Summary
Establishing priorities for the development of vaccines against diseases prevalent in developing countries is complicated by large variations in the morbidity and mortality arising from such diseases, in their geographic distribution, in the extent of knowledge about relevant pathogens and host responses, in the resources and time required for vaccine development, and in anticipated vaccine efficacy and extent of use. This report presents a comprehensive method designed to help government decision makers set priorities for accelerated development of vaccines against important diseases in the developing world. The method can be used to assess new vaccine candidates or to reassess current contenders as additional information becomes available. The primary utilization of many of these vaccines would be for reducing morbidity and mortality in developing countries. Other uses would be for U.S. travelers to such countries, for military and other personnel stationed in them, and for response to the importation of these diseases (e.g., dengue fever) and their transmission in the United States.
The decision-making approach suggested uses similar kinds of information about the occurrence and importance of events that theoretically could be used in other methods of decision making. Because information is incomplete and because the method entails, in some instances, predicting the future, gaps must be filled by estimates or judgments by experts.
The procedures described in this report bear many similarities to those in the committee’s first report on vaccines for diseases of importance in the United States. However, the reader is cautioned that there are certain significant differences in the approach adopted for this assessment. In the former analysis, the calculations were aimed at estimating real, expected benefits and net costs associated with the vaccine candidates. In this analysis, benefit values represent potential benefits, and potential expenditures on vaccines are the only cost component evaluated (cost savings from treatment averted are not assessed). One consequence of these differences is that any attempt to compare values between reports is not valid.
Providing a structural framework within which information and judgments are used and combined does not of itself improve the quality of currently available information (although further research to generate new data might be guided by such a framework). Nor does it reduce the range of opinion likely to be expressed in predictions, judgments, or estimates (except as issues are more precisely defined).
The committee believes that the system it proposes is the most appropriate for the desired purpose and has implemented it with the best available data and estimates. The committee believes that the system would improve the decision-making process by making it more accessible to evaluation and reconstruction by other decision makers and by facilitating examination of the effect of alternative assumptions or estimates. However, some cautions and comments are needed to prevent misinterpretation of the power and precision of the method.
To identify the components on which quantitative information is desirable (though not necessarily available), the system (more than others) exposes areas of ignorance and uncertainty in which expert judgment, by necessity, must be used. The proposed approach uses equations to define the way in which information or estimates are combined (something not always specified in other approaches); this does not imply that the components or the results have the accuracy sometimes associated with formal mathematical calculations. The results are simply the consequence of combining both factual and uncertain quantities, both objective and subjective elements, that are an inescapable part of reaching conclusions about the preferred investments in new vaccines.
A quantitative structured model facilitates examination of the effect of uncertainty (in data and estimates) in a way that intuitive integration of such components does not. This is expressed in the sensitivity analyses reported in the study.
All processes in which diseases are ranked by importance involve value judgments on disease conditions; incorporation of value judgments may be explicit, implicit, or unrecognized. These judgments are more subjective than those of a scientific nature. Providing a specific point at which the required value judgments are described and incorporated is one means of isolating these differences of opinion (which are often incorporated into decision making in an ill-defined way) and determining if they affect the ultimate priorities.
The committee considered these problems, resolved differences of opinion, and sought agreement on the approach it would follow in this complex area. When information was incomplete or quantitative prediction was complicated by many unresolved issues, it chose what it believed was the most rational approach to selecting priorities, recognizing that exact data on all components required by the system would not be available before decisions had to be made. Because of the uncertain data and estimates used in the calculation of health benefits and costs, the final numerical rankings are useful as they relate to each other rather than because of their absolute precision. That is, the system facilitates comparison of vaccine projects in a way that is open to revision if different estimates or assumptions seem appropriate and as new data become available.
The proposed model is based on comparison of the relative potential health benefits calculated for each vaccine candidate. Calculations of potential expenditures on vaccines to achieve these benefits—representing “affordability”—are also made and can be incorporated into the decision process, if desired. This approach combines elements of decision analysis and cost-effectiveness analysis. The approach was selected by the committee because it identifies each logical component contributing to vaccine benefits and costs* without placing a monetary value on human life or suffering. The approach requires substantial amounts of information about diseases and vaccine candidates. Committee members believe that the activity of gathering this information is beneficial in itself; it strengthens the decision-making process and highlights areas in which more research is needed. Chapter 2 describes four other approaches to establishing priorities that were considered and judged less satisfactory.
It should be emphasized that the proposed system is designed as an aid to decision making and not as a definitive answer to the selection process. Rather than merely providing a single list of priorities, the committee also demonstrated with sensitivity analyses how different rankings could result from the adoption of various viewpoints on the affordability of benefits, on the undesirability of illness or death in specific age groups, or from assumptions about disease incidence, the possible effect of new treatments, and other factors that cannot be predicted with certainty.
Several other nonquantifiable issues, all of which concern the policymaker, also must be incorporated into the final judgment on vaccine priorities. These include:
-
goals of the responsible agency and its schedule for achieving them
-
ethical questions on the distribution of benefits between socioeconomic or age groups, countries, or regions
-
most appropriate time at which the agency can exert influence and the opportunity and need for such influence
-
extent of private sector activities
-
the desired balance of the development portfolio (e.g., pediatric versus adult vaccines, global versus regional diseases)
-
arguments that can be made for treating certain vaccine development projects as unique because of their potential for facilitating immunization programs in general (e.g., by eliminating constraints on delivery, such as poor stability) or by improving public confidence (e.g., by reducing adverse reactions)
-
the prospect that a particular project may serve as a useful model for a number of other desired vaccines
-
disease related factors, such as epidemiologic and clinical characteristics likely to overwhelm medical services, and the
-
availability of alternative control strategies or safe and effective therapy
-
possible synergistic interaction with other diseases
-
the immediate U.S. interest in diseases that may be imported into the United States, that threaten travelers or personnel stationed overseas, or that are existing problems in the United States
The committee sought to develop a flexible system that could be updated as necessary. This required identifying explicitly all assumptions, estimates, and predictions incorporated in each calculation. Numerical values incorporated into the calculations represent the committee’s best efforts to develop the necessary information. It is recognized that scientific opinion differs on some of the judgments and that uncertainty surrounds other factors, for example, probable vaccine efficacy and disease incidence. The final format allows users of the system to perform sensitivity analyses in which an estimate or prediction in a specific area, such as the probability of success, can be varied systematically across its plausible range to examine its impact on the final result. Some sensitivity analyses are discussed in Chapter 9.
Chapter 3 presents an overview of the approach used in this report. It also identifies certain concepts and basic assumptions that are used throughout the study. For example, if a candidate vaccine is omitted from the full analysis, no conclusions should be drawn regarding its position relative to the assessed contenders. The assessment is conducted from an aggregate perspective for the developing world as a whole, and each development project is treated as an independent investment decision. Effects of morbidity and mortality are expressed in nonmonetary terms.
This report does not make a judgment about the number of vaccines that are worthy of development. It also does not attempt to compare the benefits of basic research with those of vaccine development.
SELECTION OF CANDIDATES
The committee defined candidates for accelerated development as those for which success was reasonably foreseeable within the next decade. The criterion for inclusion was whether a reasonable consensus could be identified on the nature of potential vaccine components (protective antigens). A more detailed description of the selection process appears in Appendix A.
The diseases and vaccine candidates chosen for assessment are shown in Table 1.1. Detailed information about individual candidates is presented in Appendixes D-1 through D-19. The committee and its advisers reviewed the prospects for immunizing against a number of major diseases for which accelerated vaccine development was ultimately judged not to be feasible or appropriate at this time. That information will be included in a supplement to this volume (see Appendix I). The supplement also will briefly describe some newer techniques that are likely to be increasingly applied to vaccine
TABLE 1.1 Candidates for Accelerated Vaccine Development: Diseases of Importance in Developing Countries
|
Pathogen |
Vaccine Envisaged |
Target Populationa |
|
Dengue virus |
Attenuated live vector virus containing gene for broadly cross-reacting protective antigen |
Infants and children in endemic areas; travelers to endemic areas |
|
Escherichia coli (enterotoxigenic) |
A combination of purified colonization factor antigens and possibly other antigens |
Infants<6 months |
|
|
Genetically engineered attenuated strains |
Infants<6 months |
|
Hemophilus influenzae type b |
Conjugated polysaccharide |
Infants |
|
Hepatitis A virus |
Attenuated live virus |
Susceptibles of all ages; routine for preschool children |
|
|
Polypeptide recombinant vaccine produced in yeast |
Susceptibles of all ages; routine for preschool children |
|
Hepatitis B virus |
Polypeptide produced by recombinant DNA technology |
Areas with high perinatal infection: all infants at birth (if possible). Other areas: all infants, simultaneous with other vaccinations, at earliest possible age |
|
Japanese encephalitis virus |
Inactivated virus produced in cell culture |
Children in epidemic and endemic areas; foreign visitors to epidemic regions |
|
Mycobacterium leprae |
Armadillo-derived M.leprae |
Immuno-prophylactic: all children in endemic areas. Immuno-therapeutic: all recently infected persons |
|
Neisseria meningitidis |
Conjugated capsular polysaccharides, Groups A,C,Y, and W135 |
Infants, 3 to 6 months |
|
Parainfluenza viruses |
Trivalent, subunit vaccine (which must contain fusion proteins) |
Infants |
|
Plasmodium spp. |
Plasmodium falciparum, synthetic or recombinant sporozoite antigen preparation |
All infants at risk, military personnel, travelers |
|
|
Multivalent synthetic or recombinant sporozoite antigen preparation (P.falciparum, P.vivax, P.ovale, P.malariae) |
All infants at risk, military personnel, travelers |
|
Rabies virus |
Vero cell derived vaccine |
Persons at high risk, plus post-exposure prophylaxis |
|
|
Glycoprotein produced by rDNA technology in mammalian cells |
Persons at high risk, plus post-exposure prophylaxis |
|
|
Attenuated live vector virus containing gene for protective glycoprotein antigen |
Birth cohort in areas of high risk |
|
Pathogen |
Vaccine Envisaged |
Target Populationa |
|
Respiratory syncytial virus |
Polypeptides produced by recombinant DNA technology |
Infants at earliest possible age |
|
|
Attenuated live virus |
Infants at earliest possible age |
|
Rotavirus |
Attenuated high passage bovine RV |
Infants at earliest possible age (preferably with oral polio vaccine) |
|
|
Attenuated low passage bovine RV |
Infants at earliest possible age (preferably with oral polio vaccine) |
|
|
Rhesus monkey RV |
Infants at earliest possible age (preferably with oral polio vaccine) |
|
Salmonella typhi |
Attenuated ga1E mutant S. typhi strain TY21a |
Children; young adults at risk; travelers from developed countries to endemic areas |
|
|
Aromatic amino acid dependent strains of S. typhi |
Children; young adults at risk; travelers from developed countries to endemic areas |
|
Shigella spp. |
Probably plasmid mediated outer membrane protein invasion determinant (there are a small number of promising options needing investigation to determine best approach) |
Infants at birth or earliest possible age; elderly for epidemic strains |
|
Streptococcus A |
Synthetic M protein segment (excluding portions cross-reacting with human tissue) |
Children, <3–4 yrs |
|
Streptococcus pneumoniae |
Conjugated polysaccharides, polyvalent |
Infants |
|
Vibrio cholera |
Genetically defined live mutant V. cholerae (A−B+ or A−B−) with respect to toxin subunit synthesis |
Children, esp. <2 yrs |
|
|
Inactivated antigens |
Children, esp. <2 yrs |
|
Yellow fever virus |
Attenuated live virus produced in cell culture |
Young children |
|
aCalculations of benefits are conducted assuming delivery at ages consistent with schedules of vaccinations recommended by the World Health Organization Expanded Program on Immunization (see Chapters 6 and 7). |
||
development in the coming decade. The vaccine-disease combinations to be described in the supplement should be regularly reviewed for possible inclusion in future applications of the model.
DETERMINATION OF HEALTH BENEFITS
To compare diseases and vaccines, it was necessary to develop a system that would allow expression of the total morbidity and mortality
associated with each disease as a single number.* The system that evolved, described in Chapter 4, consolidates information on the annual numbers of illness episodes and their durations, with additional data on related complications, sequelae, and deaths. (For chronic disability, the system incorporates the annual increment to the pool of individuals affected.) It also incorporates value judgments on the undesirability (disutility) of various conditions occurring in various age groups.
Disease Burden Estimates
Whenever possible, global or regional information from the World Health Organization or other knowledgeable sources was used as the starting point for the disease burden estimates needed in this analysis. For many conditions, however, information needed to estimate disease burdens was not available or was not of the desired reliability; in these cases, calculations were based on judgments and assumptions made by committee members and staff with the aid of consultants. Table 1.2 presents an example of the format used to consolidate disease burden information.
Infant Mortality Equivalence Values
An important feature of the system is that it allows the user to change the perspective on disutility of disease consequences to any level desired and to observe the effect of this change on the rankings of candidates. The undesirability of conditions for morbidity category/ age group combinations are expressed as infant mortality equivalence (IME) values, that is, the number of acute morbidity days or chronic cases considered to be equal in undesirability to an infant death. The perspective used as an example throughout this report reflects the median of responses from public health professionals in a variety of developing countries (elicited by means of a questionnaire—see Appendix E). The median values are shown in Table 1.3. Other perspectives could be used, for example, an “age-neutral” perspective. The effect on rankings of using other perspectives is discussed. The committee, however, does not endorse any particular perspective for policy formulation in this area. Different rankings may result from the adoption of different viewpoints on the undesirability of illness and death at different stages of life.
|
* |
See Appendix F for information on the computer software used in this analysis. |
TABLE 1.2 An Example of the Format Used to Compile Information on the Burden of Illness Arising from infectious Diseases in Developing Countries: Hepatitis A Virusa
|
|
<5 Years |
5–14 Years |
15–59 Years |
60 Years and Over |
||||||
|
Morbidity Category |
Description |
Condition |
Number of Cases |
Duration (days) |
Number of Cases |
Duration (days) |
Number of Cases |
Duration (days) |
Number of Cases |
Duration (days) |
|
A |
Moderate localized pain and/or mild systemic reaction, or impairment requiring minor change in normal activities, and associated with some restriction of work activity |
|
||||||||
|
B |
Moderate pain and/or moderate impairment requiring moderate change in normal activities, e.g., housebound or in bed, and associated with temporary loss of ability to work |
Jaundice, including convalescence from category C (total) |
139,843 |
7 |
635,651 |
7 |
2,256,561 |
7 |
149,378 |
7 |
|
C |
Severe pain, severe short-term impairment, or hosptalization |
Severe hepatitis |
31,735 |
14 |
158,675 |
14 |
1,221,794 |
14 |
206,277 |
14 |
|
D |
Mild chronic disability (not requiring hospitalization, institutionalization, or other major limitation of normal activity, and resulting in minor limitation of ability to work) |
|
n.a. |
|
n.a. |
|
n.a. |
|
n.a. |
|
|
E |
Moderate to severe chronic disability (requiring hospitalization, special care, or other major limitation of normal activity, and seriously restricting ability to work) |
|
n.a. |
|
n.a. |
|
n.a. |
|
n.a. |
|
|
F |
Total impairment |
|
n.a. |
|
n.a. |
|
n.a. |
|
n.a. |
|
|
G |
Reproductive impairment resulting in infertility |
|
n.a. |
|
n.a. |
|
n.a. |
|
n.a. |
|
|
H |
Death |
Death following fulminant hepatitis |
n.a. |
1,144 |
n.a. |
5,146 |
n.a. |
8,005 |
n.a. |
|
|
aSee Appendix D-4 for derivation. |
||||||||||
TABLE 1.3 Infant Mortality Equivalence Valuesa: The Medians of Values from Health Professionals in Developing Countries
|
|
Age Group |
|||
|
Morbidity Categoryb |
Under 5 Years |
5–14 Years |
15–59 Years |
60 Years and Over |
|
A |
40,000 |
32,500 |
8,750 |
50,000 |
|
B |
23,713 |
17,500 |
5,650 |
25,000 |
|
C |
2,000 |
2,244 |
2,000 |
5,000 |
|
D |
75 |
62.5 |
30 |
550 |
|
E |
5.5 |
5.5 |
2.75 |
23.125 |
|
F |
1 |
0.4 |
0.309 |
5 |
|
G |
100 |
22.5 |
16.5 |
300 |
|
H |
1 |
0.5 |
0.4 |
5 |
|
aInfant mortality equivalence is the number of acute morbidity days or chronic cases in each morbidity category/age group combination that are considered to be equal in undesirability to the death of an infant. bMorbidity categories are defined in Table 1.2. |
||||
Total Disease Burden Values
The system that has been developed provides a means for comparing diseases, as well as a method for comparing vaccines. The total disease burden value (TDBV) indicates the relative importance of each disease expressed in units equivalent to the undesirability of an infant death (infant mortality equivalents). It is calculated in a stepwise fashion from subtotals for each morbidity category/age group combination. It incorporates the number and duration (for acute episodes) of cases and the infant mortality equivalence values. This process permits the comparison of diseases having different consequences and can be used as described in Chapter 7 to compare vaccine benefits.
VACCINE CHARACTERISTICS
Predictions of Vaccine Development
Committee discussions, supplemented by consultations with outside experts, led to the development of specific predictions or estimates for each vaccine in the following areas:
-
probability of successful vaccine development
-
time to licensure
-
time after licensure to adoption in immunization programs future cost of development up to licensure
-
protective efficacy
-
incidence of adverse side effects
-
route of administration
-
number of doses
-
cost per dose
-
delivery requirements
-
technical difficulty of production
These factors have been incorporated into the calculations of potential health benefits and expenditures on vaccines. Chapter 5 presents in tabular form the specific estimates for each of the vaccine candidates, which are discussed in Appendixes D-1 through D-19.
Definition of Probable Vaccine Target Population
The determination of a probable vaccine target population for each vaccine was based on the age and geographic distributions of the relevant disease and the relative risk of illness. Whenever possible, the target population was matched with one already employed by the World Health Organization’s Expanded Program on Immunization (WHO-EPI) , for reasons discussed below.
Treatment of Vaccine Utilization
The benefits derived from a vaccine depend, in part, on the proportion of the target population that actually receives it, which may vary among vaccines. However, for the analysis described in this report, the committee assumed that the utilization rates would be uniform across target populations, because delivery would probably be achieved through the WHO-EPI. Its vaccination schedule recommendations are intended to be adapted to local conditions and constraints. WHO-EPI flexibility in this regard may establish new opportunities for optimizing the delivery of vaccines considered in this analysis (see Chapter 6). Actual decisions to incorporate specific vaccines into EPI should be based on local assessments of disease burden, resources, and other considerations. The methods used in the committee’s previous report (Institute of Medicine, 1985) can be adapted to situations in which utilization is likely to differ between vaccines. In this report, however, utilization is not used to differentiate among vaccine candidates.
Estimation of Time to Licensure, Time to Vaccine Adoption, and Delay of Vaccination Benefits: Discounting
Various vaccines require various amounts of time for development to licensure, and after licensure before wide incorporation into general
immunization efforts (i.e., adoption). In addition, the health benefits from vaccines are realized at different times after vaccination.
These factors affect the time interval before the health benefits associated with a vaccine and certain cost outlays (such as vaccine purchase for immunization programs) will reach a steady state and are used to determine the annualized “present value” of results that will be achieved at various times in the future.
The process by which benefits and costs that are delayed for some years are converted to their current equivalent value is termed “discounting.” This procedure enhances the relative importance of effects realized after a short delay as compared with a long one. In the central analysis, the discount rate used is 0.05. The effect of discounting at different rates is examined in Chapter 9.
Estimation of Vaccine Preventable Illness
Vaccine preventable illness (VPI) is defined as that portion of the disease burden that is preventable by delivery to the entire target population, at the anticipated age of administration, of a hypothetical vaccine that is 100 percent effective.
Calculation of Potential Health Benefits for Each Vaccine
Chapter 7 describes the integration of the components outlined above to derive the annualized present value of the potential health benefits for each vaccine candidate. Figure 1.1 summarizes the basic steps used in the analysis. (When it is believed that vaccines may have different utilization rates within their respective target populations, predicted rates can be used as an adjustment in this calculation process and the values derived would represent expected, rather than potential, health benefits.)
Cost Calculations
A comprehensive assessment of the expected net costs associated with the use of vaccine candidates would require the calculation of the cost of vaccine development, the cost of the immunization program including vaccine administration, the cost savings from treatment averted, and the cost of adverse reactions. Procedures to perform these calculations are outlined in this report and in more detail in the committee’s first report (Institute of Medicine, 1985).
For this analysis, however, the committee judged that it was not practical to attempt to estimate the costs associated with treating disease or the potential savings from treatment averted by vaccines. The committee believed that it would be extremely difficult and probably unrealistic to estimate for the developing world as a whole the proportion of cases, complications, and sequelae that receives treatments, the nature of those treatments, and their average costs.
Additionally, the committee assumed that utilization of vaccines would be through the WHO-EPI and at a uniform rate. Hence, the cost of delivery and administration would not be a factor in differentiating among vaccines.
These judgments lead to simplification of the cost components of this analysis: the cost of vaccine development and the cost of vaccine for immunization programs (see Figure 1.2). These “expenditures on vaccine” can, if desired, be included as a criterion in the decision process, representing the composite “affordability” of disease control efforts.
However, these estimated expenditures are only relative, not absolute, because they exclude elements assumed to be uniform, that is, administration and utilization. Further, the omission of cost savings, which may vary between diseases, means that the estimates do not indicate the net cost of the total effort to control a given disease by immunization. Net costs may in fact be negative; that is, a vaccine can be cost saving.
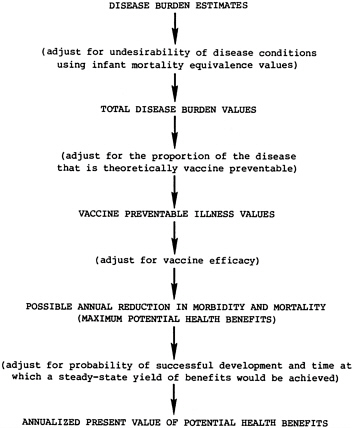
FIGURE 1.1 Calculation of potential health benefits.
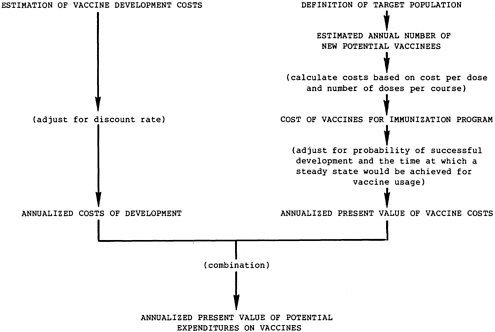
FIGURE 1.2 Calculations of expenditures on vaccines.
INTERPRETATION OF RESULTS
The process of ranking vaccines in order of desirability by using the health benefit, expenditure, and other information developed with this method depends on the ultimate goals of the exercise and constraints that limit the number of candidates that may be selected. The committee proposes that the potential global health benefit of a vaccine take precedence in determining its initial ranking for accelerated development priority. The affordability of benefits, represented by the relevant expenditures on vaccine, can also be introduced into the decision process, if desired. This is illustrated in Chapter 9 where the effect on the health benefit rankings of various levels of constraint on financial resources is examined.
Chapter 3 provides a detailed description of the procedures that can sometimes be used to establish priority rankings if two equally important selection criteria are used. These are based on the concept of dominance: if a vaccine candidate is more desirable than another on one dimension (either potential health benefits or expenditures on vaccine—lower is preferable) and at least as good on the second dimension, then it dominates the other candidate.
Chapter 8 identifies some additional issues that should be considered in the final selection of priorities for accelerated vaccine development. These include the distribution of benefits from new
vaccines among geographic or other population subgroups; various disease characteristics, such as susceptibility to control by other available means, ease and cost of treatment, synergistic interaction with other diseases, and epidemic potential; and the need and opportunity for intervention to accelerate development. The last of these will be influenced by the extent of interest from commercial companies and the possibilities for development via collaborative efforts with industry, other countries, or international organizations.
FINDINGS, CONCLUSIONS, RECOMMENDATIONS
Table 1.4, drawn from Chapter 4, shows a ranking of the burdens of illness resulting from the diseases for which vaccine candidates are assessed.
Chapter 9 presents the committee’s findings on the potential benefits and expenditures associated with the development of specific vaccines and the implications of some sensitivity analyses.* The resulting priority list is not meant to dictate the actions of government decision makers or groups (e.g., in other countries) who may be examining priorities from other perspectives. The principal focus of the committee’s efforts was to provide a flexible, reproducible technique for the assessment of vaccine development projects.
The analyses indicate that of the 29 projects considered, vaccines for S. pneumoniae, Plasmodium spp. (malaria; both monovalent and multivalent circumsporozoite protein-based approaches), rotavirus (all three candidates), S. typhi (Ty21a), and shigella consistently rank among the top 10 positions in priority lists based on potential health benefits under a wide range of assumptions and resource availabilities (Table 1.5 and Chapter 9). As willingness to pay to obtain health benefits drops to $1,000 or below per IME prevented, the rankings change more significantly.
Vaccines for hepatitis B and H. influenzae type b rank in the top 10 in the central analysis but are dislodged under certain assumptions. Vaccines for E. coli (either candidate) or the alternative candidate for S. typhi (an aromatic amino acid-requiring strain) move into the top 10 under certain assumptions.
A fairly consistent “middle-tier” is present in the potential health benefit ranking under a variety of assumptions. in addition to the candidates that will contend for higher ranking under certain assumptions, this group includes vaccines for Streptococcus group A, M. leprae, V. cholerae, respiratory syncytial virus, parainfluenza, and rabies (Vero cell derived or glycoprotein).
Table 1.6 shows the findings for a wide range of assumptions and resource availabilities. Because certain vaccines may enter other
TABLE 1.4 Ranking of Diseases by Total Disease Burden Values
|
Disease |
Total Disease Burden Value (IME units)a |
|
Streptococcus pneumoniae |
6,612,261 |
|
Hepatitis B virus |
2,394,256 |
|
Plasmodium spp. |
2,111,795 |
|
Salmonella typhi |
1,308,121 |
|
Escherichia coli |
978,248 |
|
Rotavirus |
925,042 |
|
Shigella spp. |
828,068 |
|
Streptococcus Group A |
811,477 |
|
Mycobacterium leprae |
657,349 |
|
(Escherichia coli) |
(550,248)b |
|
(Rotavirus) |
(488,542)b |
|
Hemophilus influenzae type b |
471,336 |
|
Vibrio cholera |
229,217 |
|
Respiratory syncytial virus |
183,326 |
|
Parainfluenza virus |
145,954 |
|
Neisseria meningitidis |
68,252 |
|
Rabies virus |
67,821 |
|
Dengue virus |
34,365 |
|
Yellow fever virus |
32,887 |
|
Hepatitis A virus |
30,229 |
|
Japanese encephalitis virus |
18,075 |
|
aInfant mortality equivalence units. bValues represent the anticipated disease burden from certain diarrheal pathogens if a plausible increase in oral rehydration therapy is assumed (see Appendix C) . |
|
categories if different, plausible assumptions are adopted, the assignments in Table 1.6 should not be regarded as definitive.
Most of the vaccines that consistently rank low (as compared with the other candidates in this assessment) would prevent diseases that, although often serious, are found in relatively small regions of the developing world. In such areas they may have considerable benefit relative to the more widespread diseases that rank higher when the developing world is considered as a whole.
Final decisions on the number of vaccines to be selected for accelerated development and on the ultimate choices should be addressed in a broader political/public policy forum, after consideration of the issues identified above and discussed in Chapters 8 and 9.
Scientific opinion differs on some of the judgments incorporated into the proposed method, and uncertainty surrounds certain data (e.g., on disease incidence) or the predictions (e.g., of efficacy). When data are unavailable, expert judgments are required. The attempt to be explicit about certain estimates should not be interpreted as indicating that a high degree of precision, unanimity, or certainty in
TABLE 1.5 The Effect of Resource Constraints on the Ranking of Various Vaccine Candidates
|
|
Rank Based on Annualized Present Value of Potential Health Benefits Adjusted for Opportunity Costsa |
||||
|
|
Willingness to Pay (dollars) per IME Averted |
||||
|
Vaccine |
Unrestricted |
100,000 |
10,000 |
1,000 |
500 |
|
S. pneumoniae |
1 |
1 |
1 |
5 |
–b |
|
Rotavirus (HPBRV) |
2 |
2 |
2 |
– |
– |
|
Malaria (monovalent) |
3 |
3 |
6 |
– |
– |
|
Rotavirus (LPBRV) |
4 |
4 |
4 |
– |
– |
|
Rotavirus (RMRV) |
5 |
5 |
5 |
– |
– |
|
S. typhi (Ty21a) |
6 |
6 |
3 |
3 |
– |
|
Malaria (multivalent) |
7 |
7 |
7 |
– |
– |
|
Shigella |
8 |
8 |
8 |
1 |
2 |
|
Hepatitis B |
9 |
13 |
– |
– |
– |
|
H. influenzae b |
10 |
9 |
10 |
– |
– |
|
S. typhi (aa-strain) |
11 |
10 |
9 |
6 |
– |
|
Streptococcus group A |
12 |
11 |
12 |
– |
– |
|
E. coli (attenuated live) |
13 |
12 |
11 |
2 |
3 |
|
E. coli (purified antigens) |
14 |
14 |
16 |
– |
– |
|
V. cholera (attenuated live) |
15 |
15 |
13 |
4 |
1 |
|
M. leprae |
16 |
16 |
14 |
– |
– |
|
V. cholera (inactivated) |
17 |
17 |
15 |
7 |
– |
|
RSV (attenuated live virus) |
18 |
18 |
– |
– |
– |
|
RSV (glycoprotein) |
19 |
21 |
– |
– |
– |
|
Parainfluenza viruses |
20 |
22 |
– |
– |
– |
|
Rabies (Vero cell derived) |
21 |
19 |
17 |
– |
– |
|
Rabies (glycoprotein) |
22 |
20 |
18 |
– |
– |
|
Hepatitis A (attenuated live virus) |
23 |
27 |
– |
– |
– |
|
Hepatitis A (polypeptide) |
24 |
– |
– |
– |
– |
|
N. meningitidis |
25 |
26 |
– |
– |
– |
|
Yellow fever virus |
26 |
23 |
20 |
– |
– |
|
Dengue virus |
27 |
25 |
– |
– |
– |
|
Rabies (live vector virus) |
28 |
24 |
19 |
– |
– |
|
Japanese encephalitis virus |
29 |
– |
– |
– |
– |
|
aRankings are based on values shown in Table 9.3. b– denotes not affordable at indicated willingness to pay. |
|||||
comparisons is possible in this situation. Hence, additional sensitivity analyses are suggested (see Chapter 9) to provide further information on key elements that may alter decisions. These include study of alternative IME profiles and variation in other factors for individual vaccines, such as the number of required vaccine doses or the probability of success. Assessments involving alternative assumptions on the choice of target populations also are desirable.
Data needed for disease comparisons are lacking in some areas and are of variable quality in others. Additionally, data on the pathogen serotypes prevalent in particular regions may be lacking. Better data bases in these matters would facilitate rational choices on vaccine development priorities and vaccine formulations. Therefore, NIAID and
TABLE 1.6 Summary of Findings: Rankings of Various Vaccine Candidates Based on Their Potential Health Benefits Under a Variety of Assumptions and Resource Constraints
other national and international organizations should consider means to improve available epidemiological data on infectious diseases.
As a group, the vaccines assessed in this report are generally further from licensure than those evaluated in the first volume of the committee’s report (Institute of Medicine, 1985). Additionally, for most there appears to be less commercial interest in their development (although this is sometimes difficult to ascertain). The committee therefore recommends to NIAID and other federal agencies the careful examination of opportunities to accelerate the development and availability of vaccines identified here as meriting high priority. Although this report nominally addresses vaccines for the developing world, many of those assessed (e.g., S. pneumoniae) would considerably benefit the population in the United States.
The committee believes that a major strength of this analysis is that it encourages those using it to examine all judgments and assumptions in the decision process. The committee recommends use of the proposed system by government decision makers. New candidates should be assessed as they become technically feasible and new data should be incorporated as they become available.
After the committee achieved consensus on vaccine development predictions (late summer 1985) preliminary unpublished results from certain ongoing studies came to their attention. These results, if confirmed, may slightly alter the predictions on some vaccine candidates, particularly on candidates targeted against the same pathogen relative to each other, e.g., as for cholera and rotavirus. The committee did not conduct calculations based on the preliminary information but believes it would not significantly alter the overall conclusions described above; it recommends early reappraisal of candidate ranking as data from ongoing studies is publicly reported.
An improved vaccine for hepatitis B virus (a polypeptide produced by recombinant DNA technology), predicted by the committee to be licensed in 1 year or less, was in fact licensed on July 24, 1986.
REFERENCE
Institute of Medicine. 1985. New Vaccine Development: Establishing Priorities, Volume I. Diseases of Importance in the United States. Washington, D.C.: National Academy Press.


















