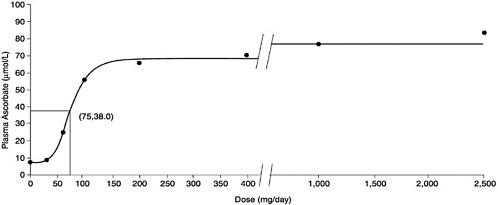
5
Vitamin C
SUMMARY
Vitamin C functions physiologically as a water-soluble antioxidant by virtue of its high reducing power. It is a cofactor for enzymes involved in the biosynthesis of collagen, carnitine, and neurotransmitters in vitro, and it can quench a variety of reactive oxygen species and reactive nitrogen species in aqueous environments. Evidence for in vivo antioxidant functions of ascorbate include the scavenging of reactive oxidants in activated leukocytes, lung, and gastric mucosa, and diminished lipid peroxidation as measured by urinary isoprostane excretion. To provide antioxidant protection, a Recommended Dietary Allowance (RDA) of 90 mg/day for adult men and 75 mg/day for adult women is set based on the vitamin C intake to maintain near-maximal neutrophil concentration with minimal urinary excretion of ascorbate. Because smoking increases oxidative stress and metabolic turnover of vitamin C, the requirement for smokers is increased by 35 mg/day. Estimates of median dietary intakes of vitamin C for adults are 102 mg/day and 72 mg/day in the United States and Canada, respectively. The Tolerable Upper Intake Level (UL) for adults is set at 2 g/day; the adverse effects upon which the UL is based are osmotic diarrhea and gastrointestinal disturbances.
BACKGROUND INFORMATION
Vitamin C is a water-soluble vitamin that is essential for all humans and a few other mammals that lack the ability to biosynthesize the compound from glucose because they lack the enzyme gulono-
lactone oxidase. The term vitamin C refers to both ascorbic acid and dehydroascorbic acid (DHA), since both exhibit anti-scorbutic activity. Ascorbic acid, the functional and primary in vivo form of the vitamin, is the enolic form of an α-ketolactone (2,3-didehydr L -threo-hexano-1,4-lactone). The two enolic hydrogen atoms give the compound its acidic character and provide electrons for its function as a reductant and antioxidant. Its one-electron oxidation product, the ascorbyl radical, readily dismutates to ascorbate and DHA, the two-electron oxidation products. Both the ascorbyl radical and DHA are readily reduced back to ascorbic acid in vivo. However, DHA can be hydrolyzed irreversibly to 2,3-diketogulonic acid. The molecular structure of ascorbic acid contains an asymmetric carbon atom that allows two enantiomeric forms, of which the L form is naturally occurring (the D -form, isoascorbic or erythorbic acid, provides antioxidant but little or no anti-scorbutic activity), as shown in Figure 5-1.
Function
The biological functions of ascorbic acid are based on its ability to provide reducing equivalents for a variety of biochemical reactions. Because of its reducing power, the vitamin can reduce most physiologically relevant reactive oxygen species (Buettner, 1993). As such, the vitamin functions primarily as a cofactor for reactions requiring a reduced iron or copper metalloenzyme and as a protective antioxidant that operates in the aqueous phase both intra- and extracellularly (Englard and Seifter, 1986; Halliwell and Whiteman, 1997; Tsao, 1997). Both the one- and the two-electron oxidation products of the vitamin are readily regenerated in vivo—chemically and enzymatically—by glutathione, nicotinamide adenine dinucleotide (NADH), and nicotinamide adenine dinucleotide phosphate (NAD-PH) dependent reductases (May et al., 1998; Park and Levine, 1996).
Vitamin C is known to be an electron donor for eight human enzymes. Three participate in collagen hydroxylation; two in carnitine biosynthesis; and three in hormone and amino acid biosynthesis. The three enzymes that participate in hormone and amino acid biosynthesis are dopamine-β-hydroxylase, necessary for the biosynthesis of the catecholamines norepinephrine and epinephrine; peptidyl-glycine monooxygenase, necessary for amidation of peptide hormones; and 4-hydroxyphenylpyruvatedioxygenase, involved in tyrosine metabolism. Ascorbate's action with these enzymes in-
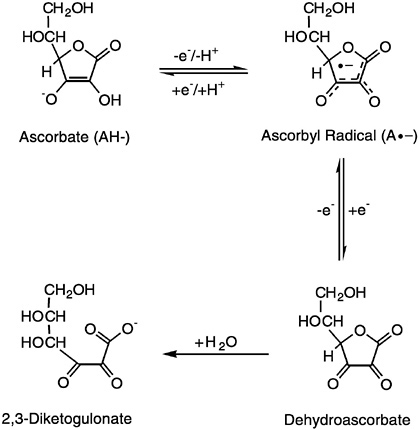
FIGURE 5-1 Chemical structure of ascorbic acid.
volves either monooxygenase or dioxygenase activities (Levine et al., 1996b).
As a cofactor for hydroxylase and oxygenase metalloenzymes, ascorbic acid is believed to work by reducing the active metal site, resulting in reactivation of the metal-enzyme complex, or by acting as a co-substrate involved in the reduction of molecular oxygen. The
best known of these reactions is the posttranslational hydroxylation of peptide-bound proline and lysine residues during formation of mature collagen. In these reactions, ascorbate is believed to reactivate the enzymes by reducing the metal sites of prolyl (iron) and lysyl (copper) hydroxylases (Englard and Seifter, 1986; Tsao, 1997).
Evidence also suggests that ascorbate plays a role in or influences collagen gene expression, cellular procollagen secretion, and the biosynthesis of other connective tissue components besides collagen, including elastin, fibronectin, proteoglycans, bone matrix, and elastin-associated fibrillin (Ronchetti et al., 1996). The primary physical symptoms of ascorbic acid's clinical deficiency disease, scurvy, which involves deterioration of elastic tissue, illustrate the important role of ascorbate in connective tissue synthesis.
Ascorbic acid is involved in the synthesis and modulation of some hormonal components of the nervous system. The vitamin is a co-factor for dopamine-β-hydroxylase, which catalyzes hydroxylation of the side chain of dopamine to form norepinephrine, and α-amidating monooxygenase enzymes, involved in the biosynthesis of neuropeptides. Other nervous system components modulated by ascorbate concentrations include neurotransmitter receptors, the function of glutamatergic and dopaminergic neurons, and synthesis of glial cells and myelin (Englard and Seifter, 1986; Katsuki, 1996).
Because of its ability to donate electrons, ascorbic acid is an effective antioxidant. The vitamin readily scavenges reactive oxygen species (ROS) and reactive nitrogen species (RNS) (e.g., hydroxyl, peroxyl, superoxide, peroxynitrite, and nitroxide radicals) as well as singlet oxygen and hypochlorite (Frei et al., 1989; Halliwell and Whiteman, 1997; Sies and Stahl, 1995). The one- and two-electron oxidation products of ascorbate are relatively nontoxic and easily regenerated by the ubiquitous reductants glutathione and NADH or NAD-PH. The relatively high tissue levels of ascorbate provide substantial antioxidant protection: in the eye, against photolytically generated free-radical damage; in neutrophils, against ROS produced during phagocytosis; and in semen, against oxidative damage to sperm deoxyribonucleic acid (DNA) (Delamere, 1996; Fraga et al., 1991; Levine et al., 1994). Ascorbic acid protects against plasma and low-density lipoprotein (LDL) oxidation by scavenging ROS in the aqueous phase before they initiate lipid peroxidation (Frei et al., 1988; Jialal et al., 1990) and possibly by sparing or regenerating vitamin E (Halpner et al., 1998). Evidence suggests that ascorbate
also provides antioxidant protection indirectly by regenerating other biological antioxidants such as glutathione and α-tocopherol back to their active state (Jacob, 1995).
Ascorbic acid functions as a reducing agent for mixed-function oxidases in the microsomal drug-metabolizing system that inactivates a wide variety of substrates, such as endogenous hormones or xenobiotics (i.e., other chemical compounds such as drugs, pesticides, or carcinogens that are foreign to humans) (Tsao, 1997). The activity of both microsomal drug-metabolizing enzymes and cytochrome P-450 electron transport is lowered by ascorbate deficiency. The vitamin is involved in the biosynthesis of corticosteroids and aldosterone and in the microsomal hydroxylation of cholesterol in the conversion of cholesterol to bile acids. In reactions similar to the hydroxylation of proline for collagen synthesis, ascorbate is required along with iron at two steps in the pathway of carnitine biosynthesis. Ascorbic acid modulates iron absorption, transport, and storage (Gosiewska et al., 1996). Limited data suggest that ascorbate modulates prostaglandin synthesis and thus exerts bronchodilatory and vasodilatory as well as anticlotting effects (Horrobin, 1996).
Physiology of Absorption, Metabolism, and Excretion
Absorption and Transport
Intestinal absorption of ascorbic acid occurs through a sodium-dependent active transport process that is saturable and dose dependent (Rumsey and Levine, 1998; Tsao, 1997). At low gastrointestinal ascorbate concentrations, active transport predominates, while simple diffusion occurs at high concentrations. Some 70 to 90 percent of usual dietary intakes of ascorbic acid (30 to 180 mg/day) are absorbed; however, absorption falls to about 50 percent or less with increasing doses above 1 g/day (Kallner et al., 1979). The bioavailabilities of the vitamin from foods and supplements are not significantly different (Johnston and Luo, 1994; Mangels et al., 1993).
Cellular transport of ascorbic acid and DHA is mediated by transporters that vary by cell type (Jacob, 1999; Tsao, 1997). DHA is the form of the vitamin that primarily crosses the membranes of blood and intestinal cells, after which it is reduced intracellularly to ascorbic acid. Accumulation of ascorbate into neutrophils and lympho-
cytes is mediated by both high- and low-affinity transporters, and the vitamin is localized mostly in the cytosol. Intracellularly and in plasma, vitamin C exists predominately in the free reduced form as ascorbate monoanion, as shown in Figure 5-1 (Levine et al., 1994).
Metabolism and Excretion
Since the immediate oxidized forms of vitamin C are readily reduced back to ascorbic acid, relatively small amounts of the vitamin are lost through catabolism. The primary products of oxidation beyond DHA include oxalic and threonic acids, L -xylose, and ascorbate 2-sulfate (Jacob, 1999). With large intakes of the vitamin, unabsorbed ascorbate is degraded in the intestine, a process that may account for the diarrhea and intestinal discomfort sometimes reported by persons ingesting large doses (see section on “Adverse Effects”).
Besides dose-dependent absorption, a second primary mechanism for regulation of body ascorbate content is renal action to conserve or excrete unmetabolized ascorbate. Recent studies have shown that little unmetabolized ascorbate is excreted with dietary intakes up to about 80 mg/day and that renal excretion of ascorbate increases proportionately with higher intakes (Blanchard et al., 1997; Melethil et al., 1986).
Body Stores
Dose-dependent absorption and renal regulation of ascorbate allow conservation of the vitamin by the body during low intakes and limitation of plasma levels at high intakes. Tissue-specific cellular transport systems allow for wide variation of tissue ascorbate concentrations. High levels are maintained in the pituitary and adrenal glands, leukocytes, eye tissues and humors, and the brain, while low levels are found in plasma and saliva (Hornig, 1975). Due to homeostatic regulation, the biological half-life of ascorbate varies widely from 8 to 40 days and is inversely related to the ascorbate body pool (Kallner et al., 1979). Similarly, catabolic turnover varies widely, about 10 to 45 mg/day, over a wide range of dietary intakes due to body pool size. A total body pool of less than 300 mg is associated with scurvy symptoms (Baker et al., 1971), while maximum body pools are limited to about 2 g (Kallner et al., 1979). At very low ascorbate intakes, essentially no ascorbate is excreted unchanged and a minimal loss occurs.
Clinical Effects of Inadequate Intake
Scurvy, the classic disease of severe vitamin C deficiency, is characterized by symptoms related to connective tissue defects. Scurvy usually occurs at a plasma concentration of less than 11 µmol/L (0.2 mg/dL). Clinical features of scurvy include follicular hyperkeratosis, petechiae, ecchymoses, coiled hairs, inflamed and bleeding gums, perifollicular hemorrhages, joint effusions, arthralgia, and impaired wound healing (Baker et al., 1971; Chazan and Mistilis, 1963; Levine et al., 1996b). Other symptoms include dyspnea, edema, Sjögren's syndrome (dry eyes and mouth), weakness, fatigue, and depression. In experimental subjects made vitamin C deficient but not frankly scorbutic, gingival inflammation (Leggott et al., 1986) and fatigue (Levine et al., 1996a) were among the most sensitive markers of deficiency. Vitamin C deficiency in infants may result in bone abnormalities such as impaired bone growth and disturbed ossification, hemorrhagic symptoms, and resultant anemia (Jacob, 1999).
Lack of ascorbate-related hydroxyproline and hydroxylysine formation needed for collagen cross-linking may explain many of the connective tissue and hemorrhagic manifestations of scurvy, however, the specific histologic defects have not been identified. Oxidative degradation of some blood coagulation factors due to low plasma ascorbate concentrations may contribute to hemorrhagic symptoms (Parkkinen et al., 1996).
Scurvy is rare in developed countries but is occasionally seen in individuals who consume few fruits and vegetables, peculiar or restricted diets, or in those who abuse alcohol or drugs. In the United States, low blood ascorbate concentrations are more prevalent in men, especially elderly men, than in women and are more prevalent in populations of lower socioeconomic status (LSRO/FASEB, 1989). Infantile scurvy is rarely seen, because human milk provides an adequate supply of vitamin C and infant formulas are fortified with the vitamin.
SELECTION OF INDICATORS FOR ESTIMATING THE REQUIREMENT FOR VITAMIN C
Antioxidant Functions
There is much support for the role of increased oxidative stress in the pathogenesis of cardiovascular disease (Jialal and Devaraj, 1996; Witztum and Steinberg, 1991). The most plausible and biologically
relevant hypothesis is that the oxidative modification of low-density lipoprotein (LDL) and other lipoproteins promote atherogenesis (Berliner and Heinecke, 1996; Devaraj and Jialal, 1996; Witztum and Steinberg, 1991). Several lines of evidence suggest that oxidized LDL (oxLDL) is pro-atherogenic. Furthermore, data support the in vivo existence of oxLDL (Berliner and Heinecke, 1996; Witztum and Steinberg, 1991). In vitro studies have clearly shown that vitamin C at concentrations greater than 40 µmol/L (0.8 mg/dL) inhibits the oxidation of isolated LDL induced by transition metals, free-radical initiators, and activated human neutrophils and macrophages (Jialal and Grundy, 1991; Jialal et al., 1990; Scaccini and Jialal, 1994). This is because vitamin C effectively scavenges aqueous reactive oxygen species (ROS) and reactive nitrogen species (RNS), which prevents them from attacking LDL. Thus, in vitro vitamin C clearly functions as an antioxidant.
Studies shown in Table 5-1 examined the effect of vitamin C supplementation alone on biomarkers of lipid peroxidation. Of the 13 studies, 7 showed that vitamin C supplementation resulted in a significant decrease in lipid oxidation products in plasma, LDL, or urine. The vitamin C supplements that resulted in positive effects ranged from 500 to 2,000 mg/day. The most convincing evidence that vitamin C functions as an antioxidant in vivo is the study by Reilly et al. (1996) showing that supplementation of smokers with 2.0 g vitamin C for 5 days was associated with a significant reduction in urinary isoprostanes, an indicator of oxidative stress. In the remaining six studies in which vitamin C was supplemented in amounts ranging from 500 to 6,000 mg/day, there was no significant effect of vitamin C supplementation on lipid oxidation products in plasma, urine, or plasma LDL.
Carr and Frei (1999) examined the effect on LDL oxidation of supplementation with vitamin C in combination with vitamin E and β-carotene. Although these investigators have clearly shown that the supplements decrease LDL oxidation, it is difficult to assess the contribution of vitamin C alone.
Vitamin C supplementation (2,000 mg/day for 4 to 12 months) in 41 patients with non-atrophic gastritis decreased gastric mucosal nitrotyrosine, a measure of RNS activity (Table 5-2) (Mannick et al., 1996). Thus, from this study and the study by Reilly et al. (1996), it can be concluded that supplementation with vitamin C results in an antioxidant effect in vivo because it significantly reduces nitrotyrosine and urinary isoprostanes.
However, with respect to the effect of vitamin C on LDL oxidation, the data are inconclusive. This could be explained by the fact
that, because vitamin C is water soluble, it does not partition into the LDL particle. Also, it must be pointed out that in one of the 13 studies summarized in Table 5-1, there was an increase in plasma thiobarbituric acid reactive substances (TBARS), an indicator of oxidative stress, with a 500-mg dose of ascorbic acid (Nyyssonen et al., 1997b).
Adhesion of mononuclear cells to endothelium is an early event in atherogenesis and may be triggered by oxidative stress. Smokers have low levels of vitamin C and increased oxidative stress. A recent study showed that monocytes of smokers display greater adhesion to endothelial cells than those of nonsmokers (Weber et al., 1996). When supplemented with 2,000 mg/day of vitamin C, the plasma ascorbate level of smokers increased, and adhesion of their monocytes to endothelium decreased to that seen in nonsmokers.
Impaired vascular function is crucial to the clinical manifestation of atherosclerosis. As depicted in Table 5-3, numerous investigators have reported a beneficial effect of high dose vitamin C administration, either orally or intraarterially, on vasodilation. This beneficial effect of vitamin C is most likely related to its antioxidant effect. Endothelium-derived relaxing factor, nitric oxide (NO), promotes vasodilation but is rapidly inactivated by superoxide. Vitamin C improves endothelial function and vasodilation, possibly by scavenging superoxide radicals, conserving intracellular glutathione, or potentiating intracellular NO synthesis. In human endothelial cells in culture, extracellular vitamin C at physiological concentrations increased cellular NO synthesis up to threefold, and the increase in NO synthesis followed a time course similar to ascorbate uptake into the cells (Heller et al., 1999).
Antioxidant Functions in Leukocytes
The content of vitamin C in leukocytes is especially important because the ROS generated during phagocytosis and neutrophil activation are associated with infectious and inflammatory stresses (Jariwalla and Harakeh, 1996; Levine et al., 1994). Along with pituitary and adrenal glands and eye lens, leukocytes contain the highest vitamin C concentrations of all body tissues (Moser, 1987). Studies with guinea pigs and monkeys show that the concentration of ascorbate in the leukocytes more accurately reflects liver and body pool ascorbate than does the concentration in plasma or erythrocytes (Omaye et al., 1987). The vitamin is transported into leukocytes by an energy-dependent transport system that concentrates the vitamin some twenty-five-, forty-, and eightyfold over plasma levels in neutr-
TABLE 5-1 Effect of Vitamin C Supplementation on Biomarkers of Lipid Oxidation in Humans
|
Reference |
Subjects |
Vitamin C Dosea (mg/d) |
|
Harats et al., 1990 |
17 smokers |
1,000 1,500 |
|
Belcher et al., 1993 |
5 healthy men |
1,000 |
|
Rifici and Khachadurian, 1993 |
4 healthy men and women |
1,000 |
|
Cadenas et al., 1996 |
21 healthy men |
1,000 |
|
Fuller et al., 1996 |
19 smokers (9 placebo) |
1,000 |
|
Mulholland et al., 1996 |
16 female smokers (8 placebo) |
1,000 |
|
Reilly et al., 1996 |
5 heavy smokers |
2,000 |
|
Anderson et al., 1997 |
48 nonsmokers (24 females) |
60 6,000 |
|
Nyyssonen et al., 1997b |
59 male smokers (19 placebo) |
500 (Pj) 500 (SRk) |
|
Samman et al., 1997 |
8 male smokers |
(40) 1,000 |
|
Wen et al., 1997 |
20 nonsmokers (9 placebo) |
1,000 |
|
Harats et al., 1998 |
36 healthy men |
(50) 500 (citrus fruit supplement) |
|
Naidoo and Lux, 1998 |
9 healthy men, 6 healthy women |
250, 500, 750 and 1,000 |
|
a Amount given in excess of variable amount consumed daily as part of the diet. b LDL = low-density lipoprotein. c TBARS = thiobarbituric acid reactive substances. d LDL oxidizability is measured by the lag time and propagation rate of in vitro lipid peroxidation. e VLDL = very low-density lipoprotein. f CD = conjugated dienes. |
||
|
Duration |
Plasma Change |
Findings |
|
2 wk |
2.0-fold |
|
|
4 wk |
2.3-fold |
↓ Plasma and LDL TBARS |
|
14 d |
Not reported |
LDL oxidationd, no change |
|
10 d |
Not reported |
↓ VLDLe and LDL oxidation (4 hour TBARS) |
|
30 d |
Not reported |
Urine TBARS, no change |
|
4 wk |
3.9-fold |
|
|
14 d |
3.0-fold |
Serum TBARS, no change |
|
5 d |
Not reported |
18 Urine 8-epi-PGF2a |
|
14 d |
1.2-fold |
|
|
14 d |
1.8-fold |
Plasma MDA/HNE, no change ↑ TAC |
|
2 mo |
1.3-fold |
LDL oxidizability, no change |
|
2 mo |
1.5-fold |
Plasma ↑ TBARS with P Vit C No ↑ with SR Vit C |
|
(2 wk) |
(baseline) |
LDL oxidizability (CD): no change |
|
2 wk |
2.0-fold |
|
|
4 wk |
2.2-fold |
↓ Plasma MDA (↑ erythrocyte Vit E and GSHl); no change LDL Vit E; no change in LDL oxidizability (TBARS and CD) |
|
(1 mo) |
(baseline) |
↓ LDL oxidizability (CD) |
|
2 mo |
3.8-fold |
|
|
2 wk |
1.5-fold (250) 2.0-fold (500) 2.0-fold (750 and 1,000) |
↓ Plasma MDA and allantoin with 500, 750 and 1,000 mg/d |
|
g MDA = malondialdehyde. h HNE = hydroxynonenal. i TAC = Total Antioxidant Capacity. j P = plain. k SR = slow release. l GSH = reduced gluthione. |
||
TABLE 5-2 Vitamin C Intake and Biomarkers of Gastric and Bladder Cancer
TABLE 5-3 Vitamin C and Endothelium-Dependent Vasodilation in Humans
|
Reference |
Subjects |
|
Heitzer et al., 1996 |
10 chronic smokers 10 control subjects |
|
Levine et al., 1996 |
46 coronary artery disease patients (20 placebo) |
|
Ting et al,, 1996 |
10 type II diabetic patients 10 control subjects |
|
Motoyama et al., 1997 |
20 smokers 20 control subjects |
|
Solzbach et al., 1997 |
22 hypertensive patients |
|
Ting et al., 1997 |
11 hypercholesterolemic patients 12 healthy control subjects |
|
Hornig et al., 1998 |
15 chronic heart failure patients 8 healthy control subjects |
|
Taddei et al., 1998 |
14 hypertensive patients 14 healthy control subjects |
|
Timimi et al., 1998 |
10 type I diabetic patients 10 control subjects |
|
Duration |
Findings |
|
5–12 d |
↓ In vivo nitrosation (N-nitrosoproline) |
|
1 wk |
↓ Urinary β-glucuronidase activity (linked to bladder cancer) |
|
4 wk |
↓ Gastric mucosa DNAa adduct formation |
|
4 wk |
↑ O6-alkyltransferase DNA repair enzyme |
|
— |
Significant (p < .001) correlation between gastric mucosa ascorbyl radical concentration and ROSb activity |
|
4–12 mo |
↓ Nitrotyrosine in gastric mucosa (measure of RNSc activity) |
|
1 d |
↓ In vivo nitrosation by urinary nitrosoproline products |
|
Vitamin C Dose |
Findings |
|
18 mg/min (infusion) |
↑ Forearm blood flow 1.6-fold (measured after acetylcholine infusion) |
|
2,000 mg (oral) |
↑ Brachial artery dilation 3.2-fold (measured after 2 h) |
|
24 mg/min (infusion) |
↑ Forearm blood flow 1.4-fold (measured after methacholine infusion) |
|
10 mg/min (infusion) |
↑ Brachial artery dilation 1.7-fold (measured after 20 min) |
|
3,000 mg (infusion) |
↓ Coronary artery vasoconstriction 2.6-fold (measured after acetylcholine infusion) |
|
24 mg/min (infusion) |
↑ Forearm blood flow 1.3-fold (measured after methacholine infusion) |
|
25 mg/min (infusion) |
↑ Radial artery dilation 1.6-fold (measured after 10 min) |
|
2,000 mg (oral) |
↑ Radial artery dilation 1.5-fold (following 4 wk supplementation) |
|
2.4 mg/min (infusion) |
↑ Forearm blood flow 1.3-fold (acetycholine) |
|
24 mg/min (infusion) |
↑ Forearm blood flow 1.4-fold (measured after methacholine infusion) |
phils, platelets, and lymphocytes, respectively (Evans et al., 1982; Jacob et al., 1992; Levine et al., 1996a). Metabolic priority for maintenance of intracellular lymphocyte ascorbate levels was demonstrated by its lower depletion rates compared to plasma and semen ascorbate levels during controlled vitamin C deficiency (intake of 5 mg/day) and faster recovery during vitamin repletion at 60 mg/day (Jacob et al., 1992). Intracellular ascorbate recycling (the intracellular regeneration of oxidized extracellular ascorbate) provides a cellular reservoir of reducing capacity (electrons) that can be transmitted both into and across the cell membrane (May et al., 1999).
The high intracellular concentration of ascorbate in leukocytes provides cellular protection against oxidant damage associated with the respiratory burst. In isolated neutrophils, ascorbate recycling is increased up to thirtyfold upon exposure of the cells to microbial pathogens (Wang et al., 1997b). Ascorbate effectively neutralizes phagocyte-derived oxidants without inhibiting the bactericidal activity of the phagosome (Anderson and Lukey, 1987). Evidence that ascorbate modulates leukocyte phagocytic action, blastogenesis, immunoglobulin production, chemotaxis, and adhesiveness has been reported in vitro, although evidence for the latter two functions has been mixed (Evans et al., 1982; Jariwalla and Harakeh, 1996).
Concentrations of ascorbate normally found in plasma (22 to 85 µmol/L [0.4 to 1.7 mg/dL]) were shown to neutralize hypochlorous acid (HOCl), one of many powerful oxidants generated by myeloperoxidase in activated neutrophils and monocytes (Halliwell et al., 1987; Heinecke, 1997). This action was hypothesized to protect α-1-antiprotease against inactivation by HOCl and thereby prevent proteolytic damage at inflamed sites such as the rheumatoid joint (Halliwell et al., 1987). Indeed, the ratio of oxidized to reduced ascorbate was found to be increased in the knee synovial fluid of active rheumatoid arthritis patients, which suggests that ascorbate is acting to scavenge phagocyte-derived oxidants in this locally inflamed area (Lunec and Blake, 1985). Similarly, increased ascorbate oxidation in the plasma of patients with adult respiratory distress syndrome (Cross et al., 1990) and in smokers (Lykkesfeldt et al., 1997) indicates protection against oxidant damage from activated neutrophils and other sources in the lung. Exposure of nine apparently healthy adults to 2,000 parts per billion (ppb) of ozone, an environmental pollutant, for 2 hours resulted in increased myeloperoxidase and decreased ascorbate concentrations in bronchoalveolar lavage fluid. These results imply that ascorbate protects against inflammatory oxidative stress induced by ozone (Mudway et al., 1999).
Ascorbate scavenging of myeloperoxidase-derived oxidants from phagocytic white cells may also be protective against in vivo LDL oxidation because HOCl-oxidized proteins have been identified in human atherosclerotic lesions (Hazell et al., 1996). In an in vitro system, ascorbate at a physiologically relevant concentration of 50 µmol/L (0.9 mg/dL) was the most effective antioxidant for preventing LDL oxidation due to myeloperoxidase-derived RNS (Byun et al., 1999).
Oxidative Deoxyribonucleic Acid and Chromosome Damage
Cellular Deoxyribonucleic Acid (DNA) Damage
Table 5-4 summarizes the results of five experimental human studies in which cellular markers of DNA damage were measured after various vitamin C intakes. Three of the studies varied vitamin C alone, while the other two studies varied vitamin C and other micronutrients.
Of the three studies that varied only vitamin C intake, one showed that 60 or 250 mg/day decreased sperm 8-hydroxy-7, 8-dihydro-2′-deoxyguanosine (8-oxodG), a measure of oxidative stress, but did not affect lymphocyte or urine 8-oxodG or DNA strand breaks (Fraga et al., 1991). In contrast, the second study showed no effect of either 60 or 6,000 mg/day vitamin C on lymphocyte DNA or chromosome damage as measured by comet assay (Anderson et al., 1997). The third study showed both decreases and increases in measures of lymphocyte DNA oxidative damage after vitamin C supplementation of 500 mg/day (Podmore et al., 1998). In a subsequent report of results from the study of Podmore et al. (1998), the investigators hypothesized that increases in serum and urine 8-oxodG following the decreases of lymphocyte 8-oxoguanine and 8-oxodG suggest a role for vitamin C in the repair of oxidant-damaged DNA (Cooke et al., 1998).
The two studies that co-supplemented with vitamin E and β-carotene (Duthie et al., 1996) or iron (Rehman et al., 1998) demonstrated mixed results in that both decreases and increases in lymphocyte DNA oxidant damage measures. Since the contribution of vitamin C alone to the results of these studies cannot be determined, these studies cannot be used to estimate a vitamin C requirement. Results of the latter study involving supplementation of apparently healthy individuals with both vitamin C and iron are discussed in the section “Tolerable Upper Intake Levels.”
Inverse correlations of lymphocyte ascorbate and glutathione con-
TABLE 5-4 Vitamin C Intake and Biomarkers of Cellular Oxidative DNA Damage in Humans
|
Reference |
Subjects |
Vitamin C Dose (mg/d) |
|
Fraga et al., 1991 |
10 males |
(250 baseline)a 5a 10 or 20a 60 or 250a |
|
Duthie et al., 1996 |
50 male smokers 50 nonsmokers |
100 +280 mg/d vitamin E +25 mg/d β-carotene 100 +280 mg/d vitamin E +25 mg/d β-carotene |
|
Anderson et al., 1997 |
48 nonsmokers (24 females) |
60 6,000 |
|
Podmore et al., 1998; Cooke et al., 1998 |
30 healthy subjects (16 females and 14 males) |
500 |
|
Rehman et al., 1998 |
10 healthy subjects 10 healthy subjects |
60 +14 mg/d Fe 260 +14 mg/d Fe |
|
a Intake from controlled diet; no supplemental doses given. b 8-oxodG = 8-oxo-7,8-dihydro-2'-deoxyguanosine. c HPLC-EC = high-performance liquid chromatography-electrochemical detection d DNA = deoxyribonucleic acid. |
||
centrations with oxidized DNA bases in another study of 105 apparently healthy adults suggest that these two intracellular antioxidants protect human lymphocytes against oxidative damage (Lenton et al., 1999). In sum, the results of studies testing the effects of vitamin C on cellular DNA damage are mixed and cannot be used for estimating the vitamin C requirement.
|
Duration |
Findings |
|
(7–14 d) 32 d 28 d 28 d |
↑ Sperm 8-oxodG (HPLC-EC) ↓ Sperm 8-oxodG (HPLC-EC) No changes in lymphocyte 8-oxodG or DNAd strand breaks |
|
20 wk |
↓ Lymphocyte DNA damage (comet assay) |
|
20 wk |
↓ Lymphocyte DNA damage (comet assay) |
|
14 d 14 d |
No change in lymphocyte DNA damage (comet assay) or chromosome breakage |
|
6 wk |
↓ Lymphocyte 8-oxoguae and 8-oxodG (GC-MSf) ↑ Serum and urine 8-oxodG (GC-MS) ↑ Lymphocyte 8-oxoadeg (GC-MS) |
|
12 wk |
↓ Leukocyte 8-oxogua (GC-MS) ↓ Leukocyte 8-oxoade (GC-MS) |
|
12 wk |
↓ Leukocyte 8-oxogua (GC-MS) ↓ Leukocyte 8-oxoade (GC-MS) ↑ Leukocyte 5-OH cytosine (GC-MS) ↑ Leukocyte thymine glycol (GC-MS) ↑ Total base damage at 6 wk, no change at 12 wk |
|
e 8-oxogua = 8-oxoguanine. f GC-MS = gas chromatography-mass spectroscopy. g 8-oxoade = 8-oxoadenine. SOURCE: Adapted from Carr and Frei (1999). |
|
Urinary Markers of DNA Damage
Urinary excretion of DNA oxidant damage products, which is thought to represent the balance of total body DNA damage and repair has been measured in the studies shown in Table 5-5. This is a nonspecific measure used to assess changes due to micronutrient status. Except for the study by Cooke et al. (1998), no relationships between vitamin C intake and urinary markers of DNA damage were
TABLE 5-5 Vitamin C Intake and Urinary Excretion of Oxidative DNA Damage Products in Humans
|
Reference |
Subjects |
Vitamin C Dose (mg/d) |
|
Fraga et al., 1991 |
10 males |
(250 baseline)a 5a 10–20a 60–250a |
|
Loft et al., 1992 |
83 subjects |
72d 5.9 mg/d vitamin Ed 1.1 mg/d vitamin Ad |
|
Witt et al., 1992 |
11 subjects |
1000 +533 mg/d vitamin E +10 mg/d β-carotene |
|
Prieme et al., 1997 |
18 male smokers 20 male smokers |
500 500 (SRe) |
|
Cooke et al., 1998 |
14 males 16 females |
500 |
|
a Intake from controlled diet; no supplemental doses given. b 8-oxodG = 8-oxo-7,8 dihydro-2′-deoxyguanosine. c HPLC-EC = high-performance liquid chromatography-electrochemical detection. d Intake estimated from diet records; no supplemental doses given. found. Thus, urinary markers of DNA damage cannot be used to determine vitamin C requirements. |
||
Ex Vivo Damage
The five studies in Table 5-6 measured DNA and chromosome damage ex vivo after supplementing the subjects with vitamin C. Single large doses of vitamin C (1 g/day or more) provided protection against lymphocyte DNA strand break damage induced ex vivo by radiation or hydrogen peroxide (H2O2) as measured by the comet assay (Green et al., 1994; Panayiotidis and Collins, 1997). In contrast, Crott and Fenech (1999) reported that a single 2-g dose of vitamin C neither caused DNA damage nor protected cells against hydrogen peroxide-induced toxicity. The two other studies measured DNA chromosome damage after treatment of lymphocytes with bleomycin, a test for genetic instability. Following vitamin C supplementation for two weeks, Pohl and Reidy (1989) found de-
|
Duration |
Findings |
|
(7–14 d) 32 d 28 d 28 d |
|
|
2 wk |
Urine 8-oxodG: not correlated (HPLC-EC) |
|
1 mo |
Urine 8-oxoG: no changes (HPLC-EC) |
|
2 mo 2 mo |
Urine 8-oxodG: no changes (HPLC-EC) Urine 8-oxodG: no changes (HPLC-EC) |
|
6 wk |
↑ Urine 8-oxodG (GC-MSf) |
|
e SR = slow release. f GC-MS = gas chromatography-mass spectroscopy. SOURCE: Adapted from Carr and Frei (1999). |
|
creased chromosome breaks and Anderson et al. (1997) reported no effects on DNA damage but increased chromosome aberrations. Since the findings of these studies were inconsistent, ex vivo damage cannot be used to estimate a vitamin C requirement.
Cancer Biomarkers
Effects of vitamin C intakes on surrogate markers and biomarkers of colorectal, gastric, and bladder cancer are shown in Table 5-2 and Table 5-7. Of six studies of patients with precancerous colon polyps, vitamin C treatment for 1 month to 3 years demonstrated variable results with regard to effect on polyp growth and cell proliferation (Table 5-7).
Biomarkers of gastric cancer after vitamin C treatment of patients with the precancerous conditions, gastritis, or Helicobacter pylori infections were measured in four studies (Table 5-2). Three studies showed positive results of vitamin C supplementation in vivo: Man-
TABLE 5-6 Vitamin C Intake and Ex Vivo Measures of Oxidative DNA Damage in Humans
TABLE 5-7 Vitamin C Intake and Colorectal Polyps
|
Reference |
Subjects |
Vitamin C Dose (mg/d) |
|
DeCosse et al., 1975 |
5 patients with familial polyps |
3,000 |
|
Bussey et al., 1982 |
36 patients with colon polyps |
3,000 |
|
McKeown-Eyssen et al., 1988 |
137 patients with colon polyps |
400 + 400 mg/d vitamin E |
|
Cahill et al., 1993 |
40 patients with colon polyps, 20 normal subjects |
750 |
|
Greenberg et al., 1994 |
380 patients with diagnosed colon adenomas |
1,000 + 400 mg vitamin E or 1,000 + 400 mg vitamin E + 25 mg β-carotene |
|
Hofstad et al., 1998 |
116 patients with colon polyps |
150 + 75 mg vitamin E + 15 mg β-carotene + 101 µg Se + 1.6 g Ca |
|
Duration |
Findings |
|
2 wk 2 wk 2 wk |
↓ Lymphocyte chromosome breaks after bleomycin treatment, average breaks per cell: 0.289 0.208 0.184 |
|
Single dose |
↓ Lymphocyte DNAa strand breaks in unirradiated and irradiated blood (comet assay) |
|
2 wk |
No effect on DNA damage (comet assay) ↑ Chromosome aberrations after bleomycin treatment |
|
Single dose |
↓ Lymphocyte DNA strand breaks in both groups after ex vivo H2O2b oxidant stress (comet assay) |
|
Single dose |
No effect on DNA damage (CBMNc assay) |
|
Duration |
Findings |
|
4–13 mo |
Complete polyp regression in two patients, partial regression in two, and increased polyps in one |
|
2 y |
↓ Polyp area |
|
2 y |
Nonsignificant in ↓ polyp recurrence |
|
1 mo |
↓ Total colonic crypt cell proliferation |
|
4 y |
No change in incidence of adenomas, polyp frequency, or size |
|
3 y |
↓ Number of new adenomas. No effect on growth of existing polyps |
nick et al. (1996) reported decreased gastric mucosal nitrotyrosine (a measure of RNS activity); Dyke et al. (1994a) reported decreased mucosal DNA damage in one group of gastric cancer patients and subsequently found increased mucosal O6-alkyltransferase, a DNA repair enzyme in a second group of patients with gastric cancer (Dyke et al. 1994b). Leaf et al. (1987) found decreased nitrosation in men after vitamin C supplementation. Drake et al. (1996) used electron paramagnetic resonance to demonstrate the presence of the ascorbyl radical in 82 unsupplemented patients with dyspepsia and showed that ascorbyl radical concentrations correlated with ROS activity. Gastric muscosal concentrations of ascorbyl radical, ROS, and malondialdehyde (a measure of lipid peroxidation) were higher in patients with gastritis and Helicobacter pylori infections compared to patients with normal mucosal histology. Young et al. (1990) found decreased β-glucuronidase activity (linked to bladder cancer) after in vivo supplementation of apparently healthy men with 1,500 mg/day of vitamin C for 1 week.
Summary
For the three studies shown in Table 5-4 in which only vitamin C intake was varied, some markers of cellular DNA damage showed no change with increased vitamin C intake, two markers decreased, and one increased. Urinary measures of oxidized DNA products showed no change attributable to vitamin C intake ( Table 5-5). Two of three studies of ex vivo DNA damage showed a benefit of vitamin C supplementation (Table 5-6); however, the relation of these results to the in vivo situation is uncertain. Studies of surrogate markers and biomarkers in precancerous colonic and gastric patients show beneficial or no effects of vitamin C supplementation. However, the interpretation of these endpoints and the relevance of the results to apparently healthy individuals are questionable. The study of dyspepsia patients indicates that vitamin C acts as an antioxidant in the gastric mucosa and prevents oxidative damage by scavenging ROS (Drake et al., 1996). This is consistent with previous findings that substantial amounts of ascorbic acid are secreted into the digestive tract (Dabrowski, 1990; Waring et al., 1996) and that vitamin C supplementation decreases gastric mucosal DNA adduct formation (Dyke et al., 1994a).
Overall, the results do not provide compelling evidence that vitamin C intakes of 60 to 6,000 mg/day reduce in vivo DNA oxidative damage in apparently healthy individuals. Hence, present data can-
not be used to estimate a vitamin C requirement using the end-point of reduction of oxidative damage to DNA and chromosomes.
Immune Function
As summarized in Table 5-8, vitamin C has been shown to affect various components of the human immune response, including antimicrobial and natural killer cell activities, lymphocyte proliferation, chemotaxis, and delayed dermal sensitivity (DDS). Except for the metabolic unit study of Jacob et al. (1991) and the study of patients with furunculosis (Levy et al., 1996), the studies involved apparently healthy free-living populations supplemented with from 200 mg/day to 6 g/day of vitamin C in addition to dietary vitamin intake. Hence, the results relate largely to the pharmacological range of vitamin C intakes rather than the nutritional range of intakes usually provided from food alone.
As seen from analysis of Table 5-8, vitamin C supplementation resulted about equally in improved or little change in frequently used measures of immune function: lymphocyte proliferation, chemotaxis, and DDS response. The decrease in DDS during vitamin C depletion of men in a metabolic unit cannot be ascribed solely to changes in ascorbate status because the DDS did not increase again upon repletion for 4 weeks with 60 to 250 mg/day of the vitamin (Jacob et al., 1991). The only negative effect of intakes in the range of 600 to 10,000 mg/day was the decrease in ex vivo bactericidal activity found after apparently healthy men received 2,000 (but not 200) mg/day of the vitamin for 4 weeks (Shilotri and Bhat, 1977).
Few controlled studies of the effect of vitamin C intake on infectious episodes in humans have been reported, except for studies of the common cold (covered later under “Common Cold” in the section “Relationship of Vitamin C Intake to Chronic Disease”). Peters et al. (1993) reported a significantly decreased incidence of post-race upper respiratory infections in marathon runners receiving 600 mg/day of vitamin C compared to control runners taking a placebo.
Results from some studies show improvement in indices of immune function due to increased vitamin C intake, whereas other studies show no effect. The lack of effect may be due to the use of subject populations whose baseline vitamin C status is already adequate, because leukocytes saturate with vitamin C at a lower intake than is required to saturate plasma, about 100 mg/day (Levine et al., 1996a). Nevertheless, the existing data do not provide convincing evidence that supplemental vitamin C has a significant effect on
TABLE 5-8 Vitamin C Intake and Measures of Immune Function in Humans
|
Reference |
Subjects |
Vitamin C Dose (mg/d) |
|
Shilotri and Bhat, 1977 |
5 healthy men, aged 23–28 y |
200 2,000 |
|
Ludvigsson et al., 1979 |
24 healthy women |
1,000–4,000 |
|
Anderson et al., 1980 |
5 healthy adults |
1,000–3,000 |
|
Panush et al., 1982 |
28 healthy young adults |
1,000–10,000 |
|
Kennes et al., 1983 |
Elderly adults, aged >70 y |
500 IMc |
|
Delafuente et al., 1986 |
15 elderly adults without acute illness |
2,000 |
|
Vogel et al., 1986 |
9 healthy men and 2 healthy women, aged 22–28 y |
1,500 |
|
Jacob et al., 1991 |
8 healthy men, aged 25–43 y |
5–20 60–250 |
|
Johnston, 1991 |
14 healthy women and men |
1,500 |
|
Johnston et al., 1992 |
10 adults |
2,000 |
|
Peters et al., 1993 |
46 runners and 46 control subjects |
600 |
|
Levy et al., 1996 |
23 patients with furunculosis (boils) |
1,000 |
|
a PMN = polymorphonuclear leukocytes. b DDS = delayed dermal sensitivity. c IM = intramuscular. immune functions in humans. Therefore, data from currently available immune function studies cannot be used to estimate the vitamin C requirement. |
||
Other Indicators
Collagen Metabolism
Ascorbic acid is required along with iron as a cofactor for the post-translational hydroxylation of proline and lysine to effect cross -
|
Duration |
Findings |
|
2 wk |
No change in bactericidal activity of leukocytes measured ex vivo |
|
2 wk |
↓ Ex vivo bactericidal activity of leukocytes |
|
5 wk |
No change in leukocyte ascorbate concentration or function |
|
1–3 wk |
↑ Mitogen-stimulated in vitro lymphocyte proliferation and PMNa chemotaxis. No change in other cellular or humoral immune functions |
|
1 wk |
↑ Mitogen-stimulated in vitro lymphocyte proliferation and DDSb response to skin antigens |
|
1 mo |
↑ Mitogen-stimulated in vitro lymphocyte proliferation and DDS response. No changes in serum immunoglobulins |
|
3 wk |
No change in mitogen-stimulated in vitro lymphocyte proliferation or DDS response |
|
90 d |
No change in PMN chemotaxis or response to experimental gingivitis |
|
60 d |
No changes in in vitro mitogen-stimulated lymphocyte proliferation |
|
4 wk |
↓ In DDS response with vitamin C intakes of 5–20 mg/d |
|
4 wk |
No change in plasma complement Clq |
|
2 wk |
No effect on PMN chemotaxis ↓ Blood histamine |
|
21 d |
↓ Incidence and severity of upper-respiratory-tract infections |
|
4–6 wk |
Improvement in PMN functions and clinical response in patients with low baseline PMN functions |
linking of mature collagen (Englard and Seifter, 1986). Lack of this function due to ascorbate deficiency results in defective collagen formation and the physical symptoms of scurvy. However, serum or urinary levels of proline or lysine, their hydroxylated forms, or other measures of collagen metabolism have not been shown to be reliable markers of ascorbate status (Hevia et al., 1990). Therefore, despite the important role of the vitamin in collagen formation, no collagen-related measures are available to use as a functional indicator for the dietary vitamin C requirement.
Carnitine Biosynthesis
Ascorbate is required along with iron at two steps in the pathway of carnitine biosynthesis in reactions similar to the hydroxylation of proline during collagen formation. Muscle carnitine is significantly depleted in scorbutic guinea pigs, suggesting that loss of energy derived from carnitine-related β-oxidation of fatty acids may explain the fatigue and muscle weakness observed in human scurvy (Jacob and Pianalto, 1997; Rebouche, 1995). However, neither guinea pig nor human studies show a consistent relationship between vitamin C status and carnitine levels (Davies et al., 1987; Jacob and Pianalto, 1997; Johnston et al., 1996). Although vitamin C deficiency appears to alter carnitine metabolism, the specific interactions and their relevance to functional carnitine status in humans are unclear. Therefore, measures of carnitine status cannot be used as an indicator for estimating the vitamin C dietary requirement.
Periodontal Health
The gingival and dental pathology that accompanies scurvy has prompted numerous investigations of the relationship between ascorbic acid and periodontal health. Epidemiological studies have failed to demonstrate an association between vitamin C intake and periodontal disease (Alvares, 1997; Russell, 1967). Controlled experimental studies of patients with gingivitis and apparently healthy adults with vitamin C intakes of 5 to 1,500 mg/day have shown mixed results with regard to the influence of vitamin C status on periodontal integrity (Leggott et al., 1986, 1991; Vogel et al., 1986; Woolfe et al., 1984). Other studies, with animals and humans, have shown that vitamin C intake can affect the structural integrity of gingival tissue, including permeability of the gingival sulcular epithelium (Alvares, 1997).
Overall, while evidence suggests that vitamin C deficiency is linked to some aspects of periodontal disease, the relationship of vitamin C intake to periodontal health in the population at large is unclear. Beyond the amount needed to prevent scorbutic gingivitis (less than 10 mg/day) (Baker et al., 1971), the results from current studies are not sufficient to reliably estimate the vitamin C requirement for apparently healthy individuals based on oral health endpoints.
Relationship of Vitamin C Intake to Chronic Disease
Cardiovascular Disease
As suggested earlier, there is reason to expect that the antioxidant vitamins should decrease the risk of cardiovascular disease (Gey, 1995; Jha et al., 1995; Simon, 1992). Several studies have considered the association between vitamin C concentrations in blood and the risk of cardiovascular disease. Singh et al. (1995) found that the risk of coronary artery disease was approximately two times less among the top compared to the bottom quintile of plasma vitamin C concentrations in Indian subjects. A prospective study of 1,605 Finnish men showed that those with increased plasma vitamin C (greater than 11.4 µmol/L [0.2 mg/dL]) had a 60 percent decreased risk of coronary heart disease (Nyyssonen et al., 1997a). The Basel Prospective Study of 2,974 Swiss men reported that plasma vitamin C concentrations greater than 23 µmol/L (0.4 mg/dL) were associated with nonsignificant reductions in the risk of coronary artery disease (Eichholzer et al., 1992) and stroke (Gey et al., 1993). In a 20-year follow-up of 730 elderly adults in Britain, plasma vitamin C concentrations greater than 28 µmol/L (0.5 mg/dL) were associated with a 30 percent decreased risk of death from stroke compared with concentrations less than 12 µmol/L (0.2 mg/dL) (Gale et al., 1995). In a similar study, cross-sectional in design, in 6,624 men and women in the Second National Health and Nutrition Examination Survey, the relative risk of coronary heart disease and stroke was decreased about 26 percent with serum vitamin C concentrations of 63 to 153 µmol/L (1.1 to 2.7 mg/dL) compared with concentrations of 6 to 23 µmol/L (0.1 to 0.4 mg/dL) (Simon et al., 1998).
In addition, several prospective cohort studies have shown that vitamin C intakes between 45 and at least 113 mg/day are associated with reduced risk of cardiovascular disease (Gale et al., 1995; Knekt et al., 1994; Pandey et al., 1995). Gale et al. (1995) reported that in 730 elderly British men and women, vitamin C intakes greater than 45 mg/day were associated with a 50 percent lower risk of stroke than were intakes less than 28 mg/day. There was a nonsignificant 20 percent decrease in the risk of coronary artery disease in this study. Knekt et al. (1994) studied more than 5,000 Finnish men and women and found that women consuming more than 91 mg/day vitamin C had a lower risk of coronary artery disease than those consuming less than 61 mg/day. However, a similar association was not found in the men. In the Western Electric
study in Chicago, a cohort of 1,556 middle-aged men consuming greater than 113 mg/day of vitamin C had a 25 percent lower risk of coronary artery disease than those consuming less than 82 mg/day (Pandey et al., 1995).
Other prospective studies have looked at higher levels of vitamin C intake and have reported similar findings. The First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study cohort of more than 11,000 adults showed a reduction in cardiovascular disease of 45 percent in men and 25 percent in women whose vitamin C intakes were approximately 300 mg/day from food and supplements (Enstrom et al., 1992). Sahyoun et al. (1996) studied 725 elderly Massachusetts adults and reported a 62 percent lower risk of cardiovascular disease in those whose vitamin C intakes were more than 388 mg/day compared to those whose intakes were less than 90 mg/day. Kritchevsky et al. (1995) reported a negative association between vitamin C intake and carotid artery wall thickness in men and women more than 55 years of age in the Atherosclerosis Risk in Communities Study. Women consuming more than 728 mg/day and men consuming at least 982 mg/day of vitamin C had decreased intima thickness compared to women with intakes of less than 64 mg/day and men with intakes of less than 56 mg/day vitamin C.
In contrast to the above studies, several studies have reported no association between vitamin C intake and risk of cardiovascular disease. In a cohort composed of 3,119 residents of Alameda County, California, vitamin C intakes were not associated with a reduction in risk for cardiovascular disease (Enstrom et al., 1986). In the Established Populations for Epidemiologic Studies of the Elderly with more than 11,000 adults 65 years of age and older (Losonczy et al., 1996) and in the Iowa Women's Heath Study of 34,486 postmenopausal women (Kushi et al., 1996b), vitamin C intake was not associated with an alteration in risk of coronary heart disease mortality in these older age groups. Similarly, the U.S. Health Professionals Follow-up Study of nearly 40,000 male health professionals found that increased intakes of vitamin C (ranging from 92 to 1,162 mg/day) were not associated with a lower risk of coronary heart disease (Rimm et al., 1993).
Although many of the above studies suggest a protective effect of vitamin C against cardiovascular disease, the data are not consistent or specific enough to estimate a vitamin C requirement based on any of these specific biomarkers for cardiovascular disease.
Cancer
As a possible protectant against cancer, vitamin C has engendered a great deal of interest. Block (1991) has reported that the epidemiologic evidence is strongly suggestive of a protective effect, especially for the non-hormone-dependent cancers. However, Ames et al. (1995) have cautioned that the evidence to date of a protective effect for any of the antioxidants is far from complete. Available studies assessing the role of vitamin C in specific cancers by site are evaluated in the following section.
Breast Cancer. A combined meta-analysis, based upon data from 12 case-control studies, found vitamin C to be the micronutrient most strongly associated with breast cancer risk (Howe et al., 1990). According to Howe and colleagues's statistical analyses, each 300-mg increase in vitamin C intake was associated with a 37 percent decrease in the risk of postmenopausal, but not premenopausal, breast cancer. The Iowa Women 's Health Study (Kushi et al., 1996a) found a 20 percent decrease in breast cancer risk with greater than 500 mg/day of vitamin C intake from supplements; in contrast, the Nurses Health Study, which used the same dietary assessment instrument, found no decreased risk of breast cancer at intakes greater than 359 mg/day (Hunter et al., 1993). Similarly, a Finnish cohort study (Jarvinen et al., 1997) of 4,697 women aged 15 years and older and the New York State Cohort Study (Graham et al., 1992) of more than 18,000 postmenopausal women with vitamin C intakes up to 498 mg/day found no association between vitamin C intake and breast cancer risk.
Cervical Cancer. In a case-control study, Wassertheil-Smoller et al. (1981) found high plasma vitamin C concentrations to be associated with decreased cervical cancer risk. Similarly Romney et al. (1985) reported a case-control study showing a negative association between increasing plasma vitamin C concentrations and cervical dysplasia.
Colorectal Cancer. In a large case-control study, Freudenheim et al. (1990) reported that increased intakes of vitamin C from food and supplements were associated with decreased risk of rectal cancer. In contrast, the Iowa Women's Cohort Study found no association between vitamin C intake and colon cancer risk at intakes from food and supplements of approximately 300 mg/day vitamin C (Bostick et al., 1993). However, in the women consuming more than 60
mg/day vitamin C from supplements compared with no supplements, the risk was decreased by 30 percent.
Pancreatic Cancer. Two separate case-control studies in Poland (Zatonski et al., 1991) and in Canada (Ghadirian et al., 1991) found that an elevated intake of vitamin C was associated with a decreased risk of pancreatic cancer. A study in the Netherlands, using a similar design, found a protective effect of vitamin C on pancreatic cancer in women but not in men (Bueno de Mesquita et al., 1991). A collaborative pooling of these and other case-control studies in 1992 found evidence overall of an inverse relationship between vitamin C and pancreatic cancer (Howe et al., 1992).
Lung Cancer. Several studies have considered whether vitamin C might be protective against lung cancer. The results of two large case-control studies in Hawaii found no association between dietary vitamin C intake and lung cancer (Hinds et al., 1984; Le Marchand et al., 1989). In contrast, Fontham et al. (1988) reported that vitamin C intake of approximately 140 mg/day was associated with protection for lung cancer among men and women in Louisiana who were non- or light smokers. Similarly, data from the First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study of more than 10,000 men and women indicated that dietary vitamin C intakes greater than 133 mg/day were inversely associated with lung cancer risk (Yong et al., 1997). There was no additional protective effect of vitamin C supplements. This association between vitamin C intake and risk of lung cancer was weaker but still in a protective direction in several studies: a Finnish cohort study of 4,538 men (Knekt et al., 1991); a Dutch cohort study of 561 men (Ocke et al., 1997); a United States prospective study of 3,102 men (Shekelle et al., 1981); and the New York State Cohort Study of 27,544 men (Bandera et al., 1997).
Gastric Cancer. Epidemiological and experimental evidence has suggested that vitamin C may protect against the development of gastric cancer by inhibiting formation of carcinogenic N -nitroso compounds or by scavenging ROS/RNS in the gastric mucosa (Fontham, 1994; Mirvish, 1994; O'Toole and Lombard, 1996). As noted earlier and summarized in Table 5-2, several experimental studies have linked increased vitamin C status to decreased ROS/RNS activity and oxidant damage products in the gastric mucosa of patients with gastritis and Helicobacter pylori infection (Drake et al., 1996; Dyke et al., 1994a; Mannick et al., 1996). Gastric juice ascorbate concentrations of patients with H. pylori infection and chronic
gastritis, risk factors for gastric cancer, are low compared to those of apparently healthy individuals and are increased by eradication of the H. pylori infection or by vitamin C supplementation (Rokkas et al., 1995; Waring et al., 1996). However, H. pylori infection and accompanying inflammation do not alter vitamin C levels or antioxidant potential in the gastroduodenal mucosa (Phull et al., 1999). Despite the epidemiological associations and the evidence that gastric juice vitamin C is protective against nitrosation and oxidant damage, the two vitamin C supplementation studies conducted to date have not shown a subsequent decrease in gastric cancer incidence (Blot et al., 1993; O'Toole and Lombard, 1996).
Although many of the above studies suggest a protective effect of vitamin C against specific cancers by site, the data are not consistent or specific enough to estimate a vitamin C requirement based on cancer.
Cataract
Ocular tissue concentrates vitamin C, which might suggest, teleologically, that the tissue needs this vitamin (Rose et al., 1998). It is reasonable to expect, therefore, that oxidative damage to ocular tissue is an important source of degenerative eye disease and that supplementation by vitamin C would be an effective means of lessening the risk of diseases such as cataract.
In a case-control comparison of 77 subjects with cataract and 35 control subjects with clear lenses, vitamin C intakes of greater than 490 mg/day were associated with a 75 percent decreased risk of cataracts compared with intakes of less than 125 mg/day (Jacques and Chylack, 1991). Similarly, vitamin C intakes greater than 300 mg/day were associated with a 70 percent reduced risk of cataracts (Robertson et al., 1989). In a second case-control comparison with 1,380 cataract patients and 435 control subjects, similar results were found: although intake numbers were not reported, above-median vitamin C intake was associated with a 20 percent decrease in the risks of cataracts (Leske et al., 1991). In contrast, an analysis of data derived from the Baltimore Longitudinal Study on Aging found no increased association between 260 mg/day of vitamin C and risk of cataracts compared to 115 mg/day (Vitale et al., 1993).
In an 8-year prospective study, Hankinson et al. (1992) evaluated the experience of more than 50,000 nurses in the Nurses Health Study. Dietary vitamin C intakes were not associated with a decreased risk of cataract, but cataract risk was 45 percent lower among the nurses who consumed vitamin C supplements for 10 or
more years. With a cohort of 247 nurses from the above study, vitamin C supplement use, in amounts ranging from less than 400 mg/day to greater than 700 mg/day for 10 years or more, was associated with a statistically significant protective effect on lens opacities (Jacques et al., 1997). Women who consumed vitamin C supplements for less than 10 years were not protected.
Although many of the above studies suggest a protective effect of vitamin C against cataracts, the data are not consistent or specific enough to estimate the vitamin C requirement based on cataracts.
Asthma and Obstructive Pulmonary Disease
It is suspected that vitamin C may decrease the risk of asthma and other related pulmonary conditions (Hatch, 1995). Two cross-sectional studies suggest that high plasma vitamin C concentrations or intakes protect or perhaps enhance respiratory function in men but not in women (Ness et al., 1996) and in both men and women (Britton et al., 1995). Similarly, dietary vitamin C intake was positively associated with enhanced pulmonary function in 2526 adult men and women participants in the First National Health and Nutrition Survey Epidemiological Follow-up Study (Schwartz and Weiss, 1994). In another study, 20 middle-aged men and women patients with mild asthma had decreased ascorbate and α-tocopherol concentrations in lung lining fluid, while blood levels were normal (Kelly et al., 1999). These findings and the presence of increased oxidized glutathione in the airways indicate an increased oxidative stress in asthma patients.
A series of small, clinical experiments reported that vitamin C supplementation of 2 g/day may be protective against airway responsiveness to viral infections, allergens, and irritants (Bucca et al., 1992). In contrast, a clinical experiment testing the blocking effect of 2 g/day vitamin C against exercise-induced asthma found little evidence of such an effect (Cohen et al., 1997).
Although many of the above studies suggest a protective effect of vitamin C against asthma and obstructive pulmonary disease, the data are not consistent or specific enough to estimate the vitamin C requirement based on asthma or pulmonary disease.
Common Cold
There has been a great deal of interest in the use of vitamin C to protect against the common cold, much of this research stimulated by the views put forth by the late Linus Pauling (Hemila and Her-
man, 1995). Reviews of numerous studies generally conclude that vitamin C megadoses have no significant effect on incidence of the common cold, but do provide a moderate benefit in terms of the duration and severity of episodes in some groups (Chalmers, 1975; Jariwalla and Harakeh, 1996; Ludvigsson et al., 1977). The often-reported improvement in severity of colds after vitamin C ingestion may be due to the antihistaminic action of the vitamin at pharmacological doses (Johnston et al., 1992). One early study comparing 44 school-aged twins in vulnerability to colds found no significant overall treatment effect of vitamin C intakes at doses of 500 to 1,000 mg/day (Miller et al., 1977). Other trials came to similar conclusions (Coulehan et al., 1976; Ludvigsson et al., 1977). Some reviews have stated that any impact of vitamin C is slight or that it is protective only among some subgroups of people (Hemila, 1996, 1997). Others view the accumulated results as so incomplete and flawed as to offer no evidence of protective effects (Herbert, 1995). Thus, the data are not consistent or specific enough to estimate the vitamin C requirement based on the common cold.
Cognitive Function and Memory
Although vitamin C's role as an antioxidant and cofactor for catecholamine biosynthesis might suggest that it protects cognitive function, there is little valid evidence that it does. One study found no association between cognitive function and vitamin C intake (range 84 to 147 mg/day) in 5,182 Dutch residents aged 55 to 95 years (Jama et al., 1996). Another study of 442 men and women, aged 65 to 94 years, reported that higher plasma ascorbate levels were associated with better memory performance (Perrig et al., 1997).
Summary
Although several studies have reported an inverse correlation between vitamin C intake and cardiovascular disease, some types of cancer, and cataracts, others have failed to do so. Very little variation in risk is seen based on the intake of vitamin C for chronic obstructive pulmonary disease, cold or infectious disease, or cognitive function and memory. Also it is important that, for all their power, human-based observational or epidemiological studies imply but do not prove cause and effect. Such studies do not rule out the impact of unidentified factors. In a recent review of epidemiological studies, Gey (1998) suggested that plasma vitamin C concentrations as low as 50 µmol/L (1.0 mg/dL) provide the optimal ben-
efits with regard to cardiovascular disease and cancer. This plasma vitamin C concentration is achieved at a dietary intake of approximately 90 mg/day vitamin C (Levine et al., 1996a). Thus, in the United States or Canada, it may be difficult to do a large-scale trial that demonstrates a health benefit for vitamin C unless the subjects are prescreened to have dietary intakes less than 90 mg/day and plasma levels less than than 50 µmol/L (1.0 mg/dL) of vitamin C.
FACTORS AFFECTING THE VITAMIN C REQUIREMENT
Bioavailability
Some 70 to 90 percent of usual dietary intakes of ascorbic acid (30 to 180 mg/day) are absorbed, although absorption decreases to about 50 percent and less with single doses above 1 g (Kallner et al., 1979; Levine et al., 1996b). The type of food consumed has not been shown to have a significant effect on absorption of either intrinsic or supplemental vitamin C. The bioavailability of the vitamin naturally found in foods or in the form of a supplement has not been shown to be significantly different from that of pure synthetic ascorbic acid (Johnston and Luo, 1994; Mangels et al., 1993).
Nutrient-Nutrient Interactions
Vitamin C participates in redox reactions with many other dietary and physiological compounds, including glutathione, tocopherol, flavonoids, and the trace metals iron and copper (Jacob, 1995).
Glutathione
Interactions of ascorbate with the endogenous antioxidant glutathione have been shown in both rodents and humans. In apparently healthy men fed a low-ascorbate diet of 5 to 20 mg/day, plasma total glutathione (reduced [GSH] and oxidized [GSSG] forms) and the ratio of GSH/GSSG, both indicators of oxidative stress, were significantly decreased (Henning et al., 1991). In apparently healthy adults supplemented with 500 mg/day of ascorbic acid, erythrocyte glutathione rose significantly (Johnston et al., 1993). The results indicate that ascorbate may contribute to antioxidant protection by maintaining reduced glutathione.
Tocopherol and Flavonoids
Evidence from in vitro and animal studies has shown that vitamin C can regenerate or spare α-tocopherol (Halpner et al., 1998), but studies in guinea pigs and humans have not confirmed that this interaction occurs to a significant extent in vivo (Jacob et al., 1996). Calculation of redox potentials indicates that ascorbate can recycle the flavonoid radical (Bors et al., 1995), and Skaper et al. (1997) showed that ascorbic acid acts synergistically with the flavonoid quercetin, to protect cutaneous tissue cells in culture against oxidative damage induced by glutathione deficiency.
Iron and Copper
A variety of interactions of ascorbate with the redox-active trace metals iron and copper have been reported (the potential pro-oxidant effects are discussed later in the section “Pro-oxidant Effects”). Ascorbic acid is involved in the regulation of iron metabolism at a number of points. Ascorbate-related reduction of iron to the ferrous state is involved in iron transfer and storage pathways. Ascorbic acid added to meals facilitates intestinal absorption of nonheme iron, possibly due to lowering of gastrointestinal iron to the more absorbable ferrous state or amelioration of the effect of dietary iron absorption inhibitors (Hallberg, 1985). However, studies in which the vitamin is added to meals over long periods have not shown significant improvement of body iron status, indicating that ascorbic acid has less effect on iron bioavailability than has been predicted from tests with single meals (Hunt et al., 1994).
Some evidence indicates that excess ascorbic acid intake may affect copper metabolism in a variety of ways, including inhibition of intestinal absorption and ceruloplasmin oxidase activity and labilization of ceruloplasmin-bound copper for cellular transport (Harris and Percival, 1991). High concentrations of plasma ascorbate in premature infants has been suggested to decrease ceruloplasmin ferroxidase activity and thereby compromise antioxidant protection (Powers et al., 1995). However, the significance of these effects in humans is questionable, because high ascorbate intakes among men on a metabolic unit did not inhibit copper absorption (Jacob et al., 1987b). In addition, the findings of decreased ceruloplasmin ferroxidase activity due to high physiologic ascorbate concentrations have been attributed to an artifact of nonphysiological assay pH (Løvstad, 1997).
Smoking
Nearly all studies show that smokers have decreased plasma and leukocyte ascorbate levels compared to nonsmokers. Part of this difference may be attributable to a lower intake of fruits and vegetables among smokers than among nonsmokers (Dallongeville et al., 1998; Marangon et al., 1998). However, studies that have adjusted for differences in vitamin C intake (Marangon et al., 1998) and those which have assessed populations with similar fruit and vegetable intakes (Lykkesfeldt et al., 2000) still find that smokers have lower plasma vitamin C concentrations than nonsmokers. This indicates that smoking per se predisposes to lower vitamin C status.
Vitamin C Turnover
The mechanism by which smoking compromises vitamin C status has not been well established. A radioisotope-labeled ascorbic acid dilution study showed that the metabolic turnover of the vitamin in smokers averaged about double that of nonsmokers: 70.0 versus 35.7 mg/day (Kallner et al., 1981). Increased ascorbate turnover in smokers is likely due to the increased oxidative stress from substances in smoke that are directly oxidizing or that stimulate oxidizing inflammatory responses (Elneihoum et al., 1997; Lehr et al., 1997; Pryor, 1997). This hypothesis is supported by the finding that the ratio of dehydroascorbic acid (DHA) to ascorbate in plasma of smokers is increased compared to that in nonsmokers (Lykkesfeldt et al., 1997).
Most studies have found that smokers suffer increased in vivo oxidation of susceptible biological molecules, including lipids (Morrow et al., 1995; Reilly et al., 1996), lipoproteins (Sasaki et al., 1997), and deoxyribonucleic acid (DNA) (Asami et al., 1997; Panayiotidis and Collins, 1997). In many but not all of these studies, intervention with administration of vitamin C or cessation of smoking decreased the oxidant damage measured. Supplementation of smokers with vitamin C (2 g/day) reduced elevated levels of urinary isoprostanes, a measure of in vivo lipid peroxidation (Reilly et al., 1996). This is consistent with earlier findings that either endogenous or in vitro added ascorbic acid uniquely protected plasma lipids against oxidative damage caused by cigarette smoke (Frei et al., 1991). Large doses of vitamin C (1 g/day or more) provided protection against lymphocyte DNA strand break damage induced ex vivo by radiation with H2O2 (hydrogen peroxide) (Green et al., 1994;
Panayiotidis and Collins, 1997). Endogenous DNA strand breaks (in the absence of added H2O2) were not different between smokers and nonsmokers; however, DNA damage due to ex vivo H2O2 addition was significantly greater in smokers than in nonsmokers. Vitamin C at 1 g/day decreased ex vivo DNA damage by about 20 percent in both groups (Panayiotidis and Collins, 1997).
A few studies have shown no effect of smoking or vitamin C supplementation on oxidizable biomolecules (Marangon et al., 1997, 1998). Supplementation of 21 male smokers with 500 mg/day of vitamin C for 2 months had no effect on urinary excretion of 8-hydroxy-7, 8-dihydro-2′-deoxyguanosine (8-oxodG), a product of oxidative DNA damage (Prieme et al., 1997).
Endothelial and Hemostatic Dysfunction
Smokers also suffer from endothelial and hemostatic dysfunctions that are reported to be ameliorated by vitamin C. Some evidence suggests that ascorbate in neurons modulates synthesis of the vasodilator nitric oxide (NO) (Millar, 1995). Since endothelium-dependent, but not endothelium-independent, vasodilation was improved by vitamin C administration in smokers, Heitzer et al. (1996) concluded that vitamin C acts to decrease oxidative stress within the vasculature of smokers by directly scavenging reactive oxygen species (ROS), thereby protecting the endogenous vasodilator NO, among other hypothesized effects. Vitamin C in physiological amounts has been shown to increase by threefold the synthesis of NO by human endothelial cells in culture (Heller et al., 1999). Motoyama et al. (1997) reported that vitamin C infusion improved impaired endothelium-dependent vasodilation in the brachial arteries of smokers, along with a decrease in plasma thiobarbitutic acid reactive substances (TBARS), a nonspecific measure of lipid peroxidation. Smokers with low levels of plasma vitamin C compared to nonsmokers also had increased monocyte adhesion to endothelial cells, which was normalized to that of nonsmokers after oral supplementation with 2 g/day of vitamin C (Weber et al., 1996). A mechanism for the effect of vitamin C on diminishing leukocyte or platelet adhesion and aggregation in smokers is suggested by findings in hamsters, in which the vitamin decreases formation of oxidized phospholipids that induce intravascular adhesion, aggregation, and inflammation (Lehr et al., 1997).
Pregnancy
Cigarette smoking also promotes oxidant damage and disturbs vitamin C nutriture in pregnant women. Although vitamin C intakes and serum concentrations were not different between third trimester smokers compared to nonsmokers; breath ethane, a measure of lipid peroxidation, was increased in the smokers and correlated inversely with serum vitamin C in the smokers but not the nonsmokers (Schwarz et al., 1995). There are more than 1015 organic free radicals per puff in gas-phase cigarette smoke (Pryor, 1992). Given the time elapsed between the last cigarette smoked and the breath collection as well as the absence of correlation between breath ethane values and hours since the last cigarette smoked, the breath ethane in pregnant smokers was thought to originate from peroxidation of the smoker's body lipids rather than the smoke itself. In Spanish women in their third trimester, serum vitamin C levels were not different between smokers and nonsmokers, but vitamin C levels were lower in the smokers' milk after parturition (Ortega et al., 1998).
Environmental Tobacco Smoke
Increased oxidative stress and ascorbate turnover have also been shown in nonsmoking individuals who are regularly exposed to tobacco smoke in their environment. Environmental or sidestream tobacco smoke provokes oxidant damage similar to mainstream cigarette smoke (Bermudez et al., 1994; Pryor et al., 1983). Plasma ascorbate concentrations of passive smokers were intermediate between those of active smokers and nonsmokers who were not exposed to environmental tobacco smoke, despite similar vitamin C intakes (Tribble et al., 1993). Hypovitaminosis C (plasma ascorbate concentrations less than 23 µmol/L [0.5 mg/dL]) was found in 24 percent of the active smokers and 12 percent of passive smokers and indicated that both passive and active smoke exposure lowered body ascorbate pools. Exposure of nonsmokers to secondhand smoke for 30 minutes in a smoke-filled room resulted in a significant decline in serum ascorbate, increased lipid peroxidation, and oxidatively modified low-density lipoprotein (LDL) (Valkonen and Kuusi, 1998). Although the above data are insufficient to estimate a special requirement for nonsmokers regularly exposed to tobacco smoke, these individuals are urged to ensure that they meet the Recommended Dietary Allowance (RDA) for vitamin C.
Gender
In both observational and intervention studies, human plasma or serum ascorbate levels are usually found to be higher in females than in males of the same population. Serum ascorbate concentrations of adult females aged 19 and older were greater than those of males in the same age category as reported in the Third National Health and Nutrition Examination Survey (NHANES III) (Appendix Table F-1). A minority of studies has reported no gender difference in plasma vitamin C levels (Johnston and Thompson, 1998). Although the reported gender differences in blood vitamin C concentrations may be attributed in part to differences in vitamin C intake, studies of elderly populations show that the difference exists over a wide range of vitamin C intakes and remains significant when males and females consuming similar amounts of the vitamin are compared (Garry et al., 1982; Itoh et al., 1989; Jacob et al., 1988; VanderJagt et al., 1987). In a population of elderly English adults (75 years and older), higher fruit consumption by women contributed to but did not entirely account for their higher plasma and leukocyte ascorbate levels compared to men (Burr et al., 1974). However, the latter finding of higher leukocyte ascorbate in women compared to men was not confirmed in a subsequent study, which found no gender differences in leukocyte ascorbate concentrations (Evans et al., 1982).
Part of the gender difference could be attributed to the larger body and fat-free mass of men compared to women (Baker et al., 1962; Blanchard, 1991a,b; Jacob et al., 1987a). However, since differences in fat-free mass accounted for only 10 to 31 percent of the variation in plasma vitamin C parameters, other unknown genderrelated variables such as hormonal or metabolic effects are needed to explain fully the observed gender differences in vitamin C metabolism (Blanchard, 1991a). The differences are not explained by renal handling of ascorbic acid, since renal clearance parameters of ascorbic acid for both young and elderly adults showed no genderrelated differences (Oreopoulos et al., 1993).
Overall, the data indicate that women maintain higher plasma ascorbate levels than men at a given vitamin C intake. Although studies were not found that directly compare the vitamin C requirements for men and women, a difference in average vitamin C requirements of men and women is assumed based on mean differences in body size, total body water, and lean body mass.
FINDINGS BY LIFE STAGE AND GENDER GROUP
Infants Ages 0 through 12 Months
Method Used to Set the Adequate Intake
No functional criteria of vitamin C status have been demonstrated that reflect response to dietary intake in infants. Thus, recommended intakes of vitamin C are based on an Adequate Intake (AI) that reflects the observed mean vitamin C intake of infants fed principally with human milk.
Human Milk. Human milk is recognized as the optimal milk source for infants throughout at least the first year of life; it is recommended as the sole nutritional milk source for infants during the first 4 to 6 months of life (IOM, 1991). Therefore determination of the AI for vitamin C for infants is based on data from infants fed human milk as the principal fluid during the periods 0 through 6 months and 7 through 12 months of age. The AI is set at the mean value for observed intakes as determined from studies in which the intake of human milk was measured by test weighing volume and the intake of food was determined by dietary records.
A number of reports of vitamin C content of human milk are available (Table 5-9). In mothers not taking vitamin C supplements, vitamin C in human milk in the first 6 months of lactation varied from 34 mg/L (Bates et al., 1982) to 83 mg/L (Byerley and Kirksey, 1985). In mothers taking vitamin C supplements ranging from 45 to greater than 1,000 mg/day, vitamin C content of human milk varied from 45 to 115 mg/L (Byerley and Kirksey, 1985; Udipi et al., 1985). Thus, the influence of maternal vitamin C intake and its effect on the vitamin C content of human milk are inconclusive (Byerley and Kirksey, 1985; Sneed et al., 1981; Thomas et al., 1979, 1980). The vitamin C content of human milk appears to decline during the first year of life so that by the twelfth month of lactation the vitamin C content is about 8 to 12 percent lower (Karra et al., 1986; Salmenpera, 1984).
In a study of infantile vitamin C intake during prolonged lactation, mean human milk vitamin C concentration decreased from 49.7 ± 10.6 mg/L (SD) at at 4 months of lactation to 44.6 ± 5.6 mg/L (SD) at 9 months of lactation (Salmenpera, 1984). Calculated from the milk concentrations and volumes, the average daily vitamin C intake by these infants was 36 mg/day at 4 and 6 months, and 42 mg/day at 9 months. The plasma concentrations of vitamin C of
all infants studied were in the normal range, greater than 34 µmol L (0.6 mg/dL) indicating that exclusively human milk-fed infants are well protected against vitamin C deficiency.
Ages 0 through 6 Months. The AI for infants 0 through 6 months is based on the average volume of milk intake of 0.78 L/day (Allen et al., 1991; Butte et al., 1984; Heinig et al., 1993), and an average concentration of vitamin C in human milk of 50 mg/L. This is the average vitamin C content of mature milk as assessed by Salmenpera (1984), Sneed et al. (1981), and George and De Francesca (1989) and is in the range of vitamin C content measured in the other studies (Table 5-9). Multiplying this amount by the average intake of human milk at 0 through 6 months, the AI would be 50 mg/L × 0.78 L/day = 39 mg/day vitamin C. Therefore the AI for vitamin C for infants 0 through 6 months of age is 40 mg/day, after rounding.
This amount is lower than the median intake of 75 mg/day of vitamin C for infants 1 through 6 months as reported in the U.S. Department of Agriculture 1994–1996 Continuing Survey of Food Intake by Individuals (CSFII) where intake data ranged from 4 to 273 mg, (Appendix Table D-1). The latter figure is probably higher than that calculated for an infant fed human milk because the data in CSFII are based on consumption of infant formula plus solid food, and the vitamin C content of proprietary infant formulas is approximately 50 mg/L (FDA, 1985). However, the proposed AI is comparable to vitamin C intakes from human milk-fed German infants whose median intakes were 41 mg/day at 6 months of age (Alexy et al., 1999). These figures are much higher than the amount of vitamin C shown to protect infants from scurvy (7 mg/day) in early studies determining amounts necessary to prevent deficiencies (Goldsmith, 1961; Rajalakshmi et al., 1965; Van Eekelen, 1953).
Ages 7 through 12 Months. During the second 6 months of life, solid foods become a more important part of the infant diet and add a significant but poorly defined amount of vitamin C to the diet. Although limited data are available for typical vitamin C intakes from foods by infants fed human milk, mean vitamin C intakes from solid foods are 22 mg/day for formula-fed infants (Montalto et al., 1985). For purposes of developing an AI for this age group, it is assumed that infants who are fed human milk have intakes of solid food similar to formula-fed infants of the same age group (Specker et al., 1997). Based on data of Dewey et al. (1984), mean human milk intake during the second 6 months of life would be 0.6 L/day. Thus, vitamin C intake from human milk with a vita-
TABLE 5-9 Vitamin C Content in Human Milk
|
Reference |
Stage of Lactation (mg/d) |
Vitamin C Content in Milk (mg/L) |
Maternal Vitamin C Intake (mg/d) |
|
Thomas et al., 1979 |
1 and 6 wk ppa |
68b 73b |
190 (diet only) 148 (diet) + 90 (supplement) |
|
Thomas et al., 1980c |
6 mo pp |
35.2 ± 12.0 (SDd) 38.4 ± 12.3 (SD) |
131 (diet only) 153 (diet) + 90 (supplement) |
|
Sneed et al., 1981 |
1 and 6 wk pp |
57b 68b |
118 (diet only)b 108 (diet) + 90 (supplement) |
|
Bates et al., 1982 |
Not Reported |
34 45 |
No diet intake data reported 35 as supplement |
|
Salmenpera, 1984 |
3–4 d pp 2 mo 4 mo 6 mo 9 mo 12 mo |
61.8 ± 0.99 (SD) 59.1 ± 1.18 (SD) 49.7 ± 1.06 (SD) 46.8 ± 1.02 (SD) 44.6 ± 0.56 (SD) 41.4 ± 1.13 (SD) |
48–277 (mean 138) (no supplements were taken) |
|
Byerley and Kirksey, 1985c |
7–13 wk lactation |
83.3 104.1 114.7 |
<100 100–999 1,000 |
|
Udipi et al., 1985 |
1–6 d lactation 7–10 d 13–15 d 20–22 d 28–31 d |
90b 95b 105b 120b 115b |
198, range 60–270 (extradietary intake only) |
|
Karra et al., 1986 |
7–12 mo lactation |
90b |
Not reported |
|
a pp = postpartum. b Values were estimated from graphs. c Lack of correlation between maternal intake and breast milk concentration of vitamin C in human milk. d SD = standard deviation. |
|||
|
Methods |
|
n = 17 healthy women, aged 18–35 y Intake was analyzed based on three 4-d diet records. There was no significant difference in milk content between the two groups. Plasma vitamin C levels were significantly lower at 7 d pp in the unsupplemented group; at 6 weeks, there was no difference. Serum concentrations were lower in the supplemented group |
|
n = 12 healthy women, aged 18–35 y Intake based on 4-d diet records |
|
n = 16 low-socioeconomic women; aged 18–32 y Intake was analyzed based on two 4-d diet records. There was no significant difference in milk content between the two groups. Also measured plasma concentration of ascorbic acid—found no significant difference |
|
n = 168 Gambian women |
|
n = 200 healthy nonsmoking mothers and full-term infants Infants were exclusively fed human milk for at least 3 mo (range 3–12 mo) Intake based on 7-d food records kept by a subset of mothers Milk volumes (mL/d) based on 3-d averages were calculated: 790 (510–1,120) at 4 mo lactation 800 (500–1,025) at 6 mo lactation 890 (655–1,100) at 9 mo lactation |
|
n = 25 healthy women aged 20–36 yrs, and their infants Milk concentration was calculated from estimates of the volume of milk intake of infants and infant intake of vitamin C |
|
n = 12 healthy women; aged 21–35 y Found significantly lower milk concentration on days 1–6 than on days 13–15 and 28–31 |
|
n = 55 women; aged 21–38 y Found 8% decrease in Vit C milk levels between 7 and 12 mo lactation |
min C concentration of about 45 mg/L at 9 months (the midpoint of this age group) of lactation (Salmenpera, 1984) would be approximately 27 mg/day. Adding the intake from milk (27 mg/day) and food (22 mg/day), the total AI for vitamin C is rounded to 50 mg/day.
An alternative method to calculate vitamin C intake is to use the method described in Chapter 3 to extrapolate from the AI for infants ages 0 through 6 months who receive human milk. Utilizing this method, the AI for the older infants is rounded up to 50 mg/day of vitamin C. This is comparable to the value calculated above utilizing human milk and solid food.
The 1994 to 1996 CSFII data for infants 7 through 12 months of age ranged from 21 to 293 mg/day, with median 106 mg/day of vitamin C (Appendix Table D-1).
Vitamin C AI Summary, Ages 0 through 12 Months
|
AI for Infants |
||
|
0–6 months |
40 mg (227 µmol)/day of vitamin C |
≈6 mg/kg |
|
7–12 months |
50 mg (256 µmol)/day of vitamin C |
≈6 mg/kg |
Children and Adolescents Ages 1 through 18 Years
Evidence Considered in Estimating the Average Requirement
No direct data were found on which to base an Estimated Average Requirement (EAR) for vitamin C for children ages 1 through 18 years. In the absence of additional information, and because vitamin C is a water-soluble vitamin and males have a larger lean body mass and total body water than women, EARs for children and adolescents have been estimated on the basis of relative body weight as described in Chapter 3 using reference weights from Chapter 1 (Table 1-1).
The Recommended Dietary Allowances (RDAs) estimated below for children 1 through 13 years of age are lower than the AIs calculated above for infants 0 through 12 months of age. The reason an AI may be higher than an RDA lies in the way they are determined (see “Differences Between the AI and the RDA” in Chapter 1). The AI is based on data on milk composition and volume of milk consumed to calculate an adequate intake of infants. The vitamin C RDA, in the case of 1- through 13-year-old children, is based on assumed differences in body weight from adults for whom there are some data. Thus, the data that are utilized to estimate the AI and RDA are different and cannot be compared.
Vitamin C EAR and RDA Summary, Ages 1 through 18 Years
|
EAR for Children |
|
|
1–3 years |
13 mg (74 µmol)/day of vitamin C |
|
4–8 years |
22 mg (125 µmol)/day of vitamin C |
|
EAR for Boys |
|
|
9–13 years |
39 mg (222 µmol)/day of vitamin C |
|
14–18 years |
63 mg (358 µmol)/day of vitamin C |
|
EAR for Girls |
|
|
9–13 years |
39 mg (222 µmol)/day of vitamin C |
|
14–18 years |
56 mg (318 µmol)/day of vitamin C |
The RDA for vitamin C is set by assuming a coefficient of variation (CV) of 10 percent (see Chapter 1) because information is not available on the standard deviation of the requirement for vitamin C; the RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for vitamin C the RDA is 120 percent of the EAR). The calculated values for RDAs have been rounded to the nearest 5 mg.
|
RDA for Children |
|
|
1–3 years |
15 mg (85 µmol)/day of vitamin C |
|
4–8 years |
25 mg (142 µmol)/day of vitamin C |
|
RDA for Boys |
|
|
9–13 years |
45 mg (256 µmol)/day of vitamin C |
|
14–18 years |
75 mg (426 µmol)/day of vitamin C |
|
RDA for Girls |
|
|
9–13 years |
45 mg (256 µmol)/day of vitamin C |
|
14–18 years |
65 mg (370 µmol)/day of vitamin C |
Adults Ages 19 through 50 Years
Evidence Considered in Estimating the Average Requirement
Although it is known that the classic disease of severe vitamin C deficiency, scurvy, is rare in the United States and Canada, other human experimental data that can be utilized to set a vitamin C requirement, based on a biomarker other than scurvy, are limited. Values recommended here are based on an amount of vitamin C that is thought to provide antioxidant protection as derived from the correlation of such protection with neutrophil ascorbate concentrations.
It is recognized that there are no human data to quantify directly the dose-response relationship between vitamin C intake and in vivo
antioxidant protection. In addition, only one study (Levine et al., 1996a) with seven apparently healthy males reported plasma, neutrophil, and urinary ascorbate concentrations during vitamin C depletion and repletion to steady state. Thus, there are wide uncertainties in the data utilized to estimate the vitamin C requirements. However, in the absence of other data, maximal neutrophil concentration with minimal urinary loss appears to be the best biomarker at the present time. It must be emphasized that research is urgently needed to explore the use of other biomarkers to assess vitamin C requirements.
Antioxidant Protection
The evidence summarized in the preceding sections indicates that vitamin C functions in vivo to scavenge reactive oxidants in activated leukocytes, lung, and gastric mucosa, and to protect against lipid peroxidation. Therefore, the determination of an EAR for vitamin C is based on an amount estimated to provide antioxidant protection. Evidence summarized in the earlier section “Antioxidant Functions in Leukocytes” indicates that the vitamin's antioxidant function in leukocytes, which includes neutrophils, lymphocytes, and monocytes, is especially important. In addition, studies with guinea pigs and monkeys show that the concentration of ascorbate in the leukocytes more accurately reflects liver and body pool ascorbate than does the concentration in plasma or erythrocytes (Omaye et al., 1987). The vitamin is transported into leukocytes by an energy-dependent transport system that concentrates the vitamin some 25, 40, and 80 times higher than plasma levels in neutrophils, platelets, and lymphocytes, respectively (Evans et al., 1982; Jacob et al., 1992; Levine et al., 1996a).
The cells actively concentrate the vitamin, which serves as a cellular reservoir of reducing capacity and scavenges damaging phagocyte-derived oxidants such as superoxide and myeloperoxidase-derived hypochlorus acid (HOCl) and reactive nitrogen species (RNS). In both the cell-free and the activated neutrophil systems described earlier, the protection of α-1-antiprotease against inactivation by HOCl (Halliwell et al., 1987) and the inhibition of super-oxide production (Anderson and Lukey, 1987) were directly proportional to ascorbate concentrations within the normal range of plasma ascorbate concentrations (22 to 85 µmol/L [0.4 to 1.5 mg/dL]). Data plotted in Figure 5-2 show that superoxide production by activated neutrophils was inhibited 29, 44, 52, and 55 percent by extracellular ascorbate concentrations of 28, 57, 114, and 284 µmol/
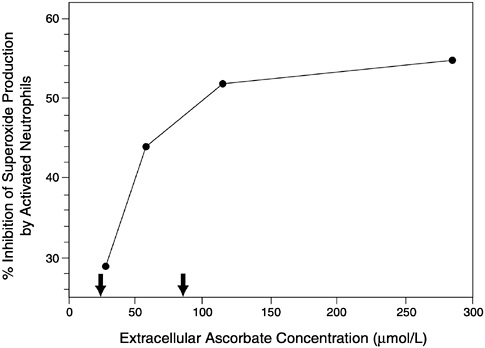
FIGURE 5-2 The effect of varying extracellular ascorbate concentrations on inhibition of superoxide produced by activated neutrophils. The range of normal human plasma ascorbate concentrations is shown within the arrows.
SOURCE: Adapted from Anderson and Lukey (1987).
L (0.5, 1.1, 2.2, and 5.0 mg/dL), respectively, without any effect on intracellular bacterial killing (Anderson and Lukey, 1987). This indicates that antioxidant protection is increasingly provided as ascorbate concentrations increase, with the greatest change in protection seen for ascorbate concentrations between 28 and 57 µmol/L (0.5 and 1.0 mg/dL).
Although similar dose-response data for leukocyte ascorbate levels are not available, the limited data from Levine et al. (1996a), seen in Figure 5-3 and Figure 5-4, show that plasma and neutrophil ascorbate concentrations are both directly related to vitamin intake between about 50 and 90 mg/day. The concentrations were measured by a sensitive high-pressure liquid chromatography assay with electrochemical detection. Therefore, increasing neutrophil ascorbate concentrations within this range should provide for increased protection against phagocyte-derived oxidant damage.
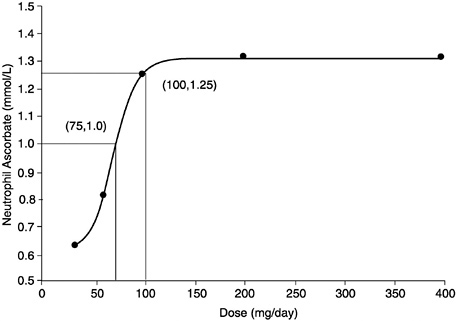
FIGURE 5-4 Neutrophil ascorbic acid concentrations (mmol/L) as a function of dose.
SOURCE: Adapted from Levine et al. (1996a).
There are no data to quantify directly the dose-response relation between vitamin C intake and in vivo antioxidant protection. Therefore, the criterion chosen for the EAR is the vitamin C intake that maintains near-maximal neutrophil vitamin C concentrations with minimal urinary loss. Since leukocyte ascorbate, which includes neutrophil ascorbate, correlates well with liver and body pool ascorbate (Omaye et al., 1987), this criterion should provide for adequate in vivo antioxidant protection to body tissues while minimizing excess urinary vitamin excretion. Vitamin C intakes greater than the urinary excretion threshold provide little or no increase in the ascorbate body pool (Baker et al., 1969; Kallner et al., 1979). A vitamin C intake that meets the above criteria is estimated from a controlled vitamin C dose-response study described below.
Depletion-Repletion Study
The requirement for vitamin C based on the above criteria can be estimated from the data reported by Levine et al. (1996a) in which
plasma, neutrophil, and urinary ascorbate concentrations were determined during vitamin C depletion and repletion to steady-state. The rigorous criteria for achieving steady-state plasma concentrations (five daily samples that varied less than or equal to 10 percent) make the Levine et al. (1996a) data unique among depletion-repletion studies.
Seven apparently healthy male volunteers, aged 20 to 26 years, were studied as in-patients for 4 to 6 months. Subjects were depleted by being fed a diet containing less than 5 mg/day vitamin C. Depletion was defined as completed when plasma vitamin C concentrations ranged from 5 to 10 µmol/L (0.1 to 0.2 mg/dL) without signs or symptoms of scurvy. For repletion, seven consecutive doses of vitamin C (30, 60, 100, 200, 400, 1,000, and 2,500 mg/day) were given sequentially until steady-state plasma and leukocyte (neutrophils, monocytes, and lymphocytes) vitamin C concentrations were achieved at each dosage. The results for plasma and neutrophil concentrations can be seen in Figure 5-3 and Figure 5-4, and Table 5-10.
As seen in Figure 5-4 and Table 5-10, the ascorbate saturation concentration in neutrophils was approximately 1.3 mmol/L. This was attained by four of the seven subjects at a vitamin C intake of 100 mg/day. Monocytes and lymphocytes also reached maximum concentrations at 100 mg/day (Levine et al., 1996a). However, at neutrophil saturation, about 25 percent of the doses were excreted in the urine, whereas at 60 percent of maximum ascorbate (dose of 60 mg/day), essentially no ascorbate was excreted.
No data from the Levine at al. (1996a) study are available for vitamin C intakes between 60 and 100 mg/day. However, because 60 percent of maximal ascorbate concentration in neutrophils would provide less antioxidant protection than 80 or 100 percent (Figure 5-2) (Anderson and Lukey, 1987), and since 25 percent of the dose is excreted at 100 percent of maximum neutrophil ascorbate concention, the midpoint 80 percent of maximum (1.0 mmol/L) was chosen. This is assuming that antioxidant protection in this range is linear. This point should better estimate an approximate neutrophil target concentration that fulfills the criteria of adequate in vivo antioxidant protection with little or no urinary loss. From the equation of Figure 5-4, 80 percent of maximal neutrophil concentration (1.0 mmol/L) is equivalent to a vitamin C intake of about 75 mg/day. This represents an EAR, because 80 percent (1.0 mmol/L) neutrophil concentration is an average value, estimated by regression analysis, for the men consuming 75 mg/day of vitamin C as shown in Table 5-10.
TABLE 5-10 Intracellular Ascorbic Acid Concentration in Neutrophils of Depleted Subjects Given Increasing Doses of Vitamin C (mmol/L)
|
Dose |
|||||||||
|
Subject |
30 mg |
60 mg |
75 mga |
100 mg |
200 mg |
400 mg |
1,000 mg |
2,500 mg |
|
|
1 |
Mean |
0.60 |
0.96 |
1.15 |
1.36 |
1.32 |
1.26 |
1.36 |
– |
|
SDb |
0.01 |
0.01 |
0.02 |
0.03 |
0.50 |
0.03 |
– |
||
|
2 |
Mean |
0.42 |
0.50 |
0.67 |
1.13 |
1.24 |
1.12 |
1.63 |
– |
|
SD |
0.01 |
0.02 |
0.06 |
0.05 |
0.08 |
0.15 |
– |
||
|
3 |
Mean |
1.18 |
0.83 |
1.19 |
1.33 |
1.43 |
– |
– |
– |
|
SD |
0.30 |
0.50 |
0.10 |
0.90 |
– |
– |
– |
||
|
4 |
Mean |
0.58 |
0.75 |
1.03 |
1.35 |
1.11 |
1.56 |
1.23 |
1.28 |
|
SD |
0.03 |
0.04 |
0.10 |
0.05 |
0.06 |
0.08 |
1.12 |
||
|
5 |
Mean |
0.57 |
1.10 |
1.21 |
1.30 |
– |
1.40 |
1.48 |
1.44 |
|
SD |
0.02 |
0.05 |
0.03 |
– |
0.05 |
0.11 |
0.13 |
||
|
6 |
Mean |
0.61 |
0.93 |
1.01 |
1.24 |
– |
0.96 |
0.95 |
– |
|
SD |
0.01 |
0.03 |
0.05 |
– |
0.14 |
0.16 |
– |
||
|
7 |
Mean |
0.42 |
0.54 |
0.77 |
1.02 |
1.46 |
– |
1.23 |
– |
|
SD |
0.01 |
0.02 |
0.05 |
0.12 |
– |
0.06 |
– |
||
|
Average |
0.63 |
0.81 |
1.00 |
1.25 |
1.31 |
1.26 |
1.31 |
1.36 |
|
|
SD |
0.26 |
0.20 |
0.12 |
0.13 |
0.23 |
0.21 |
0.80 |
||
|
a These values are neutrophil ascorbate concentrations corresponding to an intake of 75 mg/d calculated for each individual by regression analysis. b SD = standard deviation. SOURCE: Levine et al. (1996b). |
|||||||||
Relevancy of Above EAR to Other Possible Vitamin C Biomarkers
Scurvy. As discussed earlier, scurvy occurs at plasma concentrations of less than 10 µmol/L. At an EAR of 75 mg/day, scurvy would be prevented for more than a month if vitamin C ingestion were to cease suddenly (Levine et al., 1996b).
Body Pool Saturation. Kallner et al. (1979) previously reported that the body pool of vitamin C was saturated at an intake of 100 mg/day in healthy non-smoking men; thus, an average intake at the EAR of 75 mg/day would not provide body pool saturation of vitamin C.
Antioxidant Role. At a vitamin C intake of 90 mg/day, the plasma ascorbate concentration reaches 50 µmol/L which has been shown to inhibit LDL oxidation in vitro in both cellular and cell free systems (Jialal et al., 1990). Although it is not known whether vitamin C prevents LDL oxidation in vivo, if it does this might be relevant in the prevention of heart disease (Jialal et al., 1990). Also, as discussed earlier, since neutrophils are at 80 percent saturation at an EAR of 75 mg/day, this should potentially protect intracellular proteins from oxidative injury when these cells are activated during infectious and inflammatory processes (Anderson and Lukey, 1987; Halliwell et al., 1987).
Plasma Vitamin C Concentrations. Based on data from the Third National Health and Nutrition Examination Survey (NHANES III), although more than 75 percent of adult men have dietary vitamin C intakes higher than the EAR of 75 mg/day (Appendix Table C-1), only 50 percent have plasma vitamin C concentrations greater than 38 µmol/L (0.67 mg/dL) (Appendix Table F-1). This plasma concentration is estimated from the data of Levine et al. (1996a) to correspond to an intake of 75 mg/day of vitamin C (Figure 5-3). This finding is not surprising since the NHANES III vitamin C plasma concentrations are for both smokers and nonsmokers, and it is known that plasma vitamin C concentrations are reduced by about 40 percent in male smokers (Pelletier, 1977; Weber et al., 1996). In addition, as discussed in the earlier section “Environmental Tobacco Smoke,” exposure of nonsmokers to environmental tobacco smoke can result in a decline in plasma ascorbate concentrations (Tribble et al., 1993; Valkonen and Kuusi, 1998). Findings from the first three years (1988 to 1991) of NHANES III indicate that 38 percent of the participants were smokers and an additional 23 percent were nonsmokers exposed to environmental tobacco smoke at home or work (Pirkle et al., 1996).
Vitamin C EAR and RDA Summary, Ages 19 through 50 Years
Based on vitamin C intakes sufficient to maintain near-maximal neutrophil concentrations with minimal urinary loss, the data of Levine et al. (1996a) support an EAR of 75 mg/day of vitamin C for men. Since the data were based on men and no similar data are available for women at the present time, it is assumed that women will have a lower requirement due to their smaller lean body mass, total body water, and body size. This assumption is supported by the findings previously discussed that women maintain higher plasma
ascorbate concentrations than men at a given vitamin C intake. Thus, the requirement for women is extrapolated based on body weight differences from those established for men (see Table 1-1).
|
EAR for Men |
|
|
19–30 years |
75 mg (426 µmol)/day of vitamin C |
|
31–50 years |
75 mg (426 µmol)/day of vitamin C |
|
EAR for Women |
|
|
19–30 years |
60 mg (341 µmol)/day of vitamin C |
|
31–50 years |
60 mg (341 µmol)/day of vitamin C |
The RDA for vitamin C is set by assuming a coefficient of variation (CV) of 10 percent (see Chapter 1) because information is not available on the standard deviation of the requirement for vitamin C; the RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for vitamin C the RDA is 120 percent of the EAR). Due to the many assumptions and approximations involved, the RDA for women is rounded up to 75 mg from its calculated value of 72 mg/day.
|
RDA for Men |
|
|
19–30 years |
90 mg (511 µmol)/day of vitamin C |
|
31–50 years |
90 mg (511 µmol)/day of vitamin C |
|
RDA for Women |
|
|
19–30 years |
75 mg (426 µmol)/day of vitamin C |
|
31–50 years |
75 mg (426 µmol)/day of vitamin C |
Adults Ages 51 Years and Older
Evidence Considered in Estimating the Average Requirement
Some cross-sectional studies have shown that vitamin C status, as measured by plasma and leukocyte ascorbate concentrations, is lower in the elderly, especially institutionalized elderly, than in young adults (Burr et al., 1974; Cheng et al., 1985). Low blood vitamin C concentrations in institutionalized and chronically ill elderly were normalized to those of active elderly and young adults by increasing their dietary vitamin C intake, suggesting that the low levels were primarily due to poor intake (Newton et al., 1985). However, Davies et al. (1984) found that intestinal absorption of a 500-mg oral dose of ascorbic acid, as measured by urinary ascorbate excretion, was significantly less in elderly (mean age 83 years) than in younger subjects (mean age 22 years). Although this dose (500 mg/day) was about 5 times higher than the vitamin C intake of many elderly
individuals, it prompted the suggestion that impaired intestinal absorption may be an important causative factor in low blood concentrations of vitamin C in the elderly.
However, other studies, both cross-sectional and longitudinal, of apparently healthy, well-nourished elderly populations in the United States have not found evidence of a greater incidence of vitamin C deficiency among the elderly compared to young adults and no decrease in plasma ascorbate with advancing age (Garry et al., 1982, 1987; Jacob et al., 1988). Measurement of plasma, leukocyte, and urine ascorbate concentrations in a series of studies in elderly and young men and women showed no differences due to age (Blanchard, 1991a; Blanchard et al., 1989, 1990a,b). These studies included pharmacokinetic measures related to vitamin C absorption, depletion, repletion, and renal clearance. Consistent with these findings, a later study that measured maximal renal tubular reabsorption and excretion thresholds of ascorbic acid in apparently healthy elderly and young adults found no differences in renal handling of the vitamin between the two groups (Oreopoulos et al., 1993).
Older age groups, both men and women, have decreased lean body mass compared to younger individuals and thus, potentially a lower requirement for vitamin C. However, the vitamin C requirement of the elderly may be increased due to the oxidative stress of inflammatory and infectious conditions often found in this population (Cheng et al., 1985). As previously discussed, older adults have similar or lower plasma ascorbate concentrations than young adults. Therefore, the estimated requirement for vitamin C for individuals 51 years and older will remain the same as that of the younger adult.
Vitamin C EAR and RDA Summary, Ages 51 Years and Older
In summary, no consistent differences in the absorption or metabolism of ascorbic acid due to aging have been demonstrated at median vitamin C intakes. This suggests that the reports of low blood vitamin C concentrations in elderly populations may be due to poor dietary intakes, chronic disease or debilitation, or other factors, rather than an effect of aging per se. Therefore, for the older adults, no additional vitamin C allowance beyond that of younger adults is warranted.
|
EAR for Men |
|
|
51–70 years |
75 mg (426 µmol)/day of vitamin C |
|
>70 years |
75 mg (426 µmol)/day of vitamin C |
|
EAR for Women |
|
|
51–70 |
years 60 mg (341 µmol)/day of vitamin C |
|
>70 years |
60 mg (341 µmol)/day of vitamin C |
The RDA for vitamin C is set by assuming a coefficient of variation (CV) of 10 percent (see Chapter 1) because information is not available on the standard deviation of the requirement for vitamin C; the RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for vitamin C the RDA is 120 percent of the EAR). As with the RDA for younger women, the calculated RDA of 72 mg has been rounded up to 75 mg/day.
|
RDA for Men |
|
|
51–70 years |
90 mg (511 µmol)/day of vitamin C |
|
>70 years |
90 mg (511 µmol)/day of vitamin C |
|
RDA for Women |
|
|
51–70 years |
75 mg (426 µmol)/day of vitamin C |
|
>70 years |
75 mg (426 µmol)/day of vitamin C |
Pregnancy
Evidence Considered in Estimating the Average Requirement
Plasma vitamin C concentration decreases with the progression of pregnancy, probably secondary to hemodilution (Morse et al., 1975) as well as active transfer to the fetus (Choi and Rose, 1989). This decrease in plasma concentration has not been shown to be associated with poor pregnancy outcomes. The placenta apparently clears oxidized ascorbic acid from the maternal circulation and delivers it in the reduced form to the fetus (Choi and Rose, 1989). Ascorbic acid deficiency during pregnancy is associated with increased risk of infections, premature rupture of the membranes (Casanueva et al., 1993; Pfeffer et al., 1996), premature birth (Casanueva et al., 1993; Tlaskal and Novakova, 1990), and eclampsia (Jendryczko and Tomala, 1995). In addition, both serum and amniotic fluid concentrations of ascorbic acid are decreased in pregnant smokers compared to nonsmokers (Barrett et al., 1991).
Vitamin C EAR and RDA Summary, Pregnancy
Although the amount of vitamin C required by the growing fetus is unknown, it is known that maternal plasma vitamin C concentra-
tion decreases with the progression of pregnancy due to hemodilution as well as active transfer to the fetus. Therefore, in order to transfer adequate vitamin C to the fetus, additional vitamin C is needed during pregnancy. In the absence of data on near maximal neutrophil saturation during pregnancy, the method of determining the EAR for pregnancy is based on adding the EAR for near-maximal neutrophil concentration of the nonpregnant woman to the amount of vitamin C necessary to transfer adequate vitamin C to the fetus. In the absence of precise data regarding transfer of maternal vitamin C to the fetus, and with the knowledge that intakes of 7 mg/day of vitamin C will prevent young infants from developing scurvy (Goldsmith, 1961; Rajalakshmi et al., 1965; van Eekelen, 1953), the EAR for pregnancy was estimated to increase 10 mg/day over the vitamin C requirement for the nonpregnant woman.
|
EAR for Pregnancy |
|
|
14–18 years |
66 mg (375 µmol)/day of vitamin C |
|
19–30 years |
70 mg (398 µmol)/day of vitamin C |
|
31–50 years |
70 mg (398 µmol)/day of vitamin C |
The RDA for vitamin C is set by assuming a coefficient of variation (CV) of 10 percent (see Chapter 1) because information is not available on the standard deviation of the requirement for vitamin C; the RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for vitamin C the RDA is 120 percent of the EAR). The calculated values for the pregnancy RDA have been rounded up to the nearest 5 mg.
|
RDA for Pregnancy |
|
|
14–18 years |
80 mg (454 µmol)/day of vitamin C |
|
19–30 years |
85 mg (483 µmol)/day of vitamin C |
|
31–50 years |
85 mg (483 µmol)/day of vitamin C |
Special Considerations
Certain subpopulations of pregnant women may have increased requirements for vitamin C. This group includes users of street drugs and cigarettes, heavy users of alcohol, and regular users of aspirin (Flodin, 1988). Women who smoke more than 20 cigarettes per day may require twice as much vitamin C as nonsmokers to maintain a replete body pool of vitamin C (Kallner et al., 1981). It has been reported that plasma vitamin C in pregnant smokers ex-
hibited an indirect correlation with the breath content of ethane, a volatile marker of lipid peroxidation, even though the pregnant women were receiving supplements with 320 mg/day of vitamin C (Schwarz et al., 1995). Thus, pregnant women in these special sub-populations should consume additional vitamin C.
Lactation
Evidence Considered in Estimating the Average Requirement
As indicated earlier, infants fed human milk are estimated to consume on average 40 mg/day vitamin C during the first 6 months of life. Salmenpera (1984) reported that the vitamin C intake of 47 mothers during prolonged lactation ranged from 48 to 277 mg/day, mean 138 mg/day. Three mothers in this study who consumed less than 100 mg/day of vitamin C demonstrated plasma ascorbate values below the lower limit of normal [less than 10 µmol/L (0.2 mg/dL)]. Women who consumed 100 to 199 mg/day of vitamin C produced milk with 100 mg/L of vitamin C (Byerley and Kirksey, 1985). Maternal vitamin C intake in excess of 200 mg/day resulted in increased urinary excretion of vitamin C but did not increase the content of the vitamin in human milk (Byerley and Kirksey, 1985). It is thought that a regulatory mechanism in the mammary gland prevents the elevation of milk vitamin C concentrations beyond that level seen when urinary execretion increases representing blood saturation (Byerley and Kirksey, 1985).
Vitamin C EAR and RDA Summary, Lactation
To estimate the EAR for lactation, the average vitamin C produced in milk, 40 mg/day during the first 6 months of lactation, is added to the EAR for the nonlactating women. Although the vitamin C content of human milk declines with length of lactation and milk volume declines with the addition of solid foods, the EAR is not decreased for longer periods of lactation.
|
EAR for Lactation |
|
|
14–18 years |
96 mg (545 µmol)/day of vitamin C |
|
19–30 years |
100 mg (568 µmol)/day of vitamin C |
|
31–50 years |
100 mg (568 µmol)/day of vitamin C |
The RDA for vitamin C is set by assuming a coefficient of variation (CV) of 10 percent (see Chapter 1) because information is not avail-
able on the standard deviation of the requirement for vitamin C; the RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for vitamin C the RDA is 120 percent of the EAR).
|
RDA for Lactation |
|
|
14–18 years |
115 mg (653 µmol)/day of vitamin C |
|
19–30 years |
120 mg (682 µmol)/day of vitamin C |
|
31–50 years |
120 mg (682 µmol)/day of vitamin C |
Special Considerations
Smokers
Evidence that smokers have lower vitamin C status than nonsmokers, even with comparable vitamin C intakes, is summarized in the preceding section “Factors Affecting the Vitamin C Requirement.” The data also show that the metabolic turnover of ascorbate in smokers is about 35 mg/day greater than in nonsmokers (Kallner et al., 1981), apparently due to increased oxidative stress and other metabolic differences. These findings indicate that smokers need additional vitamin C to provide comparable nutriture to nonsmokers.
From analysis of NHANES II data on vitamin C intakes and serum concentrations, Schectman et al. (1991) estimated that the average intake of smokers needed to be at least 200 mg/day of vitamin C in order to attain serum ascorbate concentrations equivalent to those of nonsmokers who meet the 1989 RDA of 60 mg/day (NRC, 1989). Use of population survey data to estimate an increased ascorbate requirement for smokers is questionable, because the cause and significance of the observed differences in serum ascorbate concentrations between smokers and nonsmokers are largely unknown.
From in vitro data on the loss of ascorbate in plasma exposed to cigarette smoke, it was estimated that one cigarette may consume about 0.8 mg of ascorbate, or about 32 mg/day for a two-pack-a-day smoker (Cross and Halliwell, 1993). More precise data were obtained from an experimental study of 17 apparently healthy male smokers who were administered radiolabeled tracer ascorbic acid at steady-state intakes of 30 to 180 mg/day to allow kinetic calculations of ascorbate metabolism and body pools. Results were compared with a similar protocol for nonsmokers (Kallner et al., 1979, 1981). Metabolic turnover of the vitamin was about 35 mg/day greater in smokers than in nonsmokers. Thus, to obtain a near maximal steady-state ascorbate body pool equivalent to that of nonsmok-
ers, smokers would require an additional 35 mg/day of vitamin C over that needed by nonsmokers.
Passive Smokers
Environmental or sidestream tobacco smoke provokes oxidant damage similar to mainstream cigarette smoke (Bermudez et al., 1994; Pryor et al., 1983). Hypovitaminosis C (plasma ascorbate concentrations less than 23 µmol/L [0.5 mg/dL]) was found in 24 percent of the active smokers and 12 percent of passive smokers and indicated that both passive and active smoke exposure lowered body ascorbate pools (Tribble et al., 1993). Exposure of nonsmokers to secondhand smoke for 30 minutes in a smoke-filled room resulted in a significant decline in serum ascorbate, increased lipid peroxidation, and oxidatively modified low-density lipoprotein (LDL) (Valkonen and Kuusi, 1998). Although the above data are insufficient to estimate a special requirement for nonsmokers regularly exposed to tobacco smoke, these individuals are urged to ensure that they meet the Recommended Dietary Allowance (RDA) for vitamin C.
Exercise and Stress
The role of ascorbate as a cofactor for biosynthesis of carnitine, steroid hormones, and neurotransmitters provides a theoretical basis for increased requirements of the vitamin in persons under excessive physical and emotional stress. Studies of vitamin C status and physical activity in humans have shown mixed results, such that no definitive conclusion regarding vitamin C and exercise can be derived (Keith, 1994). For example, Fishbaine and Butterfield (1984) reported that blood vitamin C was higher in runners compared to sedentary control subjects, while a later study found that the vitamin C status of highly trained athletes was not significantly different from control subjects (Rokitzki et al., 1994). A cross-sectional study of physical activity, fitness, and serum ascorbate in 1,600 apparently healthy Irish adults provided no evidence that active people had different ascorbate status than inactive, and thus no justification for supplementation of exercisers (Sharpe et al., 1994). No substantial evidence that mental or emotional stress increases vitamin C turnover or requirement in apparently healthy persons has been reported. In sum, none of the above types of stress has been demonstrated to affect the human requirement for vitamin C.
INTAKE OF VITAMIN C
Food Sources
Almost 90 percent of vitamin C in the typical diet comes from fruits and vegetables, with citrus fruits, tomatoes and tomato juice, and potatoes being major contributors (Sinha et al., 1993). Other sources include brussel sprouts, cauliflower, broccoli, strawberries, cabbage, and spinach. Vitamin C is also added to some processed foods as an antioxidant. Values for the vitamin C content of foods can vary depending on the growing conditions, season of the year, stage of maturity, location, cooking practices, and storage time prior to consumption (Erdman and Klein, 1982).
Dietary Intake
Data from nationally representative U.S. and Canadian surveys are available to estimate vitamin C intakes (Appendix Table C-1, Table D-1, and Table E-1). In the United States, the median dietary intake of vitamin C by adult men from 1988 to 1994 was about 105 mg (596 µmol)/day and median total intake (including supplements, see Appendix Table C-2) is about 120 mg (682 µmol)/day. For women, the median intake was estimated to be 90 mg (511 µmol)/day and median total intake (including supplements) is about 108 mg (613 µmol)/day. (See Chapter 9 for vitamin C intake of men and women who smoke.) In Canada, the median dietary intake of vitamin C for adult men and woman was lower than in the United States with intake estimated to be about 70 mg (397 µmol)/day (Appendix Table E-1). Although most Americans consume fewer than the minimum of five daily servings of fruits and vegetables recommended by the U.S. Department of Agriculture and the National Cancer Institute, estimated median daily vitamin C consumption is above the Estmated Average Requirement (EAR). Five servings of most fruits and vegetables provide more than 200 mg (1,136 µmol)/day of vitamin C per day.
The Boston Nutritional Status Survey of the Elderly estimated that among this relatively advantaged group of people over aged 60, those who were not taking supplements had a median vitamin C intake of 132 mg/day for males and 128 mg/day for females (Hartz et al., 1992).
Intake from Supplements
Information from the Boston Nutritional Status Survey of the Elderly estimated that 35 and 44 percent of the males and females, respectively, took some form of vitamin C supplements; while 19 percent of males and 15 percent of females surveyed who took supplements had intakes greater than 1,000 mg (5,680 µmol)/day. Approximately 31 percent of all adults in one 1986 survey reported taking a vitamin C supplement (Moss et al., 1989). Total vitamin C intakes from food plus supplements from the Third National Health and Nutrition Examination Survey (NHANES III) are found in Appendix Table C-2.
TOLERABLE UPPER INTAKE LEVELS
The Tolerable Upper Intake Level (UL) is the highest level of daily nutrient intake that is likely to pose no risk of adverse health effects in almost all individuals. Although members of the general population should be advised not to exceed the UL routinely, intake above the UL may be appropriate for investigation within well-controlled clinical trials. In light of evaluating possible benefits to health, clinical trials of doses above the UL should not be discouraged, as long as subjects participating in these trials have signed informed consent documents regarding possible toxicity and as long as these trials employ appropriate safety monitoring of trial subjects. Also, the UL is not meant to apply to individuals who are receiving vitamin C under medical supervision.
Hazard Identification
Adverse Effects
Many people believe vitamin C to be nontoxic and beneficial to health; therefore, the vitamin is often taken in large amounts. There is no evidence suggesting that vitamin C is carcinogenic or teratogenic or that it causes adverse reproductive effects. Reviews of high vitamin C intakes have indicated low toxicity (Johnston, 1999); adverse effects have been reported primarily after very large doses (greater than 3 g/day). Data show little increase in plasma steady-state concentrations at intakes above 200 mg/day (Figure 5-3), and saturable intestinal absorption and renal tubular reabsorption data suggest that overload of ascorbic acid is unlikely in humans (Blanchard et al., 1997; Levine et al., 1996a). Possible adverse effects associated with very high intakes have been reviewed and include:
diarrhea and other gastrointestinal disturbances, increased oxalate excretion and kidney stone formation, increased uric acid excretion, pro-oxidant effects, systemic conditioning (“rebound scurvy”), increased iron absorption leading to iron overload, reduced vitamin B12 and copper status, increased oxygen demand, and erosion of dental enamel (Hornig and Moser, 1981; Rivers, 1987). The data on these adverse effects are reviewed below. The UL for vitamin C applies to intake from both food and supplements.
Gastrointestinal Effects. Gastrointestinal disturbances such as nausea, abdominal cramps, and diarrhea are the most common adverse effects of high vitamin C intake (Hoffer, 1971). These effects are attributed to the osmotic effect of unabsorbed vitamin C passing through the intestine. Intestinal absorption of ascorbic acid occurs by a saturable process (Rumsey and Levine, 1998; Tsao, 1997). The remainder is not absorbed and is eliminated in the stool. The evidence of gastrointestinal disturbances following high vitamin C intakes is primarily from uncontrolled case reports (Hoffer, 1971; Hoyt, 1980). However, some studies have been conducted to evaluate gastrointestinal effects. Cameron and Campbell (1974) reported diarrhea, transient colic, and flatulent distension in normal healthy volunteers at doses of 3 to 4 g/day. Another study, which evaluated the adverse effects of 1-, 5-, and 10-g/day supplemental ascorbate for 5 days in apparently healthy adults, reported diarrhea in 2 of 15 subjects at 10 g/day (Wandzilak et al., 1994). Stein et al. (1976) reported mild diarrhea in one of three subjects following ingestion of 4 g of ascorbic acid.
Increased Oxalate Excretion and Kidney Stone Formation. Controversy exists as to whether increased intake of vitamin C can significantly increase urinary excretion of oxalate and, therefore, lead to an increase in the potential for renal calcium oxalate stone formation. The findings from studies evaluating the effect of vitamin C intake (0.03 to 10 g/day) on urinary oxalate excretion in apparently healthy individuals are conflicting (Hughes et al., 1981; Lamden and Chrystowski, 1954; Levine et al., 1996a; Mitch et al., 1981; Schmidt et al., 1981; Tiselius and Almgard, 1977; Tsao and Salimi, 1984; Wandzilak et al., 1994). An intervention study by Hughes et al. (1981) reported significant increases in mean urinary oxalate excretion in 39 apparently healthy adults consuming 1, 3, 6, and 9 g/day of ascorbic acid. However, Tsao and Salimi (1984) reported normal plasma oxalate concentrations in healthy subjects ingesting 3–10 g/day of ascorbic acid for at least two years, and no significant
change in urinary oxalate excretion in five of six subjects who consumed 10 g/day of vitamin C over 1 day. Levine et al. (1996a) showed increased urinary oxalate excretion in apparently healthy male volunteers consuming 1 g/day of ascorbic acid; however, mean oxalate concentrations remained within the reference range. None of these studies showed oxalate excretion above normal.
Reports of kidney stone formation associated with excess ascorbic acid intake are limited to individuals with renal disease (see Sauberlich, 1994 for a review). Data from epidemiological studies do not support an association between excess ascorbic acid intake and kidney stone formation in apparently healthy individuals (Curhan et al., 1996, 1999; Fellstrom et al., 1989). A prospective cohort study by Curhan et al. (1996) of 45,000 men aged 40 to 70 years with no history of renal calculi showed that vitamin C intake was not significantly associated with the risk of stone formation. In fact, the age-adjusted relative risk for men consuming 1,500 mg/day or more compared to less than 250 mg/day was 0.78. In addition, vitamin C intake was not associated with kidney stone formation in women (Curhan et al., 1999). The lack of findings on oxalate excretion and kidney stone formation may be explained by the limited absorption of vitamin C at doses greater than 200 mg/day (Levine et al., 1996a). Because of the limited intestinal absorption, limited amounts of vitamin C are metabolized to oxalate in the urine. In addition, the large majority of excess absorbed vitamin C is excreted in the urine as ascorbic acid rather than its degradation products.
Increased Uric Acid Excretion. Similarly, the effect of high ascorbic acid intake on urate excretion has been studied (Berger et al., 1977; Fituri et al., 1983; Hatch et al., 1980; Herbert, 1978; Levine et al., 1996a; Mitch et al., 1981; Schmidt et al., 1981; Stein et al., 1976). Theoretically, increased uric acid excretion could be an important factor in the formation of uric acid stones especially in subjects who normally excrete large amounts of uric acid. The findings are conflicting. Levine et al. (1996a) reported significantly increased uric acid excretion above the normal range following ascorbic acid intakes of 1 g/day or more in 7 apparently healthy male subjects. Another study reported a 70 to 90 percent increase in the fractional clearance of uric acid following a single 4-g dose in nine subjects (Stein et al., 1976). Other studies have shown no significant effect of ascorbic acid intakes up to 12 g/day on uric acid excretion in apparently healthy subjects (Fituri et al., 1983; Hatch et al., 1980; Herbert, 1978; Mitch et al., 1981; Schmidt et al., 1981).
Excess Iron Absorption. Another possible adverse effect of high vitamin C intake is enhanced iron absorption leading to iron overload. Bendich and Cohen (1990) evaluated 24 studies to determine whether daily ascorbic acid intakes (ranging from 1 to 1,000 mg, with most in the 10- to 100-mg range) could increase iron stores above recommended levels in apparently healthy individuals. They found that vitamin C intakes did not increase the number of high iron absorbers, and limited data involving ascorbic acid intakes above 100 mg/day showed no change in iron absorption values. Another study by Cook et al. (1984) showed no increase in iron stores following vitamin C intakes up to 2 g/day (taken with meals for 20 months) in iron-replete subjects who consumed foods that contain iron. This suggests that vitamin C does not induce excess iron absorption in apparently healthy individuals. However, it is unknown if individuals with hereditary hemochromatosis, which affects between 1 in 200 and 1 in 400 persons of northern European descent (Bacon et al., 1999), could be adversely affected by long-term ingestion of large doses of vitamin C (McLaran et al., 1982).
Lowered Vitamin B12Levels. An in vitro study showed that increasing destruction of vitamin B12 was associated with increasing vitamin C levels (Herbert and Jacob, 1974). However, when this study was performed using different analytical procedures, no loss of vitamin B12 was observed (Newmark et al., 1976). In a review of the stability of cobalamins under varying conditions, Hogenkamp (1980) found that only aquocobalamin was decreased and destroyed by ascorbic acid. Aquocobalamin is not a major cobalamin in biological tissues. Furthermore, results of in vivo studies in human subjects have shown that vitamin C intakes up to 4 g/day did not induce vitamin B12 deficiency (Afroz et al., 1975; Ekvall et al., 1981).
Systemic Conditioning. Evidence of systemic conditioning (the accelerated metabolism or excretion of ascorbic acid) exists from uncontrolled observations in humans following abrupt discontinuation of prolonged, high-dose vitamin C supplementation (Rhead and Schrauzer, 1971; Siegel et al., 1982). Omaye et al. (1986) showed increased turnover of plasma ascorbic acid in apparently healthy human adults who abruptly decreased their vitamin C intake from 605 to 5 mg/day. Two other studies showed that high intakes resulted in increased clearance but did not result in blood levels lower than normal (Schrauzer and Rhead, 1973; Tsao and Leung, 1988). Other studies have reported no rebound scurvy or excessive lowering of ascorbate blood levels after cessation of high
intakes (Hoffer, 1973; Ludvigsson et al., 1979). Evidence that rebound scurvy may appear in infants whose mothers ingested large doses of vitamin C during pregnancy is limited to one anecdotal report of 2 infants (Cochrane, 1965). Overall, the evidence is inconsistent and does not suggest that systemic conditioning occurs to any significant extent in infants and adults.
Pro-oxidant Effects. Under certain conditions, ascorbate can act as a pro-oxidant by reducing iron and copper ions, which catalyze production of the hydroxyl radical via Fenton chemistry (Buettner and Jurkiewicz, 1996). The combination of ascorbic acid and redoxactive (non-protein-bound) iron can promote lipid peroxidation in vitro (Laudicina and Marnett, 1990). In vivo however, iron is bound to proteins such as transferrin and ferritin and therefore is not normally available for such catalytic functions. Nevertheless, the strong pro-oxidant nature of the iron-ascorbate complex in vitro raises concern that consumption of vitamin C supplements by individuals with high iron stores may contribute to oxidative damage in vivo. In addition, dietary ascorbic acid can enhance the intestinal absorption of nonheme iron (Hallberg, 1985).
Concerns for a possible in vivo pro-oxidant effect of the iron-ascorbate couple were heightened by the report of a fatal cardiomyopathy in a patient with hemochromatosis who ingested excessive vitamin C (McLaran et al., 1982). Also, an association between myocardial infarctions and serum ferritin levels has been reported in a Finnish population (Salonen et al., 1992). Other studies have not supported the latter finding that high iron stores were associated with increased risk of heart disease (Baer et al., 1994) and have not indicated that excess vitamin C intakes have contributed significantly to iron overload or oxidant damage in normal healthy people. Controlled human studies in which supplemental vitamin C was added to the meals of apparently healthy adults for periods of up to 2 years showed little or no change in iron status measures including serum ferritin (Cook et al., 1984; Hunt et al., 1994). Data on iron-ascorbate combinations in the plasma of normal healthy adults and preterm infants with high plasma ascorbate levels showed that high plasma ascorbate concentrations in the presence of redox-active iron did not cause either lipid or protein oxidation. In addition, the endogenous ascorbate prevented rather than promoted lipid peroxidation in iron-overloaded plasma (Berger et al., 1997).
Similarly, concern for an in vivo pro-oxidant action of vitamin C in concert with copper has been suggested but not substantiated. Possible increased oxidant damage in premature infants had been
attributed to the effect of high serum ascorbate levels inhibiting ceruloplasmin ferroxidase activity, thereby creating an excess of reactive ferrous ions (Powers et al., 1995). This result and other reports of ascorbate inhibition of ceruloplasmin ferroxidase activity (Gutteridge, 1991) have subsequently been attributed to an artifact of using a nonphysiological pH buffer in the ceruloplasmin ferroxidase assay (Løvstad, 1997).
Results of studies testing the effects of supplemental vitamin C intake on markers of oxidant damage to deoxyribonucleic acid (DNA) and chromosomes are discussed in an earlier section and are summarized in Table 5-4, Table 5-5, and Table 5-6. The results are mixed, with studies showing a decrease, increase, or no change in oxidant damage measures. A study of 30 apparently healthy adults supplemented with 500 mg/day of vitamin C for 6 weeks reported an increase in 8-oxoadenine, but a decrease in the more mutagenic DNA lesion, 8-oxoguanine (Podmore et al., 1998). Supplementation of apparently healthy volunteers with vitamin C and iron resulted in increases in some DNA damage markers, decreases in others, and a rise in total DNA base damage at 6 weeks, which disappeared at 12 weeks (Rehman et al., 1998). Other evidence from in vitro and in vivo data as well as epidemiological studies have not shown increased oxidative DNA damage or increased cancer risk associated with high intakes of vitamin C (Block, 1991; Fontham, 1994; Fraga et al., 1991; Rifici and Khachadurian, 1993).
Other Adverse Effects. Other adverse effects observed following high vitamin C intakes include diminished high-altitude resistance (Schrauzer et al., 1975), delayed-type allergic response (Metz et al., 1980), and erosion of dental enamel (Giunta, 1983). Additional studies confirming these findings were not found.
Identification of Distinct and Highly Sensitive Subpopulations. Data show that individuals with hemochromatosis, glucose-6-phosphate dehydrogenase deficiency, and renal disorders may be susceptible to adverse effects from excess vitamin C intake. Vitamin C may enhance iron absorption and exacerbate iron-induced tissue damage in individuals with hemochromatosis (McLaran et al., 1982). Individuals with renal disorders may have increased risk of oxalate kidney stone formation from excess vitamin C intake (Auer et al., 1998; Ono, 1986; Urivetzky et al., 1992). Hemolysis has been associated with ascorbic acid administration in newborns with glucose-6-phosphate dehydrogenase deficiency and in normal premature infants (Ballin et al., 1988; Mentzer and Collier, 1975). There is also anecdotal evidence of hemolysis following ascorbic acid intake in adults
with glucose-6-phosphate dehydrogenase deficiency (Campbell et al., 1975; Rees et al., 1993). However, a clinical study does not support the association (Beutler, 1991).
Summary
Based on considerations of causality, relevance, and the quality and completeness of the database, osmotic diarrhea and related gastrointestinal disturbances were selected as the critical endpoints on which to base a UL. The in vivo data do not clearly show a causal relationship between excess vitamin C intake by apparently healthy individuals and other adverse effects (i.e., kidney stone formation, excess iron absorption, reduced vitamin B12 and copper levels, increased oxygen demand, systemic conditioning, pro-oxidant effects, dental enamel erosion, or allergic response) in adults and children.
The data regarding possible vitamin C deficiency in two newborns resulting from abrupt withdrawal from mothers consuming high levels of vitamin C during pregnancy were considered too anecdotal and uncertain to warrant derivation of a separate UL for pregnant women.
Dose-Response Assessment
Adults
Data Selection. The data on osmotic diarrhea and gastrointestinal disturbances were selected as most relevant on which to base a UL for apparently healthy adults. The effects are generally not serious and are self-limiting; individuals experiencing them may easily eliminate them by reducing supplemental vitamin C intakes.
Identification of a No-Observed-Adverse-Effect Level (NOAEL) and Lowest-Observed-Adverse-Effect Level (LOAEL). A LOAEL of 3 g/day can be identified based on the data of Cameron and Campbell (1974). These investigators reported symptoms of flatulent distension, transient colic, and diarrhea at doses of 3 to 4 g/day in normal healthy volunteers (number of volunteers not stated). The volunteers increased oral ascorbic acid intake by increments of 1g/day in successive weeks. Supporting evidence is provided by case reports (Hoffer, 1971; Hoyt, 1980), a graded dose study by Stein et al. (1976), and a multiple crossover study by Wandzilak et al. (1994). Stein et al. (1976) gave three patients 8 g/day in four divided doses of 2 g for 3 to 7 days. This study reported mild diarrhea in one of three subjects following ingestion of 4 g/day of ascorbic acid. Wandzilak et al. (1994) investigated the effect of high-dose ascorbic acid in-
take on 15 apparently healthy volunteers. Subjects ingested 1, 5, and 10 g/day supplemental ascorbate at mealtime for 5 days, separated by 5 days of no supplementation. This study reported diarrhea in 2 of the 15 subjects taking 10 g/day. These subjects were unable to continue at this dose
The above human data suggest that an intake of vitamin C greater than 3 g/day is likely to cause osmotic diarrhea in many individuals, although some reports involving a few individuals suggest this may occur at 3 g/day. Thus, the 3-g/day intake is considered a LOAEL.
Uncertainty Assessment. There is little uncertainty regarding the range of vitamin C intakes that are likely to induce osmotic diarrhea. An uncertainty factor (UF) of 1.5 was selected to extrapolate the LOAEL to a NOAEL. Thus, the 3 g/day intake is considered a LOAEL, and a NOAEL of 2 g/day is estimated for adult humans. Because the database has no other significant sources of uncertainty and because of the mild, reversible nature of osmotic diarrhea caused by high vitamin C intakes, no further uncertainty factors are necessary.
Derivation of a UL. The LOAEL of 3 g/day was divided by the UF of 1.5 to obtain a NOAEL and UL value of 2 g/day.
Vitamin C UL Summary, Ages 19 Years and Older
|
UL for Adults |
|
|
19 years and older |
2,000 mg (11,360 µmol)/day of vitamin C |
Other Life Stage Groups
Infants. For infants, the UL was judged not determinable because of insufficient data on adverse effects in this age group and concern about the infant 's ability to handle excess amounts. Potential concerns for high vitamin C concentrations in infants stem from isolated reports of anecdotal rebound scurvy, oxidative damage, and hemolysis (Ballin et al., 1988; Cochrane, 1965; Powers et al., 1995). To prevent high levels of intake, the only source of intake for infants should be that available from food and formula.
Children and Adolescents. Limited data exist on vitamin C toxicity in toddlers, children, and adolescents. Ludvigsson et al. (1977) con-
ducted a double-blind, 7-week pilot study and a 3-month main study evaluating the prophylactic effect of 1,000 mg/day of vitamin C on colds in 172 and 642 children, respectively, ages 8 to 9 years. Reported side effects, including stomach pains, skin rash, headache, diarrhea, and nausea, were observed in about 3 percent of the children, which was no different from the control group and was not dose related. Therefore, this study could be used to support a NOAEL of 1,000 mg/day.
Another study tested the effectiveness of a megavitamin regimen including 3 g/day of ascorbic acid for 3 months on attention deficit disorder (ADD) in 41 children ages approximately 7 to 11 years (Haslam et al., 1984). Forty-two percent of the children developed elevation of serum aminotransferases, and it was concluded that the regimen (which was ineffective) should not be used to treat ADD. It is unlikely that the increases in serum aminotransferases were due to the high acsorbic acid intake since no such effects of high vitamin C intakes have been reported by other investigators. Nevertheless, this study appears consistent with the adult data indicating a LOEAL at intakes of 3 g/day. However, this study cannot be utilized to establish a UL for children as the vitamin C was part of a mega-vitamin and the contribution of vitamin C to the results cannot be determined.
Because the results of these studies (particularly the study by Ludvigsson et al., 1977) are consistent with the data on adverse effects in adults on a body weight basis, the UL values for toddlers, children, and adolescents are extrapolated based on body weight differences from those established for adults as described in Chapter 4 using reference weights from Chapter 1 (Table 1-1). The calculated UL is rounded to the nearest 50 mg.
Pregnancy. No evidence of maternal toxicity of excess vitamin C intakes was found. However, because vitamin C is actively transported from maternal to fetal blood, there could be a potential for maternal intake of megadoses of vitamin C during pregnancy to lead to markedly elevated concentrations of vitamin C in the fetus. There is one anecdotal report (Cochrane, 1965) of possible fetal vitamin C dependence induced in utero in two infants, whose mothers consumed 400 mg/day of vitamin C during pregnancy. Although the infants developed scurvy during the first few weeks of life, the observation was complicated by the relatively high incidence of scurvy in the region of Canada in which the infants were born. Other concerns for high vitamin C concentrations in infants stem from reports of hemolysis (Ballin et al., 1988) and possible increased
oxidative damage (Powers et al., 1995) in premature infants. However, these effects are not well documented, and do not warrant a separate UL for pregnant females.
Lactation. Byerley and Kirksey (1985) noted that the vitamin C composition of human milk was not affected by maternal vitamin C intake ranging from 156 to 1,123 mg/day and that urinary excretion increased as intake increased over 200 mg/day, suggesting that mammary tissue becomes saturated with vitamin C. One woman ingested 4,000 mg/day of vitamin C as a supplement; no toxic effects of the excess vitamin intake were noted in the mother. Her milk content of vitamin C was 100.5 mg/L, which was on the high end of values reported for human milk, but not reflective of the high intake (Anderson and Pittard, 1985). Based on these findings, the ULs for lactating adolescents and women are not different from those of nonlactating females.
Vitamin C UL Summary, Ages 1 through 18 Years, Pregnancy, Lactation
|
UL for Infants |
|
|
0–12 months |
Not possible to establish; source of intake should be formula and food only |
|
UL for Children |
|
|
1–3 years |
400 mg (2,272 µmol)/day of vitamin C |
|
4–8 years |
650 mg (3,692 µmol)/day of vitamin C |
|
9–13 years |
1,200 mg (6,816 µmol)/day of vitamin C |
|
UL for Adolescents |
|
|
14–18 years |
1,800 mg (10,224 µmol)/day of vitamin C |
|
UL for Pregnancy |
|
|
14–18 years |
1,800 mg (10,224 µmol)/day of vitamin C |
|
19 years and older |
2,000 mg (11,360 µmol)/day of vitamin C |
|
UL for Lactation |
|
|
14–18 years |
1,800 mg (10,224 µmol)/day of vitamin C |
|
19 years and older |
2,000 mg (11,360 µmol)/day of vitamin C |
Special Considerations
Individuals with hemochromatosis, glucose-6-phosphate dehydrogenase deficiency, and renal disorders may be especially susceptible to adverse effects of excess vitamin C intake and therefore should be cautious about ingesting more vitamin C than the Recommended Dietary Allowance (RDA). Vitamin C intakes of 250
mg/day or higher have been associated with false-negative results for detecting stool and gastric occult blood (Gogel et al., 1989; Jaffe et al., 1975). Therefore, high-dose vitamin C supplements should be discontinued at least 2 weeks before physical exams because they may interfere with blood and urine tests.
Intake Assessment
Based on data from the Third National Health and Nutrition Examination Survey (NHANES III), the highest mean intake of vitamin C from diet and supplements for any gender and lifestage group was estimated to be about 200 mg (1,136 µmol)/day (Appendix Table C-2). This was the intake of males aged 51 through 70 years and females aged 51 years and older. The highest reported intake at the ninety-ninth percentile was greater than 1,200 mg (6,816 µmol)/day in males aged 31 through 70 years and in females aged 51 through 70 years (Appendix Table C-2).
Risk Characterization
The risk of adverse effects resulting from excess intake of vitamin C from food and supplements appears to be very low at the highest intakes noted above. Although members of the general population should be advised not to exceed the UL routinely, intake greater than the UL may be appropriate for investigation within well-controlled clinical trials. Clinical trials of doses above the UL should not be discouraged, as long as subjects participating in these trials have signed informed consent documents regarding possible toxicity and as long as these trials employ appropriate safety monitoring of trial subjects. In addition, the UL is not meant to apply to individuals who are receiving vitamin C under medical supervision.
RESEARCH RECOMMENDATIONS FOR VITAMIN C
-
Despite the many known biochemical roles of ascorbic acid, no reliable biochemical or physiologically based functional measures of vitamin C nutriture have been established. As a result, vitamin C intake requirements in adults have been based on estimates of body pool or tissue ascorbate deemed adequate to provide anti-oxidant protection. Knowledge of vitamin C intakes needed to fulfill specific functional roles of ascorbate will allow more accurate and precise determinations of the individual and average population requirements of the vitamin. Some current candidates that
-
could be used as functional measures include pathways related to collagen and carnitine metabolism, oxidative damage, and oral health indices; however, research on new functions of the vitamin is also needed. Determination of vitamin C requirements based on antioxidant functions will require development of more reliable tests for in vivo oxidative damage and further understanding of the interactions of ascorbate with other physiological antioxidants. Additionally, a practical method for measuring the vitamin C body pool is needed as a standard of comparison against proposed functional measures and measures of health or disease endpoints.
-
Since the requirements for children ages 1 through 18 years are extrapolated from the adult Estimated Average Requirements (EARs), it is critically important to conduct large-scale studies with children using state-of-the-art biomarkers to assess their vitamin C requirement.
-
Many studies that provided vitamin C supplements to apparently healthy well-nourished populations were investigating pharmacological (at or above the point where body tissues are saturated) rather than nutritional effects of the vitamin. This may obscure possible relationships between vitamin C intake and disease risk in the range of dietary intakes. Therefore, population studies on the relationship of vitamin C nutriture and chronic disease should focus more on individuals or populations who eat few fruits and vegetables and are marginally deficient in vitamin C. Attention also has to be given to methods for sorting out the effects of vitamin C intake from those of other dietary and life-style factors that may also affect disease risk.
-
While the evidence of adverse effects due to intakes of vitamin C supplements is at this time limited to osmotic diarrhea and gastrointestinal disturbances which are self-limiting, the frequency of high intakes of the vitamin in the North American population warrants further investigation. The well known pro-oxidant effects of the iron-ascorbate couple in vitro suggest that further research be done on possible related in vivo reactions—for example, during simultaneous supplement ingestion, iron overload, and inflammation or tissue trauma where non-protein-bound iron may be released.
-
A small number of isolated reports raise concern that high vitamin C intakes during pregnancy may expose the fetus or neonate to risks of withdrawal symptoms, hemolysis, or oxidant damage. Further research is needed to confirm or refute these concerns.
REFERENCES
Afroz M, Bhothinard B, Etzkorn JR, Horenstein S, McGarry JD. 1975. Vitamins C and B12. J Am Med Assoc 232:246.
Alexy U, Kersting M, Sichert-Hellert W, Manz F, Schöch G. 1999. Vitamin intake of 3- to 36-month-old German infants and children—Results of the DONALD study. Int J Vitam Nutr Res 69:285–291.
Allen JC, Keller RP, Archer P, Neville MC. 1991. Studies in human lactation: Milk composition and daily secretion rates of macronutrients in the first year of lactation. Am J Clin Nutr 54:69–80.
Alvares O. 1997. Ascorbic acid and periodontal disease. In: Packer L, Fuchs J, eds. Vitamin C in Health and Disease. New York: Marcel Dekker. Pp. 505–516.
Ames BN, Gold LS, Willett WC. 1995. The causes and prevention of cancer. Proc Natl Acad Sci USA 92:5258–5265.
Anderson D, Phillips BJ, Yu T, Edwards AJ, Ayesh R, Butterworth KR. 1997. The effects of vitamin C supplementation on biomarkers of oxygen radical generated damage in human volunteers with “low” or “high” cholesterol levels . Environ Mol Mutagen 30:161–174.
Anderson DM, Pittard WB. 1985. Vitamin E and C concentrations in human milk with maternal megadosing. A case report. J Am Diet Assoc 85:715–717.
Anderson R, Lukey, PT. 1987. A biological role for ascorbate in the selective neutralization of extracellular phagocyte-derived oxidants. Ann NY Acad Sci 498:229–247.
Anderson R, Oosthuizen R, Maritz R, Theron A, Van Rensburg AJ. 1980. The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune function in normal volunteers. Am J Clin Nutr 33:71–76.
Asami S, Manabe H, Miyake J, Tsurudome Y, Hirano T, Yamaguchi R, Itoh H, Kasai H. 1997. Cigarette smoking induces an increase in oxidative DNA damage, 8-hydroxydeoxyguanosine, in a central site in the human lung. Carcinogenesis 18:1763–1766.
Auer BL, Auer D, Rodgers AL. 1998. Relative hyperoxaluria, crystalluria and haematuria after megadose ingestion of vitamin C. Eur J Clin Invest 28:695–700.
Bacon BR, Olynyk JK, Brunt EM, Britton RS, Wolff RK. 1999. HFE genotype in patients with hemochromatosis and other liver diseases . Ann Int Med 130:953–962.
Baer DM, Tekawa IS, Hurley LB. 1994. Iron stores are not associated with acute myocardial infarction. Circulation 89:2915–2918.
Baker EM, Sauberlich HE, Wolfskill SJ, Wallace WT, Dean EE. 1962. Tracer studies of vitamin C utilization in men: Metabolism of D -glucuronolactone-6-C14, D -glucuronic-6-C14 acid and L -ascorbic-1-C14 acid. Proc Soc Exp Biol Med 109:737–741.
Baker EM, Hodges RE, Hood J, Sauberlich HE, March SC. 1969. Metabolism of ascorbic-1-C14 acid in experimental human scurvy. Am J Clin Nutr 22:549–558.
Baker EM, Hodges RE, Hood J, Sauberlich HE, March SC, Canham JE. 1971. Metabolism of 14C- and 3H-labeled L -ascorbic acid in human scurvy. Am J Clin Nutr 24:444–454.
Ballin A, Brown EJ, Koren G, Zipursky A. 1988. Vitamin C-induced erythrocyte damage in premature infants. J Pediatr 113:114–120.
Bandera EV, Freudenheim JL, Marshall JR, Zielezny M, Priore RL, Brasure J, Baptiste M, Graham S. 1997. Diet and alcohol consumption and lung cancer risk in the New York State Cohort. Cancer Causes Control 8:828–840.
Barrett B, Gunter E, Jenkins J, Wang M. 1991. Ascorbic acid concentration in amniotic fluid in late pregnancy. Biol Neonate 60:333–335.
Bates CJ, Prentice AM, Prentice A, Paul AA, Whitehead RG. 1982. Seasonal variations in ascorbic acid status and breast milk ascorbic acid levels in rural Gambian women in relation to dietary intake . Trans Royal Soc Trop Med Hyg 76:341–347.
Belcher JD, Balla J, Balla G, Jacobs DR Jr, Gross M, Jacob HS, Vercellotti GM. 1993. Vitamin E, LDL, and endothelium. Brief oral vitamin supplementation prevents oxidized LDL-mediated vascular injury in vitro. Arterioscler Thromb 13:1779–1789.
Bendich A, Cohen M. 1990. Ascorbic acid safety: Analysis of factors affecting iron absorption . Toxicol Lett 51:189–201.
Berger L, Gerson CD, Yu TF. 1977. The effect of ascorbic acid on uric acid excretion with a commentary on the renal handling of ascorbic acid. Am J Med 62:71–76.
Berger TM, Polidori MC, Dabbagh A, Evans PJ, Halliwell B, Morrow JD, Roberts LJ II, Frei B. 1997. Antioxidant activity of vitamin C in iron-overloaded human plasma . J Biol Chem 272:15656–15660.
Berliner JA, Heinecke JW. 1996. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med 20:707–727.
Bermudez E, Stone K, Carter KM, Pryor WA. 1994. Environmental tobacco smoke is just as damaging to DNA as mainstream smoke. Environ Hlth Perspect 102:870–874.
Beutler E. 1991. Glucose-6-phosphate dehydrogenase deficiency. N Engl J Med 324:169–174.
Blanchard J. 1991a. Depletion and repletion kinetics of vitamin C in humans. J Nutr 121:170–176.
Blanchard J. 1991b. Effects of gender on vitamin C pharmacokinetics in man. J Am Coll Nutr 10:453–459.
Blanchard J, Conrad KA, Watson RR, Garry PJ, Crawley JD. 1989. Comparison of plasma, mononuclear and polymorphonuclear leucocyte vitamin C levels in young and elderly women during depletion and supplementation. Eur J Clin Nutr 43:97–106.
Blanchard J, Conrad KA, Garry PJ. 1990a. Effects of age and intake on vitamin C disposition in females. Eur J Clin Nutr 44:447–460.
Blanchard J, Conrad KA, Mead RA, Garry PJ. 1990b. Vitamin C disposition in young and elderly men. Am J Clin Nutr 51:837–845.
Blanchard J, Tozer TN, Rowland M. 1997. Pharmacokinetic perspectives on megadoses of ascorbic acid. Am J Clin Nutr 66:1165–1171.
Block G. 1991. Vitamin C and cancer prevention: The epidemiologic evidence. Am J Clin Nutr 53:270S–282S.
Blot WJ, Li J-Y, Taylor PR, Guo W, Dawsey S, Wang G-Q, Yang CS, Zheng S-F, Gail M, Li G-Y, Yu Y, Liu B-Q, Tangrea J, Sun Y-H, Liu F, Fraumeni JF Jr, Zhang Y-H, Li B. 1993. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst 85:1483–1492.
Bors W, Michel C, Schikora S. 1995. Interaction of flavonoids with ascorbate and determination of their univalent redox potentials: A pulse radiolysis study. Free Radic Biol Med 19:45–52.
Bostick RM, Potter JD, McKenzie DR, Sellers TA, Kushi LH, Steinmetz KA, Folsom AR. 1993. Reduced risk of colon cancer with high intake of vitamin E: The Iowa Women's Health Study. Cancer Res 53:4230–4237.
Britton JR, Pavord ID, Richards KA, Knox AJ, Wisniewski AF, Lewis SA, Tattersfield AE, Weiss ST. 1995. Dietary antioxidant vitamin intake and lung function in the general population. Am J Respir Crit Care Med 151:1383–1387.
Bucca C, Rolla G, Farina JC. 1992. Effect of vitamin C on transient increase of bronchial responsiveness in conditions affecting the airways. Ann NY Acad Sci 669:175–187.
Bueno de Mesquita HB, Maisonneuve P, Runia S, Moerman CJ. 1991. Intake of foods and nutrients and cancer of the exocrine pancreas: A population-based case-control study in The Netherlands. Int J Cancer 48:540–549.
Buettner GR. 1993. The pecking order of free radicals and antioxidants: Lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 300:535–543.
Buettner GR, Jurkiewicz BA. 1996. Catalytic metals, ascorbate and free radicals: Combinations to avoid . Radiat Res 145:532–541.
Burr ML, Elwood PC, Hole DJ, Hurley RJ, Hughes RE. 1974. Plasma and leukocyte ascorbic acid levels in the elderly. Am J Clin Nutr 27:144–151
Bussey HJ, DeCosse JJ, Deschner EE, Eyers AA, Lesser ML, Morson BC, Ritchie SM, Thomson JP, Wadsworth J. 1982. A randomized trial of ascorbic acid in polyposis coli. Cancer 50:1434–1439.
Butte NF, Garza C, Smith EO, Nichols BL. 1984. Human milk intake and growth in exclusively breast-fed infants. J Pediatr 104:187–195.
Byerley LO, Kirksey A. 1985. Effects of different levels of vitamin C intake on the vitamin C concentration in human milk and the vitamin C intakes of breastfed infants. Am J Clin Nutr 41:665–671.
Byun J, Mueller DM, Fabjan JS, Heinecke JW. 1999. Nitrogen dioxide radical generated by the myeloperoxidase-hydrogen peroxide-nitrite system promotes lipid peroxidation of low density lipoprotein. FEBS Lett 455:243–246.
Cadenas S, Rojas C, Méndez J, Herrero A, Barja G. 1996. Vitamin E decreases urine lipid peroxidation products in young healthy human volunteers under normal conditions. Pharmacol Toxicol 79:247–253.
Cahill RJ, O'Sullivan KR, Mathias PM, Beattie S, Hamilton H, O'Morain C. 1993. Effects of vitamin antioxidant supplementation on cell kinetics of patients with adenomatous polyps. Gut 34:963–967.
Cameron E, Campbell A. 1974. The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact 9:285–315.
Campbell GD Jr, Steinberg MH, Bower JD. 1975. Ascorbic acid-induced hemolysis in G-6-PD deficiency. Ann Intern Med 82:810.
Carr AC, Frei B. 1999. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 69:1086–1087.
Casanueva E, Polo E, Tejero E, Meza C. 1993. Premature rupture of amniotic membranes as functional assessment of vitamin C status during pregnancy. Ann NY Acad Sci 678:369–370.
Chalmers TC. 1975. Effects of ascorbic acid on the common cold. An evaluation of the evidence. Am J Med 58:532–536.
Chazan JA, Mistilis SP. 1963. The pathophysiology of scurvy. Am J Med 34:350–358.
Cheng L, Cohen M, Bhagavan HN. 1985. Vitamin C and the elderly. In: Watson RR, ed. CRC Handbook of Nutrition in the Aged. Boca Raton, FL: CRC Press. Pp. 157–185.
Choi JL, Rose RC. 1989. Transport and metabolism of ascorbic acid in human placenta. Am J Physiol 257:C110–C113.
Cochrane WA. 1965. Overnutrition in prenatal and neonatal life: A problem? Can Med Assoc J 93:893–899.
Cohen HA, Neuman I, Nahum H. 1997. Blocking effect of vitamin C in exercise-induced asthma. Arch Pediatr Adolesc Med 151:367–370.
Cook JD, Watson SS, Simpson KM, Lipschitz DA, Skikne BS. 1984. The effect of high ascorbic acid supplementation on body iron stores . Blood 64:721–726.
Cooke MS, Evans MD, Podmore ID, Herbert KE, Mistry N, Mistry P, Hickenbotham PT, Hussieni A, Griffiths HR, Lunec J. 1998. Novel repair action of vitamin C upon in vivo oxidative DNA damage . FEBS Lett 439:363–367.
Coulehan JL, Eberhard S, Kapner L, Taylor F, Rogers K, Garry P. 1976. Vitamin C and acute illness in Navajo school children. N Engl J Med 295:973–977.
Cross CE, Halliwell B. 1993. Nutrition and human disease: How much extra vitamin C might smokers need? Lancet 341:1091.
Cross CE, Forte T, Stocker R, Louie S, Yamamoto Y, Ames BN, Frei B. 1990. Oxidative stress and abnormal cholesterol metabolism in patients with adult respiratory distress syndrome. J Lab Clin Med 115:396–404.
Crott JW, Fenech M. 1999. Effect of vitamin C supplementation on chromosome damage, apoptosis and necrosis ex vivo. Carcinogenesis 20:1035–1041.
Curhan GC, Willett WC, Rimm EB, Stampfer MJ. 1996. A prospective study of the intake of vitamins C and B6, and the risk of kidney stones in men. J Urol 155:1847–1851.
Curhan GC, Willett WC, Speizer FE, Stampfer MJ. 1999. Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol 10:840–845.
Dabrowski K. 1990. Gastro-intestinal circulation of ascorbic acid. Comp Biochem Physiol 95A:481–486.
Dallongeville J, Marécaux N, Fruchart J-C, Amouyel P. 1998. Cigarette smoking is associated with unhealthy patterns of nutrient intake: A meta-analysis. J Nutr 128:1450–1457.
Davies HE, Davies JE, Hughes RE, Jones E. 1984. Studies on the absorption of L xyloascorbic acid (vitamin C) in young and elderly subjects. Hum Nutr Clin Nutr 38C:463–471.
Davies HE, Gruffudd S, Hughes RE, Jones E. 1987. Ascorbic acid and carnitine in man. Nutr Report Int 36:941–948.
DeCosse JJ, Adams MB, Kuzma JF, LoGerfo P, Condon RE. 1975. Effect of ascorbic acid on rectal polyps of patients with familial polyposis. Surgery 78:608–612.
Delafuente JC, Prendergast JM, Modigh A. 1986. Immunologic modulation by vitamin C in the elderly. Int J Immunopharmacol 8:205–211.
Delamere NA. 1996. Ascorbic acid and the eye. Subcell Biochem 25:313–329.
Devaraj S, Jialal I. 1996. Oxidized low-density lipoprotein and atherosclerosis. Int J Clin Lab Res 26:178–184.
Dewey KG, Finley DA, Lonnerdal B. 1984. Breast milk volume and composition during late lactation (7–20 months). J Pediatr Gastroenterol Nutr 3:713–720.
Drake IM, Davies MJ, Mapstone NP, Dixon MF, Schorah CJ, White KLM, Chalmers DM, Axon ATR. 1996. Ascorbic acid may protect against human gastric cancer by scavenging mucosal oxygen radicals. Carcinogenesis 17:559–562.
Duthie SJ, Ma A, Ross MA, Collins AR. 1996. Antioxidant supplementation decreases oxidative DNA damage in human lymphocytes. Cancer Res 56:1291–1295.
Dyke GW, Craven JL, Hall R, Garner RC. 1994a. Effect of vitamin C supplementation on gastric mucosal DNA damage . Carcinogenesis 15:291–295.
Dyke GW, Craven JL, Hall R, Garner RC. 1994b. Effect of vitamin C upon gastric mucosal O6-alkyltransferase activity and on gastric vitamin C levels. Cancer Lett 86:159–165.
Eichholzer M, Stahelin HB, Gey KF. 1992. Inverse correlation between essential antioxidants in plasma and subsequent risk to develop cancer, ischemic heart disease and stroke respectively: 12-year follow-up of the Prospective Basel Study. Exp Suppl 62:398–410.
Ekvall S, Chen IW, Bozian R. 1981. The effect of supplemental ascorbic acid on serum vitamin B12 levels in myelomenigocele patients. Am J Clin Nutr 34:1356–1361.
Elneihoum AM, Falke P, Hedblad B, Lindgarde F, Ohlsson K. 1997. Leukocyte activation in atherosclerosis: Correlation with risk factors . Atherosclerosis 131:79–84.
Englard S, Seifter S. 1986. The biochemical functions of ascorbic acid. Annu Rev Nutr 6:365–406.
Enstrom JE, Kanim LE, Breslow L. 1986. The relationship between vitamin C intake, general health practices, and mortality in Alameda County, California. Am J Pub Hlth 76:1124–1130.
Enstrom JE, Kanim LE, Klein MA. 1992. Vitamin C intake and mortality among a sample of the United States population. Epidemiology 3:194–202.
Erdman JW Jr, Klein BP. 1982. The influence of harvesting, processing, and cooking on vitamin C in foods. In: Seib PA, Tolbert BM, eds. Ascorbic Acid: Chemistry, Metabolism and Uses. Washington, DC: American Chemical Society. Pp. 499–532.
Evans RM, Currie L, Campbell A. 1982. The distribution of ascorbic acid between various cellular components of blood in normal individuals, and its relation to the plasma concentration . Br J Nutr 47:473–482.
FDA (Food and Drug Adminstration). 1985. Nutrient requirements for infant formulas. Fed Regis 50:45106–45108.
Fellstrom B, Danielson BG, Karlstrom B, Lithell H, Ljunghall S, Vessby B. 1989. Dietary habits in renal stone patients compared with healthy subjects . Br J Urol 63:575–580.
Fishbaine B, Butterfield G. 1984. Ascorbic acid status of running and sedentary men. Int J Vitam Nutr Res 54:273.
Fituri N, Allawi N, Bentley M, Costello J. 1983. Urinary and plasma oxalate during ingestion of pure ascorbic acid: A re-evaluation. Eur Urol 9:312–315.
Flodin NW. 1988. Pharmacology of Micronutrients. New York: Alan R. Liss. Pp. 201–244.
Fontham ET. 1994. Vitamin C, vitamin C-rich foods, and cancer: Epidemiologic studies . In: Frei B, ed. Natural Antioxidants in Human Health and Disease. San Diego: Academic Press. Pp. 157–197.
Fontham ET, Pickle LW, Haenszel W, Correa P, Lin YP, Falk RT. 1988. Dietary vitamins A and C and lung cancer risk in Louisiana. Cancer 62:2267–2273.
Fraga CG, Motchnik PA, Shigenaga MK, Helbock HJ, Jacob RA, Ames BN. 1991. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci USA 88:11003–11006.
Frei B, Stocker R, Ames BN. 1988. Antioxidant defenses and lipid peroxidation in human blood plasma . Proc Natl Acad Sci USA 85:9748–9752.
Frei B, England L, Ames BN. 1989. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA 86:6377–6381.
Frei B, Forte TM, Ames BN, Cross CE. 1991. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid. Biochem J 277:133–138.
Freudenheim JL, Graham S, Marshall JR, Haughey BP, Wilkinson G. 1990. A casecontrol study of diet and rectal cancer in western New York . Am J Epidemiol 131:612–624.
Fuller CJ, Grundy SM, Norkus EP, Jialal I. 1996. Effect of ascorbate supplementation on low density lipoprotein oxidation in smokers. Atherosclerosis 119:139–150.
Gale CR, Martyn CN, Winter PD, Cooper C. 1995. Vitamin C and risk of death from stroke and coronary heart disease in cohort of elderly people. Br Med J 310:1563–1566.
Garry PJ, Goodwin JS, Hunt WC, Gilbert BA. 1982. Nutritional status in a healthy elderly population: Vitamin C. Am J Clin Nutr 36:332–339.
Garry PJ, Vanderjagt DJ, Hunt WC. 1987. Ascorbic acid intakes and plasma levels in healthy elderly. Ann NY Acad Sci 498:90–99.
George DR, De Francesca BA. 1989. Human milk in comparison to cow milk. In: Lebenthal E, ed. Textbook of Gastroenterology and Nutrition in Infancy and Childhood , 2nd edition. New York: Raven Press. Pp. 242–243.
Gey KF. 1995. Ten-year retrospective on the antioxidant hypothesis of arterioscl rosis: Threshold plasma levels of antioxidant micronutrients related to minimum cardiovascular risk. Nutr Biochem 6:206–236.
Gey KF. 1998. Vitamins E plus C and interacting conutrients required for optimal health. A critical and constructive review of epidemiology and supplementation data regarding cardiovascular disease and cancer. Biofactors 7:113–174.
Gey KF, Stahelin HB, Eichholzer M. 1993. Poor plasma status of carotene and vitamin C is associated with higher mortality from ischemic heart disease and stroke: Basel Prospective Study. Clin Invest 71:3–6.
Ghadirian P, Boyle P, Simard A, Baillargeon J, Maisonneuve P, Perret C. 1991. Reported family aggregation of pancratic cancer within a population-based case-control study in the francophone community in Montreal, Canada . Int J Pancreatol 10:183–196.
Giunta JL. 1983. Dental erosion resulting from chewable vitamin C tablets. J Am Dent Assoc 107:253–256.
Gogel HK, Tandberg D, Strickland RG. 1989. Substances that interfere with guaiac card tests: Implications for gastric aspirate testing. Am J Emerg Med 7:474–480.
Goldsmith GA. 1961. Human requirements for vitamin C and its use in clinical medicine . Ann NY Acad Sci 92:230–245.
Gosiewska A, Mahmoodian F, Peterkofsky B. 1996. Gene expression of iron-related proteins during iron deficiency caused by scurvy in guinea pigs. Arch Biochem Biophys 325:295–303.
Graham S, Zielezny M, Marshall J, Priore R, Freudenheim J, Brasure J, Haughey B, Nasca P, Zdeb M. 1992. Diet in the epidemiology of postmenopausal breast cancer in the New York State Cohort. Am J Epidemiol 136:1327–1337.
Green MHL, Lowe JE, Waugh APW, Aldridge KE, Cole J, Arlett CF. 1994. Effect of diet and vitamin C on DNA strand breakage in freshly-isolated human white blood cells. Mutat Res 316:91–102.
Greenberg ER, Baron JA, Tosteson TD, Freeman DH, Beck GJ, Bond JH, Colacchio TA, Coller JA, Frankl HD, Haile RW, Mandel JS, Nierenberg DW, Rothstein R, Snover DC, Stevens MM, Summers RW, van Stolk RU. 1994. A clinical trial of antioxidant vitamins to prevent colorectal adenoma . N Engl J Med 331:141–147.
Gutteridge JMC. 1991. Plasma ascorbate levels and inhibition of the antioxidant activity of caeruloplasmin. Clin Sci 81:413–417.
Hallberg L. 1985. The role of vitamin C in improving the critical iron balance situation in women. Int J Vitam Nutr Res 27:177–187.
Halliwell B. 1998. Can oxidative DNA damage be used as a biomarker of cancer risk in humans? Free Radic Res 29:469–486.
Halliwell B, Whiteman M. 1997. Antioxidant and prooxidant properties of vitamin C. In: Packer L, Fuchs J, eds. Vitamin C in Health and Disease. New York: Marcel Dekker. Pp. 59–73.
Halliwell B, Wasil M, Grootveld M. 1987. Biologically significant scavenging of the myeloperoxidase-derived oxidant hypochlorous acid by ascorbic acid. FEBS Lett 213:15–17.
Halpner AD, Handelman GJ, Belmont CA, Harris JM, Blumberg JB. 1998. Protection by vitamin C of oxidant-induced loss of vitamin E in rat hepatocytes. J Nutr Biochem 9:355–359.
Hankinson SE, Stampfer MJ, Seddon JM, Colditz GA, Rosner B, Speizer FE, Willett WC. 1992. Nutrient intake and cataract extraction in women: A prospective study . Br Med J 305:335–339.
Harats D, Ben-Naim M, Dabach Y, Hollander G, Havivi E, Stein O, Stein Y. 1990. Effect of vitamin C and E supplementation on susceptibility of plasma lipopr teins to peroxidation induced by acute smoking. Atherosclerosis 85:47–54.
Harats D, Chevion S, Nahir M, Norman Y, Sagee O, Berry EM. 1998. Citrus fruit supplementation reduces lipoprotein oxidation in young men ingesting a diet high in saturated fat: Presumptive evidence for an interaction between vitamins C and E in vivo. Am J Clin Nutr 67:240–245.
Harris ED, Percival SS. 1991. A role for ascorbic acid in copper transport. Am J Clin Nutr 54:1193S–1197S.
Hartz SC, Russell RM, Rosenberg IH. 1992. Nutrition in the Elderly. The Boston Nutr tional Status Survey. London: SmithGordon. P. 38.
Haslam RH, Dalby JT, Rademaker AW. 1984. Effects of megavitamin therapy on children with attention deficit disorders. Pediatrics 74:103–111.
Hatch GE. 1995. Asthma, inhaled oxidants, and dietary antioxidants. Am J Clin Nutr 61:625S–630S.
Hatch M, Mulgrew S, Bourke E, Keogh B, Costello J. 1980. Effect of megadoses of ascorbic acid on serum and urinary oxalate . Eur Urol 6:166–169.
Hazell LJ, Arnold L, Flowers D, Waeg G, Malle E, Stocker R. 1996. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest 97:1535–1544.
Heinecke JW. 1997. Pathways for oxidation of low density lipoprotein by myelope oxidase: Tyrosyl radical, reactive aldehydes, hypochlorous acid and molecular chlorine. BioFactors 6:145–155.
Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B, Dewey KG. 1993. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: The DARLING Study. Am J Clin Nutr 58:152–161.
Heitzer T, Just H, Munzel T. 1996. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 94:6–9.
Heller R, Munscher-Paulig F, Grabner R, Till U. 1999. L -Ascorbic acid potentiates nitric oxide synthesis in endothelial cells. J Biol Chem 274:8254–8260.
Hemila H. 1996. Vitamin C, the placebo effect, and the common cold: A case study of how preconceptions influence the analysis of results. J Clin Epidemiol 49:1079–1084.
Hemila H. 1997. Vitamin C intake and susceptibility to the common cold. Br J Nutr 77:59–72.
Hemila H, Herman ZS. 1995. Vitamin C and the common cold: A retrospective analysis of Chalmers ' review. J Am Coll Nutr 14:116–123.
Henning SM, Zhang JZ, McKee RW, Swendseid ME, Jacob RA. 1991. Glutathione blood levels and other oxidant defense indices in men fed diets low in vitamin C. J Nutr 121:1969–1975.
Herbert V. 1978. Risk of oxalate stones from large doses of vitamin C. N Engl J Med 298:856.
Herbert V. 1995. Vitamin C supplements and disease—Counterpoint. J Am Coll Nutr 14:112–113.
Herbert V, Jacob E. 1974. Destruction of vitamin B12 by ascorbic acid. J Am Med Assoc 230:241–242.
Hevia P, Omaye ST, Jacob RA. 1990. Urinary hydroxyproline excretion and vitamin C status in healthy young men. Am J Clin Nutr 51:644–648.
Hinds MW, Kolonel LN, Hankin JH, Lee J. 1984. Dietary vitamin A, carotene, vitamin C and risk of lung cancer in Hawaii. Am J Epidemiol 119:227–237.
Hoffer A. 1971. Ascorbic acid and toxicity. N Engl J Med 285:635–636.
Hoffer A. 1973. Vitamin C and infertility. Lancet 2:1146.
Hofstad B, Almendingen K, Vatn M, Andersen S, Owen R, Larsen S, Osnes M. 1998. Growth and recurrence of colorectal polyps: A double-blind 3-year intervention with calcium and antioxidants. Digestion 59:148–156.
Hogenkamp HP. 1980. The interaction between vitamin B12 and vitamin C. Am J Clin Nutr 33:1–3.
Hornig B, Arakawa N, Kohler C, Drexler H. 1998. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation 97:363–368.
Hornig D. 1975. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann NY Acad Sci 258:103–118.
Hornig DH, Moser U. 1981. The safety of high vitamin C intakes in man. In: Counsell JN, Hornig DH, eds. Vitamin C (Ascorbic Acid). London: Applied Science. Pp. 225–248.
Horrobin DF. 1996. Ascorbic acid and prostaglandin synthesis. Subcell Biochem 25:109–115.
Howe GR, Hirohata T, Hislop TG, Iscovich JM, Yuan JM, Katsouyanni K, Lubin F, Marubini E, Modan B, Rohan T. 1990. Dietary factors and risk of breast cancer: Combined analysis of 12 case-control studies. J Natl Cancer Inst 82:561– 569.
Howe GR, Ghadirian P, Bueno de Mesquita HB, Zatonski WA, Baghurst PA, Miller AB, Simard A, Baillargeon J, de Waard F, Przewozniak K. 1992. A collaborative case-control study of nutrient intake and pancreatic cancer within the search programme. Int J Cancer 51:365–372.
Hoyt CJ. 1980. Diarrhea from vitamin C. J Am Med Assoc 244:1674.
Hughes C, Dutton S, Truswell AS. 1981. High intakes of ascorbic acid and urinary oxalate. J Hum Nutr 35:274–280.
Hunt JR, Gallagher SK, Johnson LK. 1994. Effect of ascorbic acid on apparent iron absorption by women with low iron stores. Am J Clin Nutr 59:1381–1385.
Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE, Willett WC. 1993. A prospective study of the intake of vitamins C, E, and A and the risk of breast cancer. N Engl J Med 329:234–240.
IOM (Institute of Medicine). 1991. Nutrition During Lactation. Washington, DC: National Academy Press. P. 179.
Itoh R, Yamada K, Oka J, Echizen H, Murakami K. 1989. Sex as a factor in levels of serum ascorbic acid in a healthy elderly population. Int J Vitam Nutr Res 59:365–372.
Jacob RA. 1995. The integrated antioxidant system. Nutr Res 15:755–766.
Jacob RA. 1999. Vitamin C. In: Shils ME, Olson JA, Shike M, Ross AC, eds. Modern Nutrition in Health and Disease, 9th edition. Baltimore, MD: Williams & Wilkins. Pp. 467–483.
Jacob RA, Pianalto FS. 1997. Urinary carnitine excretion increases during experimental vitamin C depletion of healthy men. J Nutr Biochem 8:265–269.
Jacob RA, Skala JH, Omaye ST. 1987a. Biochemical indices of human vitamin C status. Am J Clin Nutr 46:818–826.
Jacob RA, Skala JH, Omaye ST, Turnlund JR. 1987b. Effect of varying ascorbic acid intakes on copper absorption and ceruloplasmin levels of young men. J Nutr 117:2109–2115.
Jacob RA, Otradovec CL, Russell RM, Munro HN, Hartz SC, McGandy RB, Morrow FD, Sadowski JA. 1988. Vitamin C status and nutrient interactions in a healthy elderly population . Am J Clin Nutr 48:1436–1442.
Jacob RA, Kelley DS, Pianalto FS, Swendseid ME, Henning SM, Zhang Jz, Ames BN, Fraga CG, Peters JH. 1991. Immunocompetence and oxidant defense during ascorbate depletion of healthy men. Am J Clin Nutr 54:1302S–1309S.
Jacob RA, Pianalto FS, Agee RE. 1992. Cellular ascorbate depletion in healthy men. J Nutr 122:1111–1118.
Jacob RA, Kutnink MA, Csallany AS, Daroszewska M, Burton GW. 1996. Vitamin C nutriture has little short-term effect on vitamin E concentrations in healthy women. J Nutr 126:2268–2277.
Jacques PF, Chylack LT Jr. 1991. Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids in cataract prevention. Am J Clin Nutr 53:352S–355S.
Jacques PF, Taylor A, Hankinson SE, Willett WC, Mahnken B, Lee Y, Vaid K, Lahav M. 1997. Long-term vitamin C supplement use and prevalence of early age related lens opacities. Am J Clin Nutr 66:911–916.
Jaffe RM, Kasten B, Young DS, MacLowry JD. 1975. False-negative stool occult blood tests caused by ingestion of ascorbic acid (vitamin C). Ann Intern Med 83:824–826.
Jama JW, Launer LJ, Witteman JC, den Breeijen JH, Breteler MM, Grobbee DE, Hofman A. 1996. Dietary antioxidants and cognitive function in a population based sample of older persons. The Rotterdam Study. Am J Epidemiol 144:275–280.
Jariwalla RJ, Harakeh S. 1996. Antiviral and immunomodulatory activities of asco bic acid. Subcell Biochem 25:213–231.
Jarvinen R, Knekt P, Seppanen R, Teppo L. 1997. Diet and breast cancer risk in a cohort of Finnish women. Cancer Lett 114:251–253.
Jendryczko A, Tomala J. 1995. The total free radical trapping ability of blood pla ma in eclampsia . Zentralbl Gynakol 117:126–129.
Jha P, Flather M, Lonn E, Farkouh M, Yusuf S. 1995. The antioxidant vitamins and cardiovascular disease. A critical review of epidemiologic and clinical trial data. Ann Intern Med 123:860–872.
Jialal I, Devaraj S. 1996. The role of oxidized low density lipoprotein in atherogenesis. J Nutr 126:1053S–1057S.
Jialal I, Grundy SM. 1991. Preservation of the endogenous antioxidants in low density lipoprotein by ascorbate but not probucol during oxidative modification. J Clin Invest 87:597–601.
Jialal I, Vega GL, Grundy SM. 1990. Physiologic levels of ascorbate inhibit the oxidative modification of low density lipoprotein. Atherosclerosis 82:185–191.
Johnston CS. 1991. Complement component Clq unaltered by ascorbate supplementation in healthy men and women. J Nutr Biochem 2:499–501.
Johnston CS. 1999. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev 57:71–77.
Johnston CS, Luo B. 1994. Comparison of the absorption and excretion of three commercially available sources of vitamin C. J Am Diet Assoc 94:779–781.
Johnston CS, Thompson LL. 1998. Vitamin C status of an outpatient population. J Am Coll Nutr 17:366–370.
Johnston CS, Martin LJ, Cai X. 1992. Antihistamine effect of supplemental ascorbic acid and neutrophil chemotaxis. J Am Coll Nutr 11:172–176.
Johnston CS, Meyer CG, Srilakshmi JC. 1993. Vitamin C elevates red blood cell glutathione in healthy adults. Am J Clin Nutr 58:103–105.
Johnston CS, Solomon E, Corte C. 1996. Vitamin C depletion is associated with alterations in blood histamine and plasma free carnitine in adults. J Am Coll Nutr 15:586–591.
Kallner A, Hartmann D, Hornig D. 1979. Steady-state turnover and body pool of ascorbic acid in man. Am J Clin Nutr 32:530–539.
Kallner AB, Hartmann D, Hornig DH. 1981. On the requirements of ascorbic acid in man: Steady-state turnover and body pool in smokers. Am J Clin Nutr 34:1347–1355.
Karra MV, Udipi SA, Kirksey A, Roepke JL. 1986. Changes in specific nutrients in breast milk during extended lactation . Am J Clin Nutr 43:495–503.
Katsuki H. 1996. Vitamin C and nervous tissue: In vivo and in vitro aspects. Subcell Biochem 25:293–311.
Keith RE. 1994. Vitamins and physical activity. In: Wolinsky I, Hickson JF, eds. Nutrition in Exercise and Sport, 2nd edition. Boca Raton, FL: CRC Press. Pp. 159–183.
Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. 1999. Altered lung antioxidant status in patients with mild asthma. Lancet 354:482–483.
Kennes B, Dumont I, Brohee D, Hubert C, Neve P. 1983. Effect of vitamin C supplements on cell-mediated immunity in old people. Gerontology 29:305–310.
Knekt P, Jarvinen R, Seppanen R, Rissanen A, Aromaa A, Heinonen OP, Albanes D, Heinonen M, Pukkala E, Teppo L. 1991. Dietary antioxidants and the risk of lung cancer. Am J Epidemiol 134:471–479.
Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A. 1994. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol 139:1180–1189.
Kritchevsky SB, Shimakawa T, Tell G, Dennis B, Carpenter M, Eckfeldt JH, Peacher-Ryan H, Heiss G. 1995. Dietary antioxidants and carotid artery wall thickness. The ARIC Study. Circulation 92:2142–2150.
Kushi LH, Fee RM, Sellers TA, Zheng W, Folsom AR. 1996a. Intake of vitamins A, C, and E and postmenopausal breast cancer. The Iowa Women's Health Study. Am J Epidemiol 144:165–174.
Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. 1996b. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med 334:1156–1162.
Lamden MP, Chrystowski GA. 1954. Urinary oxalate excretion by man following ascorbic acid ingestion . Proc Soc Exp Biol Med 85:190–192.
Laudicina DC, Marnett LJ. 1990. Enhancement of hydroperoxide-dependent lipid peroxidation in rat liver microsomes by ascorbic acid. Arch Biochem Biophys 278:73–80.
Leaf CD, Vecchio AJ, Roe DA, Hotchkiss JH. 1987. Influence of ascorbic acid dose on N-nitrosoproline formation in humans. Carcinogenesis 8:791–795.
Leggott PJ, Robertson PB, Rothman DL, Murray PA, Jacob RA. 1986. The effect of controlled ascorbic acid depletion and supplementation on periodontal health. J Periodontol 57:480–485.
Leggott PJ, Robertson PB, Jacob RA, Zambon JJ, Walsh M, Armitage GC. 1991. Effects of ascorbic acid depletion and supplementation on periodontal health and subgingival microflora in humans. J Dent Res 70:1531–1536.
Lehr HA, Weyrich AS, Saetzler RK, Jurek A, Arfors KE, Zimmerman GA, Prescott SM, McIntyre TM. 1997. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J Clin Invest 99:2358–2364.
Le Marchand L, Yoshizawa CN, Kolonel LN, Hankin JH, Goodman MT. 1989. Vegetable consumption and lung cancer risk: A population-based case-control study in Hawaii. J Natl Cancer Inst 81:1158–1164.
Lenton KJ, Therriault H, Fulop T, Payette H, Wagner JR. 1999. Glutathione and ascorbate are negatively correlated with oxidative DNA damage in human lymphocytes. Carcinogenesis 20:607–613.
Leske MC, Chylack LT Jr, Wu SY. 1991. The Lens Opacities Case-Control Study. Risk factors for cataract. Arch Ophthalmol 109:244–251.
Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF Jr, Vita JA. 1996. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 93:1107–1113.
Levine M, Dhariwal KR, Wang Y, Park JB, Welch RW. 1994. Ascorbic acid in neutrophils. In: Frei B, ed. Natural Antioxidants in Health and Disease. San Diego: Academic Press. Pp. 469–488.
Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. 1996a. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 93:3704–3709.
Levine M, Rumsey S, Wang Y, Park J, Kwon O, Xu W, Amano N. 1996b. Vitamin C.In: Ziegler EE, Filer LJ Jr, eds. Present Knowledge in Nutrition, 7th edition. Washington, DC: ILSI Press. Pp. 146–159.
Levy R, Shriker O, Porath A, Riesenberg K, Schlaeffer F. 1996. Vitamin C for the treatment of recurrent furunculosis in patients with impaired neutrophil functions. J lnfect Dis 173:1502–1505.
Loft S, Vistisen K, Ewertz M, Tjonneland A, Overvad K, Poulsen HE. 1992. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: Influence of smoking, gender and body mass index. Carcinogenesis 13:2241–2247.
Losonczy KG, Harris TB, Havlik RJ. 1996. Vitamin E and vitamin C supplement use and risk of all-cause and coronary heart disease mortality in older persons: The Established Populations for Epidemiologic Studies of the Elderly. Am J Clin Nutr 64:190–196.
Løvstad RA. 1997. A study on ascorbate inhibition of ceruloplasmin ferroxidase activity . BioMetals 10:123–126.
LSRO/FASEB (Life Sciences Research Office/Federation of American Societies for Experimental Biology). 1989. Nutrition Monitoring in the United States: An Update Report on Nutrition Monitoring. Prepared for the U.S. Department of Agriculture and the U.S. Department of Health and Human Services. DHHS Publication No. (PHS) 89-1255 . Washington, DC: U.S. Government Printing Office.
Ludvigsson J, Hansson LO, Tibbling G. 1977. Vitamin C as a preventive medicine against common colds in children . Scand J Infect Dis 9:91–98.
Ludvigsson J, Hansson LO, Stendahl O. 1979. The effect of large doses of vitamin C on leukocyte function and some laboratory parameters. Int J Vitam Nutr Res 49:160–165.
Lunec J, Blake DR. 1985. The determination of dehydroascorbic acid and ascorbic acid in the serum and synovial fluid of patients with rheumatoid arthritis. Free Radic Res Commun 1:31–39.
Lykkesfeldt J, Loft S, Nielsen JB, Poulsen HE. 1997. Ascorbic acid and dehydroascorbic acid as biomarkers of oxidative stress caused by smoking. Am J Clin Nutr 65:959–963.
Lykkesfeldt J, Christen S, Wallock LM, Change HH, Jacob RA, Ames BN. 2000. Ascorbate is depleted by smoking and repleted by moderate supplementation: A study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am J Clin Nutr 71:530–536.
Mangels AR, Block G, Frey CM, Patterson BH, Taylor PR, Norkus EP, Levander OA. 1993. The bioavailability to humans of ascorbic acid from oranges, o ange juice and cooked broccoli is similar to that of synthetic ascorbic acid. J Nutr 123:1054–1061.
Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B, Fontham ETH, Mera R, Miller MJS, Correa P. 1996. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: Effect of antibiotics and antioxidants. Cancer Res 56:3238–3243.
Marangon K, Herbeth B, Artur Y, Esterbauer H, Siest G. 1997. Low and very low density lipoprotein composition and resistance to copper-induced oxidation are not notably modified in smokers. Clin Chim Acta 265:1–12.
Marangon K, Herbeth B, Lecomte E, Paul-Dauphin A, Grolier P, Chancerelle Y, Artur Y. 1998. Diet, antioxidant status, and smoking habits in French men. Am J Clin Nutr 67:231–239.
May JM, Cobb CE, Mendiratta S, Hill KE, Burk RF. 1998. Reduction of the ascorbyl free radical to ascorbate by thioredoxin reductase. J Biol Chem 273:23039–23045.
May JM, Mendiratta S, Qu ZC, Loggins E. 1999. Vitamin C recycling and function in human monocytic U-937 cells. Free Radic Biol Med 26:1513–1523.
McKeown-Eyssen G, Holloway C, Jazmaji V, Bright-See E, Dion P, Bruce WR. 1988. A randomized trial of vitamins C and E in the prevention of recurrence of colorectal polyps. Cancer Res 48:4701–4705.
McLaran CJ, Bett JHN, Nye JA, Halliday JW. 1982. Congestive cardiomyopathy and haemochromatosis—Rapid progression possibly accelerated by excessive ingestion of ascorbic acid. Aust NZ J Med 12:187–188.
Melethil S, Mason WD, Chang C-J. 1986. Dose-dependent absorption and excretion of vitamin C in humans. Int J Pharmaceut 31:83–89.
Mentzer WC, Collier E. 1975. Hydrops fetalis associated with erythrocyte G-6-PD deficiency and maternal ingestion of fava beans and ascorbic acid. J Pediatr 86:565–567.
Metz J, Hundertmark U, Pevny I. 1980. Vitamin C allergy of the delayed type. Contact Dermatitis 6:172–174.
Millar J. 1995. The nitric oxide/ascorbate cycle: How neurones may control their own oxygen supply. Med Hypoth 45:21–26.
Miller JZ, Nance WE, Norton JA, Wolen RL, Griffith RS, Rose RJ. 1977. Therapeutic effect of vitamin C. A co-twin control study. J Am Med Assoc 237:248–251.
Mirvish SS. 1994. Experimental evidence for inhibition of N-nitroso compound formation as a factor in the negative correlation between vitamin C consumption and the incidence of certain cancers . Cancer Res 54:1948S–1951S.
Mitch WE, Johnson MW, Kirshenbaum JM, Lopez RE. 1981. Effect of large oral doses of ascorbic acid on uric acid excretion by normal subjects. Clin Pharmcol Ther 29:318–321.
Montalto MB, Benson JD, Martinez GA. 1985. Nutrient intakes of formula-fed infants and infants fed cow's milk. Pediatrics 75:343–351.
Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ II. 1995. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. N Engl J Med 332:1198–1203.
Morse EH, Clark RP, Keyser DE, Merrow SB, Bee DE. 1975. Comparison of the nutritional status of pregnant adolescents with adult pregnant women. I. Biochemical findings. Am J Clin Nutr 28:1000–1013.
Moser U. 1987. Uptake of ascorbic acid by leukocytes. Ann NY Acad Sci 498:200–215.
Moss AJ, Levy AS, Kim I, Park YK. 1989. Use of Vitamin and Mineral Supplements in the United States: Current Users, Types of Products, and Nutrients. Advance Data, Vital and Health Statistics of the National Center for Health Statistics. Number 174. Hyattsville, MD: National Center for Health Statistics. Pp. 1–19.
Motoyama T, Kawano H, Kugiyama K, Hirashima O, Ohgushi M, Yoshimura M, Ogawa H, Yasue H. 1997. Endothelium-dependent vasodilation in the brachial artery is impaired in smokers: Effect of vitamin C. Am J Physiol 273:H1644–H1650.
Mudway IS, Krishna MT, Frew AJ, MacLeod D, Sandstrom T, Holgate ST, Kelly FJ. 1999. Compromised concentrations of ascorbate in fluid lining the respiratory tract in human subjects after exposure to ozone. Occup Environ Med 56:473–481.
Mulholland CW, Strain JJ, Trinick TR. 1996. Serum antioxidant potential, and lipoprotein oxidation in female smokers following vitamin C supplementation. Int J Food Sci Nutr 47:227–231.
Naidoo D, Lux O. 1998. The effect of vitamin C and E supplementation on lipid and urate oxidation products in plasma. Nutr Res 18:953–961.
Ness AR, Khaw KT, Bingham S, Day NE. 1996. Vitamin C status and respiratory function. Eur J Clin Nutr 50:573–579.
Newmark HL, Scheiner MS, Marcus M, Prabhudesai M. 1976. Stability of vitamin B12 in the presence of ascorbic acid. Am J Clin Nutr 29:645–649.
Newton HM, Schorah CJ, Habibzadeh N, Morgan DB, Hullin RP. 1985. The cause and correction of low blood vitamin C concentrations in the elderly. Am J Clin Nutr 42:656–659.
NRC (National Research Council). 1989. Recommended Dietary Allowances, 10th edition. Washington, DC: National Academy Press.
Nyyssonen K, Parviainen MT, Salonen R, Tuomilehto J, Salonen JT. 1997a. Vitamin C deficiency and risk of myocardial infarction: Prospective population study of men from eastern Finland. Br Med J 314:634–638.
Nyyssonen K, Poulsen HE, Hayn M, Agerbo P, Porkkala-Sarataho E, Kaikkonen J, Salonen R, Salonen JT. 1997b. Effect of supplementation of smoking men with plain or slow release ascorbic acid on lipoprotein oxidation. Eur J Clin Nutr 51:154–163.
Ocke MC, Bueno-de-Mesquita HB, Feskens EJ, van Staveren WA, Kromhout D. 1997. Repeated measurements of vegetables, fruits, beta-carotene, and vit mins C and E in relation to lung cancer. Am J Epidemiol 145:358–365.
Omaye ST, Skala JH, Jacob RA. 1986. Plasma ascorbic acid in adult males: Effects of depletion and supplementation . Am J Clin Nutr 44:257–264.
Omaye ST, Schaus EE, Kutnink MA, Hawkes WC. 1987. Measurement of vitamin C in blood components by high-performance liquid chromatography. Implication in assessing vitamin C status . Ann NY Acad Sci 498:389–401.
Ono K. 1986. Secondary hyperoxalemia caused by vitamin C supplementation in regular hemodialysis patients. Clin Nephrol 26:239–243.
Oreopoulos DG, Lindeman RD, VanderJagt DJ, Tzamaloukas AH, Bhagavan HN, Garry PJ. 1993. Renal excretion of ascorbic acid: Effect of age and sex. J Am Coll Nutr 12:537–542.
Ortega RM, Lopez-Sobaler AM, Quintas ME, Martinez RM, Andres P. 1998. The influence of smoking on vitamin C status during the third trimester of pregnancy and on vitamin C levels in maternal milk. J Am Coll Nutr 17:379–384.
O'Toole P, Lombard M. 1996. Vitamin C and gastric cancer: Supplements for some or fruit for all? Gut 39:345–347.
Panayiotidis M, Collins AR. 1997. Ex vivo assessment of lymphocyte antioxidant status using the comet assay. Free Rad Res 27:533–537.
Pandey DK, Shekelle R, Selwyn BJ, Tangney C, Stamler J. 1995. Dietary vitamin C and beta-carotene and risk of death in middle-aged men. The Western Electric Study. Am J Epidemiol 142:1269–1278.
Panush RS, Delafuente JC, Katz P, Johnson J. 1982. Modulation of certain immunologic responses by vitamin C. III. Potentiation of in vitro and in vivo ly phocyte responses. Int J Vitam Nutr Res Suppl 23:35–47.
Park JB, Levine M. 1996. Purification, cloning and expression of dehydroascorbic acid-reducing activity from human neutrophils: Identification as glutaredoxin. Biochem J 315:931–938.
Parkkinen J, Vaaranen O, Vahtera E. 1996. Plasma ascorbate protects coagulation factors against photooxidation . Thromb Haemost 75:292–297.
Pelletier O. 1977. Vitamin C and tobacco. Int J Vitam Nutr Res Suppl 16:147–170.
Perrig WJ, Perrig P, Stahelin HB. 1997. The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc 45:718–724.
Peters EM, Goetzsche JM, Grobbelaar B, Noakes TD. 1993. Vitamin C suppleme tation reduces the incidence of postrace symptoms of upper-respiratory-tract infection in ultramarathon runners. Am J Clin Nutr 57:170–174.
Pfeffer F, Valdes-Ramos R, Avila-Rosas H, Meza C, Casanueva E. 1996. Iron, zinc and vitamin C nutritional status is not related to weight gain in pregnant women. Nutr Res 16:555–564.
Phull PS, Price AB, White KL, Schorah CJ, Jacyna MR. 1999. Gastroduodenal mucosal vitamin-C levels in Helicobacter pylori infection. Scand J Gastroenterol 34:361–366.
Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. 1996. Exposure of the US population to environmental tobacco smoke: The Third National Health and Nutrition Examination Survey, 1988 to 1991 . J Am Med Assoc 275:1233–1240.
Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. 1998. Vitamin C exhibits pro-oxidant properties. Nature 392:559.
Pohl H, Reidy JA. 1989. Vitamin C intake influences the bleomycin-induced chr mosome damage assay: Implications for detection of cancer susceptibility and chromosome breakage syndromes. Mutat Res 224:247–252.
Powers HJ, Loban A, Silvers K, Gibson AT. 1995. Vitamin C at concentrations observed in premature babies inhibits the ferroxidase activity of caeruloplasmin. Free Radic Res 22:57–65.
Prieme H, Loft S, Nyyssonen K, Salonen JT, Poulsen HE. 1997. No effect of supplementation with vitamin E, ascorbic acid, or coenzyme Q10 on oxidative DNA damage estimated by 8-hydroxy-7,8-dihydro-2'-deoxyguanosine excretion in smokers. Am J Clin Nutr 65:503–507.
Pryor WA. 1992. Biological effects of cigarette smoke, wood smoke, and the smoke from plastics: The use of electron spin resonance. Free Radic Biol Med 13:659–676.
Pryor WA. 1997. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Hlth Perspect 105:875–882.
Pryor WA, Prier DG, Church DF. 1983. Electron-spin resonance study of mai stream and sidestream cigarette smoke: Nature of the free radicals in gasphase smoke and in cigarette tar. Environ Hlth Perspect 47:345–355.
Rajalakshmi R, Deodhar AD, Ramarkrishnan CV. 1965. Vitamin C secretion during lactation. Acta Paediatr Scand 54:375–382.
Rebouche CJ. 1995. Renal handling of carnitine in experimental vitamin C def ciency. Metabolism 44:1639–1643.
Rees DC, Kelsey H, Richards JDM. 1993. Acute haemolysis induced by high dose ascorbic acid in glucose-6-phosphate dehydrogenase deficiency. Br Med J 306:841–842.
Rehman A, Collis CS, Yang M, Kelly M, Diplock AT, Halliwell B, Rice-Evans C. 1998. The effects of iron and vitamin C co-supplementation on oxidative da age to DNA in healthy volunteers. Biochem Biophys Res Commun 246:293–298.
Reilly M, Delanty N, Lawson JA, Fitzgerald GA. 1996. Modulation of oxidant stress in vivo in chronic cigarette smokers . Circulation 94:19–25.
Rhead WJ, Schrauzer GN. 1971. Risks of long-term ascorbic acid overdosage. Nutr Rev 29:262–263.
Rifici VA, Khachadurian AK. 1993. Dietary supplementation with vitamins C and E inhibits in vitro oxidation of lipoproteins. J Am Coll Nutr 12:631–637.
Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. 1993. Vitamin E consumption and the risk of coronary heart disease in men . N Engl J Med 328:1450–1456.
Rivers, JM. 1987. Safety of high-level vitamin C ingestion. Ann NY Acad Sci 498:445–454.
Robertson JM, Donner AP, Trevithick JR. 1989. Vitamin E intake and risk of cataracts in humans. Ann NY Acad Sci 570:372–382.
Rokitzki L, Hinkel S, Klemp C, Cufi D, Keul J. 1994. Dietary, serum and urine ascorbic acid status in male athletes. Int J Sports Med 15:435–440.
Rokkas T, Papatheodorou G, Karameris A, Mavrogeorgis A, Kalogeropoulos N, Giannikos N. 1995. Helicobacter pylori infection and gastric juice vitamin C levels. Impact of eradication . Dig Dis Sci 40:615–621.
Romney SL, Duttagupta C, Basu J, Palan PR, Karp S, Slagle NS, Dwyer A, Wassertheil-Smoller S, Wylie-Rosett J. 1985. Plasma vitamin C and uterine cervical dysplasia. Am J Obstet Gynecol 151:976–980.
Ronchetti IP, Quaglino D Jr, Bergamini G. 1996. Ascorbic acid and connective tissue. Subcell Biochem 25:249–264.
Rose RC, Richer SP, Bode AM. 1998. Ocular oxidants and antioxidant protection. Proc Soc Exp Biol Med 217:397–407.
Rumsey SC, Levine M. 1998. Absorption, transport, and disposition of ascorbic acid in humans . J Nutr Biochem 9:116–130.
Russell AL. 1967. Epidemiology of periodontal disease. Int Dent J 17:282–296.
Sahyoun NR, Jacques PF, Russell RM. 1996. Carotenoids, vitamins C and E, and mortality in an elderly population . Am J Epidemiol 144:501–511.
Salmenpera L. 1984. Vitamin C nutrition during prolonged lactation: Optimal in infants while marginal in some mothers. Am J Clin Nutr 40:1050–1056.
Salonen JT, Salonen R, Nyyssonen K, Korpela H. 1992. Iron sufficiency is associated with hypertension and excess risk of myocardial infarction: The Kuopio Ischemic Heart Disease Risk Factor Study (KIHD). Circulation 85:864–876.
Samman S, Brown AJ, Beltran C, Singh S. 1997. The effect of ascorbic acid on plasma lipids and oxidisability of LDL in male smokers. Eur J Clin Nutr 51:472–477.
Sasaki A, Kondo K, Sakamoto Y, Kurata H, Itakura H, Ikeda Y. 1997. Smoking cessation increases the resistance of low-density lipoprotein to oxidation. Atherosclerosis 130:109–111.
Satarug S, Haswell-Elkins MR, Tsuda M, Mairiang P, Sithithaworn P, Mairiang E, Esumi H, Sukprasert S, Yongvanit P, Elkins DB. 1996. Thiocyanate-independent nitrosation in humans with carcinogenic parasite infection. Carcinogenesis 17:1075–1081.
Sauberlich HE. 1994. Pharmacology of vitamin C. Annu Rev Nutr 14371–391.
Scaccini C, Jialal I. 1994. LDL Modification by activated polymorphonuclear leukocytes: A cellular model of mild oxidative stress. Free Radic Biol Med 16:49–55.
Schectman G, Byrd JC, Hoffmann R. 1991. Ascorbic acid requirements for smokers: Analysis of a population survey. Am J Clin Nutr 53:1466–1470.
Schmidt KH, Hagmaier V, Hornig DH, Vuilleumier JP, Rutishauser G. 1981. Urinary oxalate excretion after large intakes of ascorbic acid in man. Am J Clin Nutr 34:305–311.
Schrauzer GN, Rhead WJ. 1973. Ascorbic acid abuse: Effects on long-term ingestion of excessive amounts on blood levels and urinary excretion. Int J Vitam Nutr Res 43:201–211.
Schrauzer GN, Ishmael D, Kiefer GW. 1975. Some aspects of current vitamin C usage: Diminished high-altitude resistance following overdosage. Ann NY Acad Sci 258:377–381.
Schwartz J, Weiss ST. 1994. Relationship between dietary vitamin C intake and pulmonary function in the First National Health and Nutrition Examination Survey (NHANES I). Am J Clin Nutr 59:110–114.
Schwarz KB, Cox J, Sharma S, Witter F, Clement L, Sehnert SS, Risby TH. 1995. Cigarette smoking is pro-oxidant in pregnant women regardless of antioxidant nutrient intake. J Nutr Environ Med 5:225–234.
Sharpe PC, MacAuley D, McCrum EE, Stott G, Evans AE, Mulholland C, Boreham CA, Duly E, Trinick TR. 1994. Ascorbate and exercise in the Northern Ireland population. Int J Vitam Nutr Res 64:277–282.
Shekelle RB, Lepper M, Liu S, Maliza C, Raynor WJ, Rossof AH. 1981. Dietary vitamin A and risk of cancer in the Western Electric Study . Lancet 2:1185–1190.
Shilotri PG, Bhat KS. 1977. Effect of mega doses of vitamin C on bactericidal activity of leukocytes . Am J Clin Nutr 30:1077–1081.
Siegel C, Barker B, Kunstadter M. 1982. Conditioned oral scurvy due to megavitamin C withdrawal. J Periodontol 53:453–455.
Sies H, Stahl W. 1995. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants . Am J Clin Nutr 62:1315S–1321S.
Simon JA. 1992. Vitamin C and cardiovascular disease: A review. J Am Coll Nutr 11:107–125.
Simon JA, Hudes ES, Browner WS. 1998. Serum ascorbic acid and cardiovascular disease prevalence in US adults . Epidemiology 9:316–321.
Singh RB, Ghosh S, Niaz MA, Singh R, Beegum R, Chibo H, Shoumin Z, Postiglione A. 1995. Dietary intake, plasma levels of antioxidant vitamins, and oxidative stress in relation to coronary artery disease in elderly subjects . Am J Cardiol 76:1233–1238.
Sinha R, Block G, Taylor PR. 1993. Problems with estimating vitamin C intakes. Am J Clin Nutr 57:547–550.
Skaper SD, Fabris M, Ferrari V, Carbonare MD, Leon A. 1997. Quercetin protects cutaneous tissue-associated cell types including sensory neurons from oxidative stress induced by glutathione depletion: Cooperative effects of ascorbic acid. Free Radic Biol Med 22:669–678.
Sneed SM, Zane C, Thomas MR. 1981. The effects of ascorbic acid, vitamin B6, vitamin B12, and folic acid supplementation on the breast milk and maternal nutritional status of low socioeconomic lactating women. Am J Clin Nutr 34:1338–1346.
Solzbach U, Hornig B, Jeserich M, Just H. 1997. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation 96:1513–1519.
Specker BL, Beck A, Kalkwarf H., Ho M. 1997. Randomized trial of varying mineral intake on total body bone mineral accretion during the first year of life. Pediatrics 99:e12.
Stein HB, Hasan A, Fox IH. 1976. Ascorbic acid-induced uricosuria. Ann Intern Med 84:385–388.
Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. 1998. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 97:2222–2229.
Thomas MR, Kawamoto J, Sneed SM, Eakin R. 1979. The effects of vitamin C, vitamin B6, and vitamin B12 supplementation on the breast milk and maternal status of well-nourished women. Am J Clin Nutr 32:1679–1685.
Thomas MR, Sneed SM, Wei C, Nail PA, Wilson M, Sprinkle EE. 1980. The effects of vitamin C, vitamin B6, vitamin B12, folic acid, riboflavin, and thiamin on the breast milk and maternal status of well-nourished women at 6 months postpartum. Am J Clin Nutr 33:2151–2156.
Timimi FK, Ting HH, Haley EA, Roddy MA, Ganz P, Creager MA. 1998. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol 31:552–557.
Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. 1996. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 97:22–28.
Ting HH, Timimi FK, Haley EA, Roddy MA, Ganz P, Creager MA. 1997. Vitamin C improves endothelium-dependent vasodilation in forearm resistance vessels of humans with hypercholesterolemia. Circulation 95:2617–2622.
Tiselius HG, Almgard LE. 1977. The diurnal urinary excretion of oxalate and the effect of pyridoxine and ascorbate on oxalate excretion. Eur Urol 3:41–46.
Tlaskal P, Novakova V. 1990. Vitamins C and E in neonates and their mothers. Cesk Pediatr 45:339–343.
Tribble DL, Giuliano LJ, Fortmann SP. 1993. Reduced plasma ascorbic acid concentrations in nonsmokers regularly exposed to environmental tobacco smoke. Am J Clin Nutr 58:886–890.
Tsao CS. 1997. An overview of ascorbic acid chemistry and biochemistry. In: Packer L, Fuchs J, eds. Vitamin C in Health and Disease. New York: Marcel Dekker. Pp. 25–58.
Tsao CS, Leung PY. 1988. Urinary ascorbic acid levels following the withdrawal of large doses of ascorbic acid in guinea pigs. J Nutr 118:895–900.
Tsao CS, Salimi SL. 1984. Effect of large intake of ascorbic acid on urinary and plasma oxalic acid levels. Int J Vitam Nutr Res 54:245–249.
Udipi SA, Kirksey A, West K, Giacoia G. 1985. Vitamin B6, vitamin C and folacin levels in milk from mothers of term and preterm infants during the neonatal period. Am J Clin Nutr 42:522–530.
Urivetzky M, Kessaris D, Smith AD. 1992. Ascorbic acid overdosing: A risk factor for calcium oxalate nephrolithiasis . J Urol 147:1215–1218.
Valkonen M, Kuusi T. 1998. Passive smoking induces atherogenic changes in low-density lipoprotein . Circulation 97:2012–2016.
VanderJagt DJ, Garry PJ, Bhagavan HN. 1987. Ascorbic acid intake and plasma levels in healthy elderly people. Am J Clin Nutr 46:290–294.
Van Eekelen M. 1953. Occurrence of vitamin C in foods. Proc Nutr Soc 12:228–232.
Vitale S, West S, Hallfrisch J, Alston C, Wang F, Moorman C, Muller D, Singh V, Taylor HR. 1993. Plasma antioxidants and risk of cortical and nuclear cataract. Epidemiology 4:195–203.
Vogel RI, Lamster IB, Wechsler SA, Macedo B, Hartley LJ, Macedo JA. 1986. The effects of megadoses of ascorbic acid on PMN chemotaxis and experimental gingivitis. J Periodontol 57:472–479.
Wandzilak TR, D'Andre SD, Davis PA, Williams HE. 1994. Effect of high dose vitamin C on urinary oxalate levels. J Urol 151:834–837.
Wang Y, Russo TA, Kwon O, Chanock S, Rumsey SC, Levine M. 1997. Ascorbate recycling in human neutrophils: Induction by bacteria. Proc Natl Acad Sci USA 94:13816–13819.
Waring AJ, Drake IM, Schorah CJ, White KL, Lynch DA, Axon AT, Dixon MF. 1996. Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: Effects of gastritis and oral supplementation. Gut 38:171–176.
Wassertheil-Smoller S, Romney SL, Wylie-Rosett J, Slagle S, Miller G, Lucido D, Duttagupta C, Palan PR. 1981. Dietary vitamin C and uterine cervical dysplasia. Am J Epidemiol 114:714–724.
Weber C, Wolfgang E, Weber K, Weber PC. 1996. Increased adhesiveness of isolated monocytes to endothelium is prevented by vitamin C intake in smokers. Circulation 93:1488–1492.
Wen Y, Cooke T Feely, J. 1997. The effect of pharmacological supplementation with vitamin C on low-density lipoprotein oxidation. Br J Clin Pharmacol 44:94–97.
Witt EH, Reznick AZ, Viguie CA, Starke-Reed P, Packer L. 1992. Exercise, oxidative damage and effects of antioxidant manipulation . J Nutr 122:766–773.
Witztum JL, Steinberg D. 1991. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 88:1785–1792.
Woolfe SN, Kenney EB, Hume WR, Carranza FA Jr. 1984. Relationship of ascorbic acid levels of blood and gingival tissue with response to periodontal therapy. J Clin Periodontol 11:159–165.
Yong LC, Brown CC, Schatzkin A, Dresser CM, Slesinski MJ, Cox CS, Taylor PR. 1997. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I Epidemiologic Followup Study. Am J Epidemiol 146:231–243.
Young JC, Kenyon EM, Calabrese EJ. 1990. Inhibition of beta-glucuronidase in human urine by ascorbic acid. Hum Exp Toxicol 9:165–170.
Zatonski W, Przewozniak K, Howe GR, Maisonneuve P, Walker AM, Boyle P. 1991. Nutritional factors and pancreatic cancer: A case-control study from south west Poland. Int J Cancer 48:390–394.