Achievements in Chemical Oceanography
John W. Farringtion
Woods Hole Oceanographic Institution
PREFACE
The charge given to me by the steering committee is as follows: focus on landmark achievements in chemical oceanography over the past 50 years, the individuals involved, the new technology and ideas that made these achievements possible, how one discovery built on the foundations of earlier ones, discoveries made at the intersections of disciplines, and the role that NSF programs and institutional arrangements had in making these achievements possible.
I am honored to have the opportunity to share my views on this topic of achievements in chemical oceanography since the 1950s. Given the credentials and landmark (should we call them "seamark" or "channel buoy?") contributions of the others, it is clear that I am a substitute for those much more qualified to satisfy the charge of the committee. For various reasons, those more qualified were not available to write this paper. I suspect that I am the substitute because someone on the organizing committee obtained a copy of my undergraduate transcript and learned that my grades in history and political science were reasonable and certainly much better on the average than my grades in the sciences and math. The committee must also have learned how excited and enthusiastic I am about the study of the oceans and about scientific research and education in general.
I have an apology. The space allocated for this paper is limited, and there is an abundance of significant contributions by individuals and groups deserving of explicit recognition—more than can be incorporated into this paper. Admittedly important areas of research—marine biochemistry, natural product chemistry, and contributions of marine isotopic chemistry to paleoclimate and paleoceanographic studies—that could be thought of by many as marine geochemistry or marine chemistry are not included because of space and time limitations and because they seemed to be beyond the charge given to me. I have continued to revise the paper after initial oral presentation. Those who view the videotape of the presentation and compare it with this written version will note a few significant additions. I sought advice on this paper from several colleagues, but I did not conduct a systematic survey by questionnaire. In hindsight, the lack of a more systematic survey may have been a mistake, but I have had the good fortune in my career to have met and listened to many of the chemical oceanographers and marine chemists in the United States and elsewhere. Their papers, lectures, seminars, and informal conversations inform this paper. I remain less than fully satisfied with the completeness of this paper and have yielded reluctantly to personal limits of scholarship and the requirement to submit the paper in written form by a deadline.
For the readers who are expecting a mention of their favorite element, I regret that limited space precludes a full exploration of the oceans using the periodic table of elements as a guide, although in my opinion that. would be fascinating. While delivering the oral version of this paper, I wore a tie that incorporated the periodic table o f elements to celebrate the event. Thus, I can assure you that all the elements were close to my heart. (There was .about an equal outburst of groans and laughter after this statement at the talk.) When I quote references from the years before the present, I use the language of those times and [ do not correct statements to the gender-neutral language of .our times.
INTRODUCTION
The organizing committee scheduled 'this paper between "biological oceanography" and "physical oceanography" in the symposium program. Many of the significant achievements in chemical oceanography through the 1950s might best be described as applications of chemistry to understanding biological and physical processes in the oceans. The same can be stated about chemistry applied to understanding geological processes. There is ment in organizing the study of the oceans in some manner, and doing so using the fundamental, underpinning science disciplines as a template has advantages. However, I submit that we must keep
foremost as the guiding principle of our endeavors that advancing knowledge of the oceans was the central objective of the research discussed at this symposium.
I found it difficult to define the boundaries of chemical oceanography when preparing this review. Early in the process of preparing this paper, I realized that this was not an important aspect of the undertaking. The record of accomplishments using chemistry to understand the oceans and oceanic processes involves research efforts by individuals and groups who may be primarily self-identified or generally recognized as physical oceanographers, biological oceanographers, or marine geologists. My colleague, Dr. James R. Luyten, Senior Associate Director and Director of Research at Woods Hole Oceanographic Institution (WHOI), brought to my attention a recent editorial in Science "How to Change the University" (Hazzaniga, 1998). A quote from this editorial is thought-provoking and has implications in the world of research and scholarship in general: "The modem university is partitioned along academic lines that no longer truly reflect today's intellectual life." (p. 237). Perhaps this was what the organizing committee for this symposium had in mind when it set forth the charge of "discoveries at the intersections of disciplines."
I believe that those of us studying the oceans should continue to be vigilant and take heed that we do not allow organizational boundaries among or within disciplines to frustrate significant advances in our knowledge of the oceans. Sverdrup, Johnson, and Fleming (1942) with their powerful, wide-ranging (and now venerable) text set the example for us to follow:
Oceanography embraces all studies pertaining to the sea and integrates the knowledge gained in the marine sciences that deal with such subjects as the ocean boundaries and bottom topography, the physics and chemistry of sea water, the types of currents, and the many phases of marine biology. The close interrelation and mutual dependence of the single marine sciences have long been recognized. (Sverdrup, Johnson, and Fleming, 1942, p. 1)
This is the appropriate place to acknowledge the lasting contributions of Richard H. Fleming, Professor of Oceanography, University of Washington, and the co-author of The Oceans, who was a leader in pioneering studies setting the scene for the post-1950 studies of the chemistry of the oceans.
A detailed assessment of progress in chemical oceanography for the past three decades—essentially for the 1970s, 1980s, and 1990s—was assembled recently in an effort funded by the National Science Foundation called Futures of Ocean Chemistry in the United States—an effort with the clever acronym FOCUS. Many excellent chemical oceanographers, marine chemists, and geochemists contributed to the FOCUS report and it is available on the World Wide Web (FOCUS, 1998). The 1970s through 1990s received an extensive treatment by these experts. I concentrate my effort here on the 1950s, 1960s, and early 1970s because this is the time period during which several of the most important contributions and activities occurred over the past 50 years. In fact, I will go back a bit before 1950 to set the scene, and then provide a thread of continuity from this paper to the FOCUS report by a limited discussion of important research efforts from the 1970s into the 1990s.
The Ocean Studies Board of the National Research Council organized this event, and it is appropriate to note that staff of this board and its predecessor boards and committees have provided invaluable service at the interface between the scientific community in general and the federal agencies since the early 1950s. Richard C. Vetter was a key staff person for these boards and committees, serving as Executive Secretary of the Committee on Oceanography during a significant portion of this time. When Dick retired, he advertised on an OMNET (electronic mail) bulletin board that he had a small collection of reports, books, and news clippings to be made available to anyone who would pay for the shipping and promise to keep the collection together and make it available to students in particular, as I recall. I was fortunate to be the selected recipient. This collection contained a copy of the June 1, 1964, weekly professional magazine of the American Chemical Society, Chemical and Engineering News. A part of a featured special report was "Chemistry and the Oceans."
There is an interesting statement in that report: "Chemical oceanography is an old science recently revitalized." (p. 6A) Some may question this since several folks think of oceanography as a relatively young science. Many chemical oceanography texts—for example, a compilation of papers Chemical Oceanography edited by J.P. Riley and G. Skirrow (1965); The Sea, Volume 5: Marine Chemistry, edited by Professor Edward D. Goldberg (1974) and used as keystone learning and reference guides by my generation of chemical oceanographers; and the recent text of Professor Michael E.Q. Pilson (1998)—provide guidance about the history of chemical oceanography and marine chemistry. Wallace (1974) provides a very thorough and highly recommended review of the history of chemical analysis of seawater up to the mid-1900s and then continues with a thorough review as these analyses pertained to chlorinity and salinity determinations well into the 1960s. Comprehensive reviews of various topics in chemical oceanography have been assembled by leading researchers in the volumes of Chemical Oceanography edited by J.P. Riley and R. Chester beginning in 1975 (Riley and Chester, 1975).
CHEMICAL OCEANOGRAPHY PRIOR TO 1950
In the next three paragraphs, I paraphrase or quote from the texts cited above (Riley and Skirrow, 1965; Goldberg, 1974; Wallace, 1974; Pilson, 1998).
Aristotle expounded on the possible origins of the salt in the sea (Wallace, 1974). Since Aristotle's contribution,
eminent scientists and chemists of their times have made significant contributions to understanding the chemistry of the oceans. Among them, during the 1600s and 1700s, were Robert Boyle and his "Observations and Experiment About the Saltness of the Sea" (1674), "which, in the opinion of several (modern) writers, established him as the founder of the science that is now referred to as chemical oceanography" (Wallace, 1974, p. 1). Others from those years included Edmund Halley, Count Luigi Ferdinando Marsigili, Antoine Lavoisier, and Joseph Louis Gay-Lussac. From the late 1700s through the 1800s, Alexander Marcet, Johann Forchhammer, and William Dittmar undertook painstaking analyses of seawater, which provided the heralded "Marcet' s Principle," or the constancy of ratios of several major ions in seawater. Wallace (1974, p. 121) states, "Dittmar's report on the chemistry of the 77 water samples of the 'Challenger' expedition represents the most extensive seawater analysis performed before or since." The importance of knowing the density of seawater drove a significant part of chemical oceanography during the period of 1900 to 1950 to focus on salinity measurements or surrogates, mainly chlorinity, and to affirm the constancy of the ratios of the major ions of seawater. In addition, measurements of nutrients, dissolved oxygen, and the components of the carbonate system and alkalinity were pursued. It was this combination of understanding biological systems, refining and confirming the chlorinity-salinity-density relationships, and the beginnings of the understanding of distinctive chemical compositions for distinguishing water masses that characterized chemical studies of the oceans at that time.
During the 1920s, analytical methods for nutrient substances—mainly compounds of nitrogen and phosphorus— began to appear and to be improved. Individuals such as Atkins, Harvey, and Cooper and the organizing activities of the International Council for the Exploration of the Sea were important in this effort. Harvey's (1928) book The Biological Chemistry and Physics of Seawater captures chemical oceanography of the time as it was involved with biological productivity.
Overlapping in this time frame, in the 1930s, V.M. Goldschmidt, the renowned geochemist, and his school conducted their research on crustal abundances and ionic potential classifications. Goldschmidt and his group also initiated their studies of the mass balances and geochemical cycles of elements, including the oceans in their research (e.g., Goldschmidt, 1933, 1937). Also during this time Buch of Finland and others initiated studies of the physical chemistry of carbon dioxide in seawater. Wattenberg on the Meteor expedition drew attention to the fact that some areas of the ocean were supersaturated while others were undersaturated with respect to calcium carbonate.
Elizabeth Noble Shor, in her historical account of the Scripps Institution of Oceanography, quotes Norris Rakestraw: "One of the most striking observations of marine biology is the fact that some parts of the ocean are fertile while other parts are quite barren. There must be chemical factors which determine fertility, and an explanation of this was perhaps the first serious question which oceanographers asked the chemist. In the year 1930 there were probably no more than a dozen professional chemists in the world who were actively interested in the ocean, and practically every one of them was trying to answer this question" (Shor, 1978, p. 321).
The first chemical laboratory at the Scripps Institution of Oceanography was founded by Erik G. Moberg in 1930 (Shor, 1978). This was the beginning of a tradition of excellence in chemical oceanography and marine chemistry that continues to the present. Further north on the U.S. West Coast, Thomas G. Thompson at the University of Washington labored to improve the analyses of seawater during the 1920s to 1940s. People from those times who should know (NAS, 1971a) described Thompson's laboratory as follows: "For some years this laboratory was the most productive center for chemical oceanography in the United States." (p. 10) Beginning in the 1930s, chemical work began at the Woods Hole Oceanographic Institution (WHOI) with the efforts of Redfield, Seiwell, and Rakestraw, who also conducted research on the questions of the interaction of the biology and chemistry of the sea. Rakestraw later moved to the Scripps Institution of Oceanography.
J.P. Riley (1965) notes that as early as 1935 to 1937, fluorimeteric determinations of uranium in seawater coupled with other observations of the low concentrations of radium in seawater, led to the observation that uranium and radium-226 were in disequilibrium in seawater. The explanatory hypothesis was removal of thorium-230 from the water and its incorporation into sediments.
By 1940, the complexion of chemical oceanography had changed notably. Marine geology, or the geological aspects of oceanography, had been developing through the previous decades, and it had become quite evident that the chemistry of seawater was fundamentally involved in sedimentation phenomena.
Another major division appeared—marine geochemistry— concerned not merely with the use of chemistry to solve geological problems, but also with the part the ocean plays in the broad, general weathering cycles. Since most of the chemical elements have been found in seawater. the chemist is provided with an endless number of problems concerning the source, speciation, function, and significance of these elements and their interactions." (NAS, a, Chapter 1, pp. 10-11).
The World War II years provided a focus for further understanding salinity and the major chemical components contributing to salinity because of its relationship to sound transmission in the sea. At the Woods Hole Oceanographic Institution, Alfred C. Redfield and his former graduate student at Harvard University, Bostwick H. Ketchum, conducted extensive research on antifouling paints with great
success. During the past several years in the United States, there has often been a vigorous debate about definitions and values pertaining to ''basic" and "applied" research. It is interesting that Redfield noted in a taped interview conducted in 1973 by his daughter, Elizabeth R. Marsh, "I learned one thing from [work during the war on] the paint thing, and that was that it was pretty good fun on an applied problem. Because if you had an applied problem which couldn't be solved by existing engineering principles, it meant that you didn't know what the fundamental problems were" (Marsh, 1973).
After World War II, the Office of Naval Research (ONR) continued a strong interest in ocean research, including the chemistry of the oceans (Anderson, 1973). Although this symposium was focused on the National Science Foundation and oceanography, it is important to acknowledge that ONR funding of chemical oceanography and marine chemistry was critical in the years following World War II, especially the 1950s and 1960s, as NSF funding in this arena was initiated and then increased.
Research that has had a major influence in chemical oceanography and marine geochemistry was W.F. Libby's discovery of radioactive carbon produced in the atmosphere from cosmic rays. Continuing the strong connection between biological or ecological considerations and ocean chemistry, the renowned limnologist and ecologist G. Evelyn Hutchinson wrote a provocative paper "The Problems of Oceanic Geochemistry" (Hutchinson, 1947).
The 1950s was a period of intensification of the more traditional (at the time) and mainly descriptive studies of nutrients, dissolved oxygen, and major and minor elements in general. A summary of this particular research focus is found in the report of a meeting convened by the National Academy of Sciences (NAS, 1959) on the physical and chemical properties of seawater. One glimpse of the thinking at that time is provided by this exchange, which can be found in the discussion section of the report. Professor W.T. Holsar of the Institute of Geophysics at the University of California at Los Angeles asked the question, "Can different oceans be characterized by differences in chemical composition?" Professor Richard H. Fleming of the University of Washington answered, "Yes. If a sample labeled only by depth is presented to a chemist, he can, by analyzing chlorinity, calcium, alkalinity and nutrients, distinguish whether it is from the Atlantic, Pacific or Indian Oceans." (p. 95)
An important event of the 1950s in chemical oceanography, as it was for oceanography in general, was the International Geophysical Year (IGY) of 1957-1958. From the perspective of the present, I believe that in addition to obtaining valuable data, the IGY expeditions provided experience with intensive water sampling and chemical analyses of large numbers of samples and experience with multinational collaboration. Chemical measurements were made as "routine" aspects of the hydrographic sections. Experience gained from "catching water" during the hydrographic casts of these expeditions was translated directly into practical improvements for water sampling—hydrographic casts—of the 1960s, which in turn, made possible the significant progress to come in the 1970s and later.
Early National Science Foundation Grants
The 1950s were the formative years of the National Science Foundation. I thank Dr. Michael Reeve of the Ocean Sciences Division of the National Science Foundation and his staff for making available a compilation of grants during the early years of NSF (Reeve, 1998). I have selected all the grants whose titles indicate that they pertain to marine chemistry, geochemistry, and chemical oceanography in some manner (Table 1). Many of the recipients of these grants are widely recognized today as leaders of the 1950s through the present in geochemistry, geology, and chemical oceanography or marine chemistry. Many other prominent chemical oceanographers and marine geochemists were fully funded by the Office of Naval Research and by the Atomic Energy Commission and thus may not have had the time or the inclination to submit a proposal to NSF in the early days of the 1950s.
As far as can be determined, the first NSF grant (listed under Earth Sciences) that could be described as focused in some area of chemical oceanography was awarded to T.J. Chow and T.G. Thompson of the University of Washington, "Distribution of Some Minor Elements in Seawater" (Table 1). Things picked up in 1954 and through the IGY, NSF's Earth Sciences funded research that would have profound effects on our knowledge of the chemistry of the sea and still influence our research today. As the titles of the grants in Table 1 indicate or hint, this research involved one of the major intellectual forces and practical applications of chemistry to the oceans in chemical oceanography and marine geochemistry of the past 50 years—radioactive isotope and stable isotope chemistry analyses of seawater and sediment samples to elucidate physical, biological, geochemical, and biogeochemical processes in the oceans.
Descriptive Chemical Oceanography Shifts Toward Quantifying Rates
The decade also heralded a significant move from the use of chemical measurements for descriptive oceanography to the initiation of the use of chemical measurements to quantify rates of oceanic processes. These were the early career years of several scientists who would make significant contributions to marine chemistry and chemical oceanography and the use of chemistry to understand and quantify oceanic processes: Harmon Craig and Edward D. Goldberg of the Scripps Institution of Oceanography, Wallace Broecker of Columbia University and Lamont-Doherty Geological Observatory, and Karl Turekian of Yale University, among others.
TABLE 1
NSF Grants Related to Marine Geochemistry and Chemical Oceanography in the 1950s
|
Year |
Grant |
|
1953 |
T.J. Chow and T.G. Thompson (University of Washington). Distribution of some minor elements in seawater. |
|
1954 |
C. Urey (University of Chicago). Isotopic abundances relating to geochemical research |
|
D.B. Erickson (Columbia University). Lithological and micropaleontological investigation of the Atlantic Ocean. |
|
|
J.L. Kulp (Columbia University). Time relations of ocean floor sediments . |
|
|
M.L. Keith (Pennsylvania State Univ.). Fractionation of stable isotopes in geological processes. |
|
|
1955 |
J.L. Kulp (Columbia University). Carbon-14 dating of archeological and anthropological specimens. |
|
W.H. Dennen and E. Mencher (Massachusetts Institute of Technology) Geochemical investigations of sedimentary rocks. |
|
|
D.W. Hood (Texas A&M Univ.). Calcium carbonate solubility equilibrium in sea water. |
|
|
1956 |
E.S. Barghoorn (Harvard University). Organic residues in fossil sediments . |
|
H.B. Moore (University of Miami). Oxygen-density relationships and phosphate control of Caribbean waters. |
|
|
H.D. Holland (Princeton University). Radiation damage measurements as a guide to geologic age. |
|
|
V.T. Bowen (Woods Hole Oceanographic Institution). Research instrumentation for sampling water at all depths. |
|
|
E.S. Devey (Yale University). Radiocarbon dating and other forms of geochronometry. |
|
|
1957 |
J.L. Kulp (Columbia University). Isotope geology of strontium and rubidium. |
|
F.F. Koczy (University of Miami). Distribution of radioactive elements in the oceans. |
|
|
E.K. Ralph (University of Pennsylvania). Half-life of carbon-14. |
|
|
K.O. Emery and A. Hancock (University of Southern California). Rate of deposition of sediments off Southern California. |
|
|
1958 |
B.B. Benson (Amherst College). Oxygen isotope variations in ocean water. |
|
C.C. Patterson and T.J. Chow (California Institute of Technology). Lead isotopes in the oceans. |
|
|
W.S. Broecker (Columbia University). Radiocarbon age determinations . |
|
|
T.G. Thompson (University of Washington). Organic compounds in sea water. |
|
|
K.K. Turekian (Yale University). Crustal abundance of nickel, cobalt and chromium. |
|
|
Geophysical Year Related Grants: |
|
|
Radiocarbon or Radiochemistry. (University of California-Scripps Institution of Oceanography, Columbia University, Texas A&M University, Woods Hole Oceanographic Institution) |
|
|
Procurement of Equipment for Carbon Dioxide Measurement. (University of Washington) |
|
|
SOURCE: Reeve (1998). |
|
Some publications of the 1950s would have major, lasting impact on chemical oceanography and marine geochemistry. W.W. Rubey published his very influential contribution Geologic History of Sea Water: An Attempt to State the Problem (Rubey, 1951). In the same year, Urey et al. (1951) reported on their result of measuring paleotemperatures using stable isotopes of oxygen. Harmon Craig wrote The Geochemistry of Stable Carbon Isotopes (Craig, 1953), setting the scene for many studies using stable isotopes of carbon. Edward D. Goldberg wrote Marine Geochemistry 1: Chemical Scavengers of the Sea (Goldberg, 1954), setting the scene for many studies to follow related to particle scavenging of chemicals in seawater. V.M. Goldschmidt, continuing his pioneering efforts in geochemistry over two decades, published his highly acclaimed book, Geochemistry (Goldschmidt, 1954). Goldberg and Arrhenius (1958) published their paper on residence times of elements in the oceans. According to Goldberg (1965), the important concept of residence times for elements in the oceans, as estimated from inputs from rivers (and the atmosphere) and removals to sediments (and assuming steady state conditions), was introduced by Barth (1952). As a harbinger of things to come in the 1960s and later with respect to the utilization of the uranium decay series to quantify several processes in the ocean, Goldberg and Koide (1958) published a paper about ionium-thorium chronology in sediments.
Initiation of Modern Studies of the Oceans' Role in the Carbon Dioxide-Climate Concerns
As noted previously, much of chemical oceanography in the decades prior to 1940 had been focused on biologically related problems. One of the other areas of interest was the exchange of carbon dioxide between the sea and the atmosphere, including the physical chemistry of carbon dioxide and its solution in seawater (NAS, 1971 a). The role of the oceans in the cycle of carbon and particularly the carbon dioxide exchange between the ocean and the almosphere was identified as a major research focus at the Scripps Institution of Oceanography and championed by the Scripps' director Roger Revelle, beginning in the 1950s. Not only did Revelle recognize the significance of the atmosphere-ocean exchange of carbon dioxide and its relationship to climate issues, he participated personally in the research, and he recruited a diverse group of talented chemists an d geochemists to conduct research on the problem, as has been chronicled by Shor (1978). As one example, Revelle brought Charles David Keeling (Keeling, 1958) to Scripps in 1956 and encouraged him to study carbon dioxide in the atmosphere-ocean system (Keeling, 1968). Clearly, one of the most influential papers pertaining to chemical oceanography and oceanography in general of the 1950s, and in all of the literature up to the present in oceanography, is the paper by Revelle and Suess (1957), "Carbon Dioxide Exchange Be
tween the Atmosphere and Ocean and the Question of an Increase of Atmospheric CO2 During Past Decades."
Roger Revelle had a significant influence on the future of chemical oceanography and marine geochemistry research, and ocean and environmental sciences in general, so much so that it is difficult to capture in words. The closest to an accurate description is that of MacLeisch (1982-1983) who identifies Roger Revelle by the apt designation "Senior Senator of Science." Roger Revelle received the National Medal of Science of the United States in 1990.
Tracers, Ocean Circulation and Mixing, and Global Biogeochemical Cycles
A very influential paper in chemical oceanography and marine geochemistry presented at the International Oceanographic Congress, and subsequently published in 1961, was by Broecker, Gerard, Ewing, and Heezen ( 1961) "Geochemistry and Physics of Ocean Circulation." This paper was similar to the paper published in 1960 by the same authors in the peer-reviewed journal literature (Broecker et al., 1960) and was largely the outcome of the Ph.D. thesis research of Wallace S. "Wally" Broecker, completed in 1957 at Columbia University, "Application of Radiocarbon to Oceanography and Climate Chronology" (Broecker, 1957)—the launch of a truly illustrious career by arguably one of the most influential, scholarly geoscientists of his times. Wally Broecker received the National Medal of Science of the United States in 1996 and the Blue Planet Award for his many and diverse scientific contributions.
Three additional points are worthy of mention about this specific contribution by Broecker et al. (1961). First, there is evidence of the success of an earlier NSF investment in the establishment of radiocarbon measurement capability through grants to Kulp in 1954 and 1955 (Table 1). Second, NSF continued to invest in the early career development of Wally Broecker as evidenced by its grant to him in 1958 (Table 1). Third, this was the ocean science and geoscience communities' introduction to the powerful reasoning and explanatory teaching style of Wally Broecker. Readers are invited to compare the reasoning and analogies in Broecker et al. (1961) to that found in the later influential texts Chemical Oceanography (Broecker, 1974), Tracers in the Sea (Broecker and Peng, 1982), and How to Build a Habitable Planet (Broecker, 1985).
Karl K. Turekian was also a graduate student of Professor Kulp at Columbia University, at the same time as Wally Broecker, and they collaborated on some projects (e.g., Broecker et al., 1958). Karl has been influential in many ways in his career. McElway (1983) wrote a profile of Karl Turekian, "Academic Gladiator," in which he captured the Karl Turekian I know: wide-ranging intellect, superb teacher, scrappy debater, eclectic in his significant contributions to Earth sciences—including chemical oceanography and marine geochemistry—through the use of geochemical measurements of various types. Karl' s earlier publications indicated the breadth and depth of contributions to come (e.g., Turekian, 1955, 1957, 1958; Turekian and Kulp, 1956). Karl's book Oceans (Turekian, 1968) provided many of the undergraduates and beginning graduate students of my generation with a concise, readable, important introduction to marine sciences. Karl's influence can be found in some of the most important areas of chemical oceanography from the 1950s to the present as well as in much research on global biogeochemical cycles.
Physical Chemistry of Seawater and Lars Gunnar Sillen
Other aspects of the chemistry of the oceans were receiving increased attention. In 1959, Professors Gustaf Arrenhius and Edward D. Goldberg invited Professor Lars Gunnar Sillen, one of the world's foremost inorganic chemists of the time, to give a lecture at the International Oceanographic Congress in New York, between August 31 and September 12. His paper, (another very influential paper from this decade) "The Physical Chemistry of Seawater" (Sillen, 1961) was published in the proceedings of the Congress edited by Dr. Mary Sears. Goldberg (1974) quotes from Sillen and I repeat Goldberg's quote here:
. . . it may be worthwhile to try to find out what the true equilibrium would be like, and that one might learn from a comparison with the real system. We shall often find that sufficient data are lacking to make the discussion very precise. Neither the laboratory data on chemical equilibria (needed for the model) nor the geochemical data (for the real system) are always as accurate as one might wish. Still, it may be worth while to try this approach. (p. ix)
The process described by Sillen of attempting to define equilibrium or steady-state conditions from fundamental chemical principles and laboratory experiments and then comparing the resulting chemical distributions, including detailed chemical speciation, with actual measured distributions in the oceans, is at the heart of much chemical oceanography and marine chemistry research of the past three decades and at present.
Nuclear Weapons Lest Fallout: Environmental Quality and Tracers in the Sea
The initiation of nuclear weapons testing in the Pacific Ocean in the 1950s by the United States, and elsewhere by other members of the "nuclear weapons club," was accompanied by concern for the fate and effects of several radioactive elements and led to an intensification of research concerned with "biogeochemical" cycling in the oceans (NAS, 1957). Much funding was provided from the Atomic Energy Commission to understand many aspects of oceanic processes, including chemical processes. There were major
concerns about the ultimate exposure of marine life and the critical pathways back to people through the sea. Of course, the introduction of radioactive elements from the weapons testing that continued through the mid-1960s also provided tracers that would become important in verifying and contributing to advances in our understanding of oceanic processes. In one sense, this was an experiment, albeit an experiment that rational scientists would not design and execute deliberately. However, oceanographers would have been remiss in not taking advantage of the tracers introduced by nuclear weapons testing.
Among those responding to this important challenge was Vaughan T. Bowen, a zoologist who received his Ph.D. at Yale University, studying with Professor G. Evelyn Hutchinson. Bowen had been recruited to the Woods Hole Oceanographic Institution by Alfred Redfield and had been studying the distribution of major and minor elements in marine organisms. Under his leadership, Bowen's research group began conducting research on the biogeochemical cycles of radioactive elements entering the oceans, an effort that Bowen pursued until his retirement in the mid-1980s. Bowen and Sugihara (1957) were among the first in the world to publish data on strontium-90 activity in seawater according to compilations prepared in 1971 (NAS, 1971b)—the first of many papers from this group to contribute to our knowledge of biogeochemical cycles of artificial radionuclides in the oceans. Koczy (1956) was another of the pioneers studying the geochemistry of radioactive elements in the ocean.
THE 1960S AND INTO THE EARLY 1970S
This was the decade of explosive growth and maturation in chemical oceanography, marine geochemistry, and marine chemistry. The decade began with the publication of the papers by Sillen (1961) and Broecker et al. (1960, 1961), mentioned earlier, followed by the important papers of Garrels and co-workers (Garrels et al., 1961; Garrels and Thompson, 1962). These efforts of Bob Garrels eventually led to the very productive and influential collaboration with Fred MacKensie and to the influential book Evolution of Sedimentary Rocks (Garrels and MacKensie, 1971).
In 1963, the paper that summarized the thinking and work of Alfred C. Redfield and coworkers on the influence of the chemical composition of organisms, mainly plankton, on the chemical composition of seawater—the famous Redfield or RKR (Redfield, Ketchum, and Richards) ratio (Redfield et al., 1963) was published. In an interview with his daughter (Marsh, 1973), Redfield attributes the origin of that idea to an earlier paper (Redfield, 1958).
Many more scientists were becoming engaged in analyses of seawater for nutrients and other chemicals. In an influential attempt to codify some of the important lessons learned to date, Strickland and Parsons (1965) published their first manual about seawater analysis, which would be followed by a second edition several years later (Strickland and Parsons, 1972). Francis Richards summarized the state of knowledge and importance of studying anoxic basins (Richards, 1965), stimulating several expeditions in future years to the Cariaco Trench and Black Sea (and several fjords) to study the details of biogeochemistry at the interface of oxic and anoxic waters and in anoxic waters.
Scholarship contributions are at the heart of the intellectual enterprise. In addition, organizational leaders with vision, who are also excellent scientists in their own areas of expertise, are important to move fields of research forward. John M. Hunt is this type of person. In 1964, John Hunt was hired away from Carter Oil Company (a subsidiary of Standard Oil of New Jersey) to head the newly formed Chemistry and Geology Department at the Woods Hole Oceanographic Institution. John would chair this department, and later the separate Chemistry Department, for a decade. John made his most important scholarly research contributions in the field of petroleum geochemistry (Dow, 1992). Of equal importance, John Hunt had a lasting impact on marine chemistry, geochemistry, and chemical oceanography through his efforts to build the Chemistry and Geology Department, and later the Chemistry Department, at the Woods Hole Oceanographic Institution with appointments of a diverse group of researchers to yield one of the better marine chemistry and geochemistry departments in the world (Dow, 1992).
Carbon Dioxide, the Carbon Cycle, and Climate
During the 1960s, and continuing to the present, C. David Keeling launched into a time-series measurement of carbon dioxide in the atmosphere (e.g., Keeling, 1973; Keeling et al., 1976a,b). This intense focus by Keeling and collaborators on a time-series certainly numbers among the more important individual research group efforts in marine geochemistry and atmospheric chemistry of' the entire period from 1950 to the present. His data, plotted as concentration of carbon dioxide in the atmosphere versus time at the Mauna Loa, Hawaii, sampling station. have become known worldwide among scientists and environmental policy and management practitioners, inclading heads of state.
From my perspective, Revelle and Suess sounded the alarm about the potentially serious climatic consequences of modern civilization's use of fossil fuels, the resultant increase of carbon dioxide in the atmosphere, and the role of the ocean in the global carbon cycle. Keeling and coworkers provided the data documenting the increase of carbon dioxide in the atmosphere attributable to fossil fael combustion and limestone use.
Keeling's data also begged the question of understanding the magnitude of the exchange of cartoon dioxide between the atmosphere and the ocean. This required not only an understanding of air-sea exchange processes, but also an understanding of the general circulation and mixing time of
the ocean. Broecker et al. (1961), as already noted, provided the pioneering effort to use carbon-14 as a tracer to advance knowledge of oceanic mixing times and confirm general circulation patterns.
Elucidation of the details of the carbon dioxide-carbon-ate system was, and continues to be, a critical area of research throughout the 1950s to the present. Many marine chemists and chemical oceanographers tackled this central problem, as has been documented very nicely by Gieskes (1974), Broecker and Peng (1982), and most recently by Pilson (1998). Biological productivity and remineralization of the biologically produced organic matter as part of the carbon cycle internal to the ocean were the subjects of considerable and important research efforts as reviewed and summarized by one of the main participants (Menzel, 1974).
The details of organic matter composition in seawater and the underlying surface sediments, and by interpretation the processes acting on the organic matter, began to yield to modern analytical organic chemistry methods through the pioneering efforts of Egon Degens at WHOI with his laboratory's studies of amino acids and carbohydrates; Jeffrey Bada and coworker's studies of amino acids at the Scripps Institution of Oceanography; studies of fatty acids by Peter M. Williams of the Scripps Institution of Oceanography; studies of fatty acids and sterols in sediments by Patrick L. Parker and his students at the University of Texas; the research of Max Blumer of the Woods Hole Oceanographic Institution on hydrocarbons and fatty acids in seawater, organisms, and sediments; and efforts of several other scientists (Duursma, 1965; Andersen, 1977; Kvenvolden, 1980, and references therein). I was in the group of marine organic geochemists engaged in our doctoral studies when these pioneering works appeared and they significantly influenced our research.
GEOSECS: The Most Important Chemical Oceanography-Marine Geochemistry Program of the 1950s to 1990s
I am of the opinion that the most important chemical oceanography-marine geochemistry program of the 1950s to the present was initiated in the late 1960s as part of the International Decade of Ocean Exploration: the Geochemical Ocean Sections Study (GEOSECS). One of the main participants, Dr. Peter Brewer, provides an interesting and informative account of GEOSECS in his paper later in this volume, and I will simply add an interesting story about a few of the influences that launched GEOSECS.
Henry Stommel had proposed an elegant theory about the general circulation of the oceans (Stommel, 1957, 1958, Stommel and Arons, 1960a,b; Bolin and Stommel, 1961; Arons and Stommel, 1967). The ability to use tracers such as the carbon-14 activity of the carbon dioxide-carbonate system of seawater to estimate mixing and circulation times had been demonstrated by researchers in the 1960s, following the seminal work of Broecker et al. (1960, 1961). In an interview for an Oceanus volume in honor of Hank Stommel, Wally Broecker (1992) states that it was Hank Stommel who launched GEOSECS.
Ed Goldberg (Scripps Institution of Oceanography) and I were attending some sort of meeting at WHOI during the late 1960s. Hank came to us and said that radiocarbon measurements in the sea were of great importance. He went on to gently chastise us (the geochem community) for doing only scattered stations.
What is needed, he said, is a line of stations extending the length of the Atlantic.
Gee, we said, that would cost a million dollars, a sum greater than the entire NSF annual budget for ocean chemistry.
Hank replied, "Well it would be worth more than a million."
He spurred us to propose such a venture. Soon plans were being formulated not only to do carbon-14 but also a host of other chemical and isotopic properties along Hank's Atlantic line. Boosted by the appearance of Department of Energy [initially from ERDA, DoE's predecessor] monies, Hank's dream became a reality that ultimately covered the entire world ocean and cost NSF $25 million. (p. 73)
Harmon Craig (1992), in his letter nominating Henry Stommel for the National Medal of Science, which Hank Stommel received from President George Bush in 1989, states:
Henry Stommel is the complete scientist, naturalist and sailor, with an eye to every interesting problem and observation that comes along. One of the best examples of this wide-ranging perception of new and interesting developments has been his interest in welding together the tracer geochemistry people and the physical oceanographers for a total look at oceanic circulation and mixing. One result of Henry Stommel's interest in this area was the GEOSECS (Geochemical Ocean Sections Study) Program, which was overwhelmingly considered the best of the NSF-IDOE (International Decade of Ocean Exploration) programs and is the model for the present WOCE (World Ocean Circulation Experiment) initiative, which will expand on and continue the GEOSECS Studies during the next decade.
John Edmond, a major participant in GEOSECS and a marine geochemist who has made several significant contributions to the field, recounts his view of some of the chemical oceanographic achievements that made GEOSECS a possibility (Edmond, 1980). He states, and I paraphrase, that there were several significant efforts and discoveries, such as efforts by Derek Spencer of the Woods Hole Oceanographic Institution and Karl Turekian of Yale University to overcome many obstacles and make oceanic trace-metal profile measurements a practical proposition; the pioneering efforts of Gote Ostlund and Claes Rooth of the University of Miami to measure tritium in the Atlantic Ocean; and measurement of primordial helium in the deep Pacific by Harmon
Craig of the Scripps Institution of Oceanography and Brian Clarke of McMasters University demonstrating that volcanogenic input to the deep ocean was a real thing.
GEOSECS was a monumental undertaking with extensive cruise tracks in the Atlantic, Pacific, and Indian Oceans (see Brewer paper later in this volume). The program had its trials and tribulations in analytical chemistry, among other challenges (harking back to the importance of careful, precise analyses of seawater first highlighted in the 1920s through 1950s) as recounted by Brewer. However, ultimately it was a significant success (Craig, 1972, 1974; Craig and Turekian, 1980; Edmond, 1980) and led to follow-on programs of increasing sophistication and better time and space scale resolution: TTO (Transient Tracers in the Ocean), WOCE (World Ocean Circulation Experiment), and to some extent, the JGOFS (Joint Global Ocean Flux Study) program.
Success in GEOSECS was due to the combined efforts of many people, as is the case for most advances in chemical oceanography and marine geochemistry. The importance of having first-rate sampling systems and onboard analyses systems cannot be overemphasized, and these were provided for GEOSECS by Arnold Bainbridge and the GEOSECS Operations Group. Broecker and Peng (1982) dedicate their book to Arnold Bainbridge in recognition of his important contributions.
Marine Radioactivity and Chemicals of Environmental Concern
Chemical oceanographers, marine chemists and geochemists, physicists, geologists, biologists, and ecologists intensified the investigation of the invasion of radioactive fallout into the oceans and also provided preliminary assessments of the use of artificial radionuclides as tracers of oceanic processes. Radioactivity in the marine environment was assessed in a report of that title published in 1971 (NAS, 1971 b), although the actual work on the report began in 1967. I highly recommend a careful reading of this report. The breadth and depth of advances in knowledge and the literature cited in this report are impressive. One example of information contained in the report (NAS, 1971b, Chapter 7, Figure 4) should serve to whet the reader' s intellectual appetite (Figure 1).
NSF funded much fundamental research in ocean chemistry during the 1960s alone or in partnership with the Office of Naval Research and the Atomic Energy Commission. Understanding the fundamental biogeochemical cycles of chemicals in seawater became the key to assessing some very important societal problems in addition to the role of the ocean in the carbon dioxide-related climate issues and contamination of the oceans by artificial radionuclides. Natural cycles of several elements and compounds were being modified by human activities (SCEP, 1970; NAS, 1971c). Nutrient enrichments in coastal waters were a recognized problem (NAS, 197 l c). People were poisoned with mercury in the Minimata Bay area of Japan. Rachel Carson (1962) documented, in layperson's terms, the promiscuous use of chlorinated pesticides and unintended adverse effects. Shortly thereafter, in the late 1960s, the issue of PCBs (polychlorinated biphenyls) in the environment and potential problems with these chemicals became known. The Santa Barbara oil spill of 1968 captured people's attention for a period of time. Polluted rivers and polluted air were obvious near industrialized areas. The first Earth Day would occur in 1970. All of this is chronicled in a readable book The Health of the Oceans by Edward D. Goldberg (1975a).
Earlier in this paper (see Table 1), the NSF grant to Patterson and Chow was noted. They conducted fundamental research about the biogeochemistry of lead in the marine environment. This led to a critically important finding of the evidence of lead input to the oceans from human activities (Chow and Patterson, 1966). Using isotope dilution mass spectrometry, Patterson's laboratory at California Institute of Technology set the standard for analysis for lead in marine (and other) samples. A summary of their work up to 1976 and its influence on the research of others concerned with the biogeochemistry of lead in the environment is found in Patterson et al. (1976). Claire C. Patterson was recognized for his pioneering geochemical research on lead isotopes by the award of the V.M. Goldschmidt Medal in 1980 (Epstein, 1981). I highly recommend reading Patterson's acceptance speech (Patterson, 1981) to those entering an environmental chemistry career.
Max Blumer, organic geochemist at the Woods Hole Oceanographic Institution, had been supported by both NSF and ONR to undertake fundamental investigations of organic compounds in the marine environment. Max focused on hydrocarbons and fatty acids in the contemporary environment and on pigment diagenesis products in ancient sediments. He had developed elegant and sophisticated trace analytical organic chemistry methods and applied them to the analyses of biosynthesized hydrocarbons, in marine animals, plants, seawater, and surface sediments in the 1960s (see Farrington, 1978, for a more complete review). In the fall of 1969, the barge Florida went aground and spilled No. 2 fuel oil onto the marshes and subtidal area of Buzzards Bay near West Falmouth, Massachusetts. Thus began modem studies of oil pollution in the marine environment. Max Blumer and his laboratory group applied their sophisticated methodology to analyses of surface mud and shellfish— days, weeks, months, and then two years after the visible oil slick had disappeared. They proved beyond reasonable doubt that No. 2 fuel oil persisted long after "conventional wisdom," founded in visual observations, suggested that the oil compounds would be gone from the marine environment (Blumer et al., 1970; Blumer and Sass, 1972a,b). Of equal importance, Max Blumer collaborated with WHOI colleagues in biological oceanography, Howard Sanders and John Teal, and a graduate student advised by Teal, Kathryn
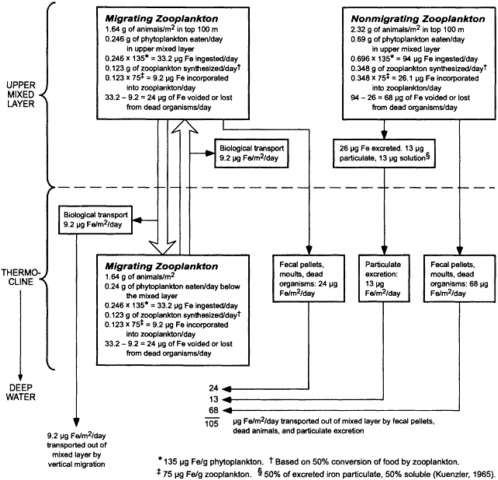
FIGURE 1 Block diagram showing the downward transport of iron by biological mechanisms in phytoplankton and zooplankton in the northeastern Pacific Ocean. SOURCE: Figure 4 in NAS (1971b).
Burns, to demonstrate a linkage between elevated concentrations of No. 2 fuel oil compounds and adverse effects in subtidal, intertidal, and marsh communities of marine organisms (e.g., Bums and Teal, 1971; Bums, 1976; Sanders, 1978; Sanders et al., 1980). Blumer, Sanders, and Teal pioneered modem oil pollution studies, along with colleagues studying the Arrow spill in Canada (e.g., Gordon and Michalik, 1971).
The results of these oil spill studies influenced early studies of the magnitude and biogeochemistry of chronic inputs of petroleum in coastal and estuarine ecosystems (Farrington and Quinn, 1973). Max Blumer shared generously of his knowledge of oil pollution with my Ph.D. thesis advisor James G. Quinn and me during a crucial phase of our studies in 1969 and 1970. I mention this because it is one example of many of how personal communications during scientific meetings and personal visits advance scientific knowledge.
The examples of Patterson's laboratory and of Blumer's research are but two of many examples of NSF-funded basic
research providing the background and underpinning for environmental quality research and the application of this research to providing crucial knowledge and solutions to critical environmental quality issues in the marine environment.
National Academy of Sciences Marine Chemistry Panel, 1969-1971
At the turn of the decade, the Marine Chemistry Panel of the Committee of Oceanography of the National Academy of Sciences met and assessed progress and the challenges ahead. The panel membership consisted of a cross section of the intellectual and organizational leaders of marine chemistry in the United States and Canada. Their names and affiliations (at that time) are Norris Rakestraw, chairman, retired from Scripps Institution of Oceanography; Richard Bader and John Bunt, Rosenstiel School of Marine and Atmospheric Sciences, University of Miami; James Carpenter, head of the Oceanography Section of the National Science Foundation; Dayton Carritt, director of the Institute for Man and His Environment, University of Massachusetts; Gordon Erdman, Phillips Petroleum Company Research Center; Robert Garrells and Edward Goldberg, Scripps Institution of Oceanography; John Hunt , Woods Hole Oceanographic Institution; David Menzel, Skidaway Institute of Oceanography; Timothy Parsons, Institute of Oceanography, University of British Columbia; and Ricardo M. Pytkowicz, Oregon State University.
Alfred C. Redfield wrote the dedication to this report at the request of the committee:
The members of the Committee responsible for this report dedicate it to their chairman, Norris W. Rakestraw, the dean of marine chemists in the United States (NAS, 1971a, p. iii).
The panel's statement in the introduction to the report poignantly outlined the role of chemistry and chemical research in the oceans and, from the perspective of almost three decades later, captured the general framework within which most of the research of the intervening years has been pursued.
There are several viewpoints from which the chemist can approach the ocean. He can consider the oceans as:
A dynamic mixing system, in which composition changes take place partly from internal processes and partly as a result of the circulation and mixing of water masses
A reservoir that is intermediate between the runoff of components from the continents and exchange reactions with the sediments
A biological system in which virtually all the biochemical changes associated with living organisms take place
A grand septic tank in which organic materials are decomposed mostly in the near-surface water or at the bottom
A vast chemical system, in which interactions. occur among an enormous number of components, both organic and inorganic, ranging in concentration through 12 orders of magnitude
An environment that is being invaded by man through his social, agricultural, and industrial activities
The key to interpreting the past history of the earth and as the custodian of its relics. (p. 5-6)
The panel went on to note:
It was said by the late Fritz Koczy of the University of Miami: Chemical reactions in the ocean... are largely determined by phenomena which occur at interfaces... seawater is bounded by two of the most extensive interfaces on earth—the one where it meets the air above, the other where it mingles with the sediment below. (p. 6)
Atmosphere-ocean interactions, in addition to the carbon dioxide exchange, and the chemistry of the surface ocean microlayer have been the subject of much innovative research since that time. Macintyre (1974) and Berg and Winchester (1978) chronicle the important contributions of Robert Duce, John Winchester, Joseph Prospero, William Barger, William Garrett, and others to this effort in the 1960s and early 1970s.
On the subject of sediment geochemistry, Professor Robert A. Berner of Yale University began his research in the 1960s and became one of the more important contributors to our understanding of marine sediment geochemistry (Siever et al., 1961; Berner, 1963, 1964) continuing to the present. Berner's book Early Diagenesis (Berner, 1980) captured much of his thinking on the subject and is one of the leading texts on this subject. Many of the researchers from the 1970s through the present, including this author, were strongly and positively influenced by Berner's work.
Many significant sediment geochemistry studies required comparisons of depth profiles of solid phase and pore water geochemistry. For studies of some important geochemical reactions, obtaining samples of pore water from deep ocean samples in a manner that avoided compromising sample integrity due to changes in temperature and pressure when bringing sediment cores up to the surface was a major challenge. The pioneering efforts of Fred Sayles and his coworkers (Sayles et al., 1976) are illustrative of how efforts to develop novel in situ sampling instrumentation by excellent scientists and careful analysts has led to significant advances in marine chemistry and geochemistry. Fred Sayles used this instrumentation, and subsequent improved versions, to make significant contributions to understanding fluxes of chemicals at the sediment-water interface (e.g., Sayles, 1979, 1981).
THE 1970S TO THE 1990S1
Physical Chemistry and Aquatic Chemistry: Theory and Experimentation
The text, Aquatic Chemistry by Werner Stumm and James J. Morgan (1970), which was aimed at the broader arena of the chemistry of all waters, brought a modern and fundamental underpinning of physical chemistry to aquatic chemistry, including chemical oceanography and marine geochemistry. This book became a required text for many of my generation and a standard reference. The text has been published in a second and third edition (Stumm and Morgan, 1981, 1995). Other excellent texts of similar aim have followed in the intervening years, but I believe that the first edition of Stumm and Morgan had a powerful positive influence on the field following in the wake of the Sillen (1961) paper.
In the arena of physical chemistry from the 1970s to the present, perhaps no other chemical oceanographer-marine geochemist has made more significant contributions than Frank Millero of the University of Miami. Early evidence of this was his contribution "Seawater as a Multicomponent Electrolyte" (Millero, 1974).
International Decade of Ocean Exploration and Chemical Oceanography-Marine Geochemistry
The International Decade of Ocean Exploration programs in chemical oceanography and marine geochemistry were more than the flagship GEOSECS effort. Several programs were grouped together under the overarching theme of environmental quality, including GEOSECS (Jennings and King, 1980). For example, the Manganese Nodules Program, which became known as MANOP in Phase II, undertook important research to better understand the geochemistry of manganese nodules—much touted in the late 1960s and early 1970s as a valuable mineral resource (Knecht, 1982).
In 1971 through early 1972, IDOE launched a one-year Baseline Data Acquisition Program for a limited survey of the extent of contamination of the marine environment by chemicals of environmental concern. The broad focus was on chemicals entering the environment as a result of human activities mobilizing both naturally occurring chemicals (e.g., trace metals and petroleum), and chemicals synthesized only by modem industrial processes (e.g., chlorinated pesticides and PCBs). A conference workshop of three days was convened in May 1972, under the leadership of Professor Edward Goldberg to assess what had been learned from the Baseline effort. I attended this conference, having contributed some of the data as a result of my ongoing postdoctoral research with Max Blumer, initiated in July 1971. Although all of us at the workshop recognized the limitations of the sparse data sets, these data were all we had. Data for trace metals, chlorinated pesticides and PCBs, and the less biodegradable petroleum hydrocarbons could be interpreted in the broadest sense within the framework of lessons learned about biogeochemical cycles from studies of the fallout radionuclides. In the forward to Radioactivity in the Marine Environment (NAS, 1971b), Dr. Philip Handler, president of the National Academy of Sciences summarized this point:
It is particularly appropriate that this contribution to our understanding of the marine environment be available at a time when man is increasingly concerned with the ways in which his own actions may affect his environment. Though this work is specifically addressed to radioactivity in the marine environment, many of the concepts that pertain to our understanding of this problem can be applied effectively to studies of other wastes discharged into the marine environment, including industrial wastes, municipal sewage, pesticides, nutrients, heavy metals and heat. It is perhaps ironic that of the many substances that man has introduced into his environment over the centuries, he understands best and controls most rigorously the radioactive materials that have been produced only during the past quarter century. We are indeed fortunate that our intense concern for public safety and protection from radioactivity since 1950 has stimulated much basic research that can be applied to other serious environmental problems that we are just beginning to recognize. (p. iii)
It was within that type of framework that Ed Goldberg led the "Baseline Conference" to consensus. The fact that we were meeting at the Brookhaven National Laboratory, the Memorial Day weekend was approaching, and Ed controlled the arrival of the buses to the airport provided one impetus for the participants to reach consensus. The consensus was important because Ed Goldberg and a dedicated secretarial staff labored over the weekend to produce the final version of the workshop report and have it printed (Goldberg, 1972). Then Goldberg delivered the report the following week to the First United Nations Conference on the Environment meeting in Stockholm, Sweden, where the report influenced that conference' s deliberations on environmental quality concerns and the ocean. In recognition of this and many other research and science-policy interactions, Ed Goldberg shared the Tyler Prize for Environmental Achievement in 1989 for his many contributions to understanding marine environmental quality issues.
The Baseline Surveys (Goldberg, 1972), and also other scientific research and survey data assessed in the very influential Workshop on Critical Problems of the Coastal Zone, under the leadership of Bostwick H. Ketchum, held May 22 to June 3, 1972, in Woods Hole, Massachusetts (funded in part by the National Science Foundation), led to the inescap
able recognition that there were some serious environmental quality problems in the coastal zone. Several of these problems were associated with chemicals of environmental concern, including trace metals, pesticides, petroleum hydrocarbons, and excessive nutrient inputs (Ketchum, 1972). This spawned many environmental quality research efforts in the coastal zone. The origin of the U.S. Environmental Protection Agency's (EPA' s) Mussel Watch Program, a prototype monitoring program during 1976-1980 for chemicals of environmental concern in coastal areas (Goldberg, 1975b; Goldberg et al., 1978; Farrington et al., 1983), can be traced directly from the experience in the IDOE Baseline Program and individual investigator research efforts funded by a mix of NSF, ONR, and the Atomic Energy Commission. The current operational National Oceanic and Atmospheric Administration (NOAA) Status and Trends Monitoring Program grew out of the U.S. EPA Mussel Watch Program prototype.
The IDOE-NSF follow-on programs to the Baseline Surveys took two pathways: one mainly biogeochemical and one mainly biological effects. In the first pathway, research on marine pollutant transfer was pursued between 1972 and 1976 and is summarized in the workshop book edited by Windom and Duce (1976). The part of this effort concerned with atmospheric inputs to the oceans eventually evolved under the leadership of Robert Duce, among others, to the SEAREX (Sea-Air Exchange) Program of the 1980s (Duce, 1989) and then to other follow-on programs assessing the atmospheric transport of chemicals to the ocean.
In the second pathway, mesocosms were used in CEPEX (Controlled Pollution Experiment) studies undertaken with large plastic enclosures hung in the sea. Within a few years, CEPEX—and mesocosm experiments at Loch Ewe in Scotland—influenced the development of the MERL (Marine Ecosystems Research Laboratory) mesocosms at the Graduate School of Oceanography, University of Rhode Island, funded by U.S. EPA (Grice and Reeve, 1982). In addition, the effect of pollutants at the organism and tissue levels was pursued in the NSF-funded PRIMA (Pollutant Responses in Marine Animals) program (Jennings and King, 1980).
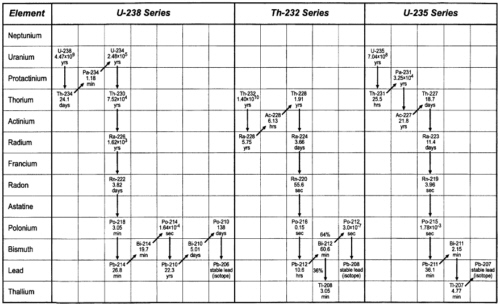
The Uranium Decay Series and Chemical Oceanography-Marine Geochemistry
A considerable number of scientists in numerous studies since the late 1960s, have utilized the uranium decay series radionuclides (Figure 2) to unravel, quantitatively, processes at the boundaries of the oceans and internal processes in the oceans. So many scientists have used this (e.g., see Broecker and Peng, 1982, and Pilson, 1998 for discussion and references), and the knowledge gained has been so important, that it is important to highlight this as an achievement.
Hydrothermal Vents, Riverine Inputs, Atmospheric Inputs and the Inner Workings—Exchanges with the Atmosphere and Deposition to Sediments
There has been amazing progress in understanding the biogeochemical cycles in the oceans since 1970. I have borrowed a cartoon from Professor Conrad Neumann of the University of North Carolina-Chapel Hill that captures many of the important aspects of biogeochemical cycles of the oceans (Figure 3). A reviewer drew my attention to the fact that my favorite part of biogeochemical cycles—organic matter (dissolved and particulate)—is missing. Nevertheless, the cartoon captures much of what needs to be qualitatively depicted.
The most exciting discovery of the 1970s in oceanography, by almost all accounts, was the discovery of the vents of hot fluids at the ridge crest valleys of the mid-ocean ridge system and the unexpected associated fauna founded in a chemosynthetic food web (Ballard, 1977; Corliss et al., 1979; Edmond, 1982). This has been described in Dick Barber's paper on biological oceanography immediately preceding this paper and in Bob Ballard's paper later in this volume. As pointed out by Corliss et al. (1979) and Edmond (1982) among several others, the vents not only were important from a biological perspective, but provided documentation of what had been suspected from analyses of altered basalt dredged from the ridge crests or obtained by submersible, that the interaction of seawater with hot and warm basalt at the ridge crests had an important influence on overall seawater chemical composition and in balancing global biogeochemical cycles on geologic time scales.
At the other end of the inputs pipeline, the flow of dissolved and particulate material into the oceans via rivers received increased and significant attention from the 1970s through the present (e.g., Martin et al., 1981; Milliman and Meade, 1983). A continuing vexing challenge was to understand the effect of increased salinity on the chemical composition of estuarine water as materials flowed from the fresh river water into the more saline estuaries. Sholkovitz and coworkers carded out a series of elegant experiments titrating river water with seawater and observing the effects on the chemistry and physical chemical forms in the resulting solutions (e.g., Sholkovitz, 1976).
William J. Jenkins began studies in W.B. Clarke's laboratory to measure helium-3:helium-4 ratios and also apply this to measuring tritium (Jenkins et al., 1972; Clarke et al., 1976). Bill Jenkins continues to make major important contributions to oceanography. As the citation for the 1997 Bigelow Medal awarded to Bill Jenkins states:
The key to Bill Jenkin's success is that he is one of those rare people who can make superb measurements and can also place the data into sound, quantitative models, allowing him to contribute to diverse fields in a unique way. Few scientists have had as much impact and achieved "recognition among so many different scientific communities.
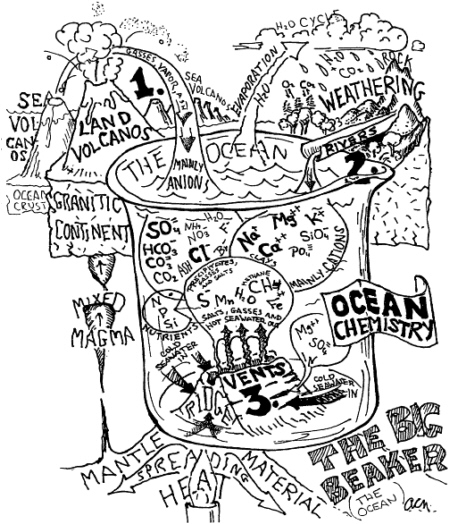
FIGURE 3 Cartoon of oceanic biogeochemical cycles ''The Big Beaker." Cartoon courtesy of Professor Conrad Neumann, University of North Carolina, Chapel Hill.
A pioneering and thoughtful paper by Oliver C. Zafiriou (1977) "previewed" the field of marine photochemistry, stimulating a fresh look at the role of photochemical reactions in the ocean. Since that time, with the efforts of Zafiriou, Zika, and others, our knowledge of marine photo-chemistry has expanded rapidly (Zika, 1987). Marine organic geochemistry moved from descriptive, qualitative studies to become more quantitative and more oceanic process oriented (e.g., see Gagosian, 1983; Farrington, 1987; Lee and Wakeham, 1989; and the review volume edited by Farrington, 1992).
The internal fluxes of materials on particulate matter in the ocean were the subject of significant efforts in chemical oceanography-marine geochemistry. Honjo Spencer, and Brewer undertook an effort using large sediment traps to assess the vertical fluxes of large particles in the oceans in their PARFLUX effort (Honjo, 1978; Spencer et al., 1978; Brewer et al., 1980). Similar efforts were undertaken simultaneously by several other investigators (e.g., Gardner, 1977; Staresinic et al., 1978; Knauer et al., 1979; and reviews by Brewer and Glover, 1987).
A very important small research group effort by Werner
G. Deuser of the Woods Hole Oceanographic Institution, funded by NSF, adopted the Honjo sediment trap design and undertook a pioneering effort to make time-series sediment trap measurements in the Sargasso Sea. Deuser and coworkers documented that there was a seasonal flux of particles to the deep Sargasso Sea (e.g., Deuser and Ross, 1980; Deuser et al., 1981). These oceanic time series measurements built on the Station S measurements off Bermuda, conducted by Hank Stommel for years, and continued by several individuals for years thereafter, and were staged from the Bermuda Biological Station for Research. This effort stimulated other measurements to assess time-variant fluxes of particles to the deep ocean and was a key to initiation of the present time-series measurements in the Joint Global Ocean Flux Study (JGOFS) program.
Dissolved Trace Metals, Biological Processes, and Paleoceanography
While the large particles were being captured and analyzed, significant efforts were underway to measure dissolved trace metals in seawater using new, improved "clean" techniques largely provoked by the work of Patterson and coworkers on measuring lead in seawater (Martin, 1991). As Pilson (1998, p. 209) describes the situation, "The first real breakthrough in attempts to learn the true concentrations of these metals in seawater came in 1975 with the publication by Boyle and Edmond of a paper showing that their data from measurements of copper in surface waters south of New Zealand made sense when plotted against another oceanographic variable, in this case nitrate" (Boyle and Edmond, 1975). Boyle continued this line of research with other examples such as relationships between cadmium and phosphate. Bruland and coworkers and others added several more examples of dissolved trace-metal depth profiles (e.g., see review by Donat and Bruland, 1995). Boyle took the connection of selected trace metal and nutrient cycles and depth profiles a step further in the significant finding that cadmium could be used as a paleoceanographic tracer (Boyle, 1988).
Progress in analytical chemistry has been crucial to many of the advances in our knowledge of trace-metal biogeochemistry, and other biogeochemical processes in the oceans, as it was in the early days of chemical oceanography-marine geochemistry (Johnson et al., 1992). Figure 4, taken from the Johnson et al. (1992) paper, provides an impressive compilation of the 15 orders of magnitude range of concentrations of seawater components now measured in studies of the oceans.
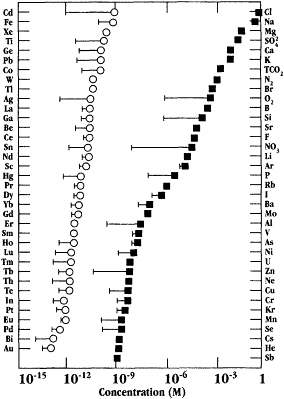
Figure 4 Plot of concentrations of seawater components spanning 15 orders of magnitude in concentration. Source Figure 1 in Johnson et al. (1992). Reproduced with permission from Analytical Chemistry, volume 68, pp. 1065-1075. Copyright 1992 by the American Chemical Society.
The Iron Hypothesis and a Return to One "Root" of Modern Chemical Oceanography
Nearly simultaneous with the sediment trap research of Honjo and Deuser and their colleagues, the VERTEX Program, led by John H. Martin of Moss Landing Marine Laboratory, and Ken Bruland and Mary Silver of the University of California-Santa Cruz, undertook efforts to study the fluxes of particles in the upper ocean and midwater regions and to couple these with both chemical and biological processes (Martin et al., 1983). From these and other studies (e.g., Martin and Fitzwater, 1988), Martin and his coworkers obtained results that led them to an important and stimulating hypothesis that iron was limiting productivity in many areas of the open ocean (Martin, 1991). This hypothesis involves atmospheric transport of dust and associated iron to the iron-limited areas of the oceans where the iron, as an essential limiting factor, stimulates biological primary production. There is even a link to carbon dioxide and climate; Martin suggested that during glacial times, atmospherically transported dust would increase in the southern ocean areas and cause higher productivity, thereby drawing down carbon dioxide levels in the atmosphere. Earlier in this volume, Dick Barber discusses this from the perspective of biological productivity.
This example from the work of Martin and coworkers returns us to one of the early and continuing themes in chemical oceanography noted in the beginning paragraphs of this
paper, the connection between chemical oceanography and biological productivity. As reported earlier in this paper, Rakestraw had noted (see Shor, 1978):
One of the most striking observations of marine biology is the fact that some parts of the ocean are fertile while other parts are quite barren. There must be chemical factors which determine fertility, and an explanation of this was perhaps the first serious question which oceanographers asked the chemist. In the year 1930 there were probably no more than a dozen professional chemists in the world who were actively interested in the ocean, and practically every one of them was trying to answer this question. (p. 231)
Have John Martin and coworkers answered the question at long last?
THE RELATIONSHIP BETWEEN CHEMISTRY, GEOLOGY, PHYSICS, AND CHEMICAL OCEANOGRAPHY/MARINE GEOCHEMISTRY
The 1950s and 1960s were periods of time when few graduate students actually formally received degrees in chemical oceanography or marine geochemistry. Instead, many who contributed to advances in this arena of research were formally educated for their graduate degrees in chemistry, geology, geochemistry, or physics. Examples from the efforts cited above are Max Blumer, Harmon Craig, Ed Goldberg, Bill Jenkins, John Hunt, Frank Millero, Claire Patterson, and Oliver Zafiriou, to name just a few. Beginning in the late 1960s, formal graduate education in chemical oceanography, marine geochemistry, and marine chemistry expanded, and now a majority of those conducting research in this arena have received formal degrees in chemical oceanography (or marine geochemistry, marine chemistry). However, it is important that research and graduate education in chemical oceanography and marine geochemistry maintain connectivity to the advances in the various areas of chemistry, physics, and geology.
I use personal experience to illustrate the point. My Ph.D. graduate education and thesis research in chemical oceanography was directed by Professor James G. Quinn. Jim was attracted to an assistant professorship position in oceanography at the Graduate School of Oceanography, University of Rhode Island, in the late 1960s because of the emergence of Sea Grant—at that time an NSF effort. He was a biochemist with no training or formal education in oceanography. I recall one of our first meetings to discuss what I would do as part of my Sea Grant-funded graduate research assistantship in the fall of 1968. Jim stated that he did not know very much about oceanography, but that he was knowledgeable about lipid biochemistry and thought that there were some exciting and important things to learn about the chemistry and biochemistry of lipids in the marine environment. He thought that perhaps we could learn about oceanography together. He was correct in both accounts! I benefited greatly in my thesis research and throughout my career, as did others of his students and associates, from Jim Quinn's knowledge of lipid biochemistry.
Although I wholeheartedly support graduate education in chemical oceanography, marine chemistry, or geochemistry, I submit that we will be much poorer in the study of the chemistry of the sea and marine sediments unless we continue to attract people such as Jim Quinn to these studies from other arenas of chemistry and biochemistry.
While on the subject of graduate education, I would be remiss if I did not acknowledge the wonderful practice initiated in 1978 by Neil Anderson and Rodger Baier of NSF and Ed Green of ONR to gather together every two years a cross section of senior graduate students (a year away from their Ph.D.) or recent Ph.D.s in chemical oceanography, geochemistry, and aquatic chemistry in a symposium to share their thesis research and ideas. These "Dissertations in Chemical Oceanography" (DISCO) symposia have enriched early careers to the betterment of chemical oceanography and marine geochemistry.
THE SUPPORT AND ENABLING PEOPLE
National Science Foundation, Office of Naval Research, Atomic Energy Commission, Department of Energy, and other agency program managers and staff people support and enable the acceptance, review, and funding of proposals submitted by scientists. More than this,, they work— often tirelessly—behind the scenes to bring the community of chemical oceanographers, marine geochemists, and others together. They have the thankless task of trying to stretch too often inadequate budgets to the maximum benefit of the science. Since this is an NSF-related activity, I confine my citation to those "career" NSF program directors and managers in the Ocean Sciences and IDOE sections with whom I have been acquainted over the years in their support of chemical oceanography and marine geochemistry research— Neil Andersen, Roger Baier, and Michael Heeley. Many scientists who spent one or two years in a temporary rotating appointment assignment at NSF ably assisted them. Dr. Neil Andersen has been recognized formally by [he ocean science community for his important and wide-ranging contributions with the 1994 Ocean Sciences Award from the American Geophysical Union.
In a similar vein, numerous people at various universities, marine laboratories, and oceanographic institutions have provided the administrative, logistical, and laboratory support to enable the research described above. Especially important among these are the officers and crews of the research vessels. I cannot pay tribute to all by name; thus, I will use just two of these many folks as examples from my personal experience. For many years, Emery, on Hiller was master of the R/V Atlantis H and then of R/V Knorr at the Woods Hole Oceanographic Institution. Most chief scien
tists I know, at least it was true for me, felt that there was no doubt that the science had first priority and that you were in "good hands" when at sea with Captain Hiller. His knowledgeable suggestions about cruise tracks and cruise execution in the face of various unforeseen challenges, and his ship handling, are legendary. Another example is Jerry Cotter the bo's'n of the R/V Knorr. There is no doubt that the Atlantic GEOSECS expedition (and many other expeditions and cruises) benefited enormously from the combined expertise and talents of these two individuals and their fellow officers and crew. There is no greater tribute to Jerry than to quote Hank Stommel about the bo's'n: "Lest we think that science is all instrumentation, data, and theory, some time when the weather is making up, and our gear is hopelessly fouled in the water over the side, and the wire has jumped the sheave, the sight of the bo's'n coming on deck is the most important of all." (Hogg and Huang, 1995, p. I-197).
CONCLUDING COMMENTS
The 1950s to the present have been years of significant advances in the application of chemistry to elucidate and quantify oceanic processes. A mix of individual investigator and larger group efforts involving innovative ideas and determined hard work has advanced the field. The development and application of sophisticated analytical methods of trace chemical measurements have been impressive. They have unlocked many secrets of natural and human-forced processes. The power of stable and radioactive isotope chemistry to elucidate and quantitatively unravel physical, chemical, and biological processes in the oceans and underlying sediments moved from concept to reality during the past 50 years and is still evolving rapidly. Mass spectrometers of all sorts have replaced titration burettes as common analytical equipment in the laboratories of chemical oceanography and marine geochemistry.
Data sets of unprecedented size and complexity are being interpreted more routinely. Both equilibrium and nonequilibrium approaches are used commonly to model data collected from the field and laboratory experiments. A rich mix of theory, experimentation, and observation has been at the heart of advances in chemical oceanography and marine geochemistry. As FOCUS (1998) notes, much more exciting and important science is already over the horizon and confronting us today. There are crucial societal needs in the global, regional, and local arenas to be served by improved knowledge in chemical oceanography-marine geochemistry. For this reason and because of the intrinsic excitement of unraveling the beauty and secrets of natural processes, let us hope that the efforts of the next 50 years will at least meet the impressive standard set by the past 50 years !
DEDICATION
In the spirit of this presentation in choosing examples to illustrate the contributions of many, I dedicate this paper to a person whom I had the privilege of collaborating with in two major efforts, the U.S. Mussel Watch Program and the VERTEX Program; I am speaking of John Holland Martin. In late 1993, at the invitation of Professor Margaret Leinen, Dean of the Graduate School of Oceanography (GSO) at the University of Rhode Island (URI), I was approached by the editors of the Bulletin of the Graduate School of Oceanography and asked as an alumnus to write a short in memoriam dedication of the bulletin for 1994-1995 to John H. Martin. John had earned his Ph.D. there in 1966. This was a great honor for me. However, I knew of a person who should aid in this venture, Dr. Donald K. Phelps, a long-standing friend and colleague of John's, who earned his Ph.D. from the GSO in 1964 and had recently retired from the U.S. EPA laboratory in Narragansett. I first met Don when he was a member of my Ph.D. thesis committee. Don offered a poem as his contribution to our effort.
Don's poem is a moving tribute to a friend and colleague who wanted a better world for all of us:
JOHN HOLLAND MARTIN
John Holland Martin, indomitable spirit.
Challenges barriers as a scythe to an emery wheel
well honed for the encounter.
Fallen from the playing field.
Incubated in an iron lung.
Emerges: wheelchair.
Braced for halting steps that bind his movement,
he travels around the world
Where many have not.
Ideas.
Data.
Image in electronic transport speeds like light to spark
controversy and awaken sleeping minds and
tradition bound visions.
Ideas spark as iron against stone.
The iron limitation hypothesis.
Now tested further.
Now closer to crystallizing that vision.
Loves much.
Family, friends, this country, its story-tellers,
The mysteries of the ocean, his students and a good time.
His legacy:
When the Fates give you a bad deal, pick up the cards
and play the game.
DONALD K. PHELPS, 1993
URI-GSO Bulletin for 1994-1995.
ACKNOWLEDGMENTS
I have many people to thank for an enjoyable career in chemical oceanography and environmental chemistry, and I hope that I am not yet finished with this career. First and foremost my family was very supportive (my wife Shirley, daughter Karen, and son Jeff) and endured countless late nights at the laboratory and weeks to months while I was away at sea—including a Thanksgiving, a Christmas, and a New Year. I wish to acknowledge my Ph.D. supervisor Professor James G. Quinn of the GSO-URI and to thank him once again for his unselfish devotion to his students and their careers. I also extend my appreciation to Professor Michael E.Q. Pilson of the GSO-URI for introducing me to chemical oceanography in his graduate class on the subject.
I am grateful to colleagues and coworkers, students and postdocs at the GSO-URI, the University of Massachusetts-Boston, and especially the Woods Hole Oceanographic Institution (where I have been in some category of appointment for twenty-six years) who enriched my life throughout my career. Professor Edward D. Goldberg of the Scripps Institution of Oceanography has provided guidance and advice for many aspects of my research and included me in many of his national and international ventures since we first met in 1972. I hope that other valued colleagues will understand my not mentioning them each by name. The list is extensive! Contribution Number 10083, Woods Hole Oceanographic Institution.
REFERENCES
Andersen, N. (ed.). 1973. Chemical Oceanographic Research: Present Status and Future Direction. Deliberations of a workshop held at Naval Postgraduate School. Monterey, California, December 11-15, 1972. Office of Naval Research, Arlington, Virginia.
Andersen, N. (ed.). 1977. Concepts in marine organic chemistry. Marine Chemistry 5:303-638.
Arons, A.B., and H. Stommel. 1967. On the abyssal circulation of the world ocean. — III. An advection-lateral mixing model of the distribution of a tracer property in an ocean basin. Deep-Sea Res. 14:441-457.
Barth, T.W. 1952. Theoretical Petrology. Wiley. New York.
Berg, W.W., Jr., and J.W. Winchester. 1978. Aerosol chemistry of the marine atmosphere. Chapter 38. Pp. 173-231 in J.P. Riley and R. Chester (eds.), Chemical Oceanography (2nd Edition) Volume 7. Academic Press, London.
Bemer, R.A. 1963. Electrode studies of hydrogen sulfide in marine sediments. Geochim. Cosmochim. Acta 27:563-575.
Berner, R.A. 1964. An idealized model of sulfate distribution in recent sediments. Geochim. Cosmochim. Acta 28:1497-1503.
Bemer, R.A. 1980. Early Diagenesis: A Theoretical Approach. Princeton University Press, Princeton, New Jersey. 241 pp.
Blumer, M., and J. Sass. 1972a. Oil pollution: Persistence and degradation of spilled fuel oil. Science 176:1120-1122.
Blumer, M., and J. Sass. 1972b. Indigenous and petroleum derived hydrocarbons in an oil polluted sediment. Mar. Pollut. Bull. 3:92-92.
Blumer, M., G. Souza, and J. Sass. 1970. Hydrocarbon pollution of edible shellfish by an oil spill. Mar. Biol. 5:195-202.
Bolin, B., and H. Stommel. 1961. On the abyssal circulation of the world ocean. IV. Origin and rate of circulation of deep ocean water as determined with the aid of tracers. Deep-Sea Res. 8:95-110.
Bowen, V.T., and T.T. Sugihara. 1957. Strontium 90 in the North Atlantic surface water. Proc. National Acad. Sci. U.S.A. 43:576-580.
Boyle, E. 1988. Cadmium: Chemical tracer of deepwater paleoceanography. Paleoceanography 3:471-489.
Boyle, E., and J.M. Edmond. 1975. Copper in surface seawaters south of New Zealand. Nature 253:107-109.
Brewer, P.G., and D.M. Glover. 1987. Ocean chemical fluxes 1983-1986. Reviews of Geophysics 25(6): 1376-1386.
Brewer, P.G., N. Yoshiyuki, D.W. Spencer, and A.P. Fleer. 1980. Sediment trap experiments in the deep North Atlantic: Isotopic and elemental fluxes. Journal of Marine Research 38(4):703-728.
Broecker, W.S. 1957. Application of Radiocarbon to Oceanography and Climate Chronology. Ph.D. Thesis. Columbia University, New York.
Broecker, W.S. 1974. Chemical Oceanography. Harcourt Brace Jovanovich, New York. 214 pp.
Broecker, W.S. 1985. How to Build a Habitable Planet. ELDIGIO Press, Columbia University, Palisades, New York. 291 pp.
Broecker, W.S. 1992. Statement. P. 73 in A Tribute to Henry Stommel. Oceanus 35 (special issue), Woods Hole Oceanographic Institution, Woods Hole, Massachusetts.
Broecker, W.S., R.D. Gerard, M. Ewing, and B.C. Heezen. 1960. Natural radiocarbon in the Atlantic Ocean. J. Geophys. Res 65:2903-2931.
Broecker, W.S., R.D. Gerard, M. Ewing, and B.C. Heezen. 1961. Geochemistry and physics of ccean circulation. Pp. 301-322 in M. Sears (ed.). Oceanography. American Association for the Advancement of Sciences, Washington, D.C.
Broecker, W.S., and T.H. Peng. 1982. Tracers in tile Sea. Columbia University, Palisades, New York. 690 pp.
Broecker, W.S., K.K. Turekian, and B.C. Heezen. 1958. The relationship of deep sea (Atlantic Ocean) sedimentation rates to variations in climate. American Journal of Science 256:503-517.
Burns, K.A. 1976. Hydrocarbon metabolism in the intertidal fidler crab, Uca pugnax. Mar. Biol. 36:5-11.
Burns, K.A., and J.M. Teal. 1971. Hydrocarbon incorporation into the salt marsh ecosystem from the West Falmouth oil spill. Woods Hole Oceanographic Institution Technical Report 71-69.
Carson, R. 1962. Silent Spring. Houghton Mifflin, Boston, Massachusetts.
Chow, T.J., and C.C. Patterson. 1966. Concentration profile of barium and lead in Atlantic waters of Bermuda. Earth Planet. Sci Lett. 1:397-400.
Clarke, W.B., W.J. Jenkins, and Z. Top. 1976. Determination of tritium by spectrometric measurement of 3He . International Journal of Applied Radioisotopes 27:515.
Corliss, J.B., J. Dymond, L.I. Gordon, J.M. Edmund, R.P von Herzen, R.D. Ballard, K. Green, D. Williams, A. Bainbridge, K Crane, T.H. van Andel. 1979. Submarine thermal springs on the Ga'apagos Rift. Science 203:1073-1083.
Craig, H. 1953. The geochemistry of stable carbon is ,topes. Geochim. Cosmochim. Acta 3:53-92.
Craig, H. 1972. The GEOSECS Program: 1970-1971. Earth Planet. Sci. Lett. 16:47-49.
Craig, H. 1974. The GEOSECS Program: 1972-1973. Earth Planet. Sci. Lett. 23:63-64.
Craig, H. 1992. Letter to the President's Committee on the National Medal of Science in support of the nomination of Henry Stommel for the National Medal of Science. Pp. 128-129 in Oceanus 35 (special issue).
Craig, H., and K.K. Turekian. 1980. The GEOSECS Program: 1976-1979. Earth Planet. Sci. Lett. 49:263-265.
Davin, E.M., and M.G. Gross. 1980. Assessing the seabed. Oceanus 23:20-32.
Deuser, W.G., and E.R. Ross. 1980. Seasonal changes in the flux of organic carbon to the deep Sargasso Sea. Nature 283:364-365.
Deuser, W.G., E.R. Ross, and R.F. Anderson. 1981. Seasonality in the supply of sediment to the deep Sargasso Sea and the implications for the rapid transfer of matter to the deep ocean. Deep-Sea Res. Part A: Oceanographic Research Papers 28:495-505.
Donat, J.R., and K.W. Bruland. 1995. Trace elements in the oceans. Pp. 247-281 in B. Salbu and E. Steinnes (eds.), Trace Elements in Natural Waters. CRC Press, Boca Raton, Florida.
Dow, W.G. 1992 Reflections on the career and times of John M. Hunt. Pp. 9-19 in J. Whelan and J. Farrington (eds.), Organic Matter. Productivity, Accumulation, and Preservation in Recent and Ancient Sediments. Columbia University Press, New York
Duce, R.A. (guest ed.). 1989. SEAREX: The Sea/Air Exchange Program. Volume 10 of J.P. Riley and R. Chester (eds.). Chemical Oceanography. Academic Press, Ltd., London.
Duursma, E.K. 1965. The dissolved organic constituents of sea water. Pp. 433-475 in J.P. Riley and G. Skirrow (eds.), Chapter 11 of Chemical Oceanography. Academic Press, New York.
Edmund, J.M. 1980. GEOSECS. Oceanus 23(1):33-39.
Edmund, J.M. 1982. Ocean hot springs: A status report. Oceanus 25:22-27.
Epstein, S. 1981. Introduction of Claire C. Patterson for the V.M. Goldschmidt Medal 1980. Geochim. Cosmochim. Acta 45:1383-1384.
Farrington, J.W. 1978. In memoriam, Dr. Max Blumer, organic geochemist and environmental scientist, 1923-77. J. Fish. Res. Board Can. 35:501-504.
Farrington, J.W. 1987. Review of marine organic geochemistry. Rev. Geophys. Space Phys. 25:1395-1416.
Farrington, J.W. (ed). 1992. Marine organic geochemistry: Review and challenges for the future. Marine Chemistry 39(1-3):1-242.
Fartington, J.W., and J.G. Quinn. 1973. Petroleum hydrocarbons in Narragansett Bay. I. Survey of hydrocarbons in sediments and clams, Mercenaria mercenaria. Est. Coast. Mar. Sci. 1:71-79.
FOCUS (1998). FOCUS Workshop Report. http://www.joss.ucar.edu/joss-psg/project/oce-workshop/focus/report/html.
Gagosian, R.B. 1983. Review of marine organic chemistry. Rev. Geophys. Space Phys. 21:1245-1258.
Gardner, W. 1977. Fluxes, Dynamics and Chemistry of Particulates in the Ocean. Ph.D. thesis, Massachusetts Institute of Technology-Woods Hole Oceanographic Institution Joint Program. 394 pp.
Garrels, R.G., and F.T. MacKensie. 1971. Evolution of Sedimentary, Rocks. W.W. Norton. New York.
Garrels, R.G., and M.E. Thompson. 1962. A chemical model for sea water at 250°C and one atmosphere total pressure. American Journal of Science 260:57-66.
Gieskes, J.M. 1974. The alkalinity-total carbon dioxide system in seawater. Pp. 123-151 in E.D. Goldberg (ed.), Marine Chemistry. Volume 5 of The Sea. John Wiley and Sons, New York.
Goldberg, E.D. 1954. Marine geochemistry. 1. Chemical scavenging of the sea. Journal of Geology 62:249-265.
Goldberg, E.D. 1965. Minor elements in sea water. Chapt. 5. Pp 163-196 in J.P. Riley and G. Skirrow (eds.)., Chemical Oceanography. Academic Press, New York.
Goldberg, E.D. (convener). 1972. Baseline Studies of Heavy Metal, Halogenated Hydrocarbon, and Petroleum Hydrocarbon Pollutants in the Marine Environment and Research Recommendations. Deliberations of the International Decade of Ocean Exploration (IDOE) Baseline Conference, May 24-26. National Science Foundation, Washington, D.C.
Goldberg, E.D. (ed.). 1974. Marine Chemistry. Volume 5 of The Sea. John Wiley and Sons, New York. 895 pp.
Goldberg, E.D. 1975a. The Health of the Oceans. UNESCO, Paris.
Goldberg, E.D. 1975b. The Mussel Watch: A first step in global marine monitoring. Mar. Pollut. Bull 6(5):111.
Goldberg, E.D., and G.O.S. Ahrrenius. 1958. Geochim. Cosmochim. Acta 13:153.
Goldberg, E.D., and M. Koide. 1958. Io-Th chronology in deep-sea sediments of the Pacific. Science 128:1003.
Goldberg, E.D., V.T. Bowen, J.W. Farrington, G.R. Harvey, J.H. Martin, P.L. Parker, R.W. Risebrough, W. Robertson, E. Schneider, and E. Gamble. 1978. The Mussel Watch. Envir. Conservation. 5:101-125.
Goldschmidt, V.M.. 1933. Grundlagen dre quantitativen Geochemie. Fortschr. Miner. 17:112-156.
Goldschmidt, V.M. 1937. The principles of distribution of chemical elements in minerals and rocks. J. Chem. Soc. Part 1:655-673.
Goldschmidt. V.M. 1954. Geochemistry. Oxford University Press, London.
Gordon, D.C., Jr., and P. Michalik. 1971. Concentration of bunker C fuel oils in waters of Chebabucto Bay, April, 1971 . J. Fish. Res. Board Can. 28:1912-1914.
Grice, G.D., and M.R. Reeve (eds.). 1982. Marine Mesocosms: Biological and Chemical Research on Experimental Ecosystems. Springer-Verlag, New York.
Harvey, H.W. 1928. Biological Chemistry and Physics of Seawater. Cambridge at the University Press, London.
Hazzigna, M.S. 1998. How to change the university. Science 282.237.
Hogg. N.G., and R.X. Huang (eds.). 1995. Pp. 1-197 in Collected Works of Henry M. Stommel. American Meteorological Society. Boston, Massachusetts.
Honjo, S. 1978. Sedimentation of materials in the Sargasso Sea at a 5,367 m deep station. J. Mar. Res. 36:469-492.
Hutchinson, G.E. 1947. The problems of oceanic geochemistry Ecological Monographs 17:299-315.
Jenkins, W.J., M.A. Beg, W.B. Clarke, P.J. Wangersky, and H. Craig. 1972. Excess He in the Atlantic. Earth Planet. Sci. Lett. 16:122.
Jennings, F.D. and L.R. King. 1980. Bureaucracy and science: The IDOE in the National Science Foundation. Oceanus 23:12-17.
Johnson, K.S., K H. Coale, and H.W. Jannasch. 1992. Analytical chemistry in oceanography. Analytical Chemistry 64:1065-1075.
Keeling, C.D. 1958. The concentration and isotopic abundance of atmospheric carbon dioxide in rural areas. Geochim. Cosmochim. Acta. 13:322-334.
Keeling. C.D. 1968. Carbon dioxide in surface ocean waters 4: Global distributions. J. Geophys. Res. 73:4543-4553.
Keeling, C.D., J.A. Adams. C.A. Ekdahl. and P.R. Guenther. 1976b. Atmospheric carbon dioxide variations at the South Pole. Tellus 28:552-564.
Keeling. C.D., R.B. Bascastow, A.E. Bainbridge, C.A. Ekdahl, P.R. Guenther, L.S. Waterman, and J.F.S. Chin. 1976a. Atmospheric carbon dioxide variations at Mauna Loa Observatory, Hawaii . Tellus 28:538-551.
Ketchurn, B.H. (ed.). 1972. The Water's Edge. The Massachusetts Institute of Technology Press, Cambridge, Massachusetts.
Knauer, G.A., J. H. Martin, and K.W. Bruland 1979. Fluxes of particulate carbon, nitrogen and phosphorous in the upper water column of the northeast Pacific. Deep-Sea Res. 26:97-108.
Knecht, R.W. 1982. Introduction: Deep ocean mining. Oceanus 25:3-11.
Koczy, F.F. 1956. Geochemistry of radioactive elements in the ocean. Deep-Sea Res. 3:93-103.
Kvenvolden, K.A. (ed.). 1980. Geochemistry of Organic Molecules. Benchmark Papers in Geology Vol. 52. Dowden, Hutchinson and Ross. Stroudsburg, Pennsylvania.
Lee, C., and S.G. Wakeham. 1989. Organic matter in seawater: Bio-geochemical processes. Pp 1-51 in J.P. Riley (ed.), Chemical Oceanography, vol. 9. Academic Press, New York.
Maclntyre, F.G. 1974. Chemical fractionation and sea-surface microlayer processes. Chapter 8. Pp. 245-299 in E.D. Goldberg (ed.), The Sea. John Wiley and Sons, New York.
MacLeisch, W.H. 1982-1983. Roger Revelle: Senior Senator of Science. Oceanus 25(4):67-70.
Marsh, E.R. 1973. Alfred C. Redfield. naturalist: His scientific career. Transcript of a taped interview with her father, by Elizabeth R. Marsh, Woods Hole, Massachusetts.
Martin, J.H. 1991. Iron. Leibig's Law and the Greenhouse. Oceanography 4: 52-55.
Martin, J.M., S.D. Burton, and D. Eisma. 1981. River Inputs to Ocean Systems. Proceedings of a SCOR/ACMR/ECOR/IAHS/CNG/IABO/ IAPSO Review and Workshop. UNEP and UNESCO, Switzerland.
Martin, J.H., and S.E. Fitzwater. 1988. Iron deficiency limits growth in the north-east Pacific subarctic. Nature 331:341-343.
Martin, J.H., G.A. Knauer, W.W. Broenkow, K.W. Bruland, D.M. Karl, L.F. Small, M.W. Silver, and M.M. Gowing. 1983. Vertical transport and exchange of materials in the upper waters of the oceans (VERTEX): Introduction to the program, hydrographic conditions and major component fluxes during VERTEX I . Moss Landing Marine Laboratory Technical Publication 83-2, 40 pp.
McElway, B. 1983. Karl K. Turekian: "Academic Gladiator." Oceanus 26(2):61-66.
Menzel, D.W. 1974. Primary productivity, dissolved and particulate organic matter, and the sites of oxidation of organic matter. Pp 659-678 in E.D. Goldberg (ed.), Marine Chemistry. Volume 5 of The Sea. John Wiley and Sons, New York.
Millero, F. 1974. Seawater as a multicomponent electrolyte solution. Pp. 3-80 in E.D. Goldberg (ed.), Marine Chemistry. Volume 5 of The Sea. John Wiley and Sons, New York.
Milliman, J.D., and R.H. Meade. 1983. World-wide delivery of river sediment to the oceans. J. Geol. 91:1-21.
National Academy of Sciences-National Research Council (NAS/NRC). 1957. The Effects of Atomic Radiation on Oceanography and Fisheries . NAS-NRC Publ. 551, Washington, D.C. 137 pp.
National Academy of Sciences (NAS). 1959. Conference of Physical and Chemical Properties of Sea Water. Committee on Oceanography. Washington, D.C. 202 pp.
National Academy of Sciences (NAS). 1971a. Marine Chemistry: A Report of the Marine Chemistry Panel of the Committee on Oceanography. Washington, D.C. 60 pp.
National Academy of Sciences (NAS). 197 lb. Radioactivity in the Marine Environment. Report of the Panel on Radioactivity in the Marine Environment of the Committee on Oceanography Washington, D.C. 272 pp.
National Academy of Sciences (NAS). 1971c. Marine Environmental Quality . Washington, D.C.
Patterson, C.C. 1981. Acceptance speech for the V.M. Goldschmidt Medal. Geochim. Cosmochim. Acta 45:1385-1388.
Patterson, C., D. Settle, B. Schaule, and M. Burnett. 1976. Transport of pollutant lead to the oceans and within ocean ecosystems. Pp. 23-28 in H.L. Windom and R.A. Duce (eds.), Marine Pollutant Transfer . Chapter 2. Lexington Books, D.C. Heath and Company, Lexington, Massachusetts.
Pilson, M.E.Q. 1998. An Introduction to the Chemistry of the Sea. Prentice Hall, Upper Saddle River, New Jersey. 431 pp.
Redfield. A.C. 1958. The biological control of chemical factors in the environment. Am. Scientist 46:205-222.
Redfield, A.C., B.W. Ketchum, and F.A. Richards. 1963. The influence of organisms on the composition of seawater. Pp. 26-87 in M.N. Hill (ed.), The Sea. Volume 2. Interscience Publishers, New York.
Reeve, M. 1998. Assessment of NSF Grants in Geosciences in the 1950s (personal communication). Ocean Sciences Division, Geosciences Directorate, National Science Foundation, Arlington, Virginia.
Revelle, R., and H.E. Suess. 1957. Carbon dioxide exchange between the atmosphere and ocean and the question of an increasing atmospheric CO2 during past decades. Tellus 9:18-27.
Richards, F.A. 1965. Anoxic basins and fjords. Pp. 611-645 in J.P. Riley and G. Skirrow (eds.), Chemical Oceanography. Chapter 13. Academic Press. New York.
Riley, J.P. 1965. Historical introduction. Pp. 1-41 in J.P. Riley and G. Skirrow (eds.), Chemical Oceanography. Chapter 1. Academic Press, New York.
Riley, J.P., and G. Skirrow (eds.). 1965. Chemical Oceanography. Academic Press, New York.
Rubey, W.W. 1951. Geologic history of seawater: An attempt to state the problem. Geological Society of America Bulletin 62:1111-1147.
SCEP. 1970. Man's Impact on the Global Environment. Report of the Study of Critical Environmental Problems. Massachusetts Institute of Technology, Cambridge, Massachusetts.
Sanders, H.L. 1978. Florida oil spill impact on Buzzards Bay benthic fauna: West Falmouth. J. Fish. Res. Board Can. 35:717-730.
Sanders, H.L., J.F. Grassle, G.R. Hampson, L.S. Morse, S. Price-Gartner, and C.C. Jones. 1980. Anatomy of an oil spill: Long term effects from the grounding of the barge Florida off West Falmouth, Massachusetts. J. Mar. Res. 38:265-380.
Sayles, F.L. 1979. The composition and diagensesis of interstitial solutions. I. Fluxes across the sediment-water interface in the Atlantic Ocean. Geochim. Cosmochim. Acta 43:527-545.
Sayles, F.L. 1981. The composition and diagensesis of interstitial solutions. II. Fluxes and diagenesis at the sediment-water interface in high latitude North and South Atlantic. Geochim. Cosmochim. Acta 45:1061-1086.
Sayles, F.L., P.C. Mangelsdorf, T.R.S. Wilson, and D.N. Hume. 1976. A sampler for the in situ collection of marine sedimentary pore waters. Deep-Sea Res. 23:259-264.
Sholkovitz, E.R. 1976. Flocculation of dissolved organic and inorganic matter during the mixing of river water and seawater. Geochim. Cosmochim. Acta 40:831-845.
Shor, E.N. 1978. Scripps Institution of. Oceanography: Probing the Oceans 1936-1976. Tofua Press, San Diego, California.
Siever, R., R.M. Garrels, J. Kanwisher, J. Willis, R.A. Berner. 1961. Interstitial waters of Recent muds off Cape Cod. Science 134:1071-1072.
Sillen, L.G. 1961. The physical chemistry of seawater. Pp. 549-581 in M. Sears (ed.), Oceanography. American Association for the Advancement of Sciences, Washington, D.C.
Spencer, D.W., P.G. Brewer, A. Fleer, S. Honjo, S. Krishnaswami, and Y. Nozaki. 1978. Chemical fluxes from a sediment trap experiment in the deep Sargasso Sea. J. Mar. Res. 36:493-523.
Staresinic, N., G.T. Rowe, D. Shaughnessey, and A.J. Williams III. 1978. Measurement of the vertical flux of particulate organic matter with a free-drifting sediment trap. Limnol. Oceanogr. 23(3) 559-563.
Stommel, H. 1957. The abyssal circulation of the ocean. Nature 180:733-734.
Stommel, H. 1958. The abyssal circulation. Deep-Sea Res. 5:80-82.
Stommel, H., and A.B. Arons. 1960a. On the abyssal circulation of the world ocean-I. Stationary planetary flow patterns or a sphere. Deep-Sea Res. 6:140-154.
Stommel, H., and A.B. Arons. 1960b. On the abyssal circulation of the world ocean-II. An idealized model of the circulation pattern and amplitude in oceanic basins. Deep-Sea Res. 6:217-233.
Strickland, J.D.H., and T.R. Parsons. 1965. Manual of .,ca water analysis with special reference to more common micronutrients and particulate organic material. Fisheries Research Board of Canada Ottawa, Canada.
Strickland, J.D.H., and T.R. Parsons. 1972. A Practical Handbook for Seawater Analysis. 2nd edition. Fisheries Research Board of Canada, Ottawa.
Stuum, W., and J.J. Morgan. 1970. Aquatic Chemistry An Introduction Emphasizing Chemical Equilibria in Natural Waters. Wiley-Interscience, New York.
Stumm, W., and J.J. Morgan. 1981. Aquatic Chemistry An Introduction Emphasizing Chemical Equilibria in Natural Waters 2nd edition John Wiley and Sons, New York.
Stuum, W., and J.J. Morgan. 1995. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. 3rd edition. John Wiley and Sons, New York.
Sverdrup, H.U., M.W. Johnson, and R.H. Fleming. 1942. The Oceans: Their Physics, Chemistry, and General Biology. Prentice-Hall, Inc.
Turekian, K.K. 1955. Paleoecological significance of the strontium-calcium ratio in fossils and sediments. Geological Society of America Bulletin 66(1):155-158.
Turekian, K.K. 1957. The significance of variations of strontium content in deep sea cores. Limnol. Oceanogr. 2:309-314.
Turekian, K.K. 1958. Rate of accumulation of nickel in Atlantic equatorial deep sea sediments and its bearing on possible extra-terrestrial sources. Nature (London) 182:1728-1729.
Turekian, K.K. 1968. Oceans. Prentice Hall, Engelwood Cliffs, New Jersey.
Turekian, K.K., and J.L. Kulp. 1956. The geochemistry of strontium. Geochem. Cosmochim. Acta 10:245-296.
Urey, H.C., H.A. Lowenstam, S. Epstein, and C.R. McKinney. 1951. Measurement of paleotemperatures and temperature of the Upper Cretaceous of England, Denmark, and the Southeastern United States. Geological Society of America Bulletin 62:399-416.
Wallace, W.J. 1974. The Development of the Chlorinity/Salinity Concept in Oceanography. Elsevier Scientific Publishing Company, New York. 227 pp.
Windom, H.L., and R.A Duce (eds.). 1976. Marine Pollutant Transfer . Lexington Books, D.C. Heath and Company, Lexington, Massachusetts.
Zafiriou, O.C. 1977. Marine organic photochemistry previewed. Mar. Chem. 5:497-522.
Zika, R.G. 1987. Advances in marine photochemistry 1983-1987. Reviews of Geophysics 25:1390-1394.