2
Conducting Basic and Clinical Research in the Managed Care Setting
Health care systems worldwide are being dramatically restructured to control costs through improved efficiency and coordination of services, reduce the level of utilization of unnecessary or inappropriate services and resources, increase the provision of preventive care, and maintain or improve the quality of care. Specifically, in the United States, the impact of health care reform on academic health centers has reduced physicians' incomes from the provision of clinical care and limited the amount of time that faculty can spend conducting research and training future scientists and physicians. In recent years, academic health centers have felt the fiscal pressure of health care restructuring as managed care organizations negotiate discounted fees with the faculty practice plans and teaching hospitals that support medical schools. The presentations described below examine the opportunities and challenges for academic health centers and managed care organizations to work together in the fields of basic and clinical research in addressing issues related to emerging infections.
PERSPECTIVE OF ASSOCIATION OF AMERICAN MEDICAL COLLEGES ON BASIC AND CLINICAL RESEARCH
Presented by David Korn, M.D.
Senior Vice President for Biomedical and Health Sciences Research, Association of American Medical Colleges
Medical schools are the intellectual hub of academic health centers (AHCs). They train physicians (M.D.s) and biomedical research scientists (Ph.D.s). They also perform a great deal of research, presently receiving just over 50 percent of the extramural research budget of the National Institutes of Health (NIH). In ad
dition to conducting research and training, academic health centers provide health care services to a large number of medically underserved populations, often indigent patients, as well as provide the bulk of specialized care. The continued provision of these important public services may be threatened by the growing dominance of managed care and cost-focused health care delivery markets.
Medical schools are heavily dependent on two primary sources of funds: clinical revenues (from the provision of patient care) and research revenues (from grants and contracts) (Figure 2-1). In private, research-intensive medical schools, the relative dependence on grants (which are mostly from federal government sources, but which also include nonfederal funds) and clinical revenues from hospitals and practice plans become proportionately larger in the absence of state and local support. These revenue sources support a wide array of education, research, and clinical care programs. In private, research-intensive medical schools, the proportion of total expenditures that come from tuition and fees, endowment earnings, and state support averages only 10 percent, whereas the proportion for public, research-intensive medical schools is about 18 percent. Thus, both private and public medical schools rely heavily on the clinical revenues generated by faculty.
The 35-year trend in the source of revenues within academic health centers is shown in Figure 2-2. In fiscal year 1961, the United States had 85 medical schools with aggregate expenditures of $430 million (current dollars), of which 40 percent was raised from federal (NIH) research funds. By 1996, there were 125 medical schools with total expenditures of over $32 billion, but only 19 percent came from federal research funds. In contrast to money from federal funding, clinical revenues rose from less than 5 percent to well over 50 percent (Figure 2-2). Some of these revenues provided cross-subsidies for academic objectives; in fiscal year 1993, for example, about 10 percent of revenues from faculty practice plans were estimated to support biomedical research.
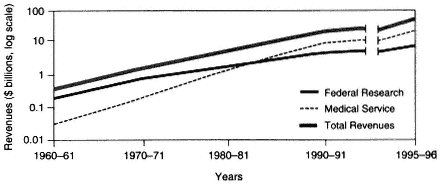
Figure 2-1 Growth in medical education in the United States from 1960 to 1996, as measured by revenues (in log billions of dollars). Source: AAMC, 1998.
Revenues derived from the provision of clinical medical services have also helped support core academic programs and undergraduate and graduate medical education and have built what are now called AHCs. in fact, the revenues from the provision of clinical services generated at AHCs over the past four decades have contributed significantly to excellent clinical care and to outstanding basic and clinical research. AHCs have also fostered the U.S. biotechnology industry and have served as an important safety net for health care delivery, particularly for those who are disadvantaged and most in need.
However, the market-driven shift away from cost reimbursement and fee-for-service revenue sources has eroded the clinical revenue margins that previously provided AHCs with substantial discretionary funds to support basic and clinical research. In addition, the ongoing transformation of health care delivery under managed care has resulted in a migration of care out of the hospital and into ambulatory-care settings. Moreover, managed care tends to emphasize general medicine as opposed to specialized care. These changes not only have affected the ability of AHCs to conduct clinical research, which is closely tied to specialty training, but also may have narrowed the scope of the research agenda. For the period from 1986 to 1995, data suggest that for the 13 medical schools in regions where managed care has achieved a high level of penetration (over 40 percent) there has been a slower rate of growth in NIH funding—and, consequently, a declining share of and rank in the proportion of NIH funding—relative to that for medical schools in markets where managed care has achieved low (under 20 percent) or medium (20 to 40 percent) levels of penetration (Figure 2-3).
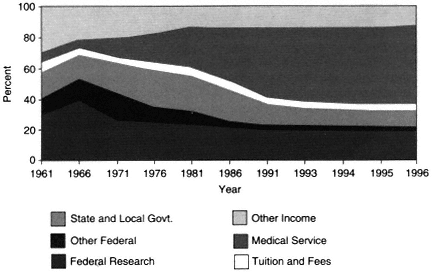
Figure 2-2 U.S. medical school revenues as a percentage of total revenues. Source: AAMC, 1998.
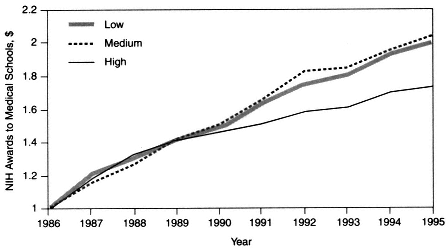
Figure 2-3 NIH awards to U.S. medical schools in different managed care markets, 1986 to 1995. Dollar amounts are relative. For instance, in 1995, markets with low levels of managed care penetration received 1.99 times more funding, markets with medium levels of managed care penetration received 2.03 times more funding, and markets with high levels of managed care penetration received 1.75 times more funding than they received in 1986. Source: Moy et al., 1997.
The modern-day structure of medical schools has evolved over time. In recent decades AHCs have provided medical education and research training, a research infrastructure, and care for the underserved. Today, however, AHCs are increasingly pressed to continue these obligations while assuming a greater proportion of their costs. As such, it has become harder to perform the type of large-scale, population-based research commonly required to control emerging infections. Managed care organizations, however, have the potential to assist in these efforts with their integrated, computerized database capabilities. These capabilities are discussed in the next section.
OPPORTUNITIES FOR POPULATION-BASED CLINICAL RESEARCH USING DATA FROM MANAGED CARE ORGANIZATIONS
Presented by Frank DeStefano, M.D., M.P.H.
Medical Epidemiologist, Vaccine Safety and Development Activity, Centers for Disease Control and Prevention
Stimulated by the shift toward managed care, health care organizations in the United States are increasingly consolidating into integrated systems that
cover large populations. Integrated systems provide data on all levels of inpatient and outpatient care, from primary care through highly specialized tertiary care. In such systems, computerized information systems are extensively used for a variety of patient care and administrative functions. The availability of computerized information covering the entire spectrum of medical care for large populations provides opportunities for many types of health research, including public health surveillance, epidemiological studies, and health services research.
The continued growth of computerized medical information systems in managed care organizations should improve the efficiency with which such studies can be conducted. At present, however, computerized databases are not sufficiently evolved to negate the need to obtain additional confirmatory or supplemental information from hard-copy records or interviews with patients. The key elements of medical information systems for use in population-based health research include (1) mechanisms for the identification and progressive tracking of individuals in a defined population, (2) procedures that ensure the accuracy of the diagnostic and other clinical data entered into an electronic database, and (3) development of uniform data sets with information on demographic variables (e.g., race and ethnicity) and other risk factors, in addition to diagnostic and treatment information.
The Centers for Disease Control and Prevention (CDC) effort to study rare adverse events that occur after the receipt of vaccines serves as an example of how computerized databases of various large health maintenance organizations (HMOs) can be linked into a single research database. The Vaccine Safety Data Link (VSD) is a project of CDC's National Immunization Program, which began in 1991 as a collaboration between four federal agencies and four long-established staff model HMOs. VSD links together the computerized databases of the four HMOs to provide primary information for research on vaccine safety and other health issues. The data fields include population and vaccination status, as well as health outcomes (diagnostic codes from hospital discharges, emergency room visits, and clinic outpatient visits), laboratory test results, and pharmacy records.
Staff model HMOs are good partners for this project because they provide and maintain detailed records on all levels of patient care, including cost data. They also have a stable, identifiable patient population from which subjects can be selected, and they are able to implement interventions (and ensure follow-up) on a systemwide basis. One of the HMO partners, for example, is conducting a systemwide study of pneumococcal polysaccharide vaccine in which revaccinated patients are compared with patients being vaccinated for the first time for immunogenicity and reactogenicity.
The databases of managed care organizations, however, also pose challenges to clinical research because of a lack of uniformity and their orientation toward administrative and management purposes rather than clinical or research purposes. Thus, adaptation of these databases for use in research can be expensive and time-consuming. In addition, many HMOs do not routinely record data on important variables, such as race or ethnicity, consanguinity, socioeconomic
status, or risk factors such as cigarette smoking. Furthermore, databases lack information on medical conditions for which the patient does not seek treatment and may not list information on deaths that occur outside the hospital (although this can be overcome by use of links to diverse local vital records offices).
Clinical researchers may also find problems with these types of integrated databases when changes in HMO policies and procedures occur, which might affect patient care, outcomes, data collection, selection bias in the patient population (because of the nature of the HMO membership), disenrollment (which complicates long-term follow-up), and the lack of research infrastructures in HMOs. For example, a proposed research project on a rotavirus vaccine would require laboratory tests that the HMOs themselves would be unlikely to conduct. Other limitations might be posed by the nature of services provided by the HMO; that is, if its emphasis is on primary care, the patient population might be skewed away from those in need of specialty care, thereby limiting the types of data collected on the enrollee population. These limitations might have implications for the development of evidence-based research or guidelines. Concerns about the privacy of the medical record may also pose an impediment to the clinical researcher (although this is not restricted to managed care organizations). For example, the Mayo Clinic reports that privacy laws in Minnesota may prevent managed health care systems from conducting medical record reviews, and laws in other states may erect barriers that would prevent the use of patient records for research. The issue of medical privacy is complex and is being examined and considered by the U.S. Congress and the Secretary of the U.S. Department of Health and Human Services.
CONDUCTING CLINICAL RESEARCH WITHIN MANAGED CARE ORGANIZATIONS
Presented by Walter Stature, M.D.
Head, Division of Allergy and Infectious Diseases, University of Washington School of Medicine
The trend toward managed care is often viewed as antithetical to research since managed care priorities often emphasize rapid patient throughput, low-cost care, limited laboratory testing, quotas for numbers of patient consults per physician, and lack of reimbursement for costly procedures. In reality, the managed care environment may be uniquely well suited for patient-oriented clinical research as well as for epidemiological and health services research. Unique advantages provided by large managed care organizations include well-defined, representative, and stable patient populations; the integration of inpatient and outpatient services within one system; centralized pharmacy services; centralized laboratory services; computerized and uniform databases, including databases containing medical, pharmacy, and laboratory records; and control of approaches to the delivery of health care at the individual practitioner level. These
characteristics can facilitate patient recruitment into large, population-based studies, patient retention, and implementation of randomized intervention trials. Elements integral to the success of clinical research conducted in the managed care setting include selection of research projects of importance to the managed care organization, participation of relevant managed care providers who are interested in a particular research topic, collaboration between academic investigators and the managed care organization, external funding of the research, publication of results in peer-reviewed publications, and incorporation of research results into the clinical practice of the managed care organization. Given these and other factors, the managed care environment can provide unique research opportunities.
Group Health Cooperative of Puget Sound (which is now merged with Kaiser-Permanente) is a large staff model HMO that has more than 500,000 enrollees and the experience and infrastructure needed to conduct research. It has collaborated with the University of Washington on numerous major research projects over the past 15 years. However, less than 25 such managed care organizations exist in the United States.
The principal advantage of working with a research-savvy managed care organization is its ability to provide a large, well-defined, and relatively stable patient population that has uniform access to care, thereby minimizing selection bias and facilitating retention and follow-up in studies. Inpatient and outpatient services are integrated, and support services such as laboratory and pharmacy services are centralized, uniform, and computerized. Moreover, standardization at the physician and clinic levels makes randomization easier. In the case of Group Health Cooperative of Puget Sound, there is also an affiliated research center with experience in supporting the infrastructure for population-based research.
An example of a collaboration between the University of Washington and Group Health Cooperative is a randomized intervention trial to screen women for cervical Chlamydia trachomatis infection for the prevention of pelvic inflammatory disease. Chlamydial infection is the most common bacterial sexually transmitted disease in the United States. The main clinical sequelae are upper genital tract infection and pelvic inflammatory disease. The first step in this project was a cross-sectional study to define the prevalence and risk factors for chlamydial infection among 1,800 female enrollees, which provided a representative demographic profile of women in the Puget Sound region. This cross-sectional study identified a subpopulation in which the disease is prevalent. The second phase of the study involved an intervention trial in which a Chlamydia screening test was randomly administered to women from the high-risk group. All women were then monitored for 12-months. The results showed that screening reduced the incidence of pelvic inflammatory disease by 60 percent.
The collaboration between the University of Washington and a large HMO was the key to success in this clinical research project. The relevance of the study to Group Health Cooperative was vital for the success of the project, as was the inclusion of Group Health Cooperative clinicians during the planning and execution stages. However, external funding was also necessary, because
the research units of managed care organizations must frequently be self-supporting. In this study, most of the funding came from NIH, which provided the financial means to pay for salaries, testing, and the collection of additional information. In this example, the results were published in a peer-reviewed journal and were subsequently incorporated into the clinical practice of Group Health Cooperative.
NIH ACTIVITIES RELATED TO CLINICAL RESEARCH AND MANAGED CARE
Presented by Lana Skirboll, Ph.D.
Associate Director for Science Policy, National Institutes of Health
In 1995, NIH convened the Clinical Research Panel, which subsequently created a subpanel to consider additional potential sources of clinical research funding, including managed care organizations. Although the panel members had received anecdotal data from academic health centers that clinical research was suffering under the current managed care system, it was difficult to obtain reliable data that addressed the magnitude of the problem or even the costs associated with conducting clinical research. To date, the effort to collect such data continues; a collaboration between the National Cancer Institute (NCI) and the RAND Corporation is attempting to provide this type of information. In addition, NCI is collaborating with the CHAMPUS program of the U.S. Department of Defense and with the Health Care Financing Administration (HCFA) to develop estimates of the patient care costs of clinical research.
Historically, there have been tensions between academic clinical researchers and HMOs. Managed care organizations complain that academics want to pursue research that is not relevant to their clinical needs, that investigators fail to consult with them early in the process of designing clinical trials, that the only concern of academic scientists is access to patients, and that these researchers do not follow through clinically with the results of their studies but merely publish them in peer-reviewed journals. On the other hand, academic clinical researchers complain that HMOs are reluctant to refer patients to academic health centers because it is perceived that care is too expensive; they demand discount rates from academic health centers, which results in lost subsidies to clinical research; and in some cases, they are reluctant to pay the costs for the routine care provided in the research context.
To bridge this gap, NIH sponsored a series of meetings to broaden the dialogue on clinical research. Participants included the Association of American Medical Colleges, the American Association of Health Plans (AAHP), and payers or purchasers of health care, including large corporations that are interested in good health care for their employees but that are not necessarily interested in clinical research. Some participants representing the corporate purchasers of
services from health care plans suggested that academic health centers need to further downsize and streamline their activities to reduce costs.
NIH has institutionalized its commitment to forging research relationships with managed care organizations by creating a Managed Care Fellowship and a Trans-NIH Managed Care Working Group. NIH has also appointed a liaison to help AAHP members better understand NIH and clinical research. The goal of these and other initiatives is to formulate a set of core principles on the nature and value of clinical research, to determine the actual costs of research, and to establish who should pay for the various costs of clinical research. In addition, a demonstration project has been proposed to HCFA. That project will study how the public sector might pay for the patient care costs of clinical investigations, an important first step in the formation of a partnership between clinical researchers and managed care organizations.
SUMMARY OF CHALLENGES AND OPPORTUNITIES
Jonathan R. Davis, Ph.D., Editor
The presentations described above and the discussion that followed during the workshop highlight several challenges and opportunities related to conducting basic and clinical research in a managed care setting. These are summarized below in the sections Supporting the Functions of Academic Health Centers, Ensuring Adequate Databases for Clinical Research, Exploiting the Unique Advantages of Managed Care for Surveillance and Research, and Promoting Collaboration.
Supporting the Functions of Academic Health Centers
The clinical revenues generated at AHCs have greatly contributed to the growth and excellence of medicine in the United States, produced outstanding basic and clinical research, promoted the training of physicians and scientists, facilitated the growth of the nation's biotechnology industry, and provided a health care safety net for those most in need. As discussed at the workshop, however, the continued provision of these important services is challenged by the growing dominance of managed care and other market forces in the health care system, in which the primary focus on cost containment can be at odds with these critically important but expensive endeavors. To address this challenge, many workshop participants emphasized the importance of involving all the various stakeholders in ensuring the continuation and support of AHCs in conducting research, training physicians and scientists, and providing access to health care.
Ensuring Adequate Databases for Clinical Research
Health care organizations are increasingly consolidating into integrated systems that provide care at all levels and that cover large populations. The computerized information systems used in these settings serve a variety of patient care and administrative functions. However, because of wide variability in these systems, their adaptation to uniformity and consistency for use in clinical research can be expensive and time-consuming. In addition, as discussed by the workshop participants, the use of integrated databases in clinical research can be compromised by policy and procedural changes adopted by managed care organizations, which can make it difficult to track trends; selection bias caused by the characteristics of the enrollee populations; and the lack of a research infrastructure within managed care organizations to collect, process, and analyze research data. An additional challenge is posed by the current trend toward less integrated models of managed care, which results in the provision of a narrower range of services and therefore the availability of a correspondingly narrower set of databases for evidence-based medicine. Use of the data in these databases by clinicians and researchers may also pose privacy concerns, and such concerns may be obstacles to investigators who rely on medical records to conduct research.
If infectious disease studies are to be conducted in the managed care setting, it was suggested that their efficiencies could be improved by using standardized, computerized medical information systems and by incorporating key elements into the development of these systems for use in population-based health research. These include mechanisms that identify and progressively track individuals in a defined population, procedures that ensure the accuracy of the diagnostic and other clinical data, and the development of uniform data sets.
Exploiting the Unique Advantages of Managed Care for Surveillance and Research
Some view managed care as antithetical to clinical research because priorities often emphasize rapid patient throughput, low-cost care, limited laboratory testing, quotas for numbers of patient consults per physician, and lack of reimbursement for costly procedures. The possibility that managed care organizations could improve surveillance and treatment of emerging infections was discussed at some length. This could be accomplished through the (1) systematic collection of relevant data, (2) standardization of computerized systems that can monitor health data, and (3) education of providers regarding their importance in accurate disease reporting.
Moreover, the managed care environment was described by many as being uniquely well suited for patient-oriented clinical research, as well as for epidemiological and health services research, because of the following characteristics: (1) a large, well-defined, and relatively stable patient population that has uniform access to care; (2) integrated inpatient and outpatient services within one
system; (3) centralized support services such as pharmacy and laboratory services; (4) uniform and computerized databases; and (5) standardized delivery of health care at the individual practitioner and clinic levels.
Promoting Collaboration
Close collaboration between academic health centers and managed care organizations is critical to the success of clinical research. As discussed in the workshop, however, managed care organizations perceive some clinical research as irrelevant to their patient needs and are not routinely consulted early in the experimental design of clinical trials. Conversely, clinical researchers at academic health centers find that some managed care organizations are reluctant to refer patients to clinical trials and are unwilling to pay for the routine patient care provided in the research context.
Close collaborations between academic health centers and managed care organizations can be achieved by (1) selecting research projects of importance to managed care, (2) seeking the active participation of relevant and interested providers, (3) identifying external funding for research protocols, (4) collaboration between academic investigators and the managed care organization, (5) facilitating publication of results in peer-reviewed publications, and (6) ensuring that the results are incorporated into managed care practice.
Summary
Although academic health centers have become dependent on clinical revenues to support a portion of their research activities, pressure from managed care is reducing both the profit margins and the faculty time that are available to support research and training. At the same time, managed care organizations have large patient populations and centralized information systems that create opportunities for epidemiological and clinical trials research. In the past 10 years, a few large and stable managed care organizations have developed the infrastructure and culture to collaborate with academic and government researchers. The creation of stronger partnerships between managed care organizations and academic health centers is one way to meet the public health need to combat emerging infections.
The system of managed care, however, is undergoing a rapid evolution that may threaten the stability and survival of these partnerships. NIH is working with the managed care industry to develop mechanisms that would continue to support clinical research in a managed care environment. Whether increases in the NIH budget or research funds from the pharmaceutical industry will replace
cutbacks from Medicaid educational opportunities or from clinical funds that formerly supported research faculty salaries remains to be determined.*
In addition, some of the discussions focused on the fact that research on emerging and reemerging infections is threatened by the decline of microbiology laboratories in academic health centers. For example, CDC has noted a decline in the quality of testing that has been performed on the samples that it receives, and this decline in quality may weaken the public health system's ability to recognize an emerging pathogen or disease outbreak. A possible solution that was discussed at the workshop and that may combat the decline would be the creation of centers of excellence in microbiology with links to the large populations in stable, research-oriented HMOs. Consequently, there is an immediate need to gauge the health of the nation's public health laboratories, a topic that will be addressed in an upcoming workshop of the Institute of Medicine Forum on Emerging Infections.












