4
DNAPLs: Technologies for Characterization, Remediation, and Containment
Chlorinated hydrocarbons are among the most common pollutants in groundwater and soils at Department of Energy (DOE) sites (Riley et al., 1992), as well as other contaminated sites across the United States (Pankow and Cherry, 1996). Other types of dense non-aqueous phase liquid (DNAPL) components, including polychlorinated biphenyls (PCBs), also may be present, but chlorinated solvents are by the far most prevalent (see Table 1-4). Therefore, this chapter focuses on technologies for remediation of chlorinated solvent DNAPLs, although many of these technologies are applicable to other types of DNAPLs as well.
The conventional strategies of excavating soil and pumping and treating contaminated groundwater are generally ineffective at restoration of DNAPL-contaminated sites (NRC, 1994; Pankow and Cherry, 1996). The innovative technologies discussed in this chapter have demonstrated potential for use in remediation of DNAPL-contaminated sites. However, data for these evaluations are limited because only a few well-documented pilot tests on DNAPL sites have been reported.
THE DNAPL PROBLEM
The chlorinated organic compounds that comprise the DNAPLs common at DOE sites have low solubilities in water (Table 4-1).1 As
Table 4-1 Properties of Select DNAPL Components Commonly Found at DOE Sites
a result, when released in the subsurface they typically do not dissolve totally in the groundwater but remain largely as a separate, nonaqueous-phase liquid (NAPL). However, solubilities of these DNAPL components are much higher than drinking water standards, so they create a persistent source of groundwater contamination as they slowly dissolve. The chlorinated solvents that comprise the most common DNAPL components are denser than water (Table 4-1) and tend to sink beneath the water table. These characteristics pose a challenge to all conventional groundwater remediation technologies (NRC, 1994; Pankow and Cherry, 1996).
In the vadose zone (the soil above the water table), DNAPL flows downward under the influence of gravity with relatively little spreading (Schwille, 1988; Pankow and Cherry, 1996). Capillary forces retain a small quantity in each pore (or fracture) through which the DNAPL flows (see Box 4-1). This fraction, which is not mobile under static conditions, is termed residual saturation. Contamination in soils above the water table therefore tends to be both laterally restricted and of relatively low saturation (saturation is defined as the fraction of the pore space filled with DNAPL). Where the water table is deep, however, as at several DOE sites, the total quantity of DNAPL retained in the vadose zone may be large.
Below the water table the distribution of DNAPLs tends to be much more irregular. Entry of DNAPL into water-filled pores requires overcoming a displacement pressure resulting from capillary forces between DNAPL and water (Box 4-1). The required entry pressure increases with decreasing grain size of the solid media in the aquifer (see Table 4-2). The downward flow of DNAPL therefore
|
BOX 4-1 Factors Influencing the Movement of DNAPLs Underground When two fluids are present in one environment, the fluid having a greater affinity for a solid surface tends to spread along the surface; this fluid is termed the wetting phase. Typically, water is the wetting phase relative to both air and DNAPLs, whereas DNAPLs are wetting relative to air. Capillary forces tend to favor the entry of the wetting phase into small pores or fractures. In contrast, capillary forces will resist the entrance of the nonwetting fluid into pores filled with the wetting phase (Pankow and Cherry, 1996). In the saturated zone (the portion of the subsurface below the water table), capillary forces resist the entry of DNAPLs into water-filled pores: the required pressure (displacement pressure) for DNAPL entry increases with decreasing grain size. This has several results, including (1) a tendency for DNAPLs to spread horizontally above finer-grained layers and thus form thin horizontal layers or lenses; (2) a tendency for DNAPLs to follow preferential pathways and thus have a highly inhomogeneous distribution; and (3) the concentration of DNAPL in larger pores. In the vadose zone (above the water table), the presence of air in soil pores allows DNAPLs to move downward without overcoming a displacement pressure. Therefore, above the water table, DNAPLs tend not to spread laterally as readily as they do in the saturated zone. Capillary forces and contact angle also affect the retention of DNAPLs. Capillary forces retain a small fraction of DNAPLs in every pore. The amount retained, referred to as residual saturation, is higher in the saturated zone, where water keeps the DNAPLs away from pore walls, than in the vadose zone. |
may be interrupted each time the DNAPL encounters a layer with a smaller grain size than the overlying one, causing the DNAPL to flow laterally above the fine-grained layer. In such cases, a DNAPL lens may accumulate until it reaches a sufficient thickness of DNAPL
Table 4-2 Examples of the Entry Pressure Required for Downward Migration of Trichloroethylene (TCE) in Different Media
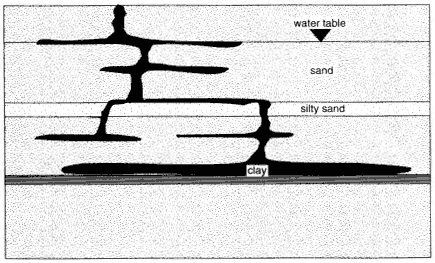
Figure 4-1
Typical distribution of a DNAPL in the subsurface.
to overcome the required displacement pressure. The result is a series of horizontal DNAPL lenses connected by narrow, vertical pathways (see Figure 4-1).
As in the vadose zone, a small amount of DNAPL is retained as residual saturation in every pore through which it flows. If the DNAPL encounters a layer that has a sufficiently high entry pressure, the DNAPL will be retained as a pool on the top of this layer. Thus, DNAPL is typically found in multiple horizontal lenses connected by sparse vertical pathways, with one or more pools above fine-grained layers. Most of the horizontal lenses and vertical pathways will be at or below residual saturation; only pools will have higher saturations. The distinction between residual saturation and pools is important, since only the DNAPL in pools is expected to be mobile. The flow of DNAPL and the resultant distribution are discussed in detail in Pankow and Cherry (1996).
A plume of dissolved contaminants, known as the dissolved-phase plume, will form when groundwater contacts either residual saturation, DNAPL pools, or DNAPL lenses. The volume of soil that contains DNAPL at or above residual saturation is termed the source zone. Removal of the source zone is required for restoration, because, if the source zone remains, groundwater will continue to be
contaminated, typically for very long periods. Some of the remediation technologies discussed in this chapter (summarized in Tables 4-3 and 4-4) are primarily for treating source zones, whereas others are for plumes of dissolved contaminants.
CHARACTERIZATION OF DNAPL CONTAMINATION
The presence of DNAPL makes site characterization both more critical and more difficult due to the irregular distribution of DNAPLs. Site characterization includes characterization of a site's hydrogeology and of contaminant distribution at the site.2 Because the distribution of DNAPLs is controlled by heterogeneities in the soil or rock and because the effectiveness of most technologies is also affected by heterogeneities, careful characterization of the hydrogeology of the site is essential. Technologies for hydrogeological characterization are not unique to DNAPL-contaminated sites and are thus not discussed in this report. Characterization of contaminant distribution includes defining the extent of both dissolved-phase contamination and the DNAPL source areas. Methods for defining dissolved-phase plumes are well established and are not discussed here. Determination of the limits of the DNAPL source zone is necessary both to ensure that all DNAPL is within the containment or treatment area and to minimize the volume to be treated (and hence cost). This section discusses emerging technologies for DNAPL source-zone characterization.
Characterization plans for DNAPL-contaminated sites must consider the risks inherent in penetrating a DNAPL pool. The high density and low viscosity of chlorinated solvent DNAPLs create a significant risk of mobilization if a pool is penetrated by drilling during site characterization. A DNAPL pool perched on a fine-grained layer may drain down a drill hole to lower, previously clean layers. In general, it is inadvisable to drill through DNAPL (Pankow and Cherry, 1996)
Direct-Push Technologies
Push-in tools, including large cone penetrometers, smaller units (e.g., geoprobes), and hand-held samplers driven by hammers, provide an extremely useful method of analyzing sites without drilling wells,
Table 4-3 Treatment Technology Options for DNAPL Source-Zone Remediation
|
Technology |
Mechanism(s) |
Objective |
Potential Application |
Limitations |
|
Steam |
Volatilization, mobilization |
Removal |
Any aquifer unit with adequate permeability |
Must be able to control steam flow and to heat units to appropriate temperatures. Heterogeneities may increase treatment time and produce tailing. Must consider implications of DNAPL mobilization. |
|
Surfactants |
Dissolution, mobilization |
Removal |
Any unit with adequate permeability |
Must be able to establish hydraulic control. Heterogeneities may increase treatment time and produce tailing. Must consider implications of DNAPL mobilization. |
|
Solvents |
Dissolution, mobilization |
Removal |
Any unit with adequate permeability |
Must be able to establish hydraulic control. Heterogeneities may increase treatment time and produce tailing. Must consider implications of DNAPL mobilization. |
|
In situ oxidation |
Chemical reaction |
Destruction |
Any unit with adequate permeability that does not react excessively with reagent |
Must be able to deliver adequate reagent to source zone. Heterogeneities may increase treatment time and produce tailing. Reaction with other compounds in aquifer may reduce effectiveness. |
|
Technology |
Mechanism(s) |
Objective |
Potential Application |
Limitations |
|
In situ vitrification |
Thermal decomposition |
Destruction |
Any site with appropriate depth and groundwater conditions |
Soil must produce appropriate melt. Groundwater volume must not be excessive. Vapor emission must be controlled. Depth limited to about 10 m (30 ft). |
|
Soil vapor extraction |
Volatilization |
Removal |
Units with low soil-water content, volatile contaminants, and adequate permeability |
Must be able to induce adequate air flow through entire source zone. Heterogeneities and high water contents may limit effectiveness. |
|
Air sparging |
Volatilization |
Removal |
Saturated units with volatile contaminants and adequate permeability |
Must be able to induce air flow through entire source zone. Heterogeneities may limit effectiveness. Contaminants must be volatile at groundwater temperatures. |
|
Electrical heating |
Volatilization |
Removal |
Typically, low-permeability units with adequate moisture to provide conductivity |
Volatilized contaminants must be removed by another technology (typically soil vapor extraction or steam). Permeability must be sufficient for vapor flow. |
Table 4-4 Technologies Primarily for Treatment of Contaminants Dissolved from DNAPLs
|
Technology |
Mechanism(s) |
Objective |
Potential Application |
Limitations |
|
In-well stripping |
Volatilization |
Removal |
Saturated units with adequate permeability |
Treatment zone depends on groundwater flow pattern established. |
|
Bioremediation |
Biologically mediated chemical reaction |
Destruction |
Electron acceptor, donor, and nutrients required; specific conditions depend on biodegradation pathways. |
Only aqueous phase can be treated |
|
Reactive barriers |
Chemical reaction |
Dechlorination |
Dissolved phase must be treatable, and water must be chemically compatible with barrier. |
Treats only aqueous phase. Wall must intercept entire plume. Specific limitations depend on barrier type. |
|
Electrokinetic systems |
Electrically induced mobilization |
Mobilization, coupled with another technology for destruction or removal |
Low-permeablity units |
Applications to DNAPL not well established. Must be coupled with technology for destruction or removal of contamination. |
|
Physical barriers |
Containment |
Containment |
Any unit in which barriers can be physically emplaced |
Limitations vary with barrier type. If not keyed into impermeable unit at base, system will be open, and contamination will not be contained. |
which is costly and invasive. Push-in tools can recover or in some cases analyze core samples of soil and samples of water taken at multiple depths during insertion. Core samples provide direct evidence of DNAPL saturation, while water samples can determine the vertical extent of contamination. A thorough characterization requires many cores, each analyzed at small vertical intervals, because of the low probability of a given core intersecting the inhomogeneously distributed DNAPL.
Many new types of push-in tools that allow direct detection of contamination, without bringing soil or water samples to the surface for analysis, have been developed in recent years. One of the most successful efforts, the site characterization and analysis penetrometer system (SCAPS) program, is discussed in detail in Chapter 5. Sensors for direct detection of contamination include the following:
-
Laser-induced fluorescence sensors. Laser-induced fluorescence sensors use a laser to cause organic components to fluoresce in the subsurface. These tools have been highly successful in hydrocarbon detection. Although hydrocarbons, including polycyclic aromatic hydrocarbons (PAHs), generally fluoresce, most chlorinated solvents do not. Therefore this technique is of limited applicability for the detection of chlorinated solvent DNAPLs.
-
Thermal desorption volatile organic compound (VOC) sampler. The SCAPS thermal desorption VOC sampler collects a small soil sample at a desired depth. The soil is heated, and volatile components are extracted and carried to the surface by a carrier gas. VOCs are analyzed on the surface by mass spectrometry. Multiple samples may be taken during a given push. This sampler provides essentially the same data as discrete-depth sampling of a conventional core for volatile compounds.
-
Hydrosparge VOC sensing system. This system is mounted on a hydropunch direct-push device and pushed to the desired depth. A water sample is obtained and sparged using helium carrier gas. The gas is routed to the surface, where the sample is analyzed with an ion trap mass spectrometer.
-
Video imaging system (GeoVIS). This system illuminates soil in contact with a sapphire window and images it with a miniature color camera. Both light nonaqueous-phase liquid (LNAPL) and DNAPL detection have been reported (Lieberman and Knowles, 1998; Lieberman et al., 1998). The NAPL phases are visible as discrete globules. Although data at this time are insufficient to establish the sensitivity of the method, the range of conditions in which it is effective, and the lower limit of NAPL saturation that can be reliably detected, the technology may offer promise for rapid source-zone delineation.
Neutron Probes
Differences in the way air and water scatter and attenuate neutrons can be used to evaluate the amount of moisture in the soil, as is done with neutron soil moisture probes. Neutron logging tools based on the difference in neutron attenuation between water and hydro-carbons are widely used to determine the saturation of oil in reservoirs. In a similar manner, the difference in attenuation of neutrons between DNAPL and water can be used to estimate DNAPL saturation. This technique is based upon the much larger neutron capture cross section of chlorine compared to water. Neutron logging tools can be used to identify intervals with DNAPL within a few centimeters of a well (Newmark et al., 1997; Daly et al., 1998).
Seismic Methods
Seismic reflection, refraction, and acoustical tomography have been used in an attempt to locate DNAPLs. Although seismic methods have been widely used for petroleum exploration and site characterization and have been highly successful in defining subsurface geology, their success in detecting DNAPLs has been limited. Generally, the differences in response due to geologic heterogeneities are greater than those due to the presence of DNAPLs. At this time, there are no well-documented successes in locating DNAPL pools using seismic methods, although research is continuing.3
Ground Penetrating Radar (GPR)
GPR has a proven capability to define shallow stratigraphy. It provides a detailed image of layering in the soil to a depth of from approximately a meter (a few feet) to 10 or so meters (a few tens of feet). It cannot penetrate clay layers and so is useful for defining confining layers. Where DNAPL is known to exist, GPR may have the potential to monitor its movement. However, with no prior data at a site, recognition of DNAPL cannot generally be accomplished, because the lithologic variations within a unit are generally greater than the variations caused by thin DNAPL pools (Grumman and Daniels, 1995; Pankow and Cherry, 1996; Young and Sun, 1996).
Electrical Resistivity
DNAPLs are generally nonconductive, so methods based on conductivity or resistivity have the potential to distinguish water-filled from DNAPL-filled pores. Results to date have shown considerable success in monitoring the change in DNAPL saturation during remediation. Direct detection of DNAPL, without previous background measurements, does not generally appear to be possible (Santamarina and Fam, 1997; Daly et al., 1998; Lien and Enfield, 1998).
Partitioning Tracers
The partitioning of an organic compound between DNAPL and water is largely a function of the solubility of the compound in water. Methanol and isopropanol, which are miscible in water, partition nearly totally into the aqueous phase. On the other hand, less soluble alcohols (pentanol, heptanol, and all heavier alcohols) have limited aqueous solubilities and hence partition into DNAPL. If a solution of conservative tracers (e.g., bromide or isopropanol) and less soluble tracers (heavier alcohols) is pumped through a contaminated zone, partitioning of the less soluble compounds into the DNAPL will slow the rate of transport of the heavier alcohols exactly as sorption slows the transport of hydrophobic organic contaminants. The fraction of pore space occupied by DNAPLs can be derived from known partition coefficients and measured breakthrough curves in a well-to-well pump test (just as the retardation factor of a compound can be converted to the fraction of organic carbon in soil if the partition coefficient between soil and water is known). This technology has been demonstrated in numerous field tests (Jin et al., 1995; Brown et al., in review).
Partitioning tracers can provide a quantitative measure of the fraction of pore space occupied by DNAPLs between an injection well and an extraction well. The test averages DNAPL saturation over the entire interval. Interpretation in nonhomogeneous units requires an accurate flow model. Tracer tests require a very large number of high-quality analyses; if DNAPL saturation is low, very precise data are needed over an extended time period.
This technology has proven invaluable at several dozen sites for determining the quantity of DNAPL present and for evaluating the performance of remediation technologies in field trials through tests conducted before and after treatment. Since cleanup goals are seldom based on the amount of DNAPL left in place, but rather are
based on the concentration of DNAPL components in soil or water, the utility of such tests in defining the performance of an actual remediation is less clear.
REMEDIATION TECHNOLOGIES FOR DNAPL SOURCE ZONES
Soil Vapor Extraction and Derivatives
Description
Soil vapor extraction (SVE) uses an induced flow of air through the unsaturated zone to remove volatile compounds from the soil in the vapor phase (EPA, 1997a; Holbrook et al., 1998; Johnson et al., 1993; Wilson and Clarke, 1994). In the most commonly practiced method of application, a vacuum source (e.g., a blower or vacuum pump) is connected to a well, which is screened across the contaminated interval of the unsaturated zone, as shown in Figure 4-2. The reduced pressure within the well bore induces air flow toward the well from the surrounding soils. As the air flows through the contaminated soils, the portion of volatile compounds present in the vapor phase flows toward the well and is removed through the well along with the extracted air. The volatile compounds associated with the soils (either adsorbed or dissolved in the soil moisture) or present as free-phase liquids will gradually partition into the surrounding soil gas and be extracted with the recovered air. The recovered air is either discharged directly to the atmosphere or treated and then discharged (Johnson et al., 1994). The requirements for treatment depend on the concentrations of the individual VOCs, the air flow rate, and state and local regulations.
Variants
Bioventing is similar to SVE except that the design emphasizes biodegradation rather than volatilization, with the intent to minimize physical removal (Dupont et al., 1998). Bioventing systems, like SVE systems, circulate air but require a much smaller volume of air than SVE systems. Bioventing eliminates or minimizes the need for treatment of off-gases containing volatilized contaminants. Where VOCs have no potential to enter buildings or other structures or where nonvolatile compounds are being treated, bioventing may be conducted through injection of air without air recovery. Bioventing of chlorinated compounds requires the introduction of methane, natural gas, or other substances that encourage cometabolism (fortuitous degradation of contaminants that occurs as microbes metabolize the in-
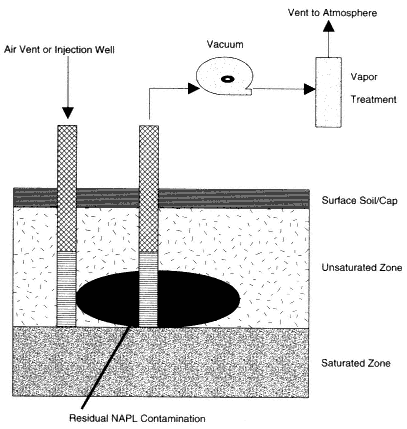
Figure 4-2
Typical design of an SVE system. Source: Adapted from NRC, 1994.
jected compound) of the chlorinated compounds. Bioventing is discussed in more detail in the bioremediation section of this chapter.
Groundwater pumping may be used to lower the water table and thus increase the depth to which SVE may be applied. Heat may be used to increase contaminant volatility; heating may be accomplished by any of the methods discussed in the thermal technologies section of this chapter.
Physical and Chemical Principles
SVE is based on the partitioning of compounds among phases: dissolved in groundwater or air or adsorbed to soil, or present in a
nonaqueous-phase liquid (NAPL) (Johnson et al., 1994; Wilson and Clarke, 1994). The concentration of a compound in the vapor phase in equilibrium with dissolved-phase contamination can be calculated from a compound's Henry's law constant. The partitioning between a NAPL and air can be calculated from a compound's vapor pressure. Due to kinetic effects, actual vapor-phase concentrations may be lower than predicted from equilibrium calculations. The vapor pressure of a compound is a function of temperature: increasing the temperature increases vapor pressure, the partitioning of a compound into the vapor phase, and thus SVE efficiency. Volatilization from nonaqueous phases and from dissolved phases is reduced if a mixture of contaminants is present. The vapor pressure of a compound in a mixture, is a function of both its pure vapor pressure and its mole fraction in the mixture as described by Raoult's law.
Because contaminants are extracted in the vapor phase, SVE performance is a function of the air movement through the soils as well as the partitioning of VOCs among phases. The amount of contaminant that can be extracted depends on the volume of air flow induced. The volume of air that SVE can induce is a function of the permeability and the water saturation of the soil.
Application
The most basic design for SVE systems uses one or more vertical wells installed by conventional drilling methods, as shown in Figure 4-2 (Johnson et al., 1994). Where there is no surface covering (such as concrete or asphalt), the tops of the well screens are located a meter or more (several feet) below the ground surface to prevent short-circuiting of air. Air is extracted from the wells in either a continuous or an intermittent mode.
Frequently, VOC recovery reaches an asymptote after an extended period of operation, making further recovery inefficient. In some instances, improved recovery rates can be achieved by operating only some of the wells at any one time using an alternating schedule. Alternatively, the system may be shut down for an extended period until a new equilibrium between the vapor phase and the combination of adsorbed, dissolved, and free-phase VOCs is established. Once the system is reactivated, recovery rates typically increase substantially.
A minor modification to the basic system is the addition of air inlet wells. These wells facilitate air entry into the subsurface, with air entering from a screened interval that matches the screened interval of the recovery wells. Wells screened in this manner can be used
alternately for recovery and air inlet. Injecting air under pressure can further enhance flow through fine-grained soils.
SVE systems can use horizontal wells where the contamination and/or water table is relatively shallow or where access for installation of vertical wells is limited (e.g., beneath buildings) (Johnson et al., 1994). Horizontal wells can be installed using trenching or horizontal drilling techniques. When using trenching, horizontal trenches are excavated, a slotted pipe is placed in the trench, the pipe is covered with a porous medium such as gravel, the porous medium is covered with a low-permeability medium, and the remaining excavation is filled to the surface. Trenches can be constructed using conventional equipment, such as backhoes, or with one-pass trenching equipment. Trenches are appropriate for shallow contamination, where short-circuiting to the surface would limit the influence of vertical wells. Trenches are not appropriate where existing site infrastructure would interfere with construction. In recent years, horizontal and directional drilling equipment has permitted wells to be drilled horizontally where there is limited access to the surface above some or all of the contaminated soils. As with vertical wells, various combinations of air extraction and air injection can be used.
A recent innovation for lightly contaminated soil uses the daily changes in barometric pressure to induce air flow through wells. Wells are fitted with valves that either close or open according to the barometric pressure. During the day, when barometric pressures are relatively lower than at night, air escapes from some wells. At night, air enters a second set of wells. The result is a low flow of air into the subsurface. This type of system is more appropriate for promoting biodegradation than physical removal of VOCs because of the low air flow volumes. It is an attractive concept for remote locations.
Heating the soil can increase SVE efficiency (EPA, 1997a). The time required for remediation depends on the extent to which the contaminants partition into the vapor phase. Several methods have been evaluated for heating soils and increasing the vapor pressure of VOCs. One constraint for all potential heating methods is that soils have a large heat content. Thus, large amounts of energy are required to achieve relatively small increases in soil temperature. Methods used successfully in field-scale trials or commercial applications include radio-frequency heating, electrical resistance heating, and steam injection.
Pumping of groundwater to lower the water table may be used to increase the effective depth of SVE (EPA, 1997a). The vacuum applied to air extraction wells lowers the air pressure in the treatment zone; hence groundwater levels are slightly higher in the region of
SVE wells than would otherwise be the case. This reduces the interval over which VOCs can be removed. Extracting some groundwater from the air extraction well (dual-phase extraction) or from a nearby well maintains the groundwater at normal levels, or lower, depending on the groundwater recovery rate and soil properties. Lowering the water table by pumping increases the depth to which SVE may be applied. In aquifers with low-permeability soils, achieving significant groundwater recovery from individual wells may be difficult. For coarser-grained soils from which the water would drain fairly rapidly, large volumes of water must be recovered to lower the ground-water table sufficiently to have any benefit, and the recovered groundwater will require treatment in most cases.
One other modification that has become widely used, although developed primarily for petroleum hydrocarbons, is high-vacuum-enhanced vapor recovery. In this method, a well is screened across the water table. An annular pipe is extended into the liquid phase. The pipe and well are sealed at the surface. A high vacuum is applied, which removes water and air. In relatively low-permeability formations, this can cause dewatering and thus removal of volatile compounds from within the unsaturated and dewatered zones (Johnson et al., 1994; Kittel et al., 1994).
An important consideration in SVE is the radius of influence (ROI), the radius at which significant flow is induced for a given well. The ROI governs the well spacing needed for complete coverage of the contaminated area and hence the number of wells required. For wells with a shallow screened interval where there is no impermeable cover on the ground surface and no layer of low-permeability soils above the screened interval, air flow will have a large vertical component, with nearly all of the air flow occurring within a few feet of the well. Under these conditions, the ROI will be small. A larger ROI will be achieved when the surface is covered with concrete or asphalt, when there is a layer of low-permeability soil above the top of the well screen, and/or when the tops of the well screens are deep below the ground surface.
Design of an SVE system typically involves conducting pilot tests in representative contaminated areas, generally using a single extraction well in conjunction with several monitoring wells or probes located at several distances and at least two directions away from the extraction well (Johnson et al., 1994). Information determined includes the ROI of individual extraction wells as a function of the air extraction rate, air flow rates through soils as a function of distance from the well and air extraction rates, estimated cleanup times, off-gas treatment requirements, optimum operating conditions, and blower requirements.
SVE operations are relatively simple, consisting of maintenance of the mechanical systems, sampling of extracted air and off-gas treatment system effluent, and acquisition of occasional soil samples. Periodic review of system performance may result in modifications to operating conditions and the monitoring program. Parameters used to evaluate performance include extraction flow rates and pressures (changes may reflect changes in flow paths and ROI), vacuums, VOC concentrations, oxygen and carbon dioxide concentrations in monitoring probes, temperature, and other performance criteria associated with the mechanical components.
Performance
Generally, SVE has been most successful for treating volatile compounds in moderately to highly permeable soils. SVE systems have been applied at a large number of sites contaminated with chlorinated solvents. A general indication of SVE performance is that as of 1997, it was used at 178 sites being cleaned up under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) (EPA, 1998). In one DOE application, SVE in combination with six-phase electrical heating reduced perchloroethylene (PCE) concentrations by 99.7 percent in a 3-m (10-ft) clay layer. The system removed approximately 180 kg of PCE (Kittel et al., 1994). The Environmental Protection Agency (EPA) has published several case histories (EPA, 1998).
Limitations
SVE is not applicable for compounds with low volatility and Henry's law constants unless the compounds are biodegradable under aerobic conditions. It also cannot be used to treat wet, clayey soils, and is generally less applicable for remediation of low-permeability soil. The limiting effect of low pneumatic permeability is exacerbated by low vapor pressure and soil heterogeneity. To some extent, both low pneumatic permeability and limited volatility can be overcome by closely spacing wells, applying heat, or increasing the operating time of the system. These alternatives, of course, add to the cost of remediation. SVE systems in some cases can be effective for low-permeability soils if the contaminants are highly volatile and have low water solubility.
Adsorption of VOCs to soils may reduce the rate of partitioning into the soil vapor phase and interfere with SVE performance. Adsorption is important for hydrophobic compounds (those with low water solubility). The effect is proportional to the soil organic con-
tent. Adsorption to soils has the greatest effect in the later stages of remediation and can extend the time required to remove the last remnants of VOCs.
Soil heterogeneity will also affect SVE performance. Air flows most easily through coarse-grained soils and very little if at all through predominantly clayey soils. Frequently, VOCs will accumulate preferentially on the surface of and within clay lenses and layers. Air flow will then be minimal in the most highly contaminated soils. However, SVE can still decrease the contamination of the aquifer and reduce the potential for exposure to contamination if the VOCs in the less permeable soils are not very mobile.
The cost of off-gas treatment, the presence of utility trenches and other infrastructure that cause short circuiting of the extracted air, and the inability to treat metals and radionuclides are other factors that can limit use of SVE.
Advantages
SVE offers several advantages over many other remediation alternatives:
-
It is an in situ technology and thus causes minimal disruption of normal site activities.
-
It can be installed beneath buildings and in the vicinity of other types of infrastructure.
-
It has been used at many sites and so is well developed. Design manuals are available and installation can be accomplished with readily available equipment.
-
It is cost-effective for many site conditions, especially when off-gas treatment is not required or can be accomplished with existing systems, and/or for very large areas; many vendors can provide prefabricated system components.
-
It is applicable to a wide variety of compounds—VOCs and aerobically biodegradable compounds.
-
It will not cause further migration of contaminants.
-
It reduces the potential for migration of vapors to basements and utility trenches.
-
It reduces the potential for VOCs to contaminate groundwater.
-
It can be used as part of a multicomponent remedial system in conjunction with a pump-and-treat system, bioremediation system, partial excavation and treatment, or natural attenuation. These methods can be implemented simultaneously with SVE or sequentially.
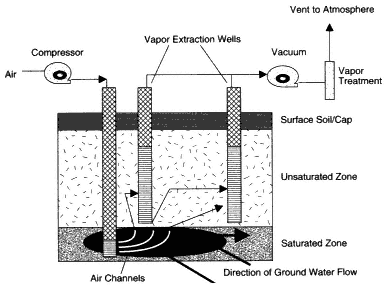
Figure 4-3
Typical process diagram for an air sparging system.
Source: Adapted from NRC, 1994.
Air Sparging
Description
Air sparging for remediation of DNAPLs involves injecting air or other gases directly into the groundwater to vaporize and recover the contaminants. Volatile components of the DNAPLs will vaporize and move upward to the atmosphere or to a vapor extraction system installed in the vadose zone (see Figure 4-3).
Physical Principles
The vaporization of volatile organic chemicals, and even mixtures of such chemicals, is well understood. Injected air moves laterally, driven by the injection pressure, and upward, due to the buoyancy of air. As the injected air moves through a formation and comes in contact with NAPLs, contaminated soil, or water containing dissolved-phase contamination, the volatile contaminants partition into the air. Partitioning from the dissolved phase is described by a compound's Henry's law constant; partitioning from DNAPLs is described by its vapor pressure. In addition, oxygen present in the
injected air will dissolve in the water, promoting in situ biodegradation of nonvolatile contaminants or those located downgradient of the sparging zone.
Application
In an idealized homogeneous geological deposit, air would be injected into the saturated zone below the DNAPL and flow upward through the source zone. Since DNAPLs at a contaminated site are found in discontinuous ganglia in the saturated zone and in low spots overlying less permeable zones in or at the bottom of aquifers, achieving a uniform air flow through the entire source zone may be difficult. Air moves through saturated media by a complex process. Air must be under sufficient pressure to displace water from the medium. Once this is achieved, the medium in the immediate vicinity of an injection well becomes unsaturated. In media consisting of particles less than 1 to 2 mm in diameter (i.e., medium or finer sands), air will travel in continuous channels rather than as discrete bubbles. Thus, the air may bypass large volumes of the medium and will not directly contact DNAPLs in these areas. Injection of air below the DNAPL-contaminated zone is difficult if not impossible at sites where contaminants occur at the bottom of the aquifer.
Even small changes in permeability will have an influence on the distribution of DNAPLs and will also influence the pathways through which injected air flows. Descending DNAPLs will flow laterally along downward-sloping layers of less permeable zones until they are trapped or move further down through a more permeable feature, while air will flow laterally along upward-sloping layers of less permeable zones until it is trapped or moves up through a more permeable feature. Thus, in an inhomogeneous stratified medium, close and complete contact between a DNAPL and injected air is unlikely. However, under some unique circumstances—for example, where DNAPL is located between two less permeable confining zones that can be dewatered to a significant extent by air injected between the zones—directly vaporizing the DNAPL may be possible.
Recently, Johnson et al. (1997) conducted a carefully controlled study of the air flow distribution in a sparging system at a Navy gasoline service station in Port Hueneme, California. At this site, a large volume of gasoline leaked onto a shallow groundwater table, resulting in a 300-m source area and a 2-km dissolved plume. Sparging with single wells was undertaken at two locations at the site, one within the source area and one in the dissolved plume. Johnson et al.
conducted sparging tests at 2.4 × 10-3, 4.7 × 10-3, and 9.4 × 10-3 m3/sec (5, 10, and 20 standard ft3/min). Each increase in flow rate produced a corresponding increase in off-gas concentrations of contaminants. However, the duration of the increase was only a few days. Air distribution measurements using neutron probes and electrical resistance showed relatively sparse air distribution at 2.4 × 10-3 m3/sec (5 ft3/min) and a more uniform air distribution at 9.4 × 10-3 m3/sec (20 ft3/min). However, nearly all of the air was contained within a 3-m radius of the well (Johnson et al., 1997). Even within this radius, there were zones that received relatively little air. As a consequence, some portions of the soil within the immediate vicinity of the injection well were effectively cleaned because of direct contact with the air, whereas other portions were apparently not cleaned at all. After 18 months of operation, a number of wells showed no apparent improvement in water quality. Similar behavior was observed at the dissolved plume site. Based on this research, high air flow rates (e.g., 9.4 × 10-3 m3/sec) are generally more effective for source-zone remediation, and close spacing of wells (e.g., 6 m) may be required to remove the bulk of the contaminants.
Performance
The majority of applications of air sparging have been for cleanup of fuel spills (Bass and Brown, 1996; Marley and Bruell, 1995). In these cases, both volatilization and enhanced biodegradation are important processes. In addition, because fuel is less dense than water, fuel source zones and groundwater plumes tend to occur near the water table. These are optimum conditions for the application of air sparging. Even so, there are relatively few documented cases in which source zones have been completely cleaned.
Air sparging also has been successful in cleaning up plumes of dissolved chlorinated solvents (Bass and Brown, 1996). In these cases, volatilization is the primary remediation mechanism. Air sparging may inhibit the anaerobic processes capable of biodegrading chlorinated solvents.
A sparging system was installed at Hill Air Force Base, Utah, to cut off a dissolved-phase trichloroethylene (TCE) plume coming from an unknown source under the runways. The injection system consisted of four wells in a line perpendicular to groundwater flow (U.S. Air Force, 1996). After about three months of operation, significant reductions in contaminant concentrations occurred at many of the monitoring points. However, the system did not achieve drinking water standards at a number of the monitoring points. As a result,
the plume was not considered captured. A groundwater pump-and-treat system was installed to ensure capture of the plume.
At the Savannah River Site, DOE conducted a field demonstration of air sparging on a site contaminated with chlorinated solvents that had leaked from an unlined sediment basin. During the demonstration, the air sparging process increased the recovery of VOCs from 49.4 kg (109 lb) per day, obtained with an SVE system alone, to 58.5 kg (129 lb) per day (EPA, 1995c). Although no reports indicate that the air sparging system directly removed DNAPLs, Gordon (1998) suggested that the system may have mobilized trapped DNAPLs, because DNAPL recovery increased at an extraction well at the site.
Limitations
In order to determine if air sparging will be effective in removing a DNAPL, a very detailed investigation is required to delineate the location of the DNAPL and the heterogeneities in the air permeability of the subsurface media. The site investigation may reveal that air sparging will be effective in only a few geological deposits at the site. Even in these deposits, however, removing enough DNAPL to bring groundwater into compliance with relevant standards may not be possible. Even where reduced concentrations of contaminants in groundwater appear to indicate that air sparging has been effective, the concentrations may increase due to remaining DNAPL after air sparging is terminated. In most instances, air sparging would have to be used in conjunction with an SVE system to capture the contaminants. Air sparging will not be effective in removing contaminants with low volatilities.
Advantages
Air sparging is relatively inexpensive. In addition, because it involves the introduction of air only, rather than other substances, regulatory approval is generally straightforward. It is commercially available, and realistic cost estimates can be obtained for a given site. It has been shown to be effective at mass removal under appropriate conditions.
Alcohol or Cosolvent Flushing
Description
Alcohol or cosolvent flushing involves pumping one or more solvents, at concentrations ranging from a few to 80 percent, through
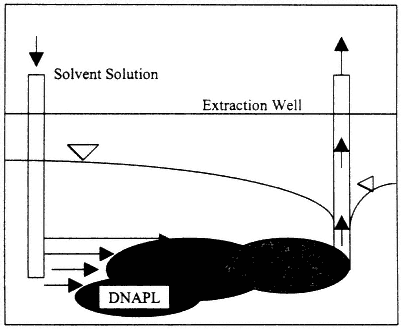
Figure 4-4
Process diagram for a cosolvent flushing system. Source: EPA, 1995b.
the DNAPL source zone to remove DNAPL by dissolution and/or mobilization (see Figure 4-4). Alcohols are the most commonly used solvents, although in principle any organic solvent may be used. An extended discussion of the technology, including phase diagrams for several relevant systems, with interpretations, is provided in Technology Practices Manual for Surfactants and Cosolvents (AATDF, 1997). The manual also includes an extensive reference list.
Physical and Chemical Principles
Alcohol and cosolvent flooding relies on an increase in solubility of hydrophobic organic compounds resulting from the addition of a solvent to water and from the reduction of interfacial tension that accompanies this increased solubility. Numerous researchers have demonstrated the ability of solvents such as short-chain alcohols (methanol, ethanol, propanol) to increase the solubility of hydrophobic organic compounds in water (e.g., Rao et al., 1985; Peters and Luthy,
1993; McCray and Falta, 1996; Lunn and Kueper, 1996). Most DNAPL components found at DOE sites are readily soluble in alcohol-water mixtures. As the solubility of the DNAPL in the solvent increases (for example, by increasing the concentration of alcohol in an alcohol-water mixture), the interfacial tension between the DNAPL and water decreases. If the system is designed so that the DNAPL is miscible with the solvent flood, the interfacial tension drops to zero. Displacement of DNAPLs may occur, as well as dissolution, as the interfacial tension decreases.
Application
A typical system consists of arrays of injection and extraction wells arranged to provide an efficient flood of the source zone. Horizontal wells, trenches, or other delivery systems may be used. Either hydraulic control or containment walls may be used to contain the solvent flood. The effluent solution produced at the extraction wells contains water, solvent, and contaminants and must be treated prior to reinjection or disposal. Recycling of solvents has not been demonstrated in the field but will be necessary to make the process cost-effective.
Performance
All field trials of solvent flooding for which results have been published involved LNAPLs, which unlike DNAPLs are less dense than water. The Technology Practices Manual for Surfactants and Cosolvents (AATDF, 1997) and Fountain (1998) describe these trials. Three key trials occurred at Hill Air Force Base in Utah:
-
In one trial at Hill, researchers from EPA and the University of Florida tested a system that pumped approximately 10 pore volumes of a mixture of 70 percent ethanol and 12 percent pentanol in water through soil in a 3 × 5 m sheet-piling cell using a line drive array of injection and extraction wells. The contaminant treated was an LNAPL consisting of a complex mixture of weathered jet fuel and other components. The sediments within the test cell were poorly sorted sands and gravels. Initial LNAPL saturation averaged about 5 percent. The system removed approximately 85 percent of the LNAPL. Dissolution was the primary recovery mechanism.
-
In a second Hill trial, researchers from EPA and Clemson University tested a system that pumped approximately 2 pore volumes of a mixture of tertiary butanol and hexanol, followed by approxi-
-
mately 2.3 pore volumes of 95 percent tertiary butanol, then 0.3 pore volume of 47 percent tertiary butanol, and finally 30 pore volumes of water. The test cell, sediment, and contaminant were similar to those in the alcohol flood described above. Dissolution was the primary recovery mechanism. The system recovered between 75 and 95 percent of the LNAPL.
-
In a third test at Hill, EPA and University of Arizona researchers circulated approximately 10 pore volumes of a 10 percent cyclodextrinin-water solution through a treatment cell with contaminants and sediments similar to those described above. Recovery ranged from 39 to 93 percent of the LNAPL originally present. Dissolution was the primary recovery mechanism.
Each of the above tests demonstrated this technology's ability to remove NAPL mass by dissolution and/or mobilization. No test achieved full recovery, although it should be noted that the Hill LNAPL is very difficult to dissolve, even in the laboratory. It is likely that these technologies would be more effective for chlorinated solvents and other easily dissolved DNAPLs such as those typically found at DOE sites.
Limitations
For any flooding technology to be effective, the entire contaminated volume of soil must be effectively flushed with treatment solutions; for solvent flooding, multiple pore volumes must be circulated. The requirement for circulation of multiple pore volumes limits application to sites with hydraulic conductivity adequate to allow large-volume pumping. The minimum conductivity will depend on the source zone size, but 10-4 cm/sec or greater is best in most situations.
As for all flushing technologies, heterogeneities in the aquifer will decrease extraction efficiency (Mackay and Cherry, 1989). In heterogeneous aquifers, some areas will be poorly swept by the flushing solution, and therefore such aquifers will require longer treatment times and larger treatment volumes than homogeneous aquifers. Standard numerical flow models can help predict the potential effects of heterogeneities.
Alcohols are generally less dense than water; therefore high-concentration alcohol flooding solutions will be less dense than ground-water, which sometimes presents problems in circulating these solutions evenly. Each solvent flood field trial required flooding with multiple pore volumes of treatment solution, with no recycling. Substantial volumes of solvent were used, and very large volumes of
extracted fluid had to be treated. Currently, the use of large volumes of flushing solutions and the production of large volumes of extracted fluids requiring treatment are the major differences between solvent flooding and surfactant flooding (discussed below) for DNAPL remediation.
The decrease in interfacial tension produced by the addition of solvents creates the risk that DNAPLs will be mobilized. If the DNAPL is perched on an impermeable layer, lowering the interfacial tension (which reduces capillary forces and hence the entry pressure) may allow the DNAPL to penetrate the aquitard and move down into previously clean zones. The amount that the interfacial tension decreases and the risk involved depend on the hydrogeology of the site, particularly the integrity of the aquitard. This risk must be evaluated for each site.
The ultimate level to which DNAPLs may be cleaned up by cosolvent flooding is unknown, since no field trials have been reported for solvent flushing of DNAPLs. In principle, the performance of these systems should be similar to that of surfactant flooding systems.
Advantages
The chemical principles of these systems are relatively simple when treating chlorinated solvents. Alcohols are effective solvents and are not sorbed significantly. The technology is suitable for removal of DNAPLs present at very high saturations.
Surfactant-Enhanced Aquifer Remediation
Description
Remediation of DNAPL-contaminated sites with surfactants involves injection of a solution of water plus surfactant into the source zone and removal of the DNAPL through a combination of dissolution and displacement (see Figure 4-5). The relative importance of dissolution compared to displacement can be controlled by formulation of the surfactant solution.
Physical and Chemical Principles
Surfactant-enhanced remediation is based on two well-established properties of surfactants: (1) their ability to decrease interfacial tension and (2) their ability to increase the solubility of hydrophobic organic compounds. When present in sufficient concentrations (above
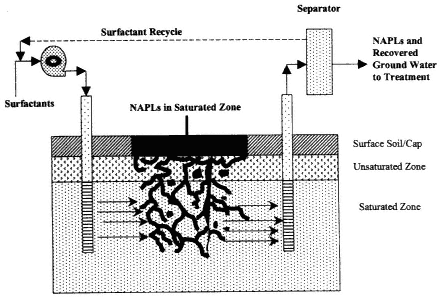
Figure 4-5
Typical process diagram for a surfactant flushing system.
Source: Adapted from NRC, 1994.
what is known as the critical micellar concentration), surfactant molecules form oriented aggregates, termed micelles. Micelles can incorporate hydrophobic molecules in their interiors, producing an apparent increase in solubility. The process of dissolving by incorporation into micelles is termed solubilization. Once solubilized, a compound is transported as if it were dissolved.
The extent of increase of solubility (solubilization) depends on the contaminant, the type of surfactant, and the surfactant concentration. Increases in solubility of more than five orders of magnitude and solubilities of hundreds of thousands of milligrams per liter have been reported for common DNAPL components (Baran et al., 1994). Early field trials used surfactants that produced modest increases in solubility (one or two orders of magnitude) and extracted the DNAPL through slow dissolution. This approach required circulation of multiple pore volumes (more than 10) of surfactant solution (Fountain et al., 1996). More recent work has emphasized higher-performance systems that would require circulation of only two to three pore volumes in a homogeneous system and would produce solubilized contaminant concentrations greater than 100,000 mg/liter (Brown et al., in review).
The interfacial tension between NAPL and water decreases as
solubilization increases. The interfacial tension may decrease by as much as four orders of magnitude at maximum solubilization. Since capillary forces decrease with decreasing interfacial tension, surfactant systems may induce DNAPL mobility (Abriola et al., 1995). This aids in recovery by increasing DNAPL flow to the extraction wells but may cause DNAPL contamination to spread if the reduced interfacial tension allows the DNAPL to penetrate underlying, previously uncontaminated layers due to high displacement pressure.
Application
A typical system involves arrays of injection and extraction wells designed to sweep the DNAPL source zone. Hydraulic controls or containment walls contain the surfactant solution. The extracted solution of water, surfactant, contaminants, and other additives must be treated prior to reinjection in the subsurface or disposal in a surface water body or sewer.
Selection of the appropriate surfactant requires consideration of performance, toxicity, biodegradability, possible chemical reactions with constituents (such as calcium) in the water, and potential for sorption. Published work has identified surfactant systems with appropriate properties that produce high solubilization of a wide range of compounds of environmental interest. Commonly, salt or a second surfactant (cosurfactant) is added to the solution to produce the desired solubilization. Alcohols may be added to optimize the phase behavior and prevent the formation of unwanted viscous phases (Lake, 1989; Pope and Wade, 1995).
After extraction, surfactants may be separated from the contaminants and reused. Air stripping has been used successfully to separate contaminants from surfactants in three field trials (at sites contaminated with PCE, carbon tetrachloride, and TCE). A permeable membrane system using solvent extraction has been developed that could be used to separate nonvolatile contaminants from the surfactant solution (AATDF, 1997). Because of the increased solubility produced by the surfactants, the extraction processes are less efficient and more costly than separation from surfactant-free water.
A modification to surfactant flooding to improve performance in heterogeneous media involves the use of mobility control agents. In surfactant floods used for enhanced oil recovery, polymers are used routinely to increase sweep efficiency (Lake, 1989). Xanthan gum, a food-grade additive, is among the most commonly used polymers. Polymers are added in low concentrations (a few hundred milligrams per liter) and produce a non-Newtonian fluid (one whose viscosity
changes with flow conditions). In high-permeability units, the polymer increases the fluid's viscosity, slowing the flow. In contrast, in low-permeability layers, the high shear conditions produce a lower viscosity. Thus, the relative flow rates in low-and high-permeability zones are more nearly equal. Foam also can be used to decrease the permeability of high-permeability zones. Foam can be injected with air into a zone where surfactant has been injected (AATDF, 1997; Hirasaki et al., 1997a,b).
Performance
Surfactant systems have been field tested at more than a dozen sites, including several with DNAPLs. Representative trials, as summarized in AATDF (1997), include the following:
-
Intera, Radian, and the University of Texas tested a surfactant flooding system at Hill Air Force Base in 1996. Approximately 2.5 pore volumes of a solution of surfactant, isopropanol, and sodium chloride were pumped through a poorly sorted sandy unit contaminated with a DNAPL composed primarily of TCE. A line drive system was used without confining walls. DNAPL was originally present at approximately 4 percent residual saturation. Reportedly, the system removed more than 99 percent of the DNAPL (Brown et al., in review). Concentrations in groundwater were approximately 10 mg/liter at the end of the test.
-
The University of Oklahoma and the EPA also conducted a surfactant flood at Hill in 1996. Approximately 6.5 pore volumes of a mixture of 4.3 percent surfactant in water were pumped through soil in a 3 × 5 m sheet-piling cell using a line drive array of injection and extraction wells. The contaminant was an LNAPL consisting of a complex mixture of weathered jet fuel and other components. The sediments were poorly sorted sands and gravels. An estimated 90 percent of the LNAPL, which was initially present at approximately 8.5 percent saturation, was removed. The surfactant system used a mixture of two surfactants to produce a system with very low interfacial tension. The primary recovery mechanism was mobilization.
-
The University of Florida and the EPA conducted a third surfactant flood in 1996. Approximately nine pore volumes of a mixture of 3 percent surfactant and 2.5 percent pentanol in water were pumped through soil within a 3 × 5 m sheet-piling cell using a line drive array of injection and extraction wells. The contaminant was an LNAPL consisting of a complex mixture of weathered jet fuel and other components. The sediments were poorly sorted sands and gravels. The surfactant system used was designed to solubilize, not mobilize the LNAPL. Soil
-
core data indicated that approximately 96 percent (24.9 mg/kg initial concentration reduced to 1.10 mg/kg) of the LNAPL was removed.
-
The University of Buffalo and DuPont tested surfactant flood of a chlorinated solvent DNAPL in 1991–1993. A total of 12.5 pore volumes of 1 percent surfactant in water were injected using six injection wells around the treatment zone and two extraction wells near the center. The DNAPL was composed primarily of carbon tetrachloride. The contaminated unit was a sand lens within a thick clay deposit. Removal was by solubilization without mobilization. Effluent was treated by air stripping, and the surfactant solution was recycled. Cores taken at the conclusion of the test from directly between an injection and an extraction well contained no residual DNAPL. A core taken from the outer portion of the treated area contained DNAPL in the fine-grained portion but none in the higher-permeability sections. This test showed that heterogeneities limit the rate of DNAPL removal by surfactant flooding systems.
These field trials have demonstrated that surfactants can rapidly remove contaminant mass from DNAPL sites, with high removals achieved in a number of tests. However, no surfactant field tests have been continued long enough to determine the ultimate level of cleanup attainable.
Limitations
Low-permeability units, heterogeneous areas, and insoluble contaminants may impose limitations. Heterogeneities result in some portions of the treated zone receiving more solution than others, requiring a longer treatment time and larger treatment volumes than are needed for homogeneous media. Use of mobility control agents (such as polymers and foam) can minimize the effect of heterogeneities. Low-permeability formations may require very long treatment times, and circulating the required volume of surfactant solution through such formations (clays and clay-rich units) may not be practical. A hydraulic conductivity of 10-4 cm/sec or greater is preferred.
Although the addition of alcohol may aid in optimizing the phase behavior of the surfactant, recycling has not been demonstrated in the presence of alcohols. The very low number of pore volumes required for high-efficiency surfactants may make recycling economically unnecessary.
The use of surfactants reduces the interfacial tension between NAPL and water, thus reducing capillary forces and creating the potential for mobilization of the DNAPL. Although mobilization can be an effective technique for rapid removal of DNAPL, it also increases
the risk of downward mobilization of the DNAPL. The resulting risk must be evaluated at each site based on the integrity of confining layers (aquitards) and the presence of water supplies at greater depth that could be contaminated by mobilized DNAPL.
None of the field trials at DNAPL sites has continued long enough to establish the ultimate cleanup levels that these systems can achieve in different circumstances. The persistence of some NAPL in every test conducted suggests that heterogeneities will inevitably result in some contamination remaining after treatment, although the level may be minimal and may be suitable for treatment by natural attenuation.
Advantages
Surfactant-enhanced aquifer remediation systems can rapidly remove mass from DNAPL source zones and remove DNAPLs nearly completely from relatively homogeneous units of moderate to high permeability. Many surfactants are FDA food-grade compounds and are readily biodegradable. Regulators have accepted the use of these surfactants at more than a dozen sites. Surfactant flushing can be done using conventional pumping equipment, so equipment costs are relatively low. The technology is not sensitive to operating parameters such as flow rates and concentrations. Existing numerical models can provide accurate simulations, allowing the prediction of performance for assessment purposes (Freeze et al., 1995). Implementation of the technology does not require significant site disruption, and the technology potentially could be applied beneath buildings and other structures.
In Situ Oxidation
Description
In situ oxidation systems work by injecting an oxidizing compound into the DNAPL source zone (see Figure 4-6). DNAPLs are destroyed through chemical reaction with the oxidizer. The system extracts excess oxidizer (if any) and then flushes water through the treatment zone. Potassium permanganate and hydrogen peroxide have been field tested as oxidizers in these systems.
Physical and Chemical Principles
The process is based on the ability of a strong oxidizer to destroy organic compounds. Virtually all organic contaminants can be oxi-
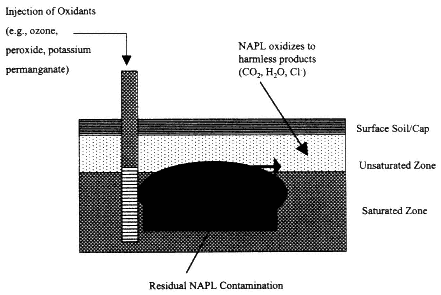
Figure 4-6
Process diagram for in situ oxidation. Source: NRC, 1994.
dized to carbon dioxide and water under sufficiently strong oxidizing conditions. The ability of a given reagent, such as potassium permanganate or hydrogen peroxide, to oxidize a specific DNAPL can be demonstrated readily in the laboratory (e.g., Gates and Siegrist, 1995; Miller et al., 1996; Schnarr et al., 1998).
Oxidation is a nonspecific process: all compounds in the system, including solid organic matter in the soil, that can be oxidized by a given reagent will react, increasing the volume of reagent required (Miller et al., 1996). Redox reactions are often also affected by the pH of the solution, requiring acid conditions for effective oxidation in some cases. This is significant for the system of hydrogen peroxide and ferrous iron (Fenton's reagent); the reaction is optimum at low pH (2–4) and less effective at higher pH (Miller et al., 1996).
Application
The reaction of potassium permanganate or hydrogen peroxide injected in source zones (with or without ferrous iron as a catalyst) with DNAPLs yields carbon dioxide and water, plus chloride and other by-products. The extent of reaction and the end products are determined by a combination of the reagents used, the DNAPL com-
ponents, and time. Potassium permanganate, or any other persistent reagent, will generally have to be washed from the treated zone by water flooding after oxidation is complete. Hydrogen peroxide spontaneously decomposes to water, with a half-life on the order of hours (Pardieck et al., 1992), so extraction of excess oxidant is not required for systems using this reagent.
Performance
A small test cell in the unconsolidated sands of Canadian Forces Base Borden was contaminated with TCE and PCE and was flushed with potassium permanganate at a concentration of 30 g/liter in a test conducted by the Solvents in Groundwater Program of the University of Waterloo. The system injected six pore volumes of permanganate, followed by clean water. VOC concentrations in water at the end of the test were near drinking water standards (Pankow and Cherry, 1996; Schnarr et al., 1998).
A field test of Fenton's reagent was conducted at DOE's Savannah River Site. In the test, 16 m3 (4,200 gallons) of hydrogen peroxide with ferrous sulfate (to generate Fenton's reagent) were injected to a depth of 43 m (140 ft) into a saturated zone contaminated with a DNAPL consisting primarily of PCE and TCE. Researchers estimated that 94 percent of the DNAPL was destroyed in a zone of approximately 15 × 15 m (50 × 50 ft) (Jerome, 1997).
DOE conducted a relatively large-scale test using potassium permanganate at its Portsmouth, Ohio, facility in 1997. Existing horizontal wells were used to inject groundwater augmented with potassium permanganate into a sand-and-gravel zone in the X-701B area, which is contaminated with a DNAPL composed primarily of TCE. The solution was injected in one well, recovered in the other, and recirculated. A total of 780 m3 (206 × 103 gallons) of solution was injected in a volume of approximately 67 × 27 × 1.5 m (220 × 90 × 5 ft). The volume injected corresponds to approximately 0.77 pore volume. The results, based on numerous analyses of TCE from cores taken before and after the test, indicated significant reductions in TCE in all locations reached by permanganate. Concentrations, originally as high as several hundred thousand micrograms per liter, were reduced to nondetectable levels in numerous monitoring wells immediately after the test and rebounded to low levels (tens to hundreds of micrograms per liter) after two weeks. Concentration reductions were not uniform, however. Apparently, heterogeneities in the flow field produced uneven flow of the oxidizing solution and hence uneven TCE removal. Permanganate did not reach the extraction wells during the
test, so recycling of permanganate solution was not attempted (Jerome, 1997)
Limitations
Laboratory data indicate that potassium permanganate is effective for oxidation of PCE and TCE but not for destruction of chlorinated compounds without double bonds (Pankow and Cherry, 1996). In addition, strong oxidizers will react with any oxidizable compound, so this method may not be practical for treating organic-rich soils because high organic content may increase the amount of reagent required. Such soils may even react violently with strong oxidizers. The use of hydrogen peroxide, as part of Fenton's reagent (hydrogen peroxide and Fe(II) ions) works best under acidic conditions. Large amounts of calcium carbonate or other acid-soluble compounds may make maintaining the appropriate pH difficult, if not impossible. In addition, hydrogen peroxide has a limited lifetime, so it can treat only a volume that can be reached within several hours. The volume of DNAPL that can be treated also may be affected by mass transfer limitations. Because the concentration of peroxide decreases with time and because a high peroxide concentration can block subsurface pores with gas and cause the ground surface to buckle, delivering a sufficient volume of reagent to a large DNAPL pool may be difficult. The range of conditions under which this technology will be effective and the ultimate cleanup levels attainable under different scenarios have not been determined.
Because this technology requires the delivery of reagent to the entire DNAPL source zone, low-permeability zones and heterogeneities may limit performance, as they do for all flushing technologies. Specifically, since the distance a reagent penetrates from an injection well is a function of the hydraulic conductivity, in source zones with a range of conductivities a large volume of reagent or a large number of injection wells might be needed to reach lower-permeability areas within the zone.
Advantages
The initial results from Base Borden and Portsmouth suggest that potassium permanganate has considerable potential for effective destruction of PCE and TCE. Fenton's reagent has long been known to oxidize common chlorinated compounds if it can be delivered to the source zone before it degrades, and at the proper pH it is also an effective oxidizer.
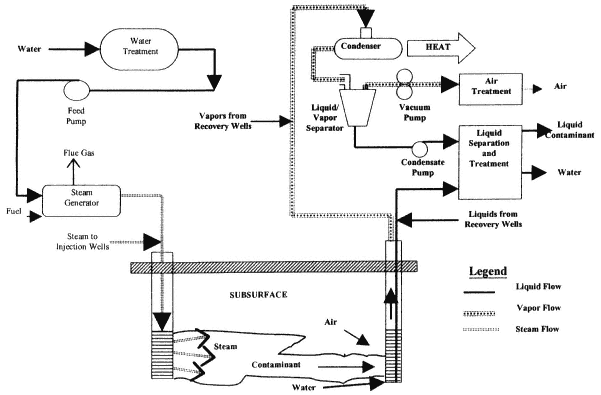
Steam Injection
Description
Steam injection involves the injection of steam into a contaminated unit to volatilize and mobilize contaminants, including DNAPLs (see Figure 4-7). Condensed steam and contaminants are recovered at extraction wells. A variant of steam injection uses hot water, with the objectives of mobilizing the contaminant through reduction of viscosity and, in a commercial application termed Contained Recovery of Oily Wastes (CROW®), reducing downward migration through reduction of DNAPL density. Another variant of the process combines steam injection with direct electrical heating of fine-grained units. Since steam requires sufficient flow to supply enough heat to the entire unit, it is less effective in fine-grained units. Electrical heating may be applied to fine-grained units to drive contaminants to the steamed zones. (The use of electrical heating as a stand-alone treatment method is described in the next section.)
Physical and Chemical Principles
Steam injection promotes contaminant recovery through several mechanisms. Contaminants with boiling points lower than that of steam will volatilize. Vapor pressures of contaminants with higher boiling points will increase greatly due to the increased temperature, promoting volatilization. Finally, the increased temperatures will lower the viscosity of DNAPLs, promoting displacement (Hunt et al., 1988).
The actual process of DNAPL recovery is complex. Volatile components will enter the vapor phase and migrate away from the injection wells, toward cooler regions (Hunt et al., 1988). Condensation will occur at the thermal front, creating a bank of contaminant in front of the advancing steam. DNAPL mobilization may also occur as a result of the decreased interfacial tension and lowered velocity accompanying the increase in temperature. The relative contributions of volatilization, condensation, and displacement depend on the contaminants, site conditions, and operating parameters (Udell, 1997).
Application
Steam at the boiling point of water under the depth being treated is injected in wells, optimally bringing the entire treated volume to the boiling point of water (at the local pressure). The recovered
fluids (hot water plus contaminants) must be treated at the surface. Steam generators and steam handling equipment are commercially available.
Performance
Researchers from Lawrence Livermore National Laboratory (LLNL) and the University of California, Berkeley, conducted a combined demonstration of steam and electrical heating, called ''dynamic underground stripping,'' at LLNL in 1992–1993. The site consisted of a sequence of sands and gravels interbedded with silt and clay units. Contamination was primarily gasoline present as an LNAPL. Researchers estimated that 25 m3 (6,500 gallons) of gasoline were present in the treatment zone prior to the start of the test. The test was conducted at and above the water table. The water table was about 30–37 m (100–120 ft) below ground surface. Steam was injected in two zones, one at 24- to 30-m (80to 100-ft) and one at 34- to 37-m (110- to 120-ft) depth, through six injection wells arranged in a circle on the outside of the treatment zone. Air was recovered through a central extraction well. Four electrodes were emplaced in the treatment zone, and electrical resistance heating was used to heat the fine-grained layers. The treatment zone was heated to boiling (93°C, or 200°F, at applied vacuum). The system recovered more gasoline (27 m3, or 7,000 gallons) than was originally estimated to be present. Subsequently, regulators determined that the site met cleanup standards and closed it (DOE, 1995).
At another site, LLNL researchers, in collaboration with a private company, demonstrated the use of steam for remediation of large volumes of creosote DNAPL. To date, an area of 1.7 ha (4.3 acres) has been treated to a depth of 30 m (100 ft). In the first six weeks of operation, the system recovered 90,000 kg (200,000 lb) of NAPLs, extracted 73,000 kg (29,000 lb) in the vapor phase and burned them, captured 8,000 kg (17,500 lb) on activated carbon, and destroyed an estimated 20,600 kg (45,500 lb) by in situ decomposition. At the time of preparation of this report, the outer portions of the site were clean, and operations were continuing in the central portion (Aines, 1997).
Limitations
Fine-grained zones may require electrical heating. A risk inherent in steam flooding of DNAPL sites is that the condensed solvent front at the leading edge of the steam bank may be more mobile than the original DNAPL. Low-permeability and heterogeneities will re-
duce the effectiveness of the process and increase the amount of contamination that remains after treatment.
Advantages
Field tests have shown that steam can effectively remove petroleum hydrocarbons; large amounts of mass have been removed relatively quickly. The limited results available to date suggest that similar performance may be achieved for DNAPLs. The thermal effects of steam, including volatilization of most common chlorinated solvent DNAPL components, will enable treatment of small-scale heterogeneities in an aquifer.
Electrical Heating
Description
A variety of electrical heating methods can be used to heat contaminated soil, with the objective of volatilizing and extracting the contaminants. As described above, heating can be coupled with SVE or steam injection. Heating methods include resistance (joule) heating, microwave heating, and radio-frequency heating (described in Figure 5-6). In each case, electrical energy is applied to the soil to produce heat. Heat increases the volatility of contaminants and may induce groundwater to boil, forming steam. Contaminants are driven out of the source zone by a combination of volatilization and thermally induced vapor-phase transport. DNAPLs will volatilize if the soil is heated to near the DNAPL boiling point and may be mobilized through a reduction of viscosity as the liquids are heated.
Physical and Chemical Principles
All heating methods rely on the increase in vapor pressure that accompanies temperature increases. As soil heats, the proportion of contaminant present in the vapor phase increases. If the temperature increases to the boiling point of water, most common DNAPL components will partition strongly into the vapor phase and can be removed through vapor extraction. Heating the vapors also increases vapor flow, as vaporization of the pore water and contaminants increases vapor pressure, promoting vapor displacement.
Application
Methods used to heat contaminated soils include the following:
-
Electrical resistance heating (joule heating). Electrical resistance heating involves inserting electrodes in the ground and passing an alternating current through the water and soil between the electrodes. The degree of heating depends on the current and the resistance of the unit. Rocks are generally nonconductive, so most current flows through soil moisture or groundwater. The current decreases as the soil dries, decreasing conductivity. The technique is thus well suited to fine-grained soils, which typically have a high soil moisture content, although vapor transport may still limit contaminant extraction.
-
Six-phase soil heating. Six-phase soil heating is a variant of electrical resistance heating, differing in the way the alternating current is applied to the soil. The reported advantages of six-phase heating are the more even distribution of heat due to splitting of the electrical energy into six phases and the ability to use conventional three-phase alternating current as the power source (DOE, 1995).
-
Radio-frequency heating. Radio-frequency heating uses an electrical field created by inserting antennas into the treatment zone and exciting the soil at approved frequencies (6.68–40.68 MHz). The technology has proven capable of heating low-permeability soils to more than 150°C (Edelstein et al., 1994).
Performance
DOE demonstrated six-phase soil heating at the Savannah River Site in 1993. The target area was a 3-m-thick (10-ft-thick) clay layer at a depth of 12 m (40 ft). The primary contaminants were PCE and TCE, present at maximum concentrations of 181 and 4,529 µg/kg, respectively. Six electrodes were placed in a circle with a diameter of 9 m (30 ft). An extraction well for SVE was placed in the center of the array. The temperature was raised to 100°C in the target zone and maintained for 17 days. The system reportedly removed 99.7 percent of the contamination (DOE, 1995). IIT Research Institute demonstrated radio-frequency heating at the Rocky Mountain Arsenal in 1992. In the test, 38 m3 (50 yd3) of clayey soils were heated to more than 250°C. Concentrations of organochlorine pesticides decreased by 97–99 percent from initial concentrations of up to 5,000 mg/kg (EPA, 1995d).
Recently, radio-frequency heating combined with SVE was demonstrated at Kirtland Air Force Base. In this field test, 0.3 m3 (10 ft3) of soil contaminated with petroleum hydrocarbons was heated for 42
days. Initial treatment by SVE alone removed organic compounds such as gasoline with fewer than 12 carbon atoms per molecule but did not remove the less volatile heavier fractions. Target contaminants for the radio-frequency heating test were the heavier organics such as diesel, with between 12 and 20 carbon atoms (C12–C20). The maximum temperature attained was 139°C. The test system removed approximately 56 percent of the diesel-range organics in the heated volume. Initial concentrations of 2,000–4,000 mg/kg were reduced to 400–1,200 mg/kg. Apparently, the heating also stimulated biodegradation.
Limitations
Because heating technologies do not recover the contaminants themselves, they must be coupled with another technology, typically SVE, for contaminant recovery. Thermal techniques are used either when the permeability of the units to air is too low to allow adequate air flow for conventional SVE or when the vapor pressure of a contaminant is too low. Limitations to the combined technology may arise from the difficulty of fully recovering mobilized vapors. Aquifer heterogeneities may create difficulties with recovery. Compounds with lower volatility will not be effectively treated. The ultimate level of cleanup possible with these systems therefore depends on the types of heterogeneities and contaminants present at the site.
Advantages
Each of the heating technologies has proven capable of heating fine-grained soils to boiling or near-boiling temperatures. At sufficiently high temperature, volatile and semivolatile compounds will volatilize, and water will be driven off as steam. Most chlorinated solvent DNAPLs are volatile enough that heating groundwater to boiling temperatures should drive off the DNAPL as a vapor phase. Thermal methods work well in fine-grained soil, which is often difficult to treat by other methods.
In Situ Vitrification
Description
In situ vitrification (ISV) is an immobilization and destruction technology designed to treat soils and other media contaminated with organic compounds, including DNAPLs, heavy metals, and/or radioac-
tive compounds. Soils are heated until they melt by applying an alternating electrical current between electrodes placed in the ground. Temperatures may exceed 1700°C; at these temperatures, organic compounds either volatilize or are destroyed. Once the target zone is melted, it is allowed. to cool, forming a glass monolith that is relatively resistant to leaching (Dragun, 1991; Oma et al., 1994; NRC, 1996;).
ISV technology was developed to immobilize radioactive isotopes, and it has been employed primarily for this purpose. The technology is discussed in detail in Chapter 3. This section focuses on the use of ISV for destruction of DNAPLs. Although the objective of ISV for treatment of metals and radionuclides is the immobilization of contaminants within the glass monolith, the objective in treating organic compounds, including DNAPLs, is their destruction by the high temperatures produced in the process.
Physical and Chemical Principles
ISV is based on joule heating. Soils have a relatively high resistance, so inducing a flow of electrical current through them generates large amounts of heat. The process is designed to produce temperatures sufficiently high to melt the soil matrix. Temperatures of approximately 1700°C are typically generated.
Organic compounds are not stable at these temperatures. As the temperature increases, organic compounds first volatilize as their boiling points are exceeded and then thermally decompose. The products of thermal decomposition depend upon oxidation conditions (whether oxidation or pyrolysis occurs). The increased vapor pressures due to heating and the creation of a low-pressure zone in the overlying hood for the system cause some organic vapors to migrate to the hood, where they are captured and treated.
As an ISV melt is conducted, the zone of melting expands gradually. A given volume of soil thus heats gradually. As the temperature exceeds the boiling point of water (at the local pressure), water vapor will be driven off, creating a zone of higher permeability to vapors on the edges of the melt. Organic material in this zone will volatilize before being thermally destroyed. The fate of the vapor (destruction in the hotter zones or flow around the melt zone) will depend on the geometry of the soil and melt.
Application
As described in Chapter 3, in the system developed by Battelle Pacific Northwest Laboratories and licensed to Geosafe, Inc., four
graphite electrodes are inserted to a shallow depth in a square pattern in the soil to be treated. An electrical current is applied to melt the contaminated soil (EPA, 1997g; Oma et al., 1994).
The extreme temperatures generated by ISV volatilize many organic compounds along the heated front before the soil melts. Organic compounds that do not volatilize are pyrolized. Volatilized compounds will move away from the melt because heating of the soil vapors increases the vapor pressure adjacent to the melt. The Geosafe, Inc. system manages the vapors by placing a hood over the melt area and applying a partial vacuum to the hood. Vapors are collected by the hood and treated. Off-gas treatment consists of a quencher, scrubber, demister, high-efficiency particulate air filter, and activated carbon. A thermal oxidizer may be incorporated downflow of the activated carbon system, depending on the contaminant(s) present in the matrix being treated (see discussion in Chapter 3 for more details on applications).
Performance
Table 4-5 shows sites at which ISV has been used to treat organic contaminants, as reported by Geosafe, Inc. (See Chapter 3 for additional sites, without organics, treated by ISV.) None of these tests included DNAPL, and no successful test involving DNAPLs has been reported.
Table 4-5 Sites at Which ISV Has Been Used for Organic Contaminants
Limitations
Limitations of ISV for treatment of DNAPLs are the same as those for treatment of metals and radionuclides, as described in Chapter 3. An additional limitation is that the application of ISV to DNAPLs has not been demonstrated. The key concern related to treating DNAPLs with ISV is the ability to contain DNAPL components as they volatilize. The ability of ISV to produce a sufficient temperature to destroy organics has been demonstrated. Containment of volatile phases will depend on site hydrogeology and system design.
Advantages
A significant advantage of ISV is that it is capable of treating complex geologic matrices containing mixtures of contaminants including organics and radionuclides in a single step, a capability that few technologies share. Another significant advantage for DOE sites is that treatment can occur without bringing radioactive materials to the surface, so the technology can decrease exposure risks and eliminate the need for transporting radioactive materials. A third advantage is that the cooled, vitrified mass can serve as a foundation for various types of construction, thus allowing a wide range of uses of the treated area.
REMEDIATION TECHNOLOGIES FOR PLUMES OF DISSOLVED DNAPL CONTAMINANTS
Treatment of plumes of contaminants dissolved from DNAPLs generally poses less of a technical challenge than treatment of undissolved DNAPLs because of the increased mobility of dissolved-phase contaminants. The treatment methods described in the remainder of this chapter apply primarily to dissolved-phase organic contaminants.
Electrokinetic Systems
Description
Electrokinetic treatment systems use a direct current electric potential, applied through electrodes inserted in the treatment zone, to induce migration of water and ions. The process mobilizes contaminants but does not destroy them. Either the contaminants must be recovered at the electrodes or the process must be coupled with an in situ contaminant treatment method. One type of electrokinetic sys-
tem, the LASAGNA® process, combines electrokinetic migration and treatment of organic contaminants, possibly including DNAPLs (see Figure 5-8). The LASAGNA® method (developed by a consortium including Monsanto, Du Pont, General Electric, and DOE) uses a treatment zone between the electrodes to capture, or break down, contaminants as the electrokinetic process transports them. Electrokinetic processes have been used to treat metals and are described in detail in Chapter 3; this section focuses on their application to contaminants from DNAPLs.
Physical and Chemical Principles
Remediation by electrokinetics is based on the migration of water and ions in an electrical field. The movement of pore water under the influence of an electrical potential is termed electroosmosis, and the movement of ions is termed electromigration (Cabrera-Guzman et al., 1990; Acar et al., 1993, 1995). Both laboratory experiments and field work have demonstrated that electric fields can cause water and dissolved ions to migrate at significant velocities. The mechanism of movement of DNAPLs and of noncharged molecules is less well defined. DNAPL molecules, which are nonionic and generally nonpolar, would not be expected to migrate in an electrical field. DNAPLs themselves are typically nonconductive. DNAPL migration may be induced by a combination of osmotic pressure produced by the flow of water, changes in relative saturation due to the removal of water, and compaction of the unit due to dewatering. In addition, substantial temperature increases that occurred during field trials where DNAPLs were suspected to be present may have enhanced volatilization. The application of electrokinetics for treatment of organic contaminants is the objective of the LASAGNA® process.
Performance
The only documented field trials of electrokinetic systems to treat DNAPL employed the LASAGNA® process at DOE's Paducah Gaseous Diffusion Plant. A 3-m by 4.5-m zone of silts and clays contaminated with TCE was treated to a depth of 4.5 m. High dissolved-phase concentrations indicated that DNAPLs might be present, although the amount was not determined. An array of electrodes was operated for 120 days, during which TCE was reduced from an average concentration of 100–500 parts per million (ppm) to 1 ppm in the soil (approximately 99 percent removal). TCE concentrations in suspected DNAPL zones were reduced to 1 ppm, except for a zone at the base of the treatment volume. Since the volume of DNAPL present at the
start was not well determined, the removal efficiency could not be estimated by mass balance. The contamination was captured on adsorbers placed between the electrodes (Ho et al., 1996).
Advantages
Data on the application of electrokinetics to contaminants from DNAPLs are insufficient to evaluate the technology's potential for this purpose. In theory, an advantage of the method is that it treats both organic contamination and metals. It is also suited to difficult-to-treat low-permeability zones.
Limitations
The mechanism of DNAPL migration is not understood, so the range of applications cannot be defined. The technology does not remove or destroy contaminants. No data are available on the levels of residual contamination that may be expected.
In Situ Bioremediation
Description
In situ bioremediation involves the in-place breakdown of contaminants by biologically mediated reactions. Bioremediation may involve no direct action to stimulate natural degradation (a method known as monitored natural attenuation, discussed later in this chapter), or it may involve addition of an electron acceptor (e.g., oxygen), nutrients, and/or an additional carbon source (a method known as engineered in situ bioremediation) (see Figure 4-8). Although organic contaminants can be degraded to carbon dioxide, water, and their component ions, biodegradation reactions may not run to completion.
At many DNAPL-contaminated sites, the DNAPL is composed of one or more common chlorinated solvents, while the dissolved-phase plume emanating from the DNAPL source zone often contains additional compounds that are metabolites of the chlorinated solvents. Common examples include the metabolites of PCE and TCE: dichloroethenes and vinyl chloride. (Ethene also may be produced but is of very limited environmental concern.) The corresponding metabolites from carbon tetrachloride are chloroform, methylene chloride, and chloromethane. Figure 4-9 shows an established metabolic pathway for PCE. The presence of metabolites that were typically not used at the sites is frequently taken as evidence that some biodegradation of
the DNAPL components has taken place. However, the persistence of halogenated organics at many sites indicates that degradation rates are slow relative to the mass of DNAPL components typically present.
Physical, Biological, and Chemical Principles
Bioremediation of many organic contaminants is known to take place naturally in groundwater and soil under both aerobic and anaerobic conditions (Vogel et al., 1987) if environmental factors are conducive to microbial growth. Much of the recent research and development in this area has been directed toward determining which environmental factors control the rate of bioremediation and the development of methods to adjust and control these factors to increase or optimize rates. Biodegradation reactions normally involve either oxidation or reduction of the contaminant and thus require both an oxidizer (electron acceptor) and a reducer (electron donor), one of which may be the contaminant itself. The compounds serving as electron donors and electron acceptors in biodegradation reactions are referred to as primary substrates.
Many organic compounds can serve as a primary substrate; organisms use them as a carbon source and obtain energy through their metabolism. An electron acceptor is required for all such reactions. Oxygen is a common electron acceptor; nitrate, sulfate, manganese, and iron can also serve as electron acceptors in the absence of oxygen. Although aerobic degradation reactions (in which oxygen acts as the electron acceptor) are highly effective at remediation of hydrocarbons as well as some less chlorinated solvents and metabolites of chlorinated solvents, most DNAPL components resist aerobic degradation (NRC, 1993).
Most common DNAPL components, such as TCE and PCE, degrade more readily under anaerobic conditions (NRC, 1993). Although recent research suggests that chlorinated compounds may serve as primary substrates during anaerobic biodegradation under certain conditions, they often do not act as primary substrates but are degraded by cometabolic anaerobic degradation processes. In a cometabolic reaction, some other compound serves as the primary substrate. The key reaction is thought to occur between the chlorinated compound and hydrogen produced through fermentation of other organic compounds, typically degradation intermediates.
Cometabolic degradation thus requires some other carbon source to serve as a primary substrate, in addition to the compound being degraded. The carbon source may be organic carbon naturally occurring in the aquifer, a co-contaminant (petroleum hydrocarbons, for
example), or a compound added by injection. This type of enhanced in situ bioremediation is in the late developmental phase. Several commercial systems have been implemented in addition to numerous field trials and demonstration projects. Organic compounds that are being tested and/or promoted by various vendors to facilitate cometabolism include benzoic acid, ethanol, propionic acid, butyric acid, lactic acid, sucrose, and molasses. Others are promoting the direct injection of hydrogen and the use of slow-release hydrogen compounds based on polymers of lactic acid.
Typically, biodegradation under anaerobic conditions is faster for more highly chlorinated compounds. As a result, cis-dichloroethylene (DCE) and vinyl chloride can accumulate. Reductive dechlorination of these compounds requires stronger reducing conditions. These by-products will, however, also degrade under aerobic and iron-reducing conditions that may occur at the downgradient edge of the plume. Whether these compounds accumulate therefore depends on the aquifer redox potential and other conditions. Accumulation of the degradation intermediates is a concern because these compounds are more mobile than the parent compounds (TCE and PCE) and because vinyl chloride is a carcinogen.
There is little doubt that the addition of electron donors will accelerate reductive dechlorination in aquifers where the requisite microorganisms are present. The formation of reductive dechlorination daughter products, microcosm studies with aquifer soils and ground-water, and/or speciation of microorganisms all can provide evidence that the microbes needed to carry out reductive dechlorination are present. For the process to occur, the chlorinated compounds of interest must be in solution. Thus, the process does not directly treat DNAPLs. Further, where chlorinated compounds are present in very high concentrations, by-products may be toxic to the microorganisms, thus causing the process to become self-limiting. In particular, chloride, if formed in sufficient amounts and not effectively diluted through advection, will cause osmotic shock to the organisms. Also, because chloride is generated as HCl, acid production can be problematic, although the aquifer's buffering capacity is typically sufficient to prevent large pH changes.
Because of these concerns, the use of enhanced anaerobic biodegradation is most likely to be effective in treating plumes dissolving from DNAPLs, rather than the DNAPLs themselves. Enhanced reductive dechlorination is not likely to be applicable to reducing the mass of DNAPL at most sites within a viable time period but can limit migration of dissolved-phase compounds.
Some chlorinated aliphatic compounds can degrade aerobically.
Vinyl chloride and dichloroethenes, for instance, can be used as the primary substrate by some microorganisms under both aerobic and iron-reducing conditions. Chlorinated ethenes, with the exception of PCE, can be aerobically biodegraded through a cometabolic process first described by Wilson and Wilson (1985). In this process, one of several compounds, including methane, butane, toluene, and phenol, is used as the primary substrate during aerobic degradation. Enzymes produced during this process fortuitously degrade certain chlorinated aliphatics.
Implementation of this process requires addition of both oxygen and a cometabolite. Cometabolites, along with oxygen, have been introduced in both gaseous and dissolved forms. Numerous field trials have been conducted. In general, these trials have shown that the extent of degradation of chlorinated compounds under cometabolic conditions has not been sufficient to be effective as a means of remediation.
Application
In practice, in situ bioremediation is implemented by introducing nutrients, typically nitrogen and/or phosphorus sources, air or other sources of oxygen (such as pure oxygen or hydrogen peroxide), and easily degraded organic substrates that can serve as a source of energy for the indigenous microorganisms. The types of additives will determine which microbial processes—aerobic (oxidation) or anaerobic (reductive dechlorination)—are active. In a few instances, bioaugmentation (the introduction of selected nonindigenous microorganisms into the subsurface environment to degrade specific organic contaminants) has been used (Criddle et al., 1996; Duba et al., 1996). In general, however, bioaugmentation has not provided any documented benefit.
Enhancement chemicals may be introduced through wells, infiltration galleries, or trenches, although typically wells have been used. In relatively low-permeability aquifers, multiple wells may be needed. Frequently, groundwater is recovered at downgradient locations in order to accelerate the movement of treatment chemicals through the aquifer, achieve hydraulic control, and remove some of the contaminant mass. Typically, the extracted water is treated, supplemented with enhancement chemicals, and then reintroduced into the aquifer.
Performance
In the past few years, several vendors have reported success in enhancing reductive dechlorination by adding electron donors to the
subsurface. One successful implementation involved a more complex process in which sodium benzoate, sulfate, and oxygen were added at different locations within the aquifer (Beeman et al., 1994). Several vendors have claimed success in inducing reductive dechlorination by adding molasses, but little peer-reviewed information is available on field trials and commercial implementations.
A field demonstration using the combined technologies of aerobic in situ bioremediation and SVE was conducted at DOE's Savannah River Site (Brockman et al., 1995; Hazen et al., 1995; Federal Remediation Technologies Roundtable, 1997). The contaminants, including TCE and PCE, had leaked from an unlined settling basin into an aquifer consisting of several layers of sand with silt and clay beds. Concentrations of TCE ranged from 10 to 1,031 mg/liter, while PCE concentrations ranged from 3 to 124 mg/liter in the groundwater. The water table was 37 m (120 ft) below the ground surface. Nitrogen as nitrous oxide, phosphorus as triethyl phosphate, air, and methane were introduced into the aquifer via horizontal wells located 54 m (176 ft) below the ground surface. Similar wells installed 23 m (75 ft) below the ground surface were used for extraction. The injected gas moved up through the aquifer and the vadose zone to the extraction wells. During 384 days of operation, the combined systems removed 7,700 kg (17,000 lb) of volatile organic compounds and lowered the residual concentrations of TCE and PCE to below 5 mg/liter. Reportedly, bioremediation removed 40 percent more VOCs than SVE alone. Overall, TCE and PCE concentrations in the groundwater decreased by up to 95 percent. Treatment was achieved in a much shorter time period than would have been anticipated for a pump-and-treat system.
In a study conducted at Moffet Naval Air Base in California, phenol and oxygen were added to the reinjected groundwater to evaluate the ability of toluene oxygenase to serve as a cometabolic enzyme for TCE and cis-DCE, which were not effectively degraded when methane was used as a substrate (Hopkins et al., 1993a,b). Phenol and oxygen were added in pulses. When phenol was injected at 12 mg/liter, 85 percent of the TCE and 90 percent of the DCE were biodegraded (Hopkins et al., 1993a,b). Trichloroethane (TCA) was not significantly transformed by either of the treatments.
As a follow-up to the pilot-scale test at Moffett, a full-scale operation was established at an Edwards Air Force Base site where groundwater was severely contaminated with TCE (McCarty et al., 1998). Injection of 7 to 13.4 mg/liter of toluene, oxygen, and hydrogen peroxide into groundwater contaminated with 500 to 1,000 mg/liter of TCE effectively promoted cometabolism of the TCE. Concentrations of TCE were lowered from 1,000 mg/liter in the incoming
groundwater to 18 to 24 mg/liter in the groundwater leaving the treatment zone. Removal rates for TCE and toluene were 97–98 percent and 99.98 percent, respectively.
A multiphase test was conducted at an industrial site in Watertown, Massachusetts, in conjunction with the EPA SITE (Superfund Innovative Technology Evaluation) program (Lewis et. al., 1998). A plume containing PCE, TCE, cis-1,2-DCE, and vinyl chloride was present in an unconfined sand and gravel aquifer. The study consisted of three phases: (1) enhanced anaerobic biodegradation using nitrogen and phosphorous sources in addition to lactic acid, (2) conversation to aerobic conditions using an oxygen release compound (ORC®), and (3) a second anaerobic phase in which hydrogen was provided using a slow-release hydrogen compound (HRC®). During the first phase, PCE was reduced from 1,500 (µ/liter to less than 100 (µ/liter, and both cis-1,2-DCE and vinyl chloride concentrations increased. During the second phase, cis-1,2-DCE and vinyl chloride concentrations decreased, and intermediate epoxides were observed. Unpublished results from the third phase indicated a 95 percent reduction in TCE and PCE concentrations and a 50 percent reduction in total mass of chlorinated ethenes.
Several reports on the application of enhanced reductive dechlorination have appeared in the non-peer-reviewed literature. For example, one report describes a site in eastern Pennsylvania, located in karst terrain 600 m (2,000 ft) upgradient of a major river (Burdick et al., 1998). Contamination resulted from metal plating wastes and sludges containing chromium and degreasing solvents. A pump-and-treat system was no longer effective in improving groundwater quality. Addition of a 2 percent molasses solution to existing wells resulted in a decrease in the oxidation-reduction potential from approximately -66 to -300 mV. A 30 percent reduction in TCE concentration, minimal increases in intermediate chlorinated ethene concentrations, and production of ethene and ethane were observed. The same authors reported a reduction of chromium to below detection limits and of TCE from 18 to 2 mg/liter at a California site.
Limitations
In situ bioremediation has several limitations. One limitation is the fact that because biodegradation occurs only in the aqueous phase, it is not suitable for direct remediation of free-phase DNAPL sources. In addition, the dechlorination of highly chlorinated hydrocarbons produces metabolites that, if not themselves degraded, are more mobile and more toxic than the original compound. In situ bioremediation
also may be limited by difficulties in delivering nutrients, electron acceptors, or electron donors to low-permeability or heterogeneous zones. Therefore, some subsurface environments may not be conducive to enhanced biodegradation.
Other possible disadvantages include the potential degradation of groundwater quality by introduced nutrients and the growth of microbial biomass that may reduce the flow of water. Labor and maintenance could be costly for systems that require long-term treatment, but these costs will be incurred over significantly shorter periods than those for pump-and-treat systems.
Advantages
The major advantage of in situ bioremediation is that it uses indigenous microorganisms to treat a wide variety of soluble organic contaminants. Contaminants treated in situ are not transferred to another medium. Treatment chemicals generally move with the plume, allowing the treatment of sorbed contaminants, or they can be placed to intercept the plume. An increasing body of knowledge is avail-able to support the systematic application of this technology in a variety of soil and geological settings. The technology can be implemented quickly, with a minimum of capital expenditure for some designs, and it is often much faster than other available options.
Phytoremediation
Description
Phytoremediation involves the use of plants to remove contaminants from soil or groundwater. As described in Chapter 3, it is an umbrella term used to describe a number of biochemical interactions that may occur among plants, microbes living on plant roots, and contaminants, and ultimately reduce contaminant concentrations (Schnoor, 1997). Potentially, it can be used to treat dissolved-phase contaminants from DNAPLs in groundwater at or near the root zone, although the use of phytoremediation for this purpose is in the very early stages of development.
Physical, Chemical, and Biological Principles
Studies have confirmed that certain plant species can take up chlorinated solvents from groundwater in the root zone (Chappell, 1997; Schnoor, 1997). Once the plant takes up the solvent, it may
store the chemical in new plant structures via covalent bonding with plant lignin (Schnoor, 1997). The plant also may metabolize the chemical to other compounds. For example, Newman et al. (1997) showed that poplar trees transformed TCE to trichloroethanol, trichloroacetic acid, and dichloroacetic acid—products similar to those produced by enzymes in the human liver on exposure to TCE. Research also has indicated that the growth of plant roots can stimulate degradation of TCE by microorganisms in the root zone via aerobic cometabolism or reductive dechlorination (Chappell, 1997); the plants exude substances through their roots that can stimulate the growth of microbes required to carry out these reactions.
Application
For potential use in treating TCE contamination, phytoremediation research to date has focused primarily on using various species of poplars to serve as natural pump-and-treat systems (Chappell, 1997; Schnoor, 1997). Researchers have bred special poplars with leaves four times as large as usual to increase the rate of water and contaminant uptake. These specially bred poplars can take up and store or transform the contaminant and, in theory, provide hydraulic control of the groundwater. Poplars can extend their roots to the water table, and research studies show that a grove of poplars can create a depression in the water table ranging from several inches to several feet (Chappell, 1997). The rate at which trees pump water depends on the number of trees, tree age, time of day, season, amount of sunlight, climate, and geographic location. In studies carried out to date, pumping rates have ranged from 6 liters per day for young trees to 200 liters per day for older trees (Chappell, 1997). Schnoor (1997) provides theoretical equations for calculating groundwater capture and contaminant uptake rates to determine whether the plants can effectively control the contaminant plume.
For phytoremediation applications using hybrid poplar trees, the planting density would have to be about 1,000 to 2,000 trees per acre (Schnoor, 1997). Trees are planted from long cuttings that root and begin growing rapidly in one season. Theoretically, trees could be harvested every six years, if desired, and sold as firewood or for pulp and paper (Schnoor, 1997). If not harvested, the typical life of a poplar is 30 years.
Performance
A number of pilot-scale studies using poplars to treat TCE are under way, but final results indicating performance levels of this
type of treatment system are not yet available (Chappell, 1997). At Aberdeen Proving Grounds in Maryland, 183 trees have been planted on a 1-acre site to treat TCE. At the Edward Sears Properties in New Gretna, New Jersey, the pilot test involves 118 trees planted on one-third of an acre. A third pilot test, at Carswell Air Force Base in Texas, involves 660 trees planted on 1 acre.
Limitations
Phytoremediation for treatment of dissolved chlorinated solvents is in a very early stage of development. Long-term performance cannot yet be determined for treatment of TCE because of the lack of full-scale applications. Further, phytoremediation is limited to application above the water table and in very shallow groundwater. Treating contaminants from DNAPLs that have migrated deep into ground-water will not be possible with this method.
Advantages
Phytoremediation eliminates the need for excavation and ex situ treatment. It is low cost relative to other treatment options because it is passive and solar driven (Chappell, 1997). Using poplars for TCE treatment does not generate secondary waste. Public acceptance is likely to be high because of the appeal of planting trees.
Permeable Reactive Barriers
Description
Permeable reactive barriers for groundwater remediation consist of subsurface units constructed of permeable reactive media placed to intercept the contaminated groundwater. As groundwater flows through the reactive media, dissolved contaminants are either immobilized or transformed into a more environmentally acceptable form. Removal mechanisms may include physical, chemical, and/or biological processes such as sorption, precipitation, dehalogenation, oxidation-reduction, and fixation. The selection of reactive media depends on site geochemistry, contaminant loading, required degree of contaminant concentration or mass reduction, and design lifetime of the permeable barrier.
Reactive barriers are typically envisioned as permanent or replaceable vertical walls, although horizontal applications have been considered for controlling the downward migration of contaminants.
In the case of vertical reactors, used to control laterally migrating plumes, the reactor system may extend the full width of the contaminant plume or be combined with sheet piling or low-permeability slurry walls to funnel the plume into the reactive wall. Barriers may be installed by excavating a trench and emplacing the reactive medium or by injecting reactive zones into the subsurface. In the latter case, the injection may be coupled with hydraulic fracturing techniques.
Reactive barriers may be used for treatment of water containing either, or both, dissolved organic contaminants and metals. The use of reactive barriers for the treatment of metal-contaminated water is discussed in Chapter 3. This section focuses on the use of reactive barriers to treat organic contaminants.
Numerous studies from the laboratory scale to the field scale are currently being conducted to demonstrate or improve the performance of reactive barrier technology and investigate alternative reactive media (see reviews by Shoemaker et al., 1995; EPA, 1995e, 1997f; and Vidic and Pohland, 1996). Two general classes of reactive media are currently under investigation: media that cause degradation of contaminants and media designed to sorb contaminants. The only reactive medium currently in the commercial stage of application is zero-valent iron, which is being used with funnel-and-gate technology. Most other reactive materials are in the laboratory study stage, although for some media, such as potassium permanganate grout and resting-state microorganisms, field-scale studies are being conducted to establish design and construction procedures.
Physical and Chemical Principles
Reactive barriers containing granular zero-valent iron are being used to degrade chlorinated hydrocarbons, the most common DNAPL components at DOE sites, by the process of reductive dechlorination (Gilham and O'Hannesin, 1994). The metal serves as a source of electrons for the reduction step, which removes chlorine atoms from the hydrocarbons and releases chloride and ferrous iron into solution. The reaction rate appears to be directly proportional to the surface area of granular iron present. Half-lives for the reductive dehalogenation of chlorinated hydrocarbons, normalized to 1 m2 iron surface per milliliter of solution, range from 0.003 to 20 hours for pure iron and 0.3 to 34 hours for commercial iron (Shoemaker et al., 1995). The end products are primarily ethene and ethane, but partially dechlorinated products may form if the reaction time is insufficient. Enhancements to granular iron that result in faster degrada-
tion are being examined but have not yet been field-tested. Promising results have been obtained with palladium-plated iron (Liang et al., 1997).
Variants
Numerous researchers have evaluated variants of, or alternatives to, zero-valent iron systems. Copper and nickel salts, when added to iron filings, increase reaction rates but raise concerns about release of these metals to the aquifer. Palladium increases reaction rates without concern for additional impact on groundwater, but it is expensive. Other researchers have investigated applying a small electrical potential (e.g., 13 V) to the iron. In one reported experiment, 10.3 µM of carbon tetrachloride (CCl4) was degraded to below the detection limit in 4 minutes compared to 3 hours without the applied voltage (Cheng and Wu, 1998). In this experiment, the pH was 7.5 and the oxidation-reduction potential was between -550 and -650 mV.
Several research groups have evaluated the use of sodium dithionite to create a permeable in situ barrier for chlorinated aliphatic hydro-carbons. Column tests conducted with aquifer materials from the Hanford Site demonstrated, like experiments conducted for hexavalent chromium reduction, that the addition of sodium dithionite could be used to create reducing conditions (Thornton et al., 1998). TCE was converted nearly completely to acetylene, with minor amounts of ethene and chloroacetylene produced. The first-order reaction was relatively slow (half-life of about 40 hours) compared to reductive dechlorination reactions with iron filings. Others found much faster degradation rates for CCl4 (Ludwig et al., 1998) but little effect on several other chlorinated aliphatic hydrocarbons. Thornton et al. (1998) conducted tests at pH 11, while the Ludwig et al. studies were conducted at pH 7.5. This may explain the differences in reaction rates. However, increasing the aquifer pH to 11 would increase costs and probably would not be acceptable to regulatory agencies without provisions to restore the pH.
Potassium permanganate is a low-cost oxidant capable of oxidizing a wide range of organic chemicals, including chlorinated hydro-carbons. It has been commonly used in water and wastewater treatment and recently was applied successfully in a field demonstration of in situ remediation of DNAPL compounds (Siegrist et al., 1997). Potassium permanganate, a purple-colored solid crystal at room temperature, readily dissolves in water. The permanganate compound slowly decomposes to form manganese dioxide, but the degradation can be minimized by keeping the solution pH between 3 and 10. In
contact with organic compounds in aqueous solution, the permanganate oxidation reactions break multiple bonds and remove the functional groups of the organic compounds. For example, double bonds in alkenes (e.g., TCE) are readily oxidized by potassium permanganate (Gates et al., 1995).
Potassium permanganate can be mixed with grout and injected or otherwise emplaced into the subsurface to form horizontal or vertical reactive barriers (Siegrist et al., 1997). The grout must be carefully chosen to ensure that it is resistant to oxidation by potassium permanganate and to provide a suitable pH for the oxidation reactions. Bentonite-based grouts appear to be the most suitable carriers for potassium permanganate.
Resting-state, indigenous microorganisms can be harnessed to create a fixed-bed biofilter, another form of reactive barrier, in which bacteria attached to aquifer material degrade chlorinated hydrocarbons (Duba et al., 1996). Microbial filters are established by biostimulation, which involves injecting electron acceptors and nutrients into the subsurface to increase the population of indigenous, contaminant-degrading microorganisms. This process is relatively simple and inexpensive: the surface operations are straightforward, and the injected compounds are generally low in cost. Creation of the biofilter may be accomplished by growing the indigenous bacteria in surface bioreactors, separating the bacteria from their growth medium, re-suspending them in an aqueous solution that is devoid of added growth nutrients, and then injecting the aqueous solution into the subsurface. After the biofilter is created, ambient or induced groundwater flow delivers contaminants to the biofilter region. The degree of contaminant degradation depends on the flux of contaminants, the attached bacterial population density, and the contaminant residence time in the biofilter. Because the bacteria in the biofilter do not receive added nutrients, the performance of the filter diminishes with time, and regular replenishment of the bacterial population by injection is required.
Performance
Commercial applications and field-scale studies of permeable reactive barriers used to remediate chlorinated hydrocarbons are listed in Table 4-6, with notes regarding the treatment efficiency achieved at each site. Successful application of this technology requires an understanding of site hydrogeology and the spatial distribution of contaminants in order to determine the optimum location for the reactive barrier. Additionally, the site geochemistry must be under-
Table 4-6 Specifications for Commercial Applications and Field-Scale Studies of Permeable Reactive Barriers
|
Treatment Medium |
Contaminants Treated |
Demonstration Location and Date |
Site and Plume Characteristics |
Treatment Efficiency (VOCs) |
Construction Design Notes |
|
Granular zero-valent iron |
Halogenated hydrocarbons: TCE, cis-1,2-DCE, VC, CFC-113 |
Sunnyvale, Calif.; February 1995 |
Semiconfined aquifer, 0.6–1.2 m |
No VOCs detected within the wall |
25-m-long slurry wall funnels; 12-m-long, 1.2-m-thick, 6-m-deep, 100% granular iron wall; 4-day residence time |
|
Granular zero-valent iron |
Halogenated hydrocarbons: TCE, PCE |
Moffet Field, Calif.; March 1996 |
Shallow alluvial aquifer |
TCE 16–45 µg/liter in samples taken in downgradient pea gravel section |
6.5-m-long sheet pile funnels; 0.6-m pea gravel, 2-m granular iron, 0.6-m pea gravel wall totaling 3.2 m thick and 8.2 m deep; concrete beneath wall |
|
Granular zero-valent iron |
Halogenated hydrocarbons: PCE, TCE, TCA, 1,2-DCE |
Coffeyville, Kans.; January 1996 |
800-m-long plume; sand-and-gravel unit above shale bedrock, 9 m below ground surface |
Concentrations of solvents in the iron zone are below maximum contaminant levels. |
150-m-long slurry wall funnels; 6-m-long, 9-m-deep 100% granular iron wall |
|
Granular zero-valent iron |
Halogenated hydrocarbons: TCE |
U.S. Coast Guard Air Station, Elizabeth City, N.C.; June 1996 |
Shallow plume, 4–6 m below ground; water table approximately 2 m below ground |
Greater than 95% reduction in chlorinated hydrocarbons |
Continuous trench; 46-m-long, 7.3-m-deep, 0.6-m-thick granular iron wall |
|
Granular zero-valent iron |
Halogenated hydrocarbons: TCE, PCE |
Borden, Ontario, Canada; 1991 |
Shallow plume 2 m wide and 1 m thick; plume was 4 m below ground and 1 m below water table |
90% TCE removal and 86% PCE removal over 4-year monitoring period; 1,2-DCE detected at downgradient monitoring well |
Rectangular cell (5.5 m long, 2.2 m deep, 1.5 m thick) placed 1 m deep; filled with 22% by weight granular iron and 78% by weight coarse sand |
stood in order to select an appropriate reactive medium that is both sufficiently reactive to effect treatment during the time the groundwater remains in contact with the medium and sufficiently stable to be effective for an economically viable period.
Advantages
Permeable reactive barriers are a promising technology for in situ contaminant remediation. They can clean up plumes even when the source of the plume cannot be located. Because they act passively, they require no ongoing energy input and only limited maintenance after installation. Monitoring wells are generally the only surface structures visible after installation. Reactive barriers also essentially eliminate disposal requirements and disposal costs for treated waste because contaminants (except any excavated during trench installation) are not brought to the surface.
Limitations
Currently, the application of permeable reactive barriers is restricted to shallow (less than 13-m-deep), well-characterized plumes. In addition, the technology is applied mainly to dissolved contaminants. The use of reactive barriers to remediate migrating contaminant sources, such as DNAPLs, has not been tested. Reactive barriers therefore are not considered a DNAPL source-zone remediation technology. Additionally, data on the longevity of barrier reactivity and the loss of permeability due to precipitation, both subjects of significant concern, are limited.
Physical Barriers
Physical barriers, such as bentonite-slurry or sheet-piling walls, may be used to contain contamination migrating from a DNAPL source zone. Such technologies are not DNAPL remediation technologies but may be used to reduce the spread of contamination or to allow aggressive source zone remediation within the wall. These technologies are summarized in Chapter 3. Their application to DNAPL contaminants is no different from their application to sites contaminated with metals, except for the risk of DNAPL mobilization that is inherent at all DNAPL-contaminated sites. Any disturbance of a site to emplace a barrier has the potential to mobilize DNAPLs if a DNAPL pool is penetrated. Thus, accurate site characterization is required.
Natural Attenuation
Description
A variety of naturally occurring physical, chemical, and biological processes in the subsurface can decrease contaminant concentrations without human intervention. The combination of these processes is known as natural attenuation. The use of natural attenuation, with monitoring to ensure that contamination is not spreading, is becoming increasingly common for both contaminant cleanup and migration control. The EPA and state regulatory agencies have approved such monitored natural attenuation in place of or in conjunction with active remedies at a large number of sites. Monitored natural attenuation is now the leading remedy for groundwater contaminated by leaking underground storage tanks containing petroleum products (EPA, 1997d). Natural attenuation, although it is the sole remedy at only a handful of these sites, is specified as a component of the remedy in records of decision at more than one-quarter of CERCLA sites (K. Lovelace, Environmental Protection Agency, unpublished data, 1998).
Physical and Chemical Principles
The EPA, in a recent policy directive on natural attenuation, identifies the following processes as active in natural attenuation: biodegradation, biostabilization, dispersion, dilution, sorption, volatilization, and chemical transformation (see Chapter 2). For chlorinated organic contaminants, natural attenuation evaluations generally focus on biodegradation since this is almost always the primary process responsible for reducing contaminant mass. Until relatively recently, scientists believed that chlorinated organic compounds were generally highly resistant to biodegradation in the environment, but in the past two decades a variety of biological processes have been discovered that can transform these compounds in nature (for review articles, see Semprini, 1997a,b). These processes are extremely complex and not fully understood but are a topic of significant research.
The biodegradation process most frequently observed to date at sites where natural degradation of chlorinated solvents has been observed is reductive dehalogenation (Semprini, 1997a). In this process, microbes use the chlorinated compound as part of their energy metabolism, and in the process a chlorine atom is removed from the contaminant. For example, reductive dehalogenation can transform PCE, which has four chlorine atoms, to TCE, which has three, and
can transform TCE to cis-DCE, with two chlorine atoms. Cis-DCE can then be reduced to vinyl chloride, which can be further reduced to ethylene (an essentially harmless compound). Buildup of some of the intermediate transformation products, especially vinyl chloride, which is more carcinogenic than the parent compounds, is a potential risk of this process. Reductive dehalogenation can occur only in anaerobic environments because it requires that the chlorinated compound serve as an electron acceptor, in place of oxygen or other electron acceptors, in microbial metabolism.
Under special conditions, some chlorinated compounds can be transformed biologically in aerobic environments (Semprini, 1997b). Aerobic transformation occurs through the process of cometabolism. In cometabolism, microorganisms do not degrade the contaminant directly, but the contaminant degrades fortuitously by enzymatic reactions that occur as the organisms metabolize other substances. Aerobic cometabolism thus requires the presence of an electron donor compound, generally methane, toluene, phenol, or some other compound that leads to production of the appropriate enzymes. The significance of aerobic cometabolism in the natural attenuation of chlorinated organic contaminant plumes is not well understood but is likely to be limited to the outer edges of the plume, where oxygen is present.
Application
Obtaining regulatory approval to use monitored natural attenuation as the sole component or as part of the remedy at a contaminated site generally requires a careful scientific study to demonstrate to regulators the extent to which natural processes are capable of controlling contaminant migration under specific conditions at the site. EPA has produced a guidance document that specifies in detail the types of evidence required at the sites it regulates (see Chapter 2 for a summary of the requirements). In addition, the Air Force has a detailed technical protocol for investigating natural attenuation of chlorinated solvents that is now widely used to guide studies of natural attenuation at non-Air Force sites (Wiedemeier et al., 1997), and EPA recently published a similar protocol based on the Air Force protocol (EPA, 1998).
Performance
A number of case studies of natural attenuation of chlorinated solvents have been conducted, some showing extensive degradation, some showing partial degradation, and some showing no degrada-
tion. One of the most extensively studied sites is a Superfund site in St. Joseph, Michigan, that is contaminated with TCE at concentrations as high as 100 mg/liter (McCarty and Wilson, 1992; Kitanidis et al., 1993; Haston et al., 1994; Wilson et al., 1994). At this site, Wilson et al. (1994) found nearly a 24-fold decrease in concentrations of chlorinated organic compounds across the site and attributed this decrease to reductive dehalogenation. Although the reductive dehalogenation was extensive, this study did not demonstrate complete transformation of the contaminants to harmless end products. The transformation products cis-DCE, vinyl chloride, and ethene were still present at the site. At another well-studied site, Edwards Air Force Base in California, detailed studies indicated that no biological transformation of TCE has occurred in 40 years (McCarty et al., 1998). Studies at this site have shown that the rate at which the TCE plume has grown is consistent with what would occur in the absence of biodegradation.
Limitations
Natural attenuation of chlorinated solvents is a slow process and thus will not be an appropriate strategy for sites at which relatively rapid cleanup of contaminants is required. Estimating the length of time required for transformation of the contaminants is often not possible due to the complexity of the microbial processes involved. In addition, the biological reactions responsible for attenuation of chlorinated solvents generally require the presence of other organic compounds to serve as electron donors or primary substrates; biodegradation will not occur in the absence of these other substances. Another limitation is that some transformation products that result during natural attenuation, such as vinyl chloride, are more harmful than the original contaminants and may accumulate at the site. Monitoring of sites for natural attenuation can be costly.
Advantages
The primary advantage of this method is that it can eliminate the need for an engineered solution that may disrupt the site, or it can reduce the size of the area requiring treatment with an engineered system. It also can be less costly than engineered methods, depending on the amount of site analysis and monitoring required.
COMMON LIMITATIONS OF DNAPL REMEDIATION TECHNOLOGIES
Regardless of the technology used, hydrologic and geochemical conditions will impose limitations on performance. Geological heterogeneities are the most significant cause of limitation of remediation technologies. The severity of problems produced by heterogeneities can often be predicted based on a thorough site assessment. Variation in hydraulic conductivity within the contaminated zones results in two types of problems: (1) regular lithologic variation produces channeling of flow, and (2) interunit heterogeneity results in unequal access to the unit.
Where some layers have higher conductivity than others, as is typical of layered sedimentary aquifer formations, flow will preferentially occur in the higher-conductivity units. Pumping any fluids, whether vapor or liquid, will require a longer time in the lower-conductivity units, resulting in much larger than necessary volumes being pumped through the high-permeability units.
Variations within a given unit, such as horizontal grain size variation, cause some areas to receive less flow than others within the same horizon. As a result, some zones will receive little or no treatment if a fluid is pumped in or out of the zone. In some cases, this uneven treatment is acceptable if natural attenuation rates are sufficient to control contaminants in less permeable zones following treatment of the permeable zones. The slow movement of groundwater in less permeable soils also can result in reagents being spent before they penetrate into the formation. Failure of reagents to penetrate the contaminated area is especially a problem for chemical oxidants that can oxidize naturally occurring organics and for Fenton's reagent, which can decompose to oxygen and water. In these cases, most of the reagent is consumed unproductively.
Other less obvious restrictions may result from the site lithology. For instance, sites at which a permeable, water-bearing interval is located immediately beneath a low-permeability unsaturated zone can be problematic. Remediation technologies that involve the injection of a gas phase, (e.g., air sparging, steam injection) or that can generate a gas phase (e.g., Fenton's reagent) are limited because the gas phase is difficult to collect for treatment.
Karst hydrogeology presents unique challenges. Groundwater movement in karst terrain occurs mostly through relatively large channels. Horizontal movement can be quite rapid. Reagents introduced in the source area, to the extent this can be defined, may be able to intercept the path followed by the contaminants; however, the reagent
will be chasing the contaminants and may not mix sufficiently with the contaminated groundwater to produce the intended reaction.
Fractured rock poses similar challenges. Flow often occurs through a few conductive fractures. Reaching the entire source area is impossible if contaminants are located in dead-end fractures, and even defining the flow pattern is difficult.
Geochemistry varies among sites and is often affected by the release of contaminants. The groundwater pH, mineral content of soils and groundwater, and presence of nutrients beneficial to microbial processes affect many processes. For example, the release of petroleum hydrocarbons and/or oxygenated solvents, such as methyl ethyl ketone, results in the depletion of oxygen through biodegradation by the indigenous microorganisms. Following consumption of dissolved oxygen, microbial processes can result in lowered oxidation-reduction potentials and increased concentrations of reduced iron (both dissolved and on the surface of minerals), reduced manganese concentrations, and reduced sulfur species (sulfide, etc.) concentrations. Remediation processes that involve reduction, such as reduction of hexavalent chromium or reductive dechlorination of chlorinated solvents, benefit from these geochemical conditions. Conversely, these conditions will create problems for remedial technologies that introduce oxidants, resulting in excess consumption of oxidants to oxidize reduced iron, manganese, and sulfide and to increase the oxidation-reduction potential.
The challenges presented by specific hydrogeologic conditions are likely to affect all remediation technologies but are likely to be less problematic for some technologies than for others. When evaluating and selecting site remedies, it is thus necessary to investigate, understand, and consider site-specific hydrogeology and geochemistry. In some cases there will be no easy answers, and remediation, if possible at all, will be more costly and require a longer time.
CONCLUSIONS
Several technologies have shown the ability to rapidly remove mass from DNAPL source zones. Other technologies have demonstrated the ability to clean up contaminants that have dissolved from these source zones. Following are brief summaries of the demonstrated capabilities of the technologies reviewed in this chapter:
-
Soil vapor extraction is effective for mass removal of volatile compounds in homogeneous, permeable soils and, with the addition of thermal processes, can be extended to semivolatile compounds. Removal of
-
DNAPLs requires sufficient flow through the entire source zone, which may be difficult to achieve.
-
Steam can remediate DNAPLs in permeable soil in both the saturated and the unsaturated zones. It may be combined with electrical heating when fine-grained layers are present. Successful application to DNAPL remediation requires adequate permeability and control of DNAPL mobility. Heterogeneities may limit efficiency.
-
Surfactants have demonstrated the ability to remove DNAPLs nearly completely from permeable units under saturated conditions. DNAPL remediation requires adequate permeability and consideration of DNAPL mobility. Heterogeneities may reduce efficiency.
-
Cosolvents have shown similar potential as surfactants for rapid removal of LNAPLs and should, in principle, be equally effective with DNAPLs. DNAPL remediation requires adequate permeability and consideration of DNAPL mobility. Performance may be limited by heterogeneities.
-
In situ oxidation has proven effective for the destruction of specific chlorinated DNAPL compounds in permeable, relatively homogeneous soils. Its application to DNAPLs requires adequate permeability and delivery of sufficient reagent to the source zone. The volume of DNAPL that may be efficiently treated may be limited by mass transfer considerations.
-
Electrical heating and electrokinetics have shown potential for remediation of DNAPLs in low-permeability units. Both must be accompanied by some form of contaminant retrieval and destruction system. Currently, data are inadequate to determine the effectiveness of electrokinetics for remediating DNAPL source zones.
-
Biodegradation of both chlorinated compounds and PAHs has been demonstrated. Degradation apparently takes place primarily in the dissolved phase, so bioremediation is not a direct DNAPL source-zone treatment method. Degradation of DNAPL source zones may require an extended time.
-
In situ vitrification has demonstrated the ability to vitrify soil and produce temperatures that should lead to the destruction or mobilization of DNAPL compounds. Data on its applicability to DNAPL sites are insufficient to provide a meaningful evaluation at this time.
-
Reactive barrier walls have shown great promise for the treatment of chlorinated solvent dissolved-phase plumes. They do not directly address the DNAPL source zone. Barrier walls, with or without reactive components, may, however, contain DNAPL source zones.
Although a range of technologies is emerging to help clean up DNAPL-contaminated sites, the number of carefully controlled field
tests is insufficient to establish the ultimate cleanup level attainable for each technology. Each technology discussed in this report is based on well-established chemical, biological, and physical principles. Performance limitations are thus more likely to be a function of the hydrogeologic conditions of the site than of the processes themselves. Since an accurate characterization of the occurrence of DNAPLs is essential for the design of a remediation system and an accurate knowledge of geological heterogeneities is vital for evaluating the hydrogeological limits on remediation, thorough site characterization is required for DNAPL sites. Once site assessment has provided the means to evaluate the applicability of the technologies discussed in this report and the probable limitations of remediation, these technologies can be compared to baseline technologies such as excavation and pump-and-treat systems.
REFERENCES
AATDF (Advanced Applied Technology Demonstration Facility). 1997. Technology Practices Manual for Surfactants and Cosolvents. Houston, Tex.: AATDF, Rice University.
Abriola, L. M., K. D. Pennell, G. A. Pope, T. J. Dekker, and D. J. Luning-Prak. 1995. Impact of surfactant flushing on the solubilization and mobilization of dense nonaqueous-phase liquids. ACS Symposium 594:10–23.
Acar, Y. B., A. N. Alshawabkeh, and R. J. Gale. 1993. Fundamentals of extracting species from soils by electrokinetics. Waste Management 13:141–151.
Acar, Y. B., R. J. Gale, A. N. Alshawabkeh, R. E. Marks, S. Puppala, M. Bricka, and R. Parker. 1995. Electrokinetic remediation: Basics and technology status. Journal of Hazardous Materials 40:117–137.
Aines, R. 1997. Results from Visalia: Rapid thermal cleanup of dense nonaqueous-phase liquids. Presentation to the National Research Council Committee on Technologies for Cleanup of Subsurface Contaminants in the DOE Weapons Complex, Third Meeting, Livermore, Calif., December 15–17.
Baran, J. R. J., G. A. Pope, W. H. Wade, V. Weerasoorlya, and A. Yapa. 1994. Microemulsion formation with chlorinated hydrocarbons of differing polarity . Environmental Science and Technology 287(7):1361–1365.
Bass, D. H., and R. A. Brown. 1996. Air sparging case study database update. In Proceedings of the 1st International Symposium on In Situ Air Sparging for Site Remediation, Las Vegas, October 24–25. Potomac, Md.: INET.
Beeman, R. E., J. E. Howell, S. H. Shoemaker, E. A. Salazar, and J. R. Butram. 1994. A field evaluation of in situ microbial reductive dehalogenation by the biotransformation of chlorinated ethylenes. Pp. 14–27 in Bioremediation of Chlorinated and Polycyclic Aromatic Hydrocarbon Compounds, R.E. Hinchee, A. Leeson, L. Semprini, and S.K. Ong, Eds. Boca Raton, Fla.: Lewis Publishers.
Brockman, F. J., W. Payne, D. J. Workman, A. Soong, S. Manley, and T. C. Hazen. 1995. Effect of gaseous nitrogen and phosphorus injection on in situ bioremediation of a trichloroethylene-contaminated site. Journal of Hazardous Materials 41:287–298.
Brown, C. L., M. Delshad, V. Dwarakanath, R. E. Jackson, J. T. Londergan, H. W. Meinardus, D. C. McKinney, T. Oolman, G. A. Pope, and W. H. Wade. In review. A successful demonstration of surfactant flooding of an alluvial aquifer contaminated with DNAPL. Submitted as a research communication to Environmental Science and Technology.
Burdick, J. S., T. Bent, and S. S. Suthersan. 1998. Field applications to demonstrate natural and enhanced transformation of chlorinated aliphatic hydrocarbons. Pp. 81–86 in Natural Attenuation: Chlorinated and Recalcitrant Compounds, E. Godage, B. Wickramanayake, and R. E. Hinchee, Eds. Columbus, Oh.: Battelle Press.
Cabrera-Guzman, D., J. T. Swartzbaugh, and A. W. Weisman. 1990. The use of electrokinetics for hazardous waste site remediation. Journal of Air and Waste Management Association 40:1670–1676.
Chappell, J. 1997. Phytoremediation of TCE Using Populus. Washington, D.C.: Environmental Protection Agency, Technology Innovation Office.
Cheng, S.-C., and S.-C. Wu. 1998. Enhancing chlorinated methane degradation by modifying the Fe reduction system. Pp. 299–304 in Remediation of Chlorinated and Recalcitrant Compounds: Physical, Chemical, and Thermal Technologies, G. B. Wickramanayake and R. E. Hinchee, Eds. Columbus, Oh.: Battelle Press.
Criddle, C. S., M. Dybas, M. Witt, M. Szafranski, C. Kelly, S. Davies, M. Sneathen, S. Mathuram, J. Tiedje, L. Forney, K. Smalla, S. Bezborodnikov, L. Sepulveda-Torres, R. Brown, R. Heine, A. Chan, T. Voice, D. Wiggert, X. Zhao, O. Kawka, M. Barcelona, and T. Mayotte. 1996. The Schoolcraft field bioaugmentation experiment: Evaluation of in-situ bioaugmentation to remediate an aquifer contaminated with carbon tetrachloride. Final report submitted to State of Michigan, Department of Environmental Quality.
Daly, W., A. Ramirez, and R. Johnson. 1998. Electrical impedance tomography of a per-chloroethylene release. Journal of Environmental and Engineering Geophysics 2:189–201.
DOE (Department of Energy). 1995. Dynamic Underground Stripping, Demonstrated at Lawrence Livermore National Laboratory Gasoline Spill Site: GSA, Livermore, CA. Innovative Technology Summary Report. Washington, D.C.: DOE, Office of Environmental Management and Office of Technology Development. Available at http://www.em.doe.gov/plumesfa/intech/dus/.
Dragun, J. 1991. Geochemistry and soil chemistry reactions occurring during in situ vitrification. Journal of Hazardous Materials 26:343–364.
Duba, A. G., K. J. Jackson, M. C. Jovanovich, R. B. Knapp, and R. T. Taylor. 1996. TCE remediation using in-situ resting-state bioaugmentation. Environmental Science and Technology 30:1982–1989.
Dupont, R. R., C. J. Bruell, D. C. Downey, S. G. Huling, M. C. Marley, R. D. Norris, and B. Pivetz. 1998. Innovative Site Remediation: Design and Application—Bioremediation. Annapolis, Md.: American Academy of Environmental Engineers.
Edelstein, W. A., I. E. T. Iben, O. M. Mueller, E. E. Uzgiris, H. R. Philipp, and P. B. Roemer. 1994. Radio frequency ground heating for soil remediation: Science and engineering. Environmental Progress 13(4):247–252.
EPA (Environmental Protection Agency). 1995a. Geosafe Corporation In Situ Vitrification, Innovative Technology Evaluation Report. EPA/540/R-94/520. Cincinnati, Oh.: U.S. EPA Risk Reduction Engineering Laboratory.
EPA. 1995b. In Situ Remediation Technology Status Report: Cosolvents. EPA 542-K-94-006. Washington, D.C.: EPA, Office of Solid Waste and Emergency Response.
EPA. 1995c. In situ air stripping of contaminated groundwater at U.S. Department of Energy, Savannah River Site—Aiken, South Carolina. In Remediation Case Studies: Groundwater Treatment. EPA/542/R-95/003. Washington, D.C.: EPA, Office of Solid Waste and Emergency Response.
EPA. 1995d. In Situ Remediation Technology Status Report: Thermal Enhancements. Washington, D.C.: Office of Solid Waste and Emergency Response.
EPA. 1995e. In Situ Remediation Technology Status Report: Treatment Walls. EPA 542-K-94-004. Washington, D.C.: EPA, Office of Solid Waste and Emergency Response.
EPA. 1996. Engineering Forum Issue Paper: Soil Vapor Extraction Implementation Experiences. EPA 540/F-95/030. Washington, D.C.: EPA, Office of Solid Waste and Emergency Response.
EPA. 1997a. Analysis of Selected Enhancements for Soil Vapor Extraction. EPA-542-R-97-007. Washington, D.C.: EPA, Office of Solid Waste and Emergency Response.
EPA. 1997b. Remediation Technologies Development Forum (RTDF) Update. EPA 542-F-97-005. Washington D.C.: EPA.
EPA. 1997c. Groundwater Currents, Developments in Innovative Groundwater Treatment. EPA 542-N-97-004. Washington D.C.: EPA
EPA. 1997d. Cleaning up the Nation's Waste Sites: Markets and Technology Trends. 1996 Edition. EPA 542-R-96-005. Washington, D.C.: EPA, Office of Solid Waste and Emergency Response.
EPA. 1997e. Use of Monitored Natural Attenuation at Superfund, RCRA Corrective Action, and Underground Storage Tank Sites. Directive No. 9200.4–17. Washington, D.C.: EPA, Office of Solid Waste and Emergency Response.
EPA. 1997f. Permeable Reactive Subsurface Barriers for the Interception and Remediation of Chlorinated Hydrocarbon and Chromium(VI) Plumes in Groundwater. EPA/600/F-97/008. Washington, D.C.: EPA.
EPA. 1997g. Remediation Case Studies: Bioremediation and Vitrification. Volume 5. PB97-177554. Springfield, Va.: National Technical Information Service.
EPA. 1998. Technical Protocol for Evaluating Natural Attenuation of Chlorinated Solvents in Groundwater. EPA/600/R-98/128. Washington, D.C.: EPA, Office of Research and Development.
Fatiadi, A. J. 1987. The classical permanganate ion: Still a novel oxidant in organic chemistry. Journal of Synthetic Organic Chemistry (2)85–206.
Federal Remediation Technologies Roundtable. 1997. Abstracts of Remediation Case Studies, Vol. 2. EPA 542-R-97-010. PB97-177570. Washington, D.C.: U.S. Environmental Protection Agency.
Fountain, J. C., R. C. Starr, T. Middleton, M. Beikirch, C. Taylor, and D. Hodge. 1996. A controlled field test of surfactant-enhanced aquifer remediation. Groundwater 34(5):910–916.
Fountain, J. C. 1998. Technologies for Dense Nonaqueous Phase Liquid Source Zone Remediation, Ground Water Remediation Technology Analysis Center, Technology Evaluation Report. 70 pp.
Freeze, G. A., J. C. Fountain, G. A. Pope, and R. E. Jackson. 1995. Modeling the surfactant-enhanced remediation of perchloroethylene at the Borden test site using UTCHEM compositional simulator. ACS Symposium Series #594: Surfactant-Enhanced Subsurface Remediation, D. A. Sabatini, R. C. Knox, and J. H. Harwell, Eds. Washington, D.C.: American Chemical Society.
Gates, D. D., S. R. Cline, and R. L. Siegrist. 1995. Chemical oxidation of volatile and semi-volatile organic compounds in soil. In Proceedings of the Air and Waste Management Association Conference, June.
Gates, D. D., and R. L. Siegrist. 1995. In-situ chemical oxidation of trichloroethylene using hydrogen peroxide. Journal of Environmental Engineering 121(9):639–644.
Gilham, R. W., and S. F. O'Hannesin. 1994. Enhanced degradation of halogenated aliphatics by zero-valent iron. Groundwater 32:958–967.
Gordon, M. J. 1998. Case history of a large-scale air sparging/soil vapor extraction system for remediation of chlorinated volatile organic compounds in groundwater . Ground-water Monitoring and Remediation 18:137–149.
Grumman, D., and D. Daniels. 1995. Experiments on the detection of organic contaminants in the vadose zone. Journal of Environmental and Engineering Geophysics 6:31–38.
Haston, Z. C., P. K. Sharma, N. N. Black, and P. L. McCarty. 1994. Enhanced reductive dechlorination of chlorinated ethenes. Pp. 11–14 in Symposium on Bioremediation of Hazardous Wastes: Research, Development, and Field Evaluation. EPA/600/R-94/075. Washington, D.C.: U.S. Environmental Protection Agency.
Hazen, T. C., K. H. Lombard, B. B. Looney, M. V. Enzien, J. M. Dougherty, C. B. Fliermans, J. Wear, and C. A. Eddy-Dilek. 1995. Summary of in-situ bioremediation demonstration (methane stimulation) via horizontal wells at the Savannah River Site Integrated Demonstration Project. Pp 137–150 in In-Situ Remediation: Scientific Basis for Current and Future Technologies, Part 1. G. W. Gee and N. R. Wing, Eds. Columbus, Oh.: Battelle Press.
Hirasaki, G. J., C. A. Miller, R. Szafranski, J. B. Lawson, and N. Akiya. 1997a. Surfactant/foam process for aquifer remediation. SPE 37257. Presented at Society of Petroleum Engineers International Symposium on Oilfield Chemistry, Houston, Tex., February 18–21.
Hirasaki, G. J., C. A. Miller, R. Szafranski, D. Tanzil, J. B. Lawson, H. Meinardus, M. Jin, J. T. Londergan, R. E. Jackson, G. A. Pope, and W. H. Wade. 1997b. Field Demonstration of the surfactant/foam process for aquifer remediation. SPE 39292. Presented at Society of Petroleum Engineers Technical Conference and Exhibition, San Antonio, Tex., Oct. 5–8.
Ho, Y.S., D. A. Wase, and C. G. Forster. 1996. Batch nickel removal from aqueous solution by sphagnum moss peat. Water Research 29:1327–1332.
Holbrook, T. B., D. Bass, P. Boersma, D. C. Di Giulio, J. Eisenbeis, N. J. Hutzler, and E. Roberts. 1998. Vapor Extraction and Air Sparging. Annapolis, Md.: American Academy of Environmental Engineers.
Hopkins, G. D., L. Semprini, and P. L. McCarty. 1993a. Microcosm and in-situ field studies of enhanced biotransformation of trichloroethylene by phenol-utilizing microorganisms. Applied and Environmental Microbiology 59:2277–2285.
Hopkins, G. D., J. Munakata, L. Semprini, and P. L. McCarty. 1993b. Trichloroethylene concentration effects on pilot field-scale in-situ groundwater bioremediation by phenol-oxidizing microorganisms. Environmental Science and Technology 27:2542–2547.
Hunt, J. R., N. Sitar, and K. S. Udell. 1988. Nonaqueous phase liquid transport and cleanup. 1: Analysis of mechanisms. Water Resources Research 24(8):1247–1258.
Jerome, K. 1997. In situ oxidation destruction of DNAPL. In EPA Groundwater Currents. Developments in Innovative Groundwater Treatment. EPA 542-N-97-004. Washington D.C.: U.S. Environmental Protection Agency.
Jin, M., M. Delshad, V. Dwarakanath, D. C. McKinney, G. A. Pope, K. Sepernoori, C. E. Tilburg, and R. E. Jackson. 1995. Partitioning tracer test for detection, estimation, and remediation performance assessment of subsurface nonaqueous phase liquids. Water Resources Research 31(5):1201–1211.
Johnson, P. C., A. Baehr, R. A. Brown, R. Hinchee, and G. Hoag. 1994. Innovative Site Remediation: Design and Application—Vacuum Vapor Extraction. Annapolis, Md.: American Academy of Environmental Engineers.
Johnson, P. C., R. L. Johnson, C. Neaville, E. E. Hansen, S. M. Stearns, and I. J. Dortch. 1997. An assessment of conventional in situ air sparging pilot tests. Groundwater 35(5):765–774.
Johnson, R. L., P. C. Johnson, A. Leeson, and C. M. Vogel. 1997. Air distribution during in situ air sparging: Tracer and geophysical measurements. In In Situ and On-Site Bioremediation, Vol. 1. Columbus, Oh.: Battelle Press.
Johnson, R. L., D. Johnson, D. McWhorter, R. Hinchee, and I. Goodman. 1993. An overview of in situ air sparging. Groundwater Monitoring and Remediation 13(4):127–135.
Kitanidis, P. K., L. Semprini, D. H. Kampbell, and J. T. Wilson. 1993. Natural anaerobic bioremediation of TCE at the St. Joseph, Michigan, Superfund site. Pp. 47–50 in Symposium on Bioremediation of Hazardous Wastes: Research, Development, and Field Evaluations. Cincinnati, Ohio: U.S. Environmental Protection Agency.
Kittel, J. A., R. E. Hinchee, R. Hoeppel, and R. Miller. 1994. Bioslurping: Vacuum-enhanced free product recovery coupled with bioventing—A case study. Presented at Petroleum Hydrocarbons and Organic Chemicals in Groundwater, Houston, Tex., November 2–4.
Lake, L. W. 1989. Enhanced Oil Recovery. Englewood Cliffs, N.J.: Prentice Hall.
Lewis, R. F., M. A. Dooley, J. C. Johnson, and W. A. Murray. 1998. Sequential anaerobic/aerobic biodegradation of chlorinated solvents: Pilot-scale field demonstration. Pp. 1–6 in Designing and Applying Treatment Technologies: Remediation of Chlorinated and Recalcitrant Compounds, E. Godage, B. Wickramanayake, and R. E. Hinchee, Eds. Columbus, Ohio: Battelle Press.
Liang, L., N. Korte, J. D. Goodlaxson, J. Clausen, Q. Fernando, and R. Muftikian. 1997. Byproduct formation during the reduction of TCE by zero-valence iron and palladized iron. Groundwater Monitoring and Remediation 17(1):122–127.
Lieberman, S. H., and D. S. Knowles. 1998. Cone penetrometer-deployable in situ video microscope for characterizing sub-surface soil properties. Field Analytical Chemistry and Technology 2(2):127–132.
Lieberman, S. H., G. W. Anderson, and V. Games. 1998. Use of a cone penetrometer deployed video-imaging system for in situ detection of NAPLs in subsurface soil environments. In Proceedings 1998 Petroleum Hydrocarbons and Organic Chemicals in Groundwater: Prevention, Detection and Remediation, Houston, Tex., November 11–13. Westerville, Oh.: National Groundwater Association.
Lien, B. K., and C. G. Enfield. 1998. Delineation of subsurface hydrocarbon contamination distribution using a direct push resistivity method. Journal of Environmental and Engineering Geophysics 2:173–179.
Ludwig, R., A. Rzeczkowska, and S. Dworatzek. 1998. Abiotic trichloroethylene dehalogenation using sodium hydrosulfite: Laboratory and field investigation. Pp. 347–352 in Physical, Chemical, and Thermal Technologies: Remediation of Chlorinated and Recalcitrant Compounds, G. B. Wickramanayake and R. E. Hinchee, Eds. Columbus, Oh.: Battelle Press.
Lunn, S. D., and B. H. Kueper. 1996. Removal of DNAPL pools using upward gradient ethanol floods. In Proceedings of NAPLs in the Subsurface Environment: Assessment and Remediation. Washington D.C.: American Society of Civil Engineers.
Mackay, D. M., and J. A. Cherry. 1989. Groundwater contamination: Pump and treat remediation. Environmental Science and Technology 23(6):630–636.
Marley, M. C., and C. J. Bruell. 1995. In Situ Air Sparging: Evaluation of Petroleum Industry Sites and Considerations for Applicability, Design and Operation. American Petroleum Industry Publication No. 4609 . Washington, D.C.
McCarty, P. L., and J. T. Wilson. 1992. Natural anaerobic treatment of a TCE plume, St. Joseph, Michigan NPL site. Pp. 47–50 in Bioremediation of Hazardous Wastes. EPA/600/R-92/126. Cincinnati, Oh.: EPA Center for Environmental Research Information.
McCarty, P. L, M. N. Goltz, G. D. Hopkins, M. E. Dolan, J. P. Allan, B. T. Kawakami, and T. J. Carrothers. 1998. Full-scale evaluation of in situ cometabolic degradation of trichloroethylene in groundwater through toluene injection. Environmental Science and Technology 32:88–100.
McCray, J. E., and R. W. Falta. 1996. Defining the air sparging radius of influence for groundwater remediation. Journal of Contaminant Hydrology. 24:25–52.
Mercer, J. W., and R. M. Cohen. 1990. A review of immiscible fluids in the subsurface. Journal of Contaminant Hydrology 6:107–163.
Miller, C. M., R. L. Valentine, M. E. Roehl, and P. J. Alvarez. 1996. Chemical and microbiological assessment of pendimethalin-contaminated soil after treatment with Fenton's reagent. Water Resources 30(11):2579–2586.
Montgomery, J. H. 1991. Groundwater Chemicals Field Guide. Chelsea, Mich.: Lewis Publishers.
Mueller, J. G., P. J. Chapman, and P. H. Pritchard. 1989. Creosote-contaminated sites. Environmental Science and Technology 23(10):1197–1201.
Newman, L. A., S. E. Strand, N. Choe, J. Duffy, G. Ekuan, M. Ruszaj, B. B. Shurleff, J. Wilmoth, P. Heilman, and M. P. Gordon. 1997. Uptake and biotransformation of trichloroethylene by hybrid poplars. Environmental Science and Technology 31(4):1062–1067.
Newmark, R. L., W. D. Daily, K. R. Kyle, and A. L. Ramirez. 1997. Monitoring DNAPL Pumping Using Integrated Geophysical Techniques. Report UCRL-ID-122215. Livermore, Calif.: Lawrence Livermore National Laboratory.
Norris, R. D., R. E. Hinchee, R. Brown, P. L. McCarty, L. Semprini, J. T. Wilson, O. H. Kampbell, M. Reinhard, E. J. Bouwer, R. C. Borden, T. M. Vogel, J. M. Thomas, and C. H. Ward. 1994. Handbook of Bioremediation. Boca Raton, Fla.: Lewis Publishers.
NRC (National Research Council). 1993. In Situ Bioremediation, When Does it Work? Washington, D.C.: National Academy Press.
NRC. 1994. Alternatives for Groundwater Cleanup. Washington, D.C.: National Academy Press.
NRC. 1996. Glass as a Waste Form and Vitrification Technology: Summary of an International Workshop. Washington, D.C.: National Academy Press.
Oma, K. H., D. J. Wilson, and A. N. Clarke. 1994. Pp. 457–492 in Hazardous Waste Soil Remediation: Theory and Application of Innovative Technologies. New York: Marcel Dekker.
Pankow, J. F., and J. A. Cherry. 1996. Dense Chlorinated Solvents and other DNAPLs in Groundwater. Portland: Waterloo Press.
Pardieck, D. L., E. J. Bouwer, and A. T. Stone. 1992. Hydrogen peroxide use to increase oxidant capacity for in situ bioremediation of contaminated soils and aquifers: A review. Journal of Contaminant Hydrology 9:221–242.
Peters, C. A., and R. G. Luthy. 1993. Coal tar dissolution in water-miscible solvents: Experimental evaluation. Environmental Science and Technology 27:2831–2343.
Pope, G. A., and W. H. Wade 1995. Lessons from enhanced oil recovery research for surfactant enhanced aquifer remediation. ACS Symposium Series 594:142–160.
Rao, P. S. C., A. G. Hornsby, D. P. Kilcrease, and P. Nkedi-Kizza. 1985. Sorption and transport of hydrophobic organic chemicals in aqueous and mixed solvent systems: Model development and preliminary evaluation. Journal of Environmental Quality 14(3):376–383.
Riley, R. G., J. M. Zachara, and F. J. Wobber. 1992. Chemical Contaminants on DOE Lands and Selection of Contaminant Mixtures for Subsurface Science Research. DOE/ER-0547T. Washington, D.C.: U.S. DOE Office of Energy Research.
Santamarina, J. C., and M. Fam. 1997. Dielectric permittivity of soils mixed with organic and inorganic fluids. Journal of Environmental and Engineering Geophysics 2.:37–51.
Schnarr, M. J., C. T. Truax, G. J. Farquhar, E. D. Hood, T. Gonullu, and B. Stickney. 1998. Experiments using potassium permanganate to remediate trichloroethylene and per-chloroethylene DNAPLs. Journal of Contaminant Hydrology 29(3):205–224.
Schnoor, J. L. 1997. Phytoremediation. TE-98-01. Pittsburgh, Pa.: Groundwater Remediation Technologies Analysis Center.
Schwille, F. 1988. Dense Chlorinated Solvents in Porous and Fractured Media, Model Experiments. Chelsea, Mich.: Lewis Publishers.
Semprini, L. 1997a. In situ transformation of halogenated aliphatic compounds under anaerobic conditions. Pp. 429–450 in Subsurface Restoration, C. H. Ward, H. A. Cherry, and M. R. Scalf, Eds. Chelsea, Mich.: Ann Arbor Press.
Semprini, L. 1997b. Strategies for the aerobic co-metabolism of chlorinated solvents. Current Opinion in Biotechnology 8(3):296–308.
Shoemaker, S. H., J. F. Greiner, and R. W. Gillham. 1995. Permeable reactive barriers. In Assessment of Barrier Containment Technologies. R. R. Rumer and J. K. Mitchel eds. PB96-180583. Springfield, Va.: National Technical Information Service.
Siegrist, R. L., K. S. Lowe, L. D. Murdoch, W. W. Slack, and T. C. Houk. 1997. X-231 A Demonstration of In Situ Remediation of DNAPL Compounds in Low Permeability Media by Soil Fracturing with Thermally Enhanced Mass Recovery of Reactive Barrier Destruction . ORNL/TM-13534. Oak Ridge, Tenn.: Oak Ridge National Laboratory.
Steeples, D.W. 1998. Shallow Seismic Special Issue, Introduction. Geophysics 63:1210–1212.
Thornton, E. C., J. E. Szecdody, K. J. Cantrell, C. J. Thompson, J. C. Evans, J. S. Fruchter, and A. V. Mitroshkov. 1991. Reductive dechlorination. of TCE by dithionite-treated sediment. Pp. 335–340 in Remediation of Chlorinated and Recalcitrant Compounds: Physical, Chemical, and Thermal Technologies, G. B. Wickramanayake and R. E. Hinchees, Eds. Columbus, Oh.: Battelle Press.
Udell, K. S. 1997. Thermally enhanced removal of liquid hydrocarbon contaminants from soils and groundwater. Chapter 16 in Subsurface Restoration, C. H. Ward, J. A. Cherry, and M. R. Scalf, Eds. Ann Arbor, Mich.: Ann Arbor Press.
U.S. Air Force. 1996. Technology Performance and Application Analysis of In Situ Air sparging, Operable Unit 6, Hill Air Force Base, Utah, 19 pp.
Vidic, R. D., and F. G. Pohland. 1996. Treatment Walls. Ground-Water Remediation Technologies Analysis Center, Technology Evaluation Report. TE-96-01. Pittsburgh, Pa.: Ground-Water Remediation Technologies Analysis Center.
Vogel, T. M., C. S. Criddle and P. L. McCarty. 1987. Transformation of halogenated aliphatic compounds. Environmental Science and Technology 21:722–736.
Wiedemeier, T. H., M. A. Swanson, D. E. Moutoux, E. K. Gordon, J. T. Wilson, B. H. Wilson, J. H. Kampbell, J. E. Hansen, P. Haas, and F. H. Chapelle. 1997. Technical Protocol for Evaluating Natural Attenuation of Chlorinated Solvents in Groundwater. San Antonio, Tex.: Air Force Center for Environmental Excellence, Brooks Air Force Base.
Wilson, D. J., and A. N. Clarke. 1994. Hazardous Waste Soil Remediation: Theory and Application of Innovative Technologies. New York: Marcel Dekker, Inc.
Wilson, J. T., J. W. Weaver, and K. D. H. Kampbell. 1994. Intrinsic bioremediation of TCE in groundwater at an NPL site in St. Joseph, Michigan. In Symposium on Intrinsic Bioremediation of Groundwater. EPA/540/R-94/515. Washington, D.C.: U.S. Environmental Protection Agency, Office of Research and Development.
Wilson, J. T., and B. H. Wilson. 1985. Biotransformation of trichloroethylene in soil. Applied and Environmental Microbiology 49:242–243.
Young R. A., and J. Sun. 1996. 3D ground penetrating radar imaging of a shallow aquifer at Hill AFB, Utah. Journal of Environmental and Engineering Geophysics 1:97–109.