained elderly men versus untrained controls (Shinkai et al., 1995). These data are interesting because T-cell function tends to diminish with age (see section on "Exercise, Aging, and Immunity").
Other Measures of Immunity
Other components of immunity have been less well studied among human athletes and nonathletes. Tomasi and coworkers (1982) reported that resting salivary IgA levels were lower in elite cross-country skiers than in age-matched controls, but this was not confirmed in a follow-up study of elite cyclists (Mackinnon et al., 1987). As reviewed by Mackinnon and Hooper (1994), the secretory immune system of the mucosal tissues of the upper respiratory tract is considered the first barrier to colonization by pathogens, with IgA the major effector of host defense. Secretory IgA inhibits attachment and replication of pathogens, preventing their entry into the body. Although several studies have shown that salivary IgA concentration decreases after a single bout of intense endurance exercise, further research is needed to determine the overall chronic effect.
Practical Applications for Exercise Prescription
These data support the concept that the innate immune system responds differentially to the chronic stress of intensive exercise, with NK cell activity tending to be enhanced while neutrophil function is suppressed (especially during periods of heavy training). The adaptive immune system,3 in general, seems to be largely unaffected (except perhaps in the highly trained elderly individuals), although the research data at present are mixed. Further research is needed with larger groups of athletes to allow a more definitive comparison.
Nonetheless, from a practical viewpoint, moderate amounts of exercise training (3–5 sessions/wk, 15–60 min/session, 40–60% ![]() O2max) appear to have little if any chronic effect on immune function (when in a state of rest). Thus any positive effects on immunosurveillance and host protection that come with moderate exercise training are probably related to changes that occur during each exercise bout.
O2max) appear to have little if any chronic effect on immune function (when in a state of rest). Thus any positive effects on immunosurveillance and host protection that come with moderate exercise training are probably related to changes that occur during each exercise bout.
For athletes, periods of heavy training have been associated with suppression of neutrophil function. Neutrophils are an important component of
the innate immune system, aiding in the phagocytosis of many bacterial and viral pathogens and in the release of immunomodulatory cytokines. Athletes should be made aware of this potential problem and urged to avoid overtraining (see "Practical Guidelines for Military Recruits and Athletes").
Exercise and Upper Respiratory Tract Infections
Among elite athletes and their coaches, a common perception is that heavy exertion lowers resistance and is a predisposing factor to URTI. There is also a common, contrasting belief among many individuals that regular exercise confers resistance against infection. For example, a survey of 750 masters athletes (ranging in age from 40 to 81 years) showed that 76 percent perceived themselves to be less vulnerable to viral illnesses than their sedentary peers (Shephard et al., 1995b).
Understanding the relationship between exercise and infection has potential implications for public health. For the athlete, it may mean the difference between being able to compete or performing at a subpar level or missing the event altogether because of illness.
The J Curve
Nieman (1994) has proposed that the relationship between exercise and URTI may be modeled in the form of a J curve (Figure 17-5). This model suggests that although the risk of URTI may decrease below that of a sedentary individual when one engages in moderate exercise training, risk may rise above average during periods of excessive amounts of high-intensity exercise. Much more research using larger subject pools and improved research designs is necessary before this model can be accepted or rejected.
Heavy Exertion and Risk of URTI
Several epidemiological reports suggest that athletes engaging in marathon-type events and/or very heavy training are at increased risk of URTI (Nieman et al., 1990a; Peters, 1990; Peters and Bateman, 1983; Peters et al., 1993) (Figure 17-6). URTI risk following a race event may depend on the distance, with an increased incidence conspicuous only following marathon or ultramarathon events. Among runners varying widely in training habits, the risk for URTI is slightly elevated for the highest distance runners, but only when several confounding factors (e.g., demographic and training variables, mental stress) are controlled (Nieman et al., 1990a).
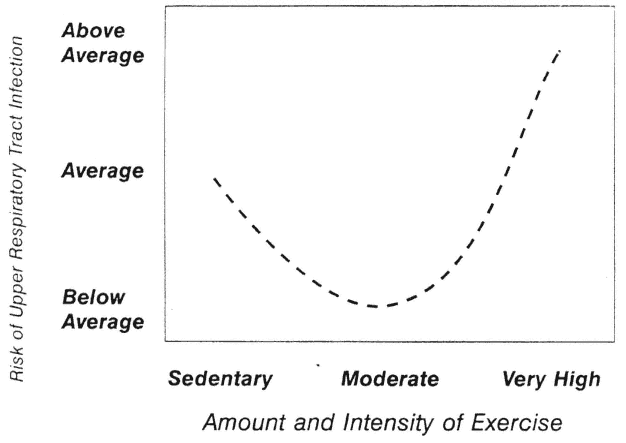
FIGURE 17-5
J-shaped model of relationship between varying amounts of exercise and risk of upper respiratory tract infection (URTI). This model suggests that moderate exercise may lower risk of URTI, while excessive amounts may increase the risk.
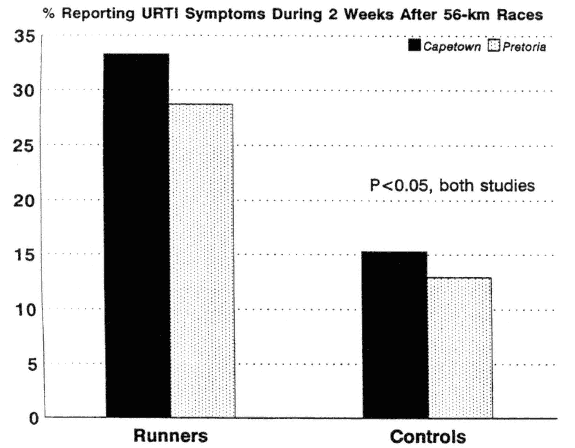
FIGURE 17-6
Incidence of upper respiratory tract infection was significantly higher in ultramarathon runners versus controls during the 2-wk period following the race event.
SOURCE: Data from Peters (1990) and Peters and Bateman (1983).
Moderate Exercise and Risk of URTI
What about the common belief that moderate physical activity is beneficial in decreasing URTI risk? Very few studies have been carried out in this area, and more research is certainly warranted to investigate this interesting question. At present, there are no published epidemiological reports that have retrospectively or prospectively compared incidence of URTI in large groups of moderately active and sedentary individuals. Two randomized experimental trials using small numbers of subjects have provided important preliminary data in support of the viewpoint that moderate physical activity may reduce URTI symptomatology (Nieman et al., 1990b, 1993b).
In one randomized, controlled study of 36 women (mean age 35 years), exercise subjects walked briskly for 45 minutes, 5 days a week, and experienced one-half the number of days with URTI symptoms during the 15-wk period compared with that of the sedentary control group (5.1 ± 1.2 vs. 10.8 ± 2.3 days, p = 0.039) (Nieman et al., 1990b). In a study of elderly women, the incidence of the common cold during a 12-wk period in the autumn was observed to be lowest in highly conditioned, lean subjects who exercised moderately each day for about 1.5 hours (8%). Elderly subjects who walked 40 minutes, 5 times/wk had an incidence of 21 percent, as compared with 50 percent for the sedentary control group (X = 6.36, p = 0.042) (Nieman et al., 1993b).
Public Health Recommendations
Although public health recommendations must be considered tentative, the data on the relationship between moderate exercise and lowered risk of URTI are consistent with guidelines urging the general public to engage in near-daily brisk walking. For athletes engaging in long-endurance events, the risk of illness is high during the 1 to 2 week recovery time period, and several precautions to lower this risk are outlined in the last section of this paper.
Infection and Exercise Performance
It is well established that various measures of physical performance capability are reduced during most types of systemic infectious episodes (Daniels et al., 1985; Friman et al., 1991, 1985; Roberts, 1985, 1986). Although causes are debated, muscle protein catabolism, circulatory deregulation, and mitochondrial abnormalities have been reported (Ilbäck et al., 1991).
Several case histories have been published demonstrating that in some individuals sudden and unexplained deterioration in athletic performance can be traced to either recent URTI or subclinical viral infections that run a protracted course (Roberts, 1985; Sharp, 1989). In some athletes, a viral infection may lead to a severely debilitating state known as post-viral fatigue syndrome (PVFS)
(Maffulli et al., 1993). The symptoms can persist for several months and include lethargy, atypical depression, excessive sleep, night sweats, easy fatiguability (made worse by exercise), and myalgia.
Exercise Recommendations During Infection
Endurance athletes are often uncertain of whether they should exercise or rest during an infectious episode. Few data are available in humans to provide definitive answers. Most clinical authorities in this area recommend that if the athlete has symptoms of a common cold with no constitutional involvement, regular training may be safely resumed a few days after the resolution of symptoms (Sharp, 1989). Mild exercise during illness with a common cold does not appear to be contraindicated, but more research in this area is needed. In one study, nasal injection with rhinovirus 16 (leading to common cold symptoms) had no effect on exercise performance (Personal communication, T. Weidner, Ball State University, Muncie, Ind., 1995). Clinicians recommend, however, if there are symptoms of systemic involvement (e.g., fever, extreme tiredness, muscle aches, swollen lymph glands), 2 to 4 weeks should probably be allowed before resumption of intensive training (Roberts, 1985; Sharp, 1989).
These recommendations are speculative, however, and are primarily based on animal studies and some case reports of humans who died following bouts of vigorous exercise during an acute viral illness. Depending on the pathogen (with some more affected by exercise than others), animal studies generally support the finding that one or two periods of exhaustive exercise following inoculation of the animal leads to a more frequent appearance of infection and a higher fatality rate (Cannon, 1993). The problem is that most individuals are unaware of the pathogen with which they are infected, which necessitates a conservative approach to prevent relapse, a worsening of the disease, myocarditis, or other types of injury. In general, if the symptoms are from the neck up, moderate exercise is probably acceptable, while bed rest and a gradual progression to normal training are recommended when the illness is systemic.
Exercise and HIV Infection
Pertinent questions have been raised regarding HIV transmission during sports that require close physical contact. Most patients diagnosed with active AIDS are acutely and chronically ill and are not likely to participate in athletic endeavors. For each patient with clinically apparent AIDS, however, there are many more who are HIV infected and free of clinical manifestations who may be capable of normal participation in sports (Calabrese and LaPerriere, 1993).
Routine social or community contact with an HIV-infected person carries no risk of transmission; only sexual exposure and exposure to blood or tissues carry a risk. Although HIV has been found in saliva, tears, urine, and bronchial
secretions, there is no evidence that the virus can be transmitted after contact with these secretions.
Several situations exist, however, in which the transmission of HIV is of concern in athletic settings (Goldsmith, 1992). During sport activities in which athletes can be cut, such as boxing or wrestling, or in other contact sports such as football, basketball, and baseball, risk of HIV transmission exists when the mucous membranes of a healthy athlete are exposed to the blood of an infected athlete. Currently, the testing of all athletes prior to sports participation is thought to be impractical, unethical, and unrealistic (Brown et al., 1994). Therefore, the team physician and athletic trainer are urged to provide information about the transmission of HIV, recommended behavior to reduce risks, and referral for care or diagnosis.
Can exercise training be used as a method to delay the progression from HIV infection to AIDS? Few investigators have published results in this area (LaPerriere et al., 1991; MacArthur et al., 1993; Rigsby et al., 1992). Rigsby and colleagues (1992) studied the effects of an exercise program (three 1-h sessions per week of strength training and aerobic exercise) on 37 HIV-infected subjects who spanned the range of HIV disease progression from asymptomatic to a diagnosis of AIDS (CD4 counts ranged from 9 to 804 cells/mm3). Subjects were randomly assigned to either a 12-week exercise training or a counseling control group. Although exercise training had the expected effect of improving both strength and cardiorespiratory fitness in exercise subjects, no significant change in CD4 cell counts or the CD4/CD8 ratio was found for either condition. These results are similar to those of LaPerriere and coworkers (1991).
This increase in strength with weight training, which has also been reported by Spence and coworkers (1990) in AIDS subjects, is noteworthy in that muscle atrophy and nervous system disorders are common among ARC (AIDS-related complex) and AIDS patients. Weight training may provide one means of retarding the wasting syndrome that accompanies AIDS and improving the quality of life for these individuals.
Practical Recommendations
One tentative conclusion that can be made from these studies is that appropriately supervised exercise training does not appear to affect HIV-infected individuals adversely (Lawless et al., 1995). Several potential benefits of both aerobic and strength training by HIV-infected individuals, especially when initiated early in the disease state, include improvement in psychological coping, maintenance of health and physical function for a longer period, and attenuation of negative immune system changes. Improved quality of life is perhaps the chief benefit of regular exercise by HIV-infected patients.
It is recommended that exercise prescriptions for all HIV-infected individuals be made on an individual basis, with appropriate initial screening.
The exercise prescription should emphasize both cardiorespiratory and musculoskeletal training components.
Exercise, Aging, and Immunity
Immune senescence or age-associated immune deficiency appears to be partly responsible for the afflictions of old age (Nieman and Henson, 1994). Elderly persons are more susceptible to many infections, autoimmune disorders, and cancers when compared with younger adults. Death rates from pneumonia and influenza, for example, are much higher among the very old (> 85 years) as compared to adults of late middle age (55–59 years) (1,361 deaths/100,000 vs. 18/100,000, respectively). Death rates for cancer also climb steeply with increasing age.
A new and growing area of research is the study of the relationship between certain lifestyle factors (in particular, physical activity and diet) and immune senescence. Regarding physical activity, national surveys have shown that older adults exercise less and have lower levels of cardiorespiratory fitness than do younger adults. Could regular physical activity attenuate the decrease in immune function with increase in age?
Very few studies have been conducted in this area (Nasrullah and Mazzeo, 1992; Nieman et al., 1993a). The most interesting results come from cross-sectional studies of highly active and lean elderly subjects and their sedentary peers (Nieman et al., 1993a; Shinkai et al., 1995). As shown in Figure 17-7, NK
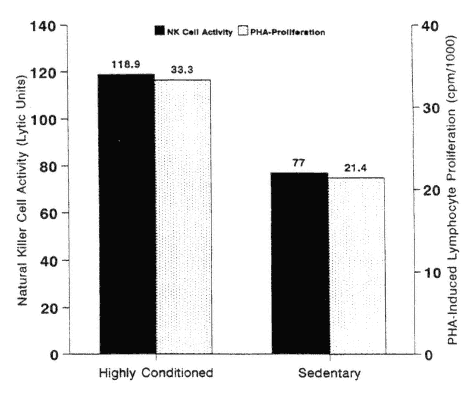
FIGURE 17-7
Natural killer (NK) cell activity was 54 percent higher and PHA (phytohemagglutinin)-induced lymphocyte proliferation 56 percent higher in trained versus untrained elderly females. * P < 0.05.
SOURCE: Data from Nieman et al. (1993a).
cell activity and PHA-induced lymphocyte proliferation were significantly higher in elderly athletes versus sedentary controls (Nieman et al., 1993a). This finding has also been confirmed in a cross-sectional study of trained and untrained elderly subjects in Japan (Shinkai et al., 1993). In a randomized study of elderly women, however, 12 weeks of moderate cardiorespiratory exercise training did not result in any improvement in NK- or T-cell function relative to sedentary controls (Nieman et al., 1993a).
Although the relative importance of high volumes of physical activity, nutrient intake, self-selection, and other confounders is impossible to determine, the data taken together do suggest that exercise training may need to be long-term (i.e., for multiple years) and of sufficient volume to induce changes in body weight and fitness before any change in immunity can be expected in old age (Nieman and Henson, 1994). In other words, because the aging process is so dominant in old age, long-term physical activity combined with leanness and other positive lifestyle habits may be necessary before immune function is enhanced.
Practical Guidelines for Military Recruits and Athletes
For military recruits and athletes who may be undergoing heavy exercise stress in preparation for combat or competition, several precautions may help them reduce their risk of URTI. Considerable evidence indicates that two other environmental factors, improper nutrition (Chandra, 1991) and psychological stress (Cohen et al., 1991) can compound the negative influence of heavy exertion on the immune system. Based on current understanding, the athlete is urged to eat a well-balanced diet, keep other life stresses to a minimum, avoid overtraining and chronic fatigue, obtain adequate sleep, and space vigorous workouts and race events as far apart as possible.
Possible indicators of overtraining include immunosuppression with loss of motivation for training and competition, depression, poor performance, and muscle soreness. However, at this time, there are no practical markers of immunosuppression (other than infection) that coaches and clinicians can use to indicate that the athlete is overtrained.
Immune system function appears to be suppressed during periods of low caloric intake and weight reduction (Kono et al., 1988; Nieman et al., 1996), so when necessary, the athlete is advised to lose weight slowly during noncompetitive training phases. Cold viruses are spread by both personal contact and breathing the air near sick people. Therefore, if at all possible, athletes should avoid being around sick people before and after important events. If the athlete is competing during the winter months, a flu shot is recommended.
Some preliminary data support that various immunomodulator drugs may afford athletes some protection against infection during competitive cycles
(Ghighineishvili et al., 1992). Much more research is needed before any of these drugs can be recommended. Indomethacin, which inhibits prostaglandin production, has been administered to athletes prior to exercise or used in vitro to determine whether the drop in NK cell activity can be countered (Nieman et al., 1995a; Pedersen et al., 1990). Following 2.5 hours of intensive running, indomethacin had no effect in countering the steep drop in NK cell activity (Nieman et al., 1995a). Other medications such as aspirin or ibuprofen are currently being studied for their effects on the immune system following heavy exertion.
Should athletes use nutrient supplements to decrease their risk of immunosuppression and infection? Glutamine is an important fuel for lymphocytes and monocytes, but glutamine supplementation in vitro has not been found capable of negating the exercise-induced decrease in T-cell function after exercise (Rohde et al., 1995).
Peters and coworkers (1993) have published data that support the use of vitamin C supplementation prior to ultramarathon race events to lower the incidence of URTI during the 2-wk recovery period. In this study, 68 percent of runners reported the development of symptoms of URTI within 2 weeks after the 90 km Comrades Ultramarathon. The incidence of URTI was greatest among the runners who trained the hardest coming into the race (85% vs. 45% of the low- or medium-training status runners). Using a double-blind placebo research design, it was also determined that only 33 percent of all runners taking a 600 mg vitamin C supplement daily for 3 weeks prior to the race developed URTI symptoms. The authors suggested that because heavy exertion enhances the production of free oxygen radicals, vitamin C, which has antioxidant properties, may be required in increased quantities. This is an interesting finding, and further research will help to determine if this finding also applies to runners racing shorter distances (for example, a typical marathon of 42.2 km). Vitamin C supplementation (double blind, placebo controlled) in a study conducted in the author's laboratory was shown to have no effect on the immune response to 2.5 hours of intense treadmill running (Nieman et al., 1997a).
Supplementation with N-acetylcysteine, a pro-glutathione free radical scavenger, has been associated with a reduction in the formation of reactive oxygen species by granulocytes following maximal graded exercise (Huupponen et al., 1995). Although antioxidant supplementation may attenuate oxidative stress following prolonged and strenuous exertion, the effect this attenuation may have on altering the exercise-induced immune response is uncertain.
The effects of electrolyte- and carbohydrate-containing solutions have also been tested. A recent double-blind, placebo, randomized study investigated the effect of drinking Gatorade on the immune response to 2.5 hours of running (Nieman et al., 1997b). In prior research, carbohydrate versus water ingestion during prolonged endurance exercise had been associated with an attenuated cortisol and epinephrine response through its effect on the blood glucose. On the
test day, following a blood sample at 0700 hours in a 12-h fasted state, subjects drank 750 ml of either Gatorade or a placebo solution. At 0730 hours, subjects began running at 75 to 80 percent ![]() O2max for 2.5 hours, and ingested 250 ml Gatorade or placebo every 15 minutes. Immediately after the 2.5-h run (1000 hours), another blood sample was taken, followed by 1.5-, 3-, and 6-h recovery samples. Subjects drank 500 ml/h of Gatorade or placebo during the first 1.5 hours of recovery and then 250 ml/h during the last 4.5 hours of recovery.
O2max for 2.5 hours, and ingested 250 ml Gatorade or placebo every 15 minutes. Immediately after the 2.5-h run (1000 hours), another blood sample was taken, followed by 1.5-, 3-, and 6-h recovery samples. Subjects drank 500 ml/h of Gatorade or placebo during the first 1.5 hours of recovery and then 250 ml/h during the last 4.5 hours of recovery.
Gatorade ingestion before, during, and after 2.5 hours of running attenuated the rise in both cortisol and the neutrophil/lymphocyte ratio. Blood glucose concentrations were significantly higher in the Gatorade versus placebo group. Recovery Con A- and PHA-induced proliferation tended to be lower in the placebo group (interaction effect, P = 0.147, P = 0.195, respectively). These data suggest that carbohydrate ingestion before, during, and after prolonged endurance exercise may help to lessen the stress on the immune system.
Author's Conclusions and Recommendations
During the last 95 years, 629 papers (60% in the 1990s) dealing specifically with exercise and immunology have been published. Major findings of practical importance in terms of public health and human performance include:
- In response to acute exercise (the most frequently studied area of exercise immunology), a rapid interchange of immune cells between peripheral lymphoid tissues and the circulation occurs. The response depends on many factors, including the intensity, duration, and mode of exercise; concentrations of hormones and cytokines; and change in body temperature, blood flow, hydration status, and body position. Of all immune cells, NK cells, neutrophils, and macrophages (of the innate immune system) appear to be most responsive to the effects of acute exercise, both in terms of numbers and function. In general, acute exercise bouts of moderate duration (< 60 minutes) and intensity (< 60%
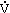 O2max) are associated with fewer perturbations and less stress to the immune system than are prolonged, high-intensity sessions.
O2max) are associated with fewer perturbations and less stress to the immune system than are prolonged, high-intensity sessions. - In response to long-term exercise training, the only finding to date reported with some congruity between investigators is a significant elevation in NK cell activity. Changes in the function of neutrophils, macrophages, and T- and B-cells in response to training have been reported inconsistently, but there is some indication that neutrophil function is suppressed during periods of heavy training.
- Limited data suggest that unusually heavy, acute, or chronic exercise may increase the risk of URTI, while regular moderate physical activity may reduce URTI symptomatology.
- Work performance tends to diminish with most systemic infections, and clinical case studies and animal data suggest that increased infection severity, relapse, and myocarditis may result when patients exercise vigorously. Military
- recruits with systemic infections such as influenza should not exercise vigorously until 2 weeks after symptoms have diminished.
- Although regular exercise has many benefits for HIV-infected individuals, helper T-cell counts and other immune measures are not enhanced significantly.
- As individuals age, they experience a decline in most cell-mediated and humoral immune responses. Two human studies suggest that immune function is superior in highly conditioned versus sedentary elderly subjects.
- Mental stress, undernourishment, quick weight loss, and improper hygiene have each been associated with impaired immunity. Military recruits who are undergoing heavy training regimens should realize that each of these factors has the potential to compound the effect of exercise stress on their immune systems.
References
Baetjer, A.M. 1932. The effect of muscular fatigue upon resistance. Physiol. Rev. 12:453-468.
Baj, Z., J. Kantorski, E. Majewska, K. Zeman, L. Pokoca, E. Fornalczyk, H. Tchorzewski, Z.Sulowska, and R. Lewicki. 1994. Immunological status of competitive cyclists before and after the training season. Int. J. Sports Med. 15:319-324.
Barger, L.K., J.E. Greenleaf, F. Baldini, and D. Huff. 1995. Effects of space missions on the human immune system: A meta-analysis. Sports Med. Train. Rehabil. 5:293-310.
Baslund, B., K. Lyngberg, V. Andersen, J. Halkjaer-Kristensen, M. Hansen, M. Klokker, and B.K. Pedersen. 1993. Effect of 8 weeks of bicycle training on the immune system of patients with rheumatoid arthritis. J. Appl. Physiol. 75:1691-1695.
Brenner, I.K.M., P.N. Shek, and R.J. Shephard. 1995. Heat exposure and immune function: Potential contribution to the exercise response. Exerc. Immunol. Rev. 1:49-80.
Brown, L.S., R.Y. Phillips, C.L. Brown, D. Knowlan, L. Castle, and J. Moyer. 1994. HIV/AIDS policies and sports: The National Football League. Med. Sci. Sports Exerc. 26:403-407.
Calabrese, L.H., and A. LaPerriere. 1993. Human immunodeficiency virus infection, exercise and athletics. Sports Med. 15:6-13.
Cannon, J.G. 1993. Exercise and resistance to infection. J. Appl. Physiol. 74:973-981.
Chandra, R.K. 1991. 1990 McCollum award lecture. Nutrition and immunity: Lessons from the past and new insights into the future. Am. J. Clin. Nutr. 53:1087-1101.
Cohen, S., D.A. Tyrrell, and A.P. Smith. 1991. Psychological stress and susceptibility to the common cold. N. Engl. J. Med. 325:606-612.
Cupps, T.R., and A.S. Fauci. 1982. Corticosteroid-mediated immunoregulation in man. Immunol. Rev. 65:133-155.
Daniels, W.L., D.S. Sharp, J.E. Wright, J.A. Vogel, G. Friman, W.R. Beisel, and J.J. Knapik. 1985. Effects of virus infection on physical performance in man. Milit. Med. 150:8-14.
Eskola J., O. Ruuskanen, E. Soppi, M.K. Viljanen, M. Järvinen, H. Toivonen, and K. Kouvalainen. 1978. Effect of sport stress on lymphocyte transformation and antibody formation. Clin. Exp. Immunol. 32:339-345.
Friman, G., N.G. Ilbäck, D.J. Crawford, and H.A. Neufeld. 1991. Metabolic responses to swimming exercise in Streptococcus pneumoniae infected rats. Med. Sci. Sports Exerc. 23:415-421.
Friman, G., J.E. Wright, N.G. Ilbäck, W.R. Beisel, J.D. White, D.S. Sharp, E.L. Stephen, W.L. Daniels, and J.A. Vogel. 1985. Does fever or myalgia indicate reduced physical performance capacity in viral infections? Acta. Med. Scand. 217:353-361.
Gabriel, H., L. Schwarz, P. Born, and W. Kindermann. 1992. Differential mobilization of leukocyte and lymphocyte subpopulations into the circulation during endurance exercise. Eur. J. Appl. Physiol. 65:529-534.
Garrey, W.E., and W.R. Bryan. 1935. Variations in white blood cell counts. Physiol. Rev. 15:597-638.
Ghighineishvili, G.R., V.V. Nicolaeva, A.J. Belousov, P.G. Sirtori, V. Balsamo, A. Miani, R. Franceschini, M. Ripani, M. Crosina, and G. Cosenza. 1992. Correction by physiotherapy of immune disorders in high-grade athletes. Clin. Ter. 140:545-550.
Goldsmith, M.F. 1992. World health organization consensus statement. Consultation on AIDS and sports. J. Am. Med. Assoc. 267:1312-1314.
Hack, V., G. Strobel, J-P. Rau, and H. Weicker. 1992. The effect of maximal exercise on the activity of neutrophil granulocytes in highly trained athletes in a moderate training period. Eur. J. Appl. Physiol. 65:520-524.
Hack, V., G. Strobel, M. Weiss, and H. Weicker. 1994. PMN cell counts and phagocytic activity of highly trained athletes depend on training period. J. Appl. Physiol. 77:1731-1735.
Hoffman-Goetz, L., and B.K. Pedersen. 1994. Exercise and the immune system: A model of the stress response? Immunol. Today 15:382-387.
Huupponen, M.R.H., L.H. Mäkinen, P.M. Hyvönen, C.K. Sen, T. Rankinen, S. Väisänen, and R. Rauramaa. 1995. The effect of N-acetylcysteine on exercise-induced priming of human neutrophils. Int. J. Sports Med. 16:399-403.
Ilbäck, N.G., G. Friman, D.J. Crawford, and H.A. Neufeld. 1991. Effects of training on metabolic responses and performance capacity in Streptococcus pneumoniae infected rats. Med. Sci. Sports Exerc. 23:422-427.
Kono, I., H. Kitao, M. Matsuda, S. Haga, H. Fukushima, and H. Kashiwagi. 1988. Weight reduction in athletes may adversely affect the phagocytic function of monocytes. Physician Sportsmed. 16(7):56-65.
LaPerriere, A.R., M.A. Fletcher, M.H. Antoni, N.G. Klimas, G. Ironson, and N. Schneiderman. 1991. Aerobic exercise training in an AIDS risk group. Int. J. Sports Med. 12(suppl. 1):S53-S57.
Lawless, D., C.G.R. Jackson, and J.E. Greenleaf. 1995. Exercise and human immunodeficiency virus (HIV-1) infection. Sports Med. 19:235-239.
Lee, D.J., R.T. Meehan, C. Robinson, T.R. Mabry, and M.L. Smith. 1992. Immune responsiveness and risk of illness in U.S. Air Force Academy cadets during basic cadet training. Aviat. Space Environ. Med. 63:517-523.
Linden, A., T. Art, H. Amory, A.M. Massart, C. Burvenich, and P. Lekeux. 1991. Quantitative buffy coat analysis related to adrenocortical function in horses during a three-day event competition. Zentralbl. Veterinärmed. 38:376-382.
MacArthur, R.D., S.D. Levine, and T.J. Birk. 1993. Supervised exercise training improves cardiopulmonary fitness in HIV-infected persons. Med. Sci. Sports Exerc. 25:684-688.
Macha, M., M. Shlafer, and M.J. Kluger. 1990. Human neutrophil hydrogen peroxide generation following physical exercise. J. Sports Med. Phys. Fitness 30:412-419.
Mackinnon, L.T., and S. Hooper. 1994. Mucosal (secretory) immune system responses to exercise of varying intensity and during overtraining. Int. J. Sports Med. 15:S179-S183.
Mackinnon, L.T., T.W. Chick, A. Van As, and T.B. Tomasi. 1987. The effect of exercise on secretory and natural immunity. Adv. Exp. Med. Biol. 216A:869-876.
Mackinnon, L.T., T.W. Chick, A. Van As, and T.B. Tomasi. 1988. Effects of prolonged intense exercise on natural killer cell number and function. Exerc. Physiol.: Current Selected Research 3:77-89.
Mackinnon, L.T., E.M. Ginn, and G.J. Seymour. 1993. Temporal relationship between decreased salivary IgA and upper respiratory tract infection in elite athletes. Aust. J. Sci. Med. Sport 25:94-99.
Maffulli, N., V. Testa, and G. Capasso. 1993. Post-viral fatigue syndrome. A longitudinal assessment in varsity athletes. J. Sports Med. Phys. Fitness 33:392-399.
Müns, G. 1993. Effect of long-distance running on polymorphonuclear neutrophil phagocytic function of the upper airways. Int. J. Sports Med. 15:96-99.
Müns, G., H. Liesen, H. Riedel, and K-Ch. Bergmann. 1989. Einfluá von langstreckenlauf auf den IgA-gehalt in nasensekret und speichel. Deutsche Zeitschr. Sportmed. 40:63-65.
Müns, G., P. Singer, F. Wolf, and I. Rubinstein. 1995. Impaired nasal mucociliary clearance in long-distance runners. Int. J. Sports Med. 16:209-213.
Nasrullah, I., and R.S. Mazzeo. 1992. Age-related immunosenescence in Fischer 344 rats: Influence of exercise training. J. Appl. Physiol. 73:1932-1938.
Nieman, D.C. 1994b. Exercise, upper respiratory tract infection, and the immune system. Med. Sci. Sports Exerc. 26:128-139.
Nieman, D.C., and D.A. Henson. 1994. Role of endurance exercise in immune senescence. Med. Sci. Sports Exerc. 26:172-181.
Nieman, D.C., and S.L. Nehlsen-Cannarella. 1994. The immune response to exercise. Semin. Hematol. 31:166-179.
Nieman, D.C., J.C. Ahle, D.A. Henson, B.J. Warren, J. Suttles, J.M. Davis, K.S. Buckley, S. Simandle, D.E. Butterworth, O.R. Fagoaga, and S.L. Nehlsen-Cannarella. 1995a. Indomethacin does not alter natural killer cell response to 2.5 hours of running. J. Appl. Physiol. 79:748-755.
Nieman, D.C., L.S. Berk, M. Simpson-Westerberg, K. Arabatzis, W. Youngberg, S.A. Tan, and W.C. Eby. 1989. Effects of long endurance running on immune system parameters and lymphocyte function in experienced marathoners. Int. J. Sports Med. 10:317-323.
Nieman, D.C., D. Brendle, D.A. Henson, J. Suttles, V.D. Cook, B.J. Warren , D.E. Butterworth, O.R. Fagoaga, and S.L. Nehlsen-Cannarella. 1995b. Immune function in athletes versus nonathletes. Int. J. Sports Med. 16:329-333.
Nieman, D.C., K.S. Buckley, D.A. Henson, B.J. Warren, J. Suttles, J.C. Ahle, S. Simandle, O.R. Fagoaga, and S.L. Nehlsen-Cannarella. 1995c. Immune function in marathon runners versus sedentary controls. Med. Sci. Sports Exerc. 27:986-992.
Nieman, D.C., D.A. Henson, D.E. Butterworth, B.J. Warren, J.M. Davis, O.R. Fagoaga, and S.L. Nehlsen-Cannarella. 1997a. Vitamin C supplementation does not alter the immune response to 2.5 hours of running. Int. J. Sport Nutr. 7:173-184.
Nieman, D.C., D.A. Henson, E.B. Garner, D.E. Butterworth, B.J. Warren, A. Utter, J.M. Davis, O.R. Fagoaga, and S.L. Nehlsen-Cannarella. 1997b. Carbohydrate affects natural killer cell redistribution but not activity after running. Med. Sci. Sports Exerc. 29:1318-1324.
Nieman, D.C., D.A. Henson, G. Gusewitch, B.J. Warren, R.C. Dotson, D.E. Butterworth, and S.L. Nehlsen-Cannarella. 1993a. Physical activity and immune function in elderly women. Med. Sci. Sports Exerc. 25:823-831.
Nieman, D.C., L.M. Johanssen, J.W. Lee, and K. Arabatzis. 1990a. Infectious episodes in runners before and after the Los Angeles Marathon. J. Sports Med. Phys. Fitness 30:316-328.
Nieman, D.C., A.R. Miller, D.A. Henson, B.J. Warren, G. Gusewitch, R.L. Johnson, J.M. Davis, D.E. Butterworth, J.L. Herring, and S.L. Nehlsen-Cannarella. 1994. Effects of high- versus moderate-intensity exercise on lymphocyte subpopulations and proliferative response. Int. J. Sports Med. 15:199-206.
Nieman, D.C., A.R. Miller, D.A. Henson, B.J. Warren, G. Gusewitch, R.L. Johnson, J.M. Davis, D.E. Butterworth, and S.L. Nehlsen-Cannarella. 1993b. The effects of high-versus moderate-intensity exercise on natural killer cell cytotoxic activity . Med. Sci. Sports Exerc. 25:1126-1134.
Nieman, D.C., S.L. Nehlsen-Cannarella, K.M. Donohue, D.B.W. Chritton, B.L. Haddock, R.W. Stout, and J.W. Lee. 1991. The effects of acute moderate exercise on leukocyte and lymphocyte subpopulations. Med. Sci. Sports Exerc. 23:578-585.
Nieman, D.C., S.L. Nehlsen-Cannarella, D.A. Henson, D.E. Butterworth, O.R. Fagoaga, B.J. Warren, and M.K. Rainwater. 1996. Immune response to obesity and moderate weight loss. Int. J. Obesity Relat. Metab. Disord. 20:353-360.
Nieman, D.C., S.L. Nehlsen-Cannarella, P.A. Markoff, A.J. Balk-Lamberton, H. Yang, D.B.W. Chritton, J.W. Lee, and K. Arabatzis. 1990b. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int. J. Sports Med. 11:467-473.
Nieman, D.C., S. Simandle, D.A. Henson, B.J. Warren, J. Suttles, J.M. Davis, K.S. Buckley, J.C. Ahle, D.E. Butterworth, O.R. Fagoaga, and S.L. Nehlsen-Cannarella. 1995d. Lymphocyte proliferation response to 2.5 hours of running. Int. J. Sports Med. 16:404-408.
Pedersen, B.K., and H. Bruunsgaard. 1995. How physical exercise influences the establishment of infections. Sports Med. 19:393-400.
Pedersen, B.K., and H. Ullum. 1994. NK cell response to physical activity: Possible mechanisms of action. Med. Sci. Sports Exerc. 26:140-146.
Pedersen, B.K., M. Kappel, M. Klokker, H.B. Nielsen, and N.H. Secher. 1994. The immune system during exposure to extreme physiologic conditions. Int. J. Sports Med. 15:S116-S121.
Pedersen, B.K., N. Tvede, L.D. Christensen, K. Klarlund, S. Kragbak, and J. Halkjaer-Kristensen. 1989. Natural killer cell activity in peripheral blood of highly trained and untrained persons. Int. J. Sports Med. 10:129-131.
Pedersen, B.K., N. Tvede, K. Klarlund, L.D. Christensen, F.R. Hansen, H. Galbo, A. Kharazmi, and J. Halkjaer-Kristensen. 1990. Indomethacin in vitro and in vivo abolishes post-exercise suppression of natural killer cell activity in peripheral blood. Int. J. Sports Med. 11:127-131.
Peters, E.M. 1990. Altitude fails to increase susceptibility of ultramarathon runners to postrace upper respiratory tract infections. S. Afr. J. Sports Med. 5:4-8
Peters, E.M. 1997. Exercise, immunology, and upper respiratory tract infections. Int. J. Sports Med. 18 (suppl. 1):S69-S77.
Peters, E.M., and E.D. Bateman. 1983. Respiratory tract infections: An epidemiological survey. S. Afr. Med. J. 64:582-584.
Peters, E.M., J.M. Goetzsche, B. Grobbelaar, and T.D. Noakes. 1993. Vitamin C supplementation reduces the incidence of postrace symptoms of upper respiratory tract infection in ultramarathon runners. Am. J. Clin. Nutr. 57:170-174.
Pyne, D.B. 1994. Regulation of neutrophil function during exercise. Sports Med. 17:245-258.
Pyne, D.B., M.S. Baker, P.A. Fricker, W.A. McDonald, and W.J. Nelson. 1995. Effects of an intensive 12-wk training program by elite swimmers on neutrophil oxidative activity. Med. Sci. Sports Exerc. 27:536-542.
Rigsby, L.W., R.K. Dishman, A.W. Jackson, G.S. Maclean, and P.B. Raven. 1992. Effects of exercise training on men seropositive for the human immunodeficiency virus-1. Med. Sci. Sports Exerc. 24:6-12.
Roberts, J.A. 1985. Loss of form in young athletes due to viral infection. Br. J. Med. 290:357-358.
Roberts J.A. 1986. Viral illnesses and sports performance. Sports Med. 3:296-303.
Rohde, T., H. Ullum, J.P. Rasmussen, J.H. Kristensen, E. Newsholme, and B.K. Pedersen. 1995. Effects of glutamine on the immune system: Influence of muscular exercise and HIV infection. J. Appl. Physiol. 79:146-150.
Sharp, J.C.M. 1989. Viruses and the athlete. Br. J. Sports Med. 23:47-48.
Shephard, R.J., T. Kavanagh, D.J. Mertens, S. Qureshi, and M. Clark. 1995b. Personal health benefits of masters athletics competition. Br. J. Sport Med. 29:35-40.
Shinkai, S., H. Kohno, K. Kimura, T. Komura, H. Asai, R. Inai, K. Oka, Y. Kurokawa, and R.J. Shephard. 1995. Physical activity and immunosenescence in elderly men. Med. Sci. Sports Exerc. 27:1516-1526.
Shinkai, S., Y. Kurokawa, S. Hino, M. Hirose, J. Torii, S. Watanabe, S. Shiraishi, K. Oka, and T. Watanabe. 1993. Triathlon competition induced a transient immunosuppressive change in the peripheral blood of athletes. J. Sports Med. Phys. Fitness 33:70-78.
Simon, H.B. 1984. The immunology of exercise: A brief review. J. Am. Med. Assoc. 252:2735-2738.
Smith, J.A., R.D. Telford, I.B. Mason, and M.J. Weidemann. 1990. Exercise, training and neutrophil microbicidal activity. Int. J. Sports Med. 11:179-187.
Spence, D.W., M.L.A. Galantino, K.A. Mossberg, and S.O. Zimmerman. 1990. Progressive resistance exercise: Effect on muscle function and anthropometry of a select AIDS population. Arch. Phys. Med. Rehabil. 71:644-648.
Tomasi, T.B., F.B. Trudeau, D. Czerwinski, and S. Erredge. 1982. Immune parameters in athletes before and after strenuous exercise. J. Clin. Immunol. 2:173-178.
Tvede, N., J. Steensberg, B. Baslund, J. Halkjaer-Kristensen, and B.K. Pedersen. 1991. Cellular immunity in highly-trained elite racing cyclists and controls during periods of training with high and low intensity. Scand. J. Sports Med. 1:163-166.
Discussion
SUSANNA CUNNINGHAM-RUNDLES: Does the natural killer cell assay use separated cells or whole blood?
DAVID NIEMAN: We are using separate cells. However, we are in transition to a whole blood flow cytometry assay, which is tricky. So we are sticking to the chromium-51 release assay until we get that under control.
HELLEN GREENBLATT: You had mentioned two factors when you mentioned vitamin C. First, you showed data that vitamin C supplementation reduced upper respiratory tract infections by about 50 percent. But then you
showed data that vitamin C did not affect cortisol levels. I was wondering if those were temporal effects where you really cannot compare those studies, or whether those are long-term effects that are more important than short-term?
DAVID NIEMAN: Well, she is asking about, first of all, the epidemiologic research by Edith Peters (Peters et al., 1993) in South Africa, where she showed that vitamin C supplementation would reduce the infection rate during the 2-wk period after an ultramarathon. Now, Edith Peters and I are good friends. She has followed that study up with another, showing the same thing (Peters, 1997). She has found, by the way, that β-carotene has no effect on infection rates. She also found that vitamin E has no effect. It is only with the vitamin C that she has found an effect. So, in light of her research, we conducted a double-blind placebo study where, for 1 week, runners were supplemented with 1,000 mg of vitamin C a day for 8 days prior to coming into the lab, and then we measured cortisol and immune response. I did not show the other slides. But we have measured every key cell of the immune system: neutrophils, monocytes, natural killer cells, B-cells, and T-cells. None of the immune cells were affected by the vitamin C relative to placebo (Nieman et al., 1997a).
Now, Edith Peters, who is an epidemiologist and does not work in the lab with the assays, has her epidemiology showing that vitamin C helps with the infection rates of ultramarathons. In the lab, we are showing it has no effect on the immune response to a simulated marathon. Well, she says that they are doing an ultramarathon and we are doing only a marathon. They are looking at epidemiology, we are looking at immune response. So we are just going to have to sort these things out. She feels that, if we would run the athletes for 5, 6 hours like hers, that maybe vitamin C would have an effect—that the vitamin C pools in the body may be sufficient to keep the immune cells operating at the proper level until you get out there to the ultra-marathon distances. That is her interpretation of the data thus far. But, so far, that is all of the research we have. So it is all speculation right now.
STEVE GAFFIN: I just wanted to mention one other piece of information, and that is that in epidemiological work in the United States, Schwartz and Weiss have shown that lung function is related to vitamin C status in normal individuals—that those individuals in the U.S. population, as part of the NHANES study, had better forced respiratory volume when they were consuming a relatively modest amount of vitamin C, 178 mg, compared with 60 mg. So maybe immune function is not the proper assay to use to look at what is happening in the respiratory area when it comes to viral infections. Maybe there are other assays that need to be looked at.
DAVID NIEMAN: Dr. Munck does these nasal lavages. He feels that if it is an upper respiratory tract problem, so then that is what he studies. I showed you three of his studies showing that there is a marked suppression of immunity in that area. So the question would be would vitamin C help that area? We will have to see. I am not a supplement person. I fight it as much as possible. I am hard to convince that it has an effect. Maybe when you get out to ultramarathon distances, it may become more important in that arena.
WILLIAM BEISEL: It would be nice to find a unifying theory. One thing that cortisol does is it is a very important hormone for gluconeogenesis. In these heavy activities, the only fuel you are going to have is neutrophil production. So it would be interesting to make certain or see that the mechanism for the cortisol is just purely a way to mobilize the fuel rather than being a component of a stress response that is not due to a cytokine. Because it would then suggest that that would be the mechanism to deal with it. It is purely just a mobilization or substrate line.
There was some earlier work with vitamin C and upper respiratory infections (URIs) with nonexercising individuals. Are you using as an index for infection just symptoms of URIs or actual infections. . .
DAVID NIEMAN: Self-report symptoms.
WILLIAM BEISEL: Self-report symptoms? There is good evidence that vitamin C will reduce self-reported symptoms. There is no evidence that I am aware of, though, that it actually reduces infection.
DAVID NIEMAN: I agree with you on that part.
WILLIAM BEISEL: I think when you start talking about ''immune'' you really should make certain of your definition because, if vitamin C does not prevent infection, then reduction in immunity may not be a factor. The decrease in symptoms may have an entirely different etiology.
DAVID NIEMAN: I agree with you 100 percent. When we are at the epidemiology level, it takes many more years of research to figure it out.
Now, I do want to respond to the first question there, which was on the mechanism of a carbohydrate supplement. There have now been a growing number of both animal and human studies showing very carefully that the stress hormone response carefully tracks the blood glucose response to exercise. So we feel that anything that can keep the blood glucose response at a near flat-line
level, should attenuate the stress hormone response and then, in our hands, we have shown that then the immune system is less suppressed. So everything is multifactorial. But the data thus far point towards a blood glucose response.
WILLIAM BEISEL: You are saying it is very important to define that. I would certainly measure that. But I would also measure the other major hormones for gluconeogenesis, which under that setting would be glucagon, and see whether the glucagon levels are also reproducible during this period of time.
DAVID NIEMAN: Dr. John Smith from Australia has looked at glucagon. Indeed, it has the same lessened response with the carbohydrate.
WILLIAM BEISEL: Because what it [measuring glucagon] would then do for you is it would indicate whether this is a separate phenomenon from what we would need to look at if people started talking about lymphocytes causing the release of IL-1 and so forth, which also causes cortisol release. But it is that cortisol release in that setting, which has a very, very different implication.
RANJIT CHANDRA: My question relates to the data on chronic exercise on resting immune responses. I feel that it partly depends on the subject at issue. For instance, if you are dealing with young individuals, with near normal responses to begin with, then you do not expect them to enhance that, even though they are sedentary to begin with. If you deal with, say, a population of elderly individuals who for a variety of reasons, including a sedentary lifestyle, have reduced immune responses, then chronic exercise for 4 to 6 months, regular exercise does have a partial or significant effect on resting immune responses and on the incident of common infection.
DAVID NIEMAN: Yes.
RANJIT CHANDRA: The question I have is whether in the studies that you have done or reviewed, coincident with chronic exercise, nutrient intakes were measured?
DAVID NIEMAN: Yes.
RANJIT CHANDRA: You did refer to one study where mood changes and other psychological indices were looked at. Both of those factors, among others, may have an effect on the immune response.
DAVID NIEMAN: Yes. We have conducted three randomized, controlled training studies for 12 to 15 weeks, one with elderly women and now two with women in their 30s and 40s, who are overweight. In all of those studies, we measured psychological mood changes and dietary intake, along with immunity and infection. Even with the elderly women, 12 weeks of walking 40 minutes 5 days a week, was an insufficient stimulus to do anything to resting immunity. It had no effect whatsoever. But, when we compared those highly conditioned, lean elderly women with their sedentary peers, where the ![]() O2max of the highly conditioned group was 67 percent higher, which is a huge separation, then we found the natural killer cell activity was elevated. We feel that the natural killer cell activity is only elevated in the most extreme comparisons [of highly active vs. sedentary individuals] and that, as soon as you get into the middle there is nothing.
O2max of the highly conditioned group was 67 percent higher, which is a huge separation, then we found the natural killer cell activity was elevated. We feel that the natural killer cell activity is only elevated in the most extreme comparisons [of highly active vs. sedentary individuals] and that, as soon as you get into the middle there is nothing.
ROBERT NESHEIM: We will have one last question, and then we could continue the discussion at the end of the afternoon's program. We will take one more and then we will move on.
GERALD KEUSCH: I want to focus in on one issue. It gets at the functional significance of some of these measurements, the neutrophil. You measured neutrophil number and circulation under circumstances in which the hormonal environment had altered the number of circulating neutrophils. Those of us who come from a background of infectious disease do not believe that the neutrophil does anything in circulation. It acts on the surface or within tissue. So changes in the number do not mean anything to me without knowing what the redistribution is unless there is an activation event that has altered the functional capacity of those cells. Measuring the number alone is not a marker to me of anything of importance, although I believe the changes are obviously real.
It might be worthwhile to try and focus on things that you could measure in the circulation like primary and secondary granular products. You can measure lactoferrin in the circulation. There should not be very much there under normal circumstances; but if you are activating the neutrophils in a way that is altering their functional capacity, you might find elevations in lactoferrin or elastase or something of that nature.
I think that just looking at the number is not going to get us very far in understanding what is going on. Under no circumstances do I believe that the changes in neutrophils would have anything to do with viral infection.
DAVID NIEMAN: Well, we agree 100 percent. You see, with the neutrophil/lymphocyte ratio, trainers of race horses have used that index as a simple way to see how stressed the animal is. Because of the pretty good correlation between rises in cortisol and that ratio, it is used as an index of the
stress that the animal or the human is going through. So that is the only purpose there.
Now, I did not show slides. We have measured neutrophil and monocyte phagocytic function and oxidative burst activity in response to 2 1/2 hours of running. The phagocytic response goes way up, 85 percent elevation in phagocytic response of Staphylococcus aureus bacteria using flow cytometry. Whereas, the oxidative burst activity is significantly reduced. So we personally feel that this represents the inflammatory response.
At the same time, IL-6, which we have measured, goes up sixfold. So the IL-6 is way up. The phagocytic response is way up. Oxidative burst activity is down. We feel that that is just representing the inflammatory response that is going on.
| This page in the original is blank. |




























