8
β-Carotene and Other Carotenoids
SUMMARY
Blood concentrations of carotenoids are the best biological markers for consumption of fruits and vegetables. A large body of observational epidemiological evidence suggests that higher blood concentrations of β-carotene and other carotenoids obtained from foods are associated with lower risk of several chronic diseases. This evidence, although consistent, cannot be used to establish a requirement for β-carotene or carotenoid intake because the observed effects may be due to other substances found in carotenoidrich food, or to other behavioral correlates of increased fruit and vegetable consumption. While there is evidence that β-carotene is an antioxidant in vitro, its importance to health is not known. The one clear function of certain carotenoids that is firmly linked to a health outcome is the provitamin A activity of some dietary carotenoids (α-carotene, β-carotene, and β-cryptoxanthin) and their role in the prevention of vitamin A deficiency. Establishment of a requirement for carotenoids based upon vitamin A activity must be done in concert with the evaluation of Dietary Reference In-takes (DRIs) for vitamin A, which was not included in this report, but will be addressed in a subsequent DRI report. Although no DRIs are proposed for β-carotene or other carotenoids at the present time, existing recommendations for increased consumption of carotenoid-rich fruits and vegetables are supported. Based on evidence that β-carotene supplements have not been shown to confer any benefit for the prevention of the major chronic diseases and may cause harm in certain subgroups, it is concluded that β-carotene supplements are not advisable, other than as a provitamin
A source and for the prevention and control of vitamin A deficiency in at-risk populations.
BACKGROUND INFORMATION
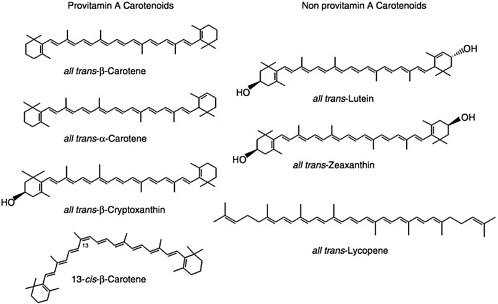
The most prevalent carotenoids in North American diets include the following: α-carotene, β-carotene, lycopene, lutein, zeaxanthin, and β-cryptoxanthin. The structures of these carotenoids are shown in Figure 8-1. Three of these carotenoids, namely α-carotene, β-carotene, and β-cryptoxanthin, can be converted into retinol and are thus referred to as provitamin A carotenoids. Lycopene, lutein, and zeaxanthin have no vitamin A activity and are thus referred to as nonprovitamin A carotenoids. Most naturally occurring carotenoids are in the all-trans-configuration; but under conditions of heating, for example, cis-isomers such as 13-cis-β-carotene (Figure 8-1) are formed.
Functions and Actions
The various biological effects of carotenoids can be classified into functions, actions, and associations. Carotenoids function in plants and in photosynthetic bacteria as accessory pigments in photosynthesis and protect against photosensitization in animals, plants, and bacteria. In humans, the only known function of carotenoids is vitamin A activity (provitamin A carotenoids only).
Carotenoids also are thought to have a variety of different actions, including possible antioxidant activity, immunoenhancement, inhibition of mutagenesis and transformation, inhibition of premalignant lesions, quenching of nonphotochemical fluorescence, and activity as a pigment in primate macula (Olson, 1999). Carotenoids have also been associated with various health effects: decreased risk of macular degeneration and cataracts, decreased risk of some cancers, and decreased risk of some cardiovascular events (Olson, 1999).
However, as described above, the only known function of carotenoids in humans is to act as a source of vitamin A in the diet. This function, as well as carotenoid actions and associations, is reviewed elsewhere (Krinsky, 1993; Olson, 1989) and discussed in subsequent sections.
Physiology of Absorption, Metabolism, and Excretion
Absorption
The intestinal absorption of dietary carotenoids is facilitated by
the formation of bile acid micelles. The hydrocarbon backbone of the carotenoids makes them insoluble in water, and like other non-polar lipids, they must be solubilized within micelles in the gastrointestinal tract to allow for absorption. Micellar solubilization facilitates the diffusion of lipids across the unstirred water layer. The presence of fat in the small intestine stimulates the secretion of bile acids from the gall bladder and improves the absorption of carotenoids by increasing the size and stability of micelles, thus allowing more carotenoids to be solubilized. The uptake of tene by the mucosal cell is believed to occur by passive diffusion (Hollander and Ruble, 1978). Uptake by these cells, however, is not sufficient for absorption to be completed. Once inside the mucosal cell, carotenoids or their metabolic products (e.g., vitamin A) must also be incorporated into chylomicrons and released into the lymphatics. When mucosal cells are sloughed off due to cell turnover, spilling their contents into the lumen of the gastrointestinal tract, carotenoids that have been taken up by the cells but not yet incorporated into chylomicrons are lost into the lumen (Boileau et al., 1999).
Metabolism, Transport, and Excretion
Carotenoids may be either absorbed intact, or in the case of those possessing vitamin A activity, cleaved to form vitamin A prior to secretion into lymph. Portal transport of carotenoids is minimal due to the lipophilic nature of their structures. Some portal transport of more polar metabolites, such as retinoic acid, can occur (Olson, 1999).
Carotenoid cleavage is accomplished either by the intestinal mucosal enzyme β-carotene 15,15′-dioxygenase (EC 1.13.11.21) or by noncentral cleavage mechanisms (Boileau et al., 1999; Olson, 1999; Parker, 1996; Wang, 1994). The extent of conversion of a highly bioavailable source of dietary β-carotene to vitamin A in humans has been shown to be between 60 and 75 percent, with an additional 15 percent of the β-carotene absorbed intact (Goodman et al., 1966). However, absorption of most carotenoids from foods is considerably lower and can be as low as 2 percent (Rodriguez and Irwin, 1972). The effects of dietary and nondietary factors on the efficiency of carotenoid absorption are reviewed later.
Noncentral (or excentric) cleavage of carotenoids yields a wide variety of metabolic products, some of which are further metabolized. These cleavage products include aldehyde, acid, alcohol, and epoxide derivatives (Parker, 1996; Wang, 1994). Isomerization of
carotenoids or their metabolic products may occur in vivo because isomers have been found upon extraction of carotenoids from human tissues (Clinton et al., 1996). Although little attention has been given to the study of carotenoid excretion pathways, epoxides and carotenoid metabolic products with less than 15 carbon chain lengths would presumably have no vitamin A activity. It is assumed that bile and urine would be excretion routes for metabolites (Olson, 1999).
The carotenoids are transported in blood exclusively by lipoproteins. The carotenoid content of individual lipoprotein classes is not homogeneous. In the fasted state, the hydrocarbon carotenoids such as α-carotene, β-carotene, and lycopene are carried predominantly by low-density lipoprotein. The remaining carotenoids, including the more polar xanthophylls such as lutein and zeaxanthin, are carried by high-density lipoprotein (HDL) and, to a lesser extent, by very low-density lipoprotein (Johnson and Russell, 1992; Parker, 1996; Traber et al., 1994). It is thought that β-carotene and other hydrocarbon carotenoids reside in the hydrophobic core of the particles, whereas the more polar xanthophylls reside closer to the surface (Parker, 1996).
β-Carotene is the most studied carotenoid in terms of metabolism and its potential effects on health. Lycopene, lutein, zeaxanthin, and α-carotene have received increasing attention in recent years. Much remains to be learned, however, about the relative metabolic effects of these carotenoids.
Body Stores
Recently, 34 carotenoids were identified in human serum and milk (Khachik et al., 1997b). Of these, 13 were geometrical isomers of their all-trans parent structures and 8 were metabolites. This finding is in contrast to the up to 50 carotenoids that have been identified in the U.S. diet and the more than 600 found in nature. The most prevalent carotenoids in human serum (Khachik et al., 1997b) are the same as those most commonly found in the diet: β-carotene, lycopene, and lutein (Nebeling et al., 1997). Cis-isomers of lycopene are commonly found in the serum and in fact have been shown to constitute more than 50 percent of the total serum lycopene (Stahl et al., 1992). In contrast, cis-isomers of β-carotene are considerably less common in serum with the trans-isomers being more common. In addition to these forms of α-carotene, β-carotene, lycopene, and zeaxanthin are also major serum carotenoids. The concentrations of various carotenoids in human serum and
tissues are highly variable and likely depend on a number of factors such as food sources, efficiency of absorption, amount of fat in the diet, and so forth (Table 8-1).
The serum concentration of carotenoids after a single dose peaks at 24 to 48 hours post dose (Johnson and Russell, 1992). The earliest postprandial serum appearance of carotenoids is in the chylomicron fraction. It has been proposed that the increase in carotenoids in the triglyceride-rich lipoprotein fraction (primarily chylomicrons) be used for quantitating carotenoid absorption (van Vliet et al., 1995). This would provide a more direct measure of absorption because total serum carotenoid content is not an exclusive measure of newly absorbed carotenoids.
Data from the Third National Health and Nutrition Examination Survey (NHANES III) demonstrate the variability of normal serum carotenoid concentrations (Appendix Table F-4, Table F-5, Table F-6, Table F-7, through Table F-8). This variability is attributed to a variety of life-style and physiological factors. In a recent population-based study, Brady et al. (1996) reported that lower serum concentrations of α-carotene, β-carotene, β-cryptoxanthin, lutein, and zeaxanthin, but not lycopene, were generally associated with male gender, smoking, younger age, lower non-HDL cholesterol, greater ethanol consumption, and higher body mass index.
The delivery of carotenoids to extrahepatic tissue is accomplished through the interaction of lipoprotein particles with receptors and the degradation of lipoproteins by extrahepatic enzymes such as lipoprotein lipase. Carotenoids are present in a number of human tissues including adipose, liver, kidney, and adrenal, but adipose tissue and liver appear to be the main storage sites (Parker, 1996). However, based on a wet tissue weight, the liver, adrenal gland, and testes contain the highest per-gram concentrations (Stahl et al., 1992). Similar to what is reported in serum, β-carotene, lutein, and lycopene are the main tissue carotenoids, although α-carotene, β-cryptoxanthin, and zeaxanthin are also present (Boileau et al., 1999). In contrast to serum profiles, 9-cis-β-carotene is consistently present in storage tissues. In both serum and tissue storage, lycopene cis-isomers constitute greater than 50 percent of the total lycopene present (Clinton et al., 1996; Stahl et al., 1992).
Clinical Effects of Inadequate Intake
If adequate retinol is provided in the diet, there are no known clinical effects of consuming diets low in carotenes over the short term. One study of premenopausal women consuming low-carotene
diets in a metabolic ward reported skin lesions (Burri et al., 1993). However, this effect was not observed after 60 days of depletion in a subsequent β-carotene depletion study by the same group of investigators (Lin et al., 1998). These studies of carotene-deficient diets were reported to increase various measures of oxidative susceptibility (Dixon et al., 1994, 1998; Lin et al., 1998), but as discussed below, this is of uncertain relevance with regard to clinical outcomes.
SELECTION OF POSSIBLE INDICATORS FOR ESTIMATING THE REQUIREMENT FOR β-CAROTENE AND OTHER CAROTENOIDS
Vitamin A Equivalency
Vitamin A equivalency is a possible indicator for establishing requirements for provitamin A carotenoids. However, any such establishment of requirements for carotenoids based on vitamin A activity must be considered in concert with the evaluation of requirements for vitamin A. This information will be presented in a later Dietary Reference Intakes report.
Markers of Antioxidant Activity
The effect of increasing β-carotene intake on several markers of antioxidant activity has been investigated in a series of studies involving humans. These studies have examined antioxidant marker activity in apparently healthy men and women as well as in subjects who were physiologically challenged (i.e., smokers and patients with coronary disease or cystic fibrosis).
Studies of the effect of β-carotene intake on measures of antioxidant activity are summarized in Table 8-2. The dietary source of β-carotene ranged from modification of diets with normally consumed foods to giving supplements that provided as much as 120 mg/day of a highly bioavailable preparation. In general, subjects in most studies consumed β-carotene in amounts that would be difficult to achieve from foods alone and, as a result, relate to the pharmacological range of intakes.
The findings reported in Table 8-2 indicate that β-carotene supplementation did not alter, or inconsistently alter, markers of antioxidant activity, which were somewhat dependent on β-carotene intake. In studies in which subjects were fed less than 25 mg/day of β-carotene, either from foods or as a supplement, changes in the markers for antioxidant activity were minimal. Exceptions noted
TABLE 8-1 Concentrations of Selected Carotenoids in Human Serum and Tissues
|
Carotenoid |
Serum (µmol/L) |
Liver (µmol/g) |
|
α-Carotene |
0.02–0.47 |
0.075–10.8 |
|
(1.0–25.3 µg/dL) |
(0.04–5.8 µg/g) |
|
|
β-Carotene |
0.04–2.26 |
0.39–19.4 |
|
(2.2–122.7 µg/dL) |
(0.21–6.3 µg/g) |
|
|
β-Cryptoxanthin |
0.03–0.70 |
0.037–20.0 |
|
(1.4–38.2 µg/dL) |
(0.05–11.0 µg/g) |
|
|
Lutein |
0.10–1.23 |
0.10–3.0 |
|
(5.8–69.8 µg/dL) |
(0.06–6.9 µg/g) |
|
|
Lycopene |
0.05–1.05 |
0.20–17.2 |
|
(2.7–54.6 µg/dL) |
(0.11–11.1 µg/g) |
|
|
SOURCE: Data from Schmitz et al. (1991) and Kaplan et al. (1990) for tissues and Iowa State University Department of Statistics (1999) for serum. |
||
were decreased deoxyribonucleic acid strand breaks observed when 22 mg/day of β-carotene was administered as carrot juice (Pool-Zobel et al., 1997) and lowered copper-induced oxidation of low-density lipoprotein when 12 or 24 mg/day of β-carotene was given along with vitamins C and E (Mosca et al., 1997). As shown in Table 8-2, feeding β-carotene in amounts greater than 25 mg/day generally resulted in inconsistent responses of the biological markers monitored. Administration of β-carotene to subjects with increased oxidative stress (e.g., smoking, cystic fibrosis) was associated with more consistent evidence of decreased lipid peroxidation compared to studies in which subjects without known additional oxidative stress were given β-carotene. In studies that involved depletion followed by repletion of body stores of β-carotene, as indicated by plasma concentrations, the biological markers that were negatively altered as a result of depleted body stores of β-carotene were restored to baseline values as a consequence of repletion (Table 8-2).
In summary, results from some studies show improvement of measures of antioxidant activity due to intake of relatively high levels of β-carotene, while studies that investigated low to modest levels of β-carotene show no or inconsistent changes in the same activities.
|
Kidney(µmol/g) |
Lung(µmol/g) |
|
0.037–1.5 |
0.1–1.0 |
|
(0.02–0.80 µg/g) |
(0.05–0.54 µg/g) |
|
0.093–2.8 |
0.1–1.6 |
|
(0.05–1.5 µg/g) |
(0.05–0.86 µg/g) |
|
0.019–3.9 |
0.1–2.5 |
|
(0.05–2.2 µg/g) |
(0.05–1.4 µg/g) |
|
0.037–2.1 |
0.1–2.3 |
|
(0.05–5.9 µg/g) |
(0.05–1.3 µg/g) |
|
0.093–2.4 |
0.1–1.0 |
|
(0.05–1.3 µg/g) |
(0.05–2.3 µg/g) |
Some benefit of feeding increased amounts of β-carotene was observed for several markers of antioxidant activity when body stores were relatively low or when an oxidant-type stress was present. These observations suggest that the lack of effect in some studies may be due to study populations whose baseline β-carotene status was already adequate. Nevertheless, current data do not provide convincing evidence that substantially increasing β-carotene intake above current dietary intakes has a significant effect on measures of antioxidant status. Also, none of these markers has been validated to be predictive of any known health outcomes. Therefore, these data are inadequate for the estimation of a requirement for β-carotene.
Gap Junctional Communication
Appropriate communication among cells is essential for the coordination of biochemical functions in complex, multicellular organisms. One theory suggests that failure of signaling is one cause of cell overgrowth and eventually cancer. Two research groups have demonstrated that carotenoids stimulate gap junction communication between cells in vitro (Sies and Stahl, 1997; Zhang et al., 1991).
TABLE 8-2 β-Carotene Intake and Measures of Antioxidant Activity in Selected Studies
|
Reference, Country |
Subjects |
β-Carotene Dose |
|
Richards et al., 1990 South Africa |
40 smokers, average age 33 y; received placebo and 20 received treatment |
40 mg/d, Roche prep |
|
Mobarhan et al., 1990; Gottlieb et al., 1993 United States |
15 healthy men, aged 19–30 y; randomly assigned repletion levels |
Carotene-free diet (depletion); Repletion: 15 mg/d or 120 mg/d, Roche prep |
|
Van Poppel et al., 1992a, 1992b, 1995 Holland |
143 male smokers, average age 39 y; randomly assigned to placebo or treatment |
40 mg/d first 2 wk 20 mg/d next 12 wk |
|
Allard et al., 1994 Canada |
38 male nonsmokers, 25 male smokers, aged 20–75 y; randomly assigned to placebo or treatment |
20 mg/d, Roche prep |
|
Calzada et al., 1995 United States |
12 healthy men and 7 women, aged 21–50 y; randomly assigned to placebo or treatment |
15 mg/d, Roche prep |
|
Gaziano et al., 1995 United States |
4 healthy men and 12 women, aged 25–47 y; randomly assigned to either synthetic (BASF) or natural (Henkel) β-carotene |
100 mg/d load dose; natural treatment, 66 or 100 mg/2d; synthetic treatment, 50 mg/2 d |
|
Winklhofer-Roob et al., 1995 Switzerland |
CFm patients, 32 boys and girls; average age 10.8 y |
0.5 mg/kg BWn/d, 3M Medica, Ltd. |
|
Clevidence et al., 1997 United States |
5 healthy men and 7 women, aged 27–61 y |
18 mg/d additional as foods; kale, tomato juice, sweet potato |
|
Duration |
Plasma β-Carotene (µmol/L) |
Findings |
|
6 wk |
Baseline—0.50 (27 µg/dL) Trta—2.06 (111 µg/dL) |
No change in leukocyte sister chromatid exchange |
|
2 wk 4 wk |
Baseline—0.24 (13 µg/dL) Depletion—0.09 (5 µg/dL) 15 mg/d—3.32 (178 µg/dL) 120 mg/d—8.74 (469 µg/dL) |
↓ Breath pentane on 120 mg/d only; ↓ Serum lipid peroxide levels, both repletion levels |
|
2 wk 12 wk |
Baseline—0.33 (18 µg/dL) Trt at 14 wk—4.36 (234 µg/dL) |
↓ Sputum nuclei No change in lymphocyte sister chromatid exchange or urinary 8-oxodG b |
|
4 wk |
Placebo/NSc—0.38 (20 µg/dL) Placebo/Sd—0.27 (14 µg/dL) Trt/NS—3.50 (188 µg/dL) Trt/S—3.38 (181 µg/dL) |
↓ Breath pentane in smokers No change in breath pentane in nonsmokers No change in breath ethane, RBCe, MDAf or plasma SeGSHPxg in either group |
|
14 d 56 d |
Baseline—0.87 (47 µg/dL) Trt—3.07 (165 µg/dL) |
No change in plasma Trolox equivalent antioxidant activity |
|
6 d load; followed by 21 d treatment |
Baseline—0.25 (13 µg/dL) Both Trts—1.39 (75 µg/dL) |
↑ Cu2+-induced LDLk oxidation No change in AAPHl -induced LDL oxidation |
|
3 m |
Baseline—0.09 (5 µg/dL) Trt—1.07 (57 µg/dL) |
↓ Plasma MDA and Cu2+-induced LDL oxidation |
|
3 wk |
Baseline—0.29 (15 µg/dL) Trt—0.76 (40.2 µg/dL) |
No change in plasma ORACo, plasma hydroperoxides, LDL TBARS, or 8-oxodG |
TABLE 8-2 Continued
|
Duration |
Plasma β-Carotene (µmol/L) |
Findings |
|
2 wk |
Baseline/NS—0.95 (51 µg/dL) Baseline/S—0.58 (31 µg/dL) Trt/NS—1.13 (61 µg/dL) Trt/S—0.82 (44 µg/dL) |
↑ RBC CuZn-SOD activity; No change in plasma MDA, GSHp, GSSG, -SH groups, carbonyls, or Se-GSHPx activity |
|
12 wk |
Baseline—0.30 (16 µg/dL) 12 mg/d—1.99 (107 µg/dL) 24 mg/d—3.01 (162 µg/dL) |
↓ Copper-induced LDL oxidation |
|
2 wk depletion; 4 wk added food containing β-carotene |
Not reported |
↓ DNA strand breaks (COMET) and oxidized pyrimidine bases in lymphocytes |
|
0.56 (29.8 µg/dL) as criteria for establishing high/low intake |
No difference in lymphocyte DNA adducts between high and low plasma β-carotene groups |
|
|
100 d depletion; 20 d repletion |
Baseline—0.76 (40.2 µg/dL) Depletion—0.33 (17.5 µg/dL) Repletion—1.73 (91.5 µg/dL) |
Depletion ↑ plasma MDA and LDL oxidation rate (carbonyl production). Repletion ↓ LDL oxidation rate below baseline |
|
12 wk |
Baseline—0.08 (4.8 µg/dL) 12 wk—0.60 (31.7 µg/dL) |
↓ Plasma MDA on high-dose βC |
|
4 wk |
Baseline—0.23 (12.3 µg/dL) Trt—1.21 (64.9 µg/dL) |
↓ Breath pentane, LDL oxidation No change in plasma total peroxyl radical trapping |
|
i SOD = superoxide dismutase. j GSSG = erythrocyte oxidized glutathione. k LDL = low-density lipoprotein. l AAPH = 2,2′-azobis[2-amidinopropane]dihydrochloride. m CF = cystic fibrosis. n BW = body weight. o ORAC = oxygen radical absorbance capacity. p GSH = glutathione. |
||
It is not known whether the parent carotenoids or their metabolites are the active factors (Hanusch et al., 1995), nor is it known whether carotenoids influence this communication process in vivo. More study is needed to ascertain whether carotenoids play a direct role in cell-cell communication and, if so, what health outcomes are influenced by this action.
Immune Function
There has been great interest in the potential role of carotenoids in enhancement of the immune response. Children with vitamin A deficiency suffer from compromised immunity and have difficulty protecting themselves from infections. It is important to remember, however, that studies conducted with provitamin A carotenoids may yield results that are attributable to the conversion of carotenoids to vitamin A or other retinoids, not to the effects of the intact carotenoid.
Santos et al. (1996) showed that long-term β-carotene supplementation enhanced natural killer cell activity in men 65 to 86 years of age, but not in men 51 to 64 years of age; enhancement by β-carotene in this age group was confirmed in a subsequent study (Santos et al., 1998). Hughes et al. (1997) evaluated mechanisms by which β-carotene might enable immune cells to act more efficiently. Subjects were supplemented for 26 days with either 15 mg of β-carotene or a placebo. Subjects receiving the β-carotene treatment had increases in expression of adhesion molecules by monocytes, in ex vivo secretion of tumor necrosis factor-α, and in the percentage of monocytes expressing major histocompatibility complex II, a cell surface molecule responsible for presenting antigen to T-helper cells.
Other immunological effects that carotenoids are reported to increase are lymphocyte response to mitogens (Kramer and Burri, 1997) and total white blood cells and helper T cells in human immunodeficiency virus-infected humans (Coodley et al., 1993). Whether these and the other effects noted are specific to carotenoids and are important in overall immunity is not confirmed. Therefore the usefulness of these as markers for disease has yet to be established.
Relationship of Carotenoid Intake to Chronic Disease
A vast number of observational studies, including both case-control and cohort studies, of carotenoids and chronic disease risk have
been conducted. Many of the studies are based upon estimated intake of carotenoids in the diet, while many include biochemical evaluation of carotenoid concentrations in blood. Because the dietary intake data are generally obtained via food frequency questionnaires, they do not provide quantitative estimates of carotenoid intake, but rather allow for relative ranking of carotenoid intakes within a population. The blood concentration data, however, are more quantitative and generally more comparable across studies.
Prospective blood carotenoid concentration studies may be particularly informative because blood samples are generally obtained several years prior to the clinical detection of disease. Thus, for the purposes of evaluating the association between quantitative carotenoid exposure and risk of chronic disease, the prospective blood concentration studies are most useful and are given the greatest weight in the analysis that follows. The studies in which food intakes were the basis for evaluating risk of disease are less useful due to the inherent problems in adequately estimating carotenoid intake. These studies, however, may give support to the overall evaluation of the role of carotenoids in chronic disease. The following section briefly summarizes some key research findings from observational studies of the relationship between carotenoids and chronic disease risk.
Mortality
Greenberg et al. (1996) obtained blood samples from 1,188 men and 532 women enrolled in a skin cancer prevention trial and examined the relationship between plasma β-carotene concentrations at entry and subsequent mortality over a median follow-up period of 8.2 years (Table 8-3). Persons in the lowest quartile of plasma β-carotene had a significant increase in their risk of dying compared to those with higher plasma concentrations of β-carotene. The adjusted relative risk was lowest for persons with plasma β-carotene concentrations in the range of 0.34 to 0.53 µmol/L (18 to 28 µg/dL) (quartile 3), with a risk reduction (compared to the lowest quartile) of 43 percent for total deaths, 43 percent for cardiovascular disease deaths, and 51 percent for cancer deaths. The relative risk for overall mortality was 38 percent lower for persons who had plasma β-carotene concentrations in the highest quartile compared to the lowest quartile (relative risk [RR] = 0.62; 95 percent confidence interval [CI] = 0.44−0.87). Thus, these results suggest that plasma β-carotene concentrations in the range of 0.34 to 0.53 µmol/L (18 to 28 µg/dL) are associated with the lowest risk of all-cause
TABLE 8-3 Concentrations of β-Carotene and Total Carotenoids in Plasma or Serum Associated with a Lower Risk of Various Health Outcomes in Selected Studies
|
Author |
Population |
|
Nomura et al., 1985 |
Japanese men |
|
Menkes et al., 1986 |
U.S. men and women |
|
Connett et al., 1989 |
MRFITb cohort men |
|
Greenberg et al., 1996 |
U.S. men and women, 24–84 y |
|
Jacques and Chylack, 1991 |
U.S. men and women, 40–70 y |
|
Riemersma et al., 1991 |
British men |
|
Stahelin et al., 1991 |
Swiss men |
|
Batieha et al., 1993 |
U.S. women |
|
EDCCSG, 1993 |
U.S. men and women |
|
Eichholzer et al., 1992; Gey et al., 1993b |
Swiss men |
|
Zheng et al., 1993 |
U.S. men and women |
|
Morris et al., 1994 |
U.S. men |
|
West et al., 1994 |
U.S. men and women, ≥40 y |
|
Sahyoun et al., 1996 |
U.S. men and women, >60 y |
|
Bonithon-Kopp et al., 1997 |
French men, >58 y French women, >58 y |
|
a Concentration in the quartile/quantile where the risk reduction was of the greatest magnitude. For studies that only report mean or median concentrations in the diseased and disease-free groups, the concentration is the level in the group that remained free of disease. SI Conversion factor used for β-carotene and total carotenoids = 0.01863 µg/dL to µmol/L, with the exception of Greenberg et al., 1996. |
|
mortality in U.S. adults. Note that these blood concentrations reflect levels in the absence of supplementation with β-carotene. Thus, this prospective study emphasizes the inverse association between β-carotene-rich foods and the risk of all-cause mortality.
Another cohort study of carotenoids and mortality examined both dietary intake of total carotenoids and plasma concentrations of
|
Endpoint |
β-Carotene Concentration (µmol/L)a |
Total Carotenoid Concentration (µmol/L)a |
|
Lung cancer |
≥0.54 (29 µg/dL) |
|
|
Lung cancer |
≥0.54 (29 µg/dL) |
|
|
Lung cancer |
≥0.22 (12 µg/dL)c |
≥1.84 (99 µg/dL)c |
|
All-cause mortality |
0.34–0.53 (18–28 µg/dL) |
|
|
Cataract |
>3.3 (177 µg/dL) |
|
|
Angina |
>0.54 (29 µg/dL)d |
|
|
Total cancers |
≥0.34 (18 µg/dL)d |
|
|
Lung cancer |
≥0.34 (18 µg/dL)d |
|
|
Cervical cancer |
≥0.26 (14 µg/dL) |
≥1.73 (93 µg/dL) |
|
Macular degeneration |
≥0.74 (40 µg/dL) |
≥2.39 (128 µg/dL) |
|
Ischemic heart disease |
≥0.18 (10 µg/dL)d |
|
|
Oropharyngeal cancers |
≥0.28 (15 µg/dL) |
≥1.75 (94 µg/dL) |
|
CHDe |
>3.16 (170 µg/dL) |
|
|
Macular degeneration |
>0.88 (47 µg/dL) |
|
|
Cancer mortality |
>3.13 (168 µg/dL) |
|
|
CHD mortality |
1.73–3.13 (93–168 µg/dL) |
|
|
All other causes, mortality |
>3.13 (168 µg/dL) |
|
|
Intima-media thickness |
>2.1 (113 µg/dL) |
|
|
Intima-media thickness |
>3.7 (199 µg/dL) |
|
|
b MRFIT = Multiple Risk Factor Intervention Trial. c Samples were stored at −50°C. d Assumes value given for carotene is 80% β-carotene. e CHD = coronary heart disease. |
||
total carotenoids as predictors of mortality (Sahyoun et al., 1996). Results indicated that mortality from cancer and all causes other than coronary heart disease (CHD) was lowest at a plasma concentration of 3.13 µmol/L (168 µg/dL) total carotenoids or greater; mortality from CHD was lowest at plasma concentrations of 1.73 to 3.13 µmol/L (93 to 168 µg/dL). Overall mortality was lowest at
dietary carotenoid intake levels of 8.6 mg/day (RR = 0.68 compared to those consuming 1.1 mg/day of carotenoids).
In the Western Electric cohort study, all-cause mortality was lowest for men who consumed the highest tertile of dietary β-carotene (RR = 0.80 for more than 4.1 mg/day of β-carotene versus less than 2.9 mg/day of β-carotene; p for trend = 0.01) (Pandey et al., 1995).
Cancer
Because there are literally hundreds of studies of carotenoids and cancer risk, this section emphasizes the results of epidemiological studies of all cancers combined, studies of carotenoids and lung cancer, and a few other selected tumor sites for which an inverse association with carotenoids is commonly seen.
Observational Epidemiological Studies. The Basel Prospective Study evaluated the relationship between plasma carotene concentrations in blood samples obtained in 1971–1973 and subsequent cancer mortality up to 1985 (Stahelin et al., 1991). Results showed that persons who went on to develop any cancer had significantly lower prediagnostic carotene concentrations than persons who remained alive and free of cancer in 1985 (mean plasma total carotenoid concentration 0.34 µmol/L [18 µg/dL] in those with cancer versus 0.43 µmol/L [23 µg/dL] in those free of cancer). The authors state that the reported carotene values represent approximately 80 percent β-carotene and 20 percent α-carotene; thus, plasma β-carotene concentrations of approximately 0.34 µmol/L (0.43 µmol/L × 0.8) (18 µg/dL [23 µg/dL × 0.8]) were typical for the survivors of this cohort. This concentration is within the range associated with lower risk elsewhere as shown in Table 8-3.
Numerous epidemiological studies have shown that individuals who consume a relatively large quantity of carotenoid-rich fruits and vegetables have a lower risk of cancer at several tumor sites (Block et al., 1992). The consistency of the results from observational studies is particularly striking for lung cancer, where carotenoid and fruit and vegetable intake has been associated with lower lung cancer risk in 8 of 8 prospective studies and 18 of 20 retrospective studies reviewed (Ziegler et al., 1996b).
Focusing on prospective blood analyses studies, the study with the largest number of cases (n = 99) was reported by Menkes et al. (1986) as part of the Washington County, Maryland, cohort. The risk of lung cancer increased in a linear fashion with decreasing serum concentrations of β-carotene, with the greatest risk at the
lowest quintile (cutpoint not stated). The mean concentration of serum β-carotene in persons who subsequently developed lung cancer was 0.47 µmol/L (25 µg/dL), compared to 0.54 µmol/L (29 µg/dL) in persons who remained free of disease.
Nomura et al. (1985) conducted a prospective study of 6,860 men of Japanese ancestry in Hawaii; 74 men subsequently developed lung cancer. Men who later developed lung cancer had lower serum β-carotene concentrations (0.37 µmol/L [20 µg/dL]) than control subjects (0.54 µmol/L [29 µg/dL]). Similar results were reported in the Basel Prospective Study. Men who later developed lung cancer (n = 68) had α- plus β-carotene serum concentrations of 0.30 µmol/L (16 µg/dL) versus 0.43 µmol/L (23 µg/dL) in survivors (Stahelin et al., 1991). The Multiple Risk Factor Intervention Trial (MRFIT) cohort study had prediagnostic serologic data on 66 lung cancer cases and 131 control subjects (Connett et al., 1989). Lung cancer cases had lower serum β-carotene concentrations (mean of 0.17 µmol/L [9 µg/dL]) and total carotenoid concentrations (1.62 µmol/L [87 µg/dL]) compared to the control subjects (0.22 µmol/L [12 µg/dL] and 1.84 µmol/L [99 µg/dL]), respectively. The absolute carotenoid concentrations in this study are lower than those in the previous studies, which may be a consequence of long-term storage of the samples at −50°C, rather than at −70°C or colder as is recommended for carotenoids.
As for dietary studies, the majority of the studies of carotenoids and lung cancer risk have relied upon the U.S. Department of Agriculture (USDA) Nutrient Database for Standard Reference, Release 13, which does not contain estimates of the amount of carotenoids in various food items, but simply contains estimates of provitamin A activity. With the release of a new carotenoid database in 1993 (Mangels et al., 1993), quantitative studies relating consumption of individual carotenoids to lung cancer risk are now available. Le Marchand et al. (1993) found that higher dietary intake of α-carotene, β-carotene, and lutein was significantly associated with lower lung cancer risk in both men and women. Optimal levels of intake for each of these three carotenoids were as follows: β-carotene more than 4.0 mg/day for men and more than 4.4 mg/day for women; α-carotene more than 0.6 mg/day for men and more than 0.7 mg/day for women; and lutein more than 3.3 mg/day for both males and females. Ziegler et al. (1996a) also found significant inverse trends for dietary α- and β-carotene and a marginally significant effect for lutein and zeaxanthin with risk of lung cancer. Optimal levels in this study were as follows: β-carotene 2.5–5.9 mg/day; α-carotene more than 1.5 mg/day; and lutein and zeaxanthin more than 4.2 mg/day.
As reviewed elsewhere, retrospective and prospective epidemiological studies of diet and serum carotenoids strongly indicate that greater consumption of fruits, vegetables, and carotenoids is inversely associated with risk of cancers of the oral cavity, pharynx, and larynx (Mayne, 1996; Mayne and Goodwin, 1993). In a review (Block et al., 1992), 13 of 13 studies indicated that fruit and vegetable intake was associated with reduced risk of cancers of the oral cavity, pharynx, and larynx. As for prospective serologic studies, Zheng et al. (1993) conducted a nested case-control study of serum micronutrients and subsequent risk of oral and pharyngeal cancer. Blood samples were collected and stored in 1974 from a cohort of 25,802 adults in Maryland. Over the next 15 years, 28 individuals developed oral or pharyngeal cancer. Serum analyses indicated that prediagnostic serum concentrations of all the major individual carotenoids, particularly β-carotene, were lower among the case group than among control subjects selected from the same cohort. β-Carotene concentrations in persons who later developed these cancers were 0.21 µmol/L (11 µg/dL) versus 0.28 µmol/L (15 µg/dL) in control subjects (mean; p = 0.03). Adjustment for smoking, which is known to be associated with lower serum carotenoid concentrations, attenuated the protective association slightly. The unadjusted and adjusted relative odds of oral or pharyngeal cancer, comparing the upper tertile of serum β-carotene concentrations (cutpoints not given) versus the lower tertile, were 0.50 and 0.69, respectively.
One recent prospective cohort study (Giovannucci et al., 1995) evaluated 47,894 participants in the Health Professionals Follow-up Study, 812 of whom were diagnosed with prostate cancer during the 6-year follow-up. Intake of tomato-based foods (tomato sauce, tomatoes, and pizza—but not tomato juice) and lycopene, which is found predominantly in tomato products, was associated with significantly lower prostate cancer risk. Risk was lowest for those who were estimated to consume more than 6.46 mg/day of lycopene. The lack of association for tomato juice may reflect the fact that lycopene is more bioavailable from processed tomato products than from fresh tomatoes (Gartner et al., 1997).
A prospective study of serum micronutrients and prostate cancer in Japanese men in Hawaii, however, found no difference in prediagnostic serum lycopene concentrations in 142 cases versus 142 matched control subjects (Nomura et al., 1997). The lack of effect seen in this study could possibly relate to the fact that serum lycopene concentrations were relatively low in this population (median 0.25 µmol/L [13 µg/dL]). This is likely a consequence of the fact
that tomato products are not widely consumed in the Asian diet (thus the range of exposure may have been limited). Comprehensive reviews of the relationship between lycopene and prostate cancer have been published elsewhere (Clinton, 1998; Giovannucci, 1999).
Consumption of fruits and vegetables also has been reported to be inversely associated with cervical cancer risk in a number of studies. Batieha et al. (1993) conducted a nested case-control study, analyzing a variety of carotenoids in sera stored from 50 women who had developed either invasive cervical cancer or carcinoma in situ during a 15-year follow-up and in 99 control women pair-matched to the cases. The risk of cervical cancer was significantly higher among women with the lowest prediagnostic serum concentrations of total carotenoids (odds ratio [OR] = 2.7; 95 percent CI = 1.1−6.4), α-carotene (OR = 3.1; 95 percent CI = 1.3−7.6), and β-carotene (OR = 3.1; 95 percent CI = 1.2−8.1) compared to women in the upper tertiles. Mean serum concentrations of β-cryptoxanthin were also lower among cases relative to control subjects (p = 0.03). Optimal concentrations of these carotenoids for reducing the risk of cervical cancer were as follows: total carotenoids greater than 1.88 µmol/L (101 µg/dL); α-carotene greater than 0.05 µmol/L (2.7 µg/dL); β-carotene greater than 0.26 µmol/L (14 µg/dL); and cryptoxanthin greater than 0.17 µmol/L (9 µg/dL).
Intervention Trials. Three major double-blind, randomized intervention trials have been conducted using high-dose β-carotene supplements, either alone or in combination with other agents, in an attempt to evaluate any protective role in the development of lung or total cancers. In none of these studies was there any evidence of a protective role for supplementary β-carotene.
In current smokers participating in the Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study, supplementation with 20 mg/day of β-carotene (with or without 50 mg of α-tocopherol) for 5 to 8 years led to a higher incidence in lung cancer but had no effect on the incidence of other major cancers occurring in this population (prostate, bladder, colon or rectum, or stomach) (ATBC Cancer Prevention Study Group, 1994). In addition, the Carotene and Retinol Efficacy Trial (CARET) used a nutrient combination of β-carotene (30 mg/day) plus retinyl palmitate (25,000 international units [IU]/day) versus placebo in asbestos workers and smokers (Omenn et al., 1996a, 1996b). This study reported more lung cancer cases in the supplemented group. The Physicians' Health Study (PHS) of supplemental β-carotene versus placebo in 22,071 male
U.S. physicians reported no significant effect of 12 years of supplementation of β-carotene (50 mg every other day) on cancer or total mortality (Hennekens et al., 1996).
Summary. Higher consumption of carotenoid-containing fruits and vegetables and higher plasma concentrations of several carotenoids, including β-carotene, are associated with a lower risk of many different cancers, especially lung, oral cavity, pharyngeal, laryngeal, and cervical cancers. These prospective blood concentration studies show that β-carotene concentrations in the range of 0.28 µmol/L (15 µg/dL) or less are associated with higher risk of many cancers (Table 8-3), whereas concentrations greater than 0.28 to 0.37 µmol/L (15 to 20 µg/dL) are associated with reduced risk of many cancers. This approximate threshold for cancer risk reduction is concordant with that for the prevention of all-cause mortality, given above. Furthermore, these studies show that increased consumption of foods containing these carotenoids, including carotenoids lacking vitamin A activity, is associated with risk reduction. However, in three large randomized clinical trials using high-dose β-carotene supplements (20 or 30 mg/day or 50 mg given every other day) for 4 to 12 years, no protection was reported with respect to lung cancer, or any other cancer.
Cardiovascular Disease
Epidemiological studies, including descriptive, cohort, and case-control studies, suggest that carotenoid- and β-carotene-rich diets are associated with a reduced risk of cardiovascular disease (Gaziano and Hennekens, 1993; Kohlmeier and Hastings, 1995; Manson et al., 1993). Beginning with biochemical epidemiological studies of plasma carotenoids, Gey et al. (1993a) reported data from the Vitamin Substudy of the World Health Organization's Monitoring Cardiovascular (WHO/MONICA) Project, in which plasma was obtained from approximately 100 apparently healthy men from each of 16 study sites within Europe. A comparison between median plasma β-carotene concentrations and ischemic heart disease mortality revealed no association when all 16 study sites were considered (r2 = 0.04). However, a reasonably strong inverse association was evident (r2 = 0.50) when three study sites, all apparent outliers (and all Finnish sites), were excluded from the analysis.
Men in the Basel Prospective Study, who had low blood concentrations of β-carotene and vitamin C initially and who were followed for 12 years, had a significantly higher risk of subsequent ischemic
heart disease (RR = 1.96; p = 0.022) and stroke (RR = 4.17; p = 0.002) (Eichholzer et al., 1992; Gey et al., 1993b). Based upon these and other data, Gey et al. (1993a) proposed that more than 0.4 to 0.5 µmol/L (21 to 27 µg/dL) α-plus β-carotene or 0.3 to 0.4 µmol/L (16 to 21 µg/dL) β-carotene is needed to reduce the risk of ischemic heart disease.
Total serum carotenoids, measured at baseline in the placebo group of the Lipid Research Clinics Coronary Primary Prevention Trial, were inversely related to subsequent coronary heart disease events (Morris et al., 1994). Men in the highest quartile of total serum carotenoids (more than 3.16 µmol/L [172 µg/dL]) had an adjusted relative risk of 0.64 (95 percent CI = 0.44−0.92); among those who never smoked, the relative risk was 0.28 (95 percent CI = 0.11−0.73). Riemersma e t al. (1991) reported that persons with plasma carotene concentrations in the lowest quintile (less than 0.26 µmol/L [14 µg/dL]) had 2.64 times the risk of angina pectoris. Adjustment for smoking reduced the magnitude of risk. However, because smoking may be part of the causal path, adjustment may not be appropriate.
The U.S. Health Professionals Follow-up Study of over 39,000 men reported a relative risk for coronary heart disease of 0.71 (95 percent CI = 0.55−0.92) for those at the top quintile of total carotene intake relative to the lowest quintile of intake (Rimm et al., 1993). The effect of β-carotene varied by smoking status: among current smokers, the relative risk was 0.30 (95 percent CI = 0.11−0.82); among former smokers, the risk was 0.60 (95 percent CI = 0.38−0.94), and among nonsmokers, the risk was 1.09 (95 percent CI = 0.66−1.79). A prospective cohort study of postmenopausal women found that the lowest risk of coronary heart disease was found for dietary carotenoid intakes greater than 8,857 IU/day (RR = 0.77; p = NS) (Kushi et al., 1996). A case-control study in 10 European countries found that lycopene concentrations, but not other carotenoid concentrations, in adipose tissue were inversely associated with the risk of myocardial infarction (Kohlmeier et al., 1997).
Cardiovascular epidemiology studies are now pursuing the use of intermediate endpoints, such as intima-media thickness, which can be estimated via ultrasonography as a measure of atherosclerosis. Bonithon-Kopp et al. (1997) reported a decrease in the intima-media thickness of the common carotid arteries with increasing concentrations of total plasma carotenoids in both men and women. Plasma carotenoid concentrations in excess of 2.07 µmol/L (111 µg/dL) were optimal for men; concentrations in excess of 3.73 µmol/L (200 µg/dL) were optimal for women. Salonen et al. (1993)
evaluated the change in the intima-media thickness as a measure of atherosclerotic progression and reported that progression was 92 percent greater in the lowest (less than or equal to 0.27 µmol/L [14 µg/dL]) versus the highest (more than or equal to 0.64 µmol/L [34 µg/dL]) quartile of plasma β-carotene.
Age-Related Macular Degeneration
Dietary carotenoids have been suggested to decrease the risk of age-related macular degeneration (AMD), the most common cause of irreversible blindness in people over age 65 in the United States, Canada, and Europe (Seddon et al., 1994; Snodderly, 1995). The macula lutea (macula) is a bright yellow spot in the center of the retina and is specialized and functions to maintain acute central vision. Of all the carotenoids circulating in the body, only two polar species, lutein and zeaxanthin, are contained in the macula (Bone et al., 1985; Handelman et al., 1988). Two groups of investigators have suggested pathways by which these two carotenoids are biochemically interchanged in the macula (Bone et al., 1993; Khachik et al., 1997a).
The potential role of carotenoids in the prevention of AMD has been comprehensively reviewed (Snodderly, 1995). Seddon et al. (1994) analyzed the association between carotenoid intake and advanced AMD in a large, multicenter, case-control study involving 356 cases and 520 control subjects with other ocular conditions. Those in the highest quintile of dietary carotenoid intake had a 43 percent lower risk for macular degeneration compared with those in the lowest (OR = 0.57; 95 percent CI = 0.35−0.92). Among the specific carotenoids, intake of lutein and zeaxanthin (grouped in the carotenoid food composition database) was most strongly associated with decreased risk. Those in the highest quintile of intake had a 60 percent lower risk compared to the lowest quintile of intake.
Some, but not all, studies using blood carotenoid concentrations also suggest protective effects against risk of AMD. The Eye Disease Case-Control Study (EDCCSG, 1993) measured serum carotenoids in 391 cases with neovascular AMD and 577 control subjects. The study reported protective effects of total carotenoids, α-carotene, β-carotene, β-cryptoxanthin, and lutein and zeaxanthin, with odds ratios ranging from 0.3 to 0.5 for the high group (more than the eightieth percentile) versus the low group (less than the twentieth percentile). Carotenoid concentrations associated with the lowest risk are shown in Table 8-4.
TABLE 8-4 Example of Plasma Carotenoid Concentrations Associated with Lowest Risk of Age-Related Macular Degeneration
|
Carotenoids |
Concentrations (µmol/L)a |
|
Total carotenoids |
≥2.39 (128 µg/dL) |
|
α-Carotene |
≥0.19 (10 µg/dL) |
|
β-Carotene |
≥0.74 (40 µg/dL) |
|
β-Cryptoxanthin |
≥0.32 (18 µg/dL) |
|
Lutein and zeaxanthin |
≥0.67 (38 µg/dL) |
|
Lycopene |
≥0.61 (33 µg/dL) |
|
a SI conversion factor used for total caroteniods, α- and β-carotene, and lycopene = 0.01863 µg/dL to µmol/L; for β-cryptoxanthin = 0.01809; and for lutein and zeaxanthin = 0.01758. SOURCE: EDCCSG (1993). |
|
Mares-Perlman et al. (1994) examined the association between serum carotenoid concentrations and age-related maculopathy in 167 case-control pairs and reported no association for any of the carotenoids, except lycopene, with persons in the lowest quintile of lycopene having a doubling in risk of maculopathy (cutpoint not stated). West et al. (1994) examined the relationship between plasma β-carotene concentration and AMD in 226 subjects and found the risk was lowest for the highest quartile of plasma β-carotene (more than 0.88 µmol/L [47 µg/dL] ) (OR high quartile versus low = 0.62). Plasma lutein and zeaxanthin were not measured in this study.
Hammond and Fuld (1992) developed an optical system that, in situ, measures the intensity of the unique yellow color of the macula and presumably estimates the levels of lutein and zeaxanthin. This measure is known as Macular Pigment Optical Density (MPOD). Dietary intake of carotenoids, fat, and iron, as well as plasma concentrations of lutein and zeaxanthin, were positively related with MPOD in men, but only plasma concentrations of lutein and zeaxanthin were associated with MPOD values for women (Hammond et al., 1996). In the same studies, men had significantly higher MPOD readings than women despite similar plasma carotenoid concentrations and similar dietary intake, except for fat. These investigators also demonstrated that the MPOD of most subjects could be substantially increased by the addition of relatively small amounts of foods to the diet that are high in lutein (1/2 cup
spinach per day) or lutein and zeaxanthin (1 cup of corn per day) (Hammond et al., 1997). Interestingly, when MPOD was enhanced following dietary modification, it was maintained at that level for several months despite resumption of an unmodified diet.
In summary, results of studies that have investigated MPOD as a biological indicator of carotenoid adequacy suggest that it has substantial potential as an indicator for estimating the requirements for lutein and zeaxanthin. Because of the unique metabolism of carotenoids in the macula, this technique will be useful in associating dietary intakes of lutein and zeaxanthin with the health of the macula. However, insufficient MPOD studies have been conducted to date to make recommendations relative to the dietary intakes of lutein and zeaxanthin.
Cataracts
Cataracts are also problematic, with cataract extraction being the most frequently performed surgical procedure in the elderly (Taylor, 1993). Although the etiology of this condition is not known, oxidative processes may play a role. Cataracts are thought to result from photo-oxidation of lens proteins, resulting in protein damage, accumulation, aggregation, and precipitation in the lens (Taylor, 1993). The cornea and lens filter out ultraviolet light, but visible blue light reaches the retina and may contribute to photic damage or other oxidative insults (Seddon et al., 1994).
Higher dietary intake of carotenoids or higher blood concentrations of carotenoids have been found to be inversely associated with the risk of various forms of cataract in some, but not all, studies. Jacques and Chylack (1991) reported that subjects with low plasma carotenoid concentrations (those with concentrations less than the twentieth percentile: less than 1.7 µmol/L [90 µg/dL]) had a 5.6-fold increased risk of any senile cataract and a 7.2-fold increased risk of cortical cataract, compared with subjects with high plasma total carotenoid concentrations (greater than the eightieth percentile; more than 3.3 µmol/L [177 µg/dL]). Mares-Perlman et al. (1995) performed a cross-sectional analysis of serum α-carotene, β-carotene, β-cryptoxanthin, lutein and zeaxanthin, and lycopene versus the severity of nuclear and cortical opacities, and found that higher concentrations of individual or total carotenoids were not associated with the severity of nuclear or cortical opacities overall. However, higher serum β-carotene (highest quintile median concentration 0.32 µmol/L [17 µg/dL] ) was associated with less opacity in men, and higher concentrations of α-carotene (highest quintile
median 0.14 µmol/L [7.5 µg/dL]), β-cryptoxanthin (highest quintile median 0.31 µmol/L [17 µg/dL]), and lutein (highest quintile median 0.44 µmol/L [25 µg/dL]) were associated with less nuclear sclerosis in men who smoked. In women, however, higher concentrations of some carotenoids (highest quintile median 2.19 µmol/L [118 µg/dL]) were associated with an increased severity of nuclear sclerosis.
Recently, the U.S. Health Professionals Follow-up Study reported a relative risk for cataract extraction in men of 0.81 (95 percent CI = 0.65−1.01) for those at the top quintile of lutein and zeaxanthin intake (median intake of 6.87 mg/day) relative to the lowest quintile of intake (Brown et al., 1999). Similar inverse associations for dietary lutein and zeaxanthin were seen in the Nurses' Health Study cohort, with a relative risk of 0.78 (95 percent CI = 0.63−0.95) for those at the top quintile of total lutein and zeaxanthin intake (median intake of 11.68 mg/day) relative to the lowest quintile of intake (Chasan-Taber et al., 1999). This decreased risk of cataracts (severe enough to require extraction) with higher intakes of lutein and zeaxanthin was not found with higher intakes of other carotenoids (α-carotene, β-carotene, lycopene, and β-cryptoxanthin) in either of these studies.
Plasma and Tissue Concentrations
As just detailed, plasma and tissue concentrations of carotenoids have been associated with a variety of health outcomes; that is, higher concentrations are associated with a lower risk of cancer, coronary heart disease, and all-cause mortality. This could be used as a possible indicator for establishing requirements for carotenoids. However, the limitation of this approach is that it is not clear whether observed health benefits are due to carotenoids per se or to other substances found in carotenoid-rich foods.
Thus, these data are suggestive of prudent intake levels, not required levels of intake. Recommendations have been made by a number of federal agencies and other organizations with regard to fruit and vegetable intake. Nutrient analysis of menus adhering to the U.S. Dietary Guidelines and the National Cancer Institute's Five-a-Day for Better Health Program, for example, indicates that persons following these diets would be consuming approximately 5.2 to 6.0 mg/day provitamin A carotenes on average if a variety of fruits and vegetables were consumed (Lachance, 1997). Similar levels would be obtained by following Canada's Food Guide for
Healthy Eating which specifies a minimum of five servings of vegetables and fruit (Health Canada, 1997). Other food-based dietary patterns recommended for the prevention of cancer and other chronic diseases would provide approximately 9 to 18 mg/day of carotenoids (WCRF/AICR, 1997).
The current U.S. and international guidelines encourage plant-based dietary patterns with less emphasis on foods of animal origin. With this type of dietary pattern, approximately 90 percent of the total ingested vitamin A would be in the form of provitamin A carotenoids (Lachance, 1997). This pattern is in stark contrast to current intake patterns in the United States, where less than 40 percent of vitamin A in the diet is derived from provitamin A carotenoids in fruits and vegetables (Figure 8-2), or to the intake patterns found in native Americans in some arctic regions of the United States and Canada (Kuhnlein et al., 1996).
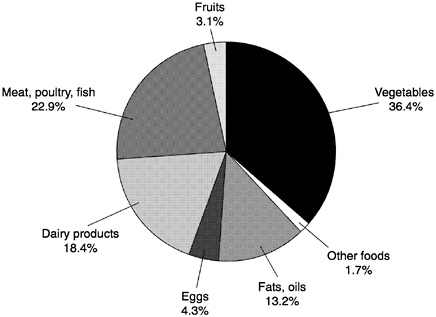
FIGURE 8-2 Contributors to Vitamin A intake in the U.S. food supply. The “other foods” category includes grain products (0.5 percent) and miscellaneous foods (1.2 percent).
SOURCE: LSRO/FASEB (1995).
An examination of human studies using dietary interventions with carotenoid-containing foods is necessary to determine the plasma carotene concentrations that an optimal diet would be expected to produce. In a controlled diet study (Micozzi et al., 1992), plasma β-carotene concentrations in the men who received the low carotenoid diet (less than 2 mg/day) to which broccoli had been added to provide 6 mg/day of carotenoids (3 mg of β-carotene, 3 mg of lutein) were raised significantly from 0.30 µmol/L (16 µg/dL) at baseline to 0.49 µmol/L (26 µg/dL) after six weeks, as were plasma lutein concentrations (from 0.38 µmol/L [22 µg/dL] to 0.63 µmol/L [36 µg/dL]). Plasma lycopene declined with this intervention because the baseline diet as well as broccoli was low in the content of lycopene and other carotenoids.
The Minnesota Cancer Prevention Research Unit feeding studies evaluated three experimental diets (two of which included carotenoids) and one control diet given for 9 days each to 23 young nonsmoking men and women. Persons on the control diet had a plasma β-carotene concentration of 0.26 µmol/L (14 µg/dL); 5 mg/day β-carotene from food increased plasma β-carotene to 0.37 µmol/L (19.5 µg/dL). When β-carotene from food was increased to 42 mg/day, plasma β-carotene increased further to 0.83 µmol/L (44 µg/dL) (Martini et al., 1995). Yong et al. (1994) studied dietary carotenoid intake and plasma carotenoids cross-sectionally in premenopausal nonsmoking women; the population had a geometric mean β-carotene intake of approximately 3 mg/day and a geometric mean plasma β-carotene concentration of 0.30 µmol/L (15.8 µg/dL). For total carotenoids, the geometric mean level of intake was 6.6 to 8.1 mg/day, with a total carotenoid concentration in plasma of approximately 1.51 µmol/L (80 µg/dL). A randomized, controlled trial on the effect of increasing fruit and vegetable intake for 8 weeks on plasma micronutrient concentrations was conducted with 87 subjects in New Zealand (Zino et al., 1997). β-Carotene intake increased from about 2.0 mg/day at baseline to 4.7 mg/day at week 4. This resulted in a mean plasma β-carotene increase from 0.34 µmol/L (18 µg/dL) at baseline to 0.48 µmol/L (25 µg/dL) at 4 weeks.
These data, although in varying populations, suggest that 3 to 6 mg/day of β-carotene from food sources is prudent to maintain plasma β-carotene concentrations in the range associated with a lower risk of various chronic disease outcomes (see Table 8-3).
FACTORS AFFECTING CAROTENOID BIOAVAILABILITY
Bioavailability
Bioavailability of carotenoids from food, concentrated extracts, or synthetic products is quite variable (Figure 8-3) because a complex set of factors affects carotenoid bioavailability. Erdman et al. (1993) and Castenmiller and West (1998) described the events necessary for adequate absorption of carotenoids from the diet: (1) digestion of the food matrix, (2) formation of lipid micelles in the gastrointes
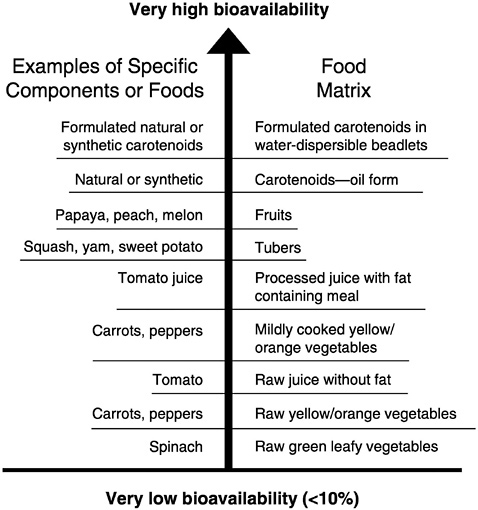
FIGURE 8-3 Effect of food matrix and processing on bioavailability of carotenoids.
SOURCE: Adapted from Boileau et al (1999).
tinal tract, (3) uptake of carotenoids by intestinal mucosal cells, and (4) transport of carotenoids and their metabolic products to the lymph or portal circulation.
Food Matrix
Of the factors that affect carotenoid bioavailability, the food matrix effects on carotenoid absorption are generally the most critical. The absorption of β-carotene supplements that are solubilized with emulsifiers and protected by antioxidants can be 70 percent or more. In contrast, less than 5 percent bioavailability of carotenes has been reported from raw foods such as carrots (Rodriguez and Irwin, 1972). Recently, van her Hof et al. (1999) reported substantial differences between the relative bioavailabilities of β-carotene (14 percent) compared to lutein (67 percent) when feeding a high-vegetable diet (490 g of vegetables without supplements) and comparing it to a low-vegetable diet (130 g of vegetables) supplemented with β-carotene (6 mg/day) or lutein (9 mg/day), both of which were assumed to be 100 percent bioavailable. These differences were based on changes in plasma concentration of β-carotene or lutein.
Daily supplementation of dark-green leafy vegetables rich in carotenoids to lactating Indonesian women with low vitamin A status did not increase vitamin A status, whereas a similar amount of β-carotene given in a wafer supplement led to a significant increase in plasma retinol (de Pee et al., 1995). More recently, the same group (de Pee et al., 1998) studied anemic school children in Indonesia and calculated the relative vitamin A equivalency of β-carotene from different food sources. The calculated equivalencies were as follows: 26 µg of β-carotene from leafy vegetables and carrots corresponded to 12 µg of β-carotene from fruit, and equaled 1 µg of preformed vitamin A in vitamin A-rich foods. In contrast, Mahapatra and Manorama (1997), in a small study with vitamin A-deficient school children in India, concluded that β-carotene from red palm oil was as bioavailable as preformed vitamin A.
β-Carotene in the form of supplements has a much higher bioavailablity than β-carotene from foods. Micozzi et al. (1992) demonstrated that 30 mg/day of supplemental all-trans β-carotene produced more than a fivefold increase in plasma β-carotene compared to 29 mg/day of β-carotene from carrots. The relatively low bioavailability of plant carotenoids may be due to the fact that they can be bound in carotenoproteins and are often associated with the plant matrix. Typically in green leafy vegetables, carotenoids are found bound in chloroplasts where they play roles in photosynthesis. In
carrot root, α- and β-carotene are largely in crystal forms. In both cases, the carotenoids are not easily solubilized out of these tissues by the digestive process.
Cooking
The hypothesis that cooking may improve the bioavailability of carotenoids has been tested. The bioavailability of lycopene from tomato juice is vastly improved by heat treatment in the presence of oil (Gartner et al., 1997; Stahl and Sies, 1992). When subjects consumed tomato juice (equivalent to a single lycopene dose of 2.5 µmol/kg body weight) that had been heated at 100°C for 1 hour with oil, they experienced a serum lycopene peak at 24 to 48 hours. In contrast, equivalent doses that were not heat treated did not result in an increase in serum lycopene. Steaming has also been shown to increase the amount of extractable carotenoids in spinach and carrots (Dietz et al., 1988). In contrast to steaming, more prolonged exposure to high temperatures (boiling) can reduce the carotenoid availability of vegetables by increasing the production of isomers or oxidation products. For example, canned carrots contain 73 percent all-trans β-carotene, 19 percent 13-cis-β-carotene, and 8 percent 9-cis-β-carotene, while fresh carrots contain 100 percent of the β-carotene in the all-trans configuration (Chandler and Schwartz, 1987). The relative vitamin A values of cis isomers of β-carotene compared to all-trans β-carotene is an active area of research.
Dietary Fat
Many research groups have shown that to optimize carotenoid absorption, dietary fat must be consumed during the same eating period as the carotenoid. Roels et al. (1958) demonstrated that in boys with vitamin A deficiency in an African village, supplementation of their carotene-sufficient but low-fat diets with 18 g/day of olive oil improved carotene absorption from 5 to 25 percent. More recently, Jalal et al. (1998) studied the roles of β-carotene-rich meals (mostly red sweet potatoes), extra dietary fat (15 g/day), and deworming on serum retinol concentrations of children in Sumatra. Prior to the intervention, these children all had intestinal infestations and were consuming diets with about 7 percent of calories from fat. A 3-week intervention of β-carotene-rich meals alone improved vitamin A status without added fat or deworming, but the combination of all three measures—β-carotene meals, added fat, and deworming—provided the greatest increase in serum retinol.
Other Factors
Lipid-lowering drugs have been shown to decrease serum carotenoids dramatically (Elinder et al., 1995). In a double-blind, randomized trial, treatment with cholestyramine (a lipid-lowering resin) for 4 months and probucol (antioxidant and lipid-lowering drug) for 2 months resulted in a 65 percent reduction in serum β-carotene and a 51 percent reduction in lycopene. The reductions were attributed to reduced intestinal absorption of lipids by cholestyramine and reduced lipoprotein particle number and size by probucol. Sucrose polyester (olestra), the nonabsorbable fat substitute, lowered carotenoid absorption when consumed at the same time as carotenoids (Koonsvitsky et al., 1997; Weststrate and van het Hof, 1995). Plant sterol-enriched margarines (Weststrate and Meijer, 1998) and dietary pectin supplementation also decreased β-carotene absorption (Rock and Swendseid, 1992).
Nutrient-Nutrient Interactions
Competitive interactions among different carotenoids during the absorptive process have been studied. Recipients of daily β-carotene supplements in either 12-mg or 30-mg capsules for 6 weeks had significantly lower plasma lutein concentrations than subjects who consumed both β-carotene and lutein from food sources (Micozzi et al., 1992). In addition, plasma β-carotene was higher in the subjects receiving β-carotene as supplements rather than as food, demonstrating the greater bioavailability of this source. Interactions between β-carotene and lutein have also been described by other investigators. When subjects were given purified crystalline β-carotene and crystalline lutein in a combined dose, β-carotene significantly reduced the serum area under the curve (AUC) value (a measure of total absorption) for lutein (Kostic et al., 1995). Lutein in a combined dose with β-carotene significantly enhanced β-carotene AUC in those subjects whose AUC for β-carotene (when dosed alone) was the lowest.
These studies (White et al., 1994) indicate that two carotenoids administered concurrently in controlled settings can affect the absorption of each other. Several investigators have examined the effect of daily supplementation with high-dose β-carotene on plasma concentrations of other carotenoids in participants in multiyear cancer prevention intervention trials (Albanes et al., 1997; Mayne et al., 1998; Nierenberg et al., 1997; Wahlqvist et al., 1994). These studies suggest no overall adverse effect on other carotenoids with
high-dose supplementation of β-carotene daily for several years. This finding is not inconsistent with the results of the metabolic studies, because the trials were done in free-living individuals taking a supplement of β-carotene each day, which most likely is not consumed concurrently with an entire day's intake of other carotenoids from food.
FINDINGS BY LIFE STAGE AND GENDER GROUP
As discussed elsewhere in this document, this report does not establish a requirement for β-carotene or other carotenoids for any gender or life stage group. This issue will be considered in a subsequent report when addressing vitamin A. However, the following summarizes findings regarding carotenoid status, as measured by serum carotenoid concentrations, in different groups of the population.
Special Populations
If plasma carotenoid concentrations are considered as an indicator of adequacy with regard to reducing risk of chronic disease, it becomes apparent that certain subgroups of the population are known to have notably lower circulating concentrations of carotenoids. Thus, consumption of carotenoid-containing foods may have to be greater in these groups in order to achieve plasma carotenoid concentrations that are associated with a reduced risk of chronic disease (Table 8-3).
Adolescents
Serum carotenoid concentrations were measured in the Third National Health and Nutrition Examination Survey (NHANES III). As shown in Appendix Table F-4, serum β-carotene concentrations were lower during the period of adolescence and early adulthood in this U.S. population survey. The average concentration in children was approximately 0.34 µmol/L (18 µg/dL), which dropped to 0.28 µmol/L (15 µg/dL) or less in teenagers and did not return to childhood concentrations until the fourth decade (the thirties) for women, and the fifth decade (the forties) for men. This lower level during adolescence is also evident for α-carotene (Appendix Table F-5), β-cryptoxanthin (Appendix Table F-6), and lutein/zeaxanthin (Appendix Table F-7), but not lycopene (Appendix Table F-8). This may reflect relatively greater consumption of tomato products compared to other vegetables by adolescents in the United States.
Smoking
Many investigators have reported that those who smoke, on average, have lower plasma carotenoid concentrations compared to individuals that don't smoke (Brady et al., 1996; Chow et al., 1986; Comstock et al., 1988; Fukao et al., 1996; Herbeth et al., 1990; Margetts and Jackson, 1996; Pamuk et al., 1994; Stryker et al., 1988; Witter et al., 1982). The greater the intensity of smoking (cigarettes per day), the greater is the decrease in serum carotenoid concentrations. Fukao et al. (1996) studied 1,902 Japanese men in a cohort study and showed a dose-dependent decline in geometric mean serum β-carotene with greater smoking intensity (Table 8-5).
While smokers ingest less β-carotene than nonsmokers, it is unclear at present whether or not the lower serum concentrations seen can be fully explained by the reduced β-carotene intakes of smokers, as discussed recently by Brady et al. (1996). Many studies find differences in serum carotenoid concentrations even after adjusting for intake. However, because dietary intake is necessarily measured with some error, it is unclear whether full adjustment is possible. Tobacco smoke is known to be highly oxidative, and the gas phase of tobacco smoke has been shown to destroy β-carotene and other carotenoids in in vitro studies of human plasma (Handelman et al., 1996). As demonstrated recently by Baker et al. (1999), both smoke and gas-phase smoke oxidize β-carotene to carbonyls, epoxides, and nitro derivatives. Thus, it is possible that the smoke oxidatively degrades β-carotene in vivo and thus contributes to the reduction in circulating levels.
TABLE 8-5 Serum β-Carotene in Men in Relation to Smoking
|
Meana Serum β-Carotene (µmol/L) |
|
|
Nonsmokers |
0.39 (20.7 µg/dL) |
|
Ex-smokers |
0.31 (16.6 µg/dL) |
|
Smokers |
|
|
1–10 cigarettes/d |
0.25 (13.6 µg/dL) |
|
11–20 cigarettes/d |
0.23 (12.1 µg/dL) |
|
21–100 cigarettes/d |
0.20 (10.5 µg/dL) |
|
a Geometric mean. SOURCE: Fukao et al. (1996). |
|
A first report in the area of putative mechanisms to explain the increase in lung cancer risk observed in heavy smokers taking high-dose supplements indicates that ferrets exposed to cigarette smoke and supplemented with β-carotene developed squamous metaplasia in their lungs as well as altered retinoid signaling (Wang et al., 1999). Another report suggests that oxidation products of tene stimulate the binding of metabolites of benzo [a] pyrene to deoxyribonucleic acid (Salgo et al., 1999). These very new data await confirmation and further development.
Although smoking may result in a need for higher intakes of dietary carotenoids to achieve optimal plasma carotenoid concentrations, caution is warranted because β-carotene supplements, but not β-carotene-rich foods, have been suggested as causing adverse effects in smokers (see “ Tolerable Upper Intake Levels ”). Thus, any recommendations need to state clearly that those who smoke, in particular, may benefit from even higher average intakes of carotenoids from foods.
Alcohol Consumption
Alcohol intake, like tobacco, is inversely associated with serum β-carotene and carotenoid concentrations (Brady et al., 1996; Fukao et al., 1996; Herbeth et al., 1988, 1990; Stryker et al., 1988). Brady et al. (1996) reported that higher ethanol intake was associated with a decrease in all serum carotenoids measured, with the exception of lycopene. The inverse association appears to be dose dependent as shown by the cohort study in men of Fukao et al. (1996) in Table 8-6. It should be noted that in this study, the effects of smoking and alcohol consumption independently affected serum β-carotene concentrations in men.
Persons who consume large quantities of ethanol typically consume diets that are micronutrient deficient. Therefore, as is the case for smoking, it is not clear whether the observed decrements are fully attributable to reduced intakes or also reflect metabolic consequences of chronic ethanol ingestion.
INTAKE OF CAROTENOIDS
Food Sources
A database of values for α-carotene, β-carotene, β-cryptoxanthin, lutein plus zeaxanthin, and lycopene for 120 foods has been assembled (Mangels et al., 1993) and was recently updated and released
TABLE 8-6 Serum β-Carotene in Men in Relation to Alcohol Consumption
|
Meana Serum β-carotene (µmol/L) |
|
|
Nondrinkers |
0.38 (20.1 µg/dL) |
|
Ex-drinkers |
0.32 (16.9 µg/dL) |
|
Drinkers |
|
|
1–15 g ethanol/d |
0.33 (17.9 µg/dL) |
|
15–28 g ethanol/d |
0.30 (16.2 µg/dL) |
|
29–56 g ethanol/d |
0.19 (10.0 µg/dL) |
|
56–140 g ethanol/d |
0.15 (8.2 µg/dL) |
|
a Geometric mean. SOURCE: Fukao et al. (1996). |
|
(Holden et al., 1999). Using an expansion of the earlier database and based on the 1986 U.S. Department of Agriculture Continuing Survey of Food Intake by Individuals (CSFII), Chug-Ahuja et al. (1993) reported that carrots were the major contributor of β-carotene to the diet of women of reproductive age (25 percent) with lesser contributions from the following food categories: cantaloupe, broccoli, vegetable beef or chicken soup, and spinach or collard greens. Similarly, the major contributors for α-carotene, β-cryptoxanthin, lycopene, and lutein and zeaxanthin were, respectively, carrots, followed by the categories of orange juice and its blends, tomatoes and tomato products, and spinach or collard greens.
A summary of the carotenoid content of human milk is shown in Table 8-7. It should be noted that the β-carotene content and the concentrations of other carotenoids in human milk are highly variable and appear to be altered easily by manipulation of the carotenoid content of the mother's diet. Most infant formulas, either milk or soy based, do not have carotenoids added to them and, as a result, would be expected to contain very low levels of β-carotene and other carotenoids.
Dietary Intake
Data for intakes of carotenoids (β-carotene, α-carotene, β-cryptoxanthin, lutein and zeaxanthin, and lycopene) from the 1988 –1992 Third National Health and Nutrition Examination Survey (NHANES III) based on an expanded food composition database
TABLE 8-7 Carotenoid Content in Human Milka
|
Author, Year |
Country |
Number of Subjects |
Stage of Lactationb |
Maternal Carotenoid Intake |
|
Gebre-Medhin et al., 1976 |
Sweden |
66 |
0.5–1.5 mo 1.5–3.5 mo 3.5–6.5 mo |
Not reported |
|
Butte and Calloway, 1981 |
U.S.d |
23 |
19–62 d |
Suboptimal |
|
Chappell et al., 1985 |
Canada |
24f |
1 d 4 d 37 d |
Not reported |
|
Ostrea et al., 1986 |
U.S. |
19f |
1–5 d |
Not reported |
|
Patton et al., 1990 |
U.S. |
11 |
Colostrum |
Not reported |
|
Giuliano et al., 1992 |
U.S. |
3 |
1 mo |
Not reported |
|
Giuliano et al., 1994 |
U.S. |
18 |
>1 mo |
Not reported |
|
Canfield et al., 1997 |
U.S. |
12 |
≤6 mo |
Dietary intake: β-carotene: 5.08 ± 2.5 mg/d α-carotene: 13.0 ± 0.8 mg/d Lycopene: 2.8 ± 2.6 mg/d β-Carotene supplements: Group 1: 60 mg/wk × 10 wk Group 2: 210 mg/wk × 3 wk |
|
Carotenoid Content in Milk (µg/dL)c |
Methods |
|
β-Carotene: 16.3 ± 7.5 β-Carotene: 17.1 ± 7.5 β-Carotene: 20.8 ± 10.2 |
Spectrophotometric |
|
Carotene: 19.7 ± 6.3e |
Spectrophotometric |
|
Carotene: 200 ± 12 Carotene: 100 ± 4 Carotene: 23 ± 5 |
HPLCg |
|
β-Carotene: day 1: 213 ± 167 day 2: 117 ± 112 day 3: 120 ± 63 day 4: 50 ± 20 day 5: 39 ± 35 |
Spectrophotometric |
|
α-Carotene: 16 ± 17 β-Carotene: 66 ± 76 β-Cryptoxanthin: 71 ± 61 |
TLCh, HPLC, and spectrophotometric |
|
Lycopene: 96 ± 85 |
|
|
α-Carotene: 0.32 ± 0.02 β-Carotene: 1.01 ± 0.02 Lutein: 1.06 ± 0.03 Lycopene: 2.73 ± 0.13 |
HPLC |
|
α-Carotene: 0.6 ± 0.4 β-Carotene: 2.5 ± 1.6i β-Cryptoxanthin: 1.1 ± 0.4 Lycopene: 1.7 ± 0.9 |
HPLC |
|
Initial β-carotene: 1.9 ± 0.3 (group 1) 2.7 ± 0.9 (group 2) |
HPLC |
|
Postsupplementation: β-carotene milk concentrations were 4 times the initial for both groups. No significant increases in α-carotene, β-cryptoxanthin, lutein/zeaxanthin, or lycopene noted |
TABLE 8-7 Carotenoid Content in Human Milka
|
Author, Year |
Country |
Number of Subjects |
Stage of Lactationb |
Maternal Carotenoid Intake |
|
Johnson et al., 1997 |
U.S. |
12 |
1–8 mo |
Supplement: 64 mg all-trans-β-carotene + 69 mg 9-cis-β-carotene (1 dose/d × 7 d) Fed a low-carotenoid diet |
|
Canfield et al., 1998 |
U.S. |
5 |
>1 mo |
Mean dietary intake of β-carotene at baseline = 4.0 ± 3.5 mg/d (measured by three 24-h dietary intake records) |
|
Supplemented with 30 mg β-carotene for 28 d |
||||
|
NOTE: For Guiliano et al. (1994), Canfield et al. (1997, 1998), and Johnson et al. (1997), milk carotenoid values were converted from nmol/L or µmol/L to µg/dL. The conversion factor used for β-carotene, α-carotene, lutein/zeaxanthin: µg/dL × 0.01863 = µmol/L. The conversion factor used for β-cryptoxanthin: µg/dL × 0.01809 = µmol/L. a Unless noted otherwise, milk content was based on studies of healthy women with full-term pregnancies. b pp = Postpartum. c Mean ± standard deviation. d U.S. = United States. e Mean milk volume was reported as 634 ± 113 mL/d. |
||||
for carotenoids are presently being analyzed and are not available to be included in this report. Thus, they will be included in the appendix of the next DRI report that will include vitamin A.
However, dietary recall data from 1,102 adult women participating in the 1986 Continuing Survey of Food Intake by Individuals indicate mean intakes of β-carotene, α-carotene, lutein, and lycopene of 1.8, 0.4, 1.3, and 2.6 mg/day, respectively, with total carotenoid intake from β-carotene, α-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene of approximately 6 mg/day (Chug-Ahuja et al., 1993). Later food frequency data from the 8,341 adults participating in the 1992 National Health Interview Survey indicate that mean intakes of β-carotene, lutein, and lycopene for men were 2.9,
|
Carotenoid Content in Milk (µg/dL)c |
Methods |
|
all-trans-β-Carotenej prestudy: ≈ 42.9 d 8: ≈ 225.4 |
HPLC |
|
9-cis-β-Carotene prestudy: ≈ 1.3 d 8: ≈ 3.2 |
|
|
α-carotene: 0.7 ± 0.2 β-carotene: 3.6 ± 1.0 β-cryptoxanthin : 1.4 ± 0.2 Lutein/zeaxanthin: 1.2 ± 0.2 Lycopene: 2.6 ± 0.3 |
HPLC |
|
Postsupplementation: β-carotene concentrations increased, on average, 6.4-fold over initial and remained elevated (≈ 2-fold over initial) 1 mo after supplementation |
|
|
f Milk samples were obtained from both preterm and full-term pregnancies. g HPLC = high-performance liquid chromatography. h TLC = thin-layer chromatography. i Large intra- and interindividual variability in milk carotenoid concentration. j One month after the study, milk concentrations remained higher than baseline in the supplemented women. No changes in milk concentrations were seen in the placebo group. k Mean milk volume was reported as 62.9 mL per human-feeding episode (Canfield et al., 1998). |
|
2.2, and 2.3 mg/day, respectively, and for women 2.5, 1.9, and 2.1 mg/day, respectively (Nebeling et al., 1997). Another survey, the Nutritional Factors in Eye Disease Study, with 2,152 adults responding to a food frequency questionnaire, reported median dietary carotenoid intakes or ranges of 1.3 mg/day of β-carotene, 0.2 mg/day of α-carotene, 0.02–0.07 mg/day of β-cryptoxanthin, 0.7–0.8 mg/day of lutein and zeaxanthin, and 0.6–1.6 mg/day of lycopene (VandenLangenberg et al., 1996).
Intake levels of β-carotene for infants can be estimated using data on human milk concentrations of β-carotene (Table 8-7). Human milk β-carotene concentrations obtained at more than 1 month postpartum varied from 1 to 21 µg/dL. Assuming that infants re-
ceiving human milk consume 0.78 L/day on average in the first 6 months (Chapter 2); this would result in β-carotene intake levels of 8 to 163 µg/day.
Intake from Supplements
β-Carotene, α-carotene, lutein and zeaxanthin, and lycopene are available as dietary supplements. There are no reliable estimates of the amount of these dietary supplements consumed by individuals in the United States or Canada.
TOLERABLE UPPER INTAKE LEVELS
Hazard Identification
Adverse Effects
No adverse effects other than carotenodermia have been reported from the consumption of β-carotene or other carotenoids in food. Carotenodermia is a harmless but clearly documented biological effect of high carotenoid intake. It is characterized by a yellowish discoloration of the skin that results from an elevation of carotene concentrations.
β-Carotene is used therapeutically, at extremely high doses (approximately 180 mg/day), for the treatment of erythropoietic protoporphyria, a photosensitivity disorder. No toxic side effects have been observed at these doses. There is no evidence that β-carotene or other carotenoids are teratogenic, mutagenic, or carcinogenic in long-term bioassays in experimental animals (Heywood et al., 1985). In addition, long-term supplementation with β-carotene to persons with adequate vitamin A status does not increase the concentration of serum retinol (Nierenberg et al., 1997). However, two recent clinical trials reported an increase in lung cancer associated with supplemental β-carotene in current smokers (ATBC Cancer Prevention Study Group, 1994; Omenn et al., 1996a,b). These effects are discussed below.
Lung Cancer. The Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study showed a significantly higher incidence of lung cancer (relative risk [RR] = 1.18; 95 percent confidence interval [CI] = 1.03−1.36) and total mortality (RR = 1.08; 95 percent CI = 1.01−1.16) in current smokers supplemented with 20 mg/day β-carotene (with or without 50 mg of α-tocopherol) for 5 to 8 years
compared to the placebo group (ATBC Cancer Prevention Study Group, 1994). Supplemental β-carotene had no significant effect on the incidence of other major cancers occurring in this population (prostate, bladder, colon or rectum, stomach). In addition, the Carotene and Retinol Efficacy Trial (CARET), a multicenter lung cancer prevention trial of a nutrient combination versus placebo in asbestos workers and smokers (Omenn et al., 1996a,b) reported more lung cancer cases in the supplemented group. The nutrient combination used in CARET included supplemental β-carotene (30 mg/day) plus retinol (25,000 international units [IU]/day). Both CARET and ATBC included β-carotene in the intervention and reported similar effects on lung cancer. However, it should be noted that CARET used a nutrient combination, without a factorial design, and it is not clear whether the reported effects were attributable to β-carotene, retinol, or both acting in concert.
In contrast, the Physicians' Health Study of supplemental β-carotene versus placebo in 22,071 male U.S. physicians reported no significant effect of 12 years of supplementation with β-carotene (50 mg every other day) on cancer or total mortality (Hennekens et al., 1996). With regard to lung cancer, there was no indication of excess lung cancer in the β-carotene-supplemented individuals, even among smokers who took the supplements for up to 12 years.
One additional trial, which was not designed as a lung cancer prevention trial, nonetheless produced results that are of relevance to the topic of lung cancer prevention. The trial tested the efficacy of four different nutrient combinations in inhibiting the development of esophageal and gastric cancers in 30,000 men and women aged 40 to 69 years living in Linxian County, China (Blot et al., 1993). One of the nutrient supplements was a combination of β-carotene, selenium, and vitamin E. After a 5-year intervention period, those who were given this combination had a 13 percent reduction in cancer deaths (RR = 0.87; 95 percent CI = 0.75−1.00), a 9 percent reduction in total deaths (RR = 0.91; 95 percent CI = 0.84−0.99), a 4 percent reduction in esophageal cancer deaths (RR = 0.96; 95 percent CI = 0.78−1.18), and a 21 percent reduction in gastric cancer deaths (RR = 0.79; 95 percent CI = 0.64−0.99). For lung cancer, this trial had limited statistical power, with only 31 total lung cancer deaths (Blot et al., 1994). However, the relative risk of death from lung cancer was 0.55 (95 percent CI = 0.26−1.14) among those receiving the combination of β-carotene, α-tocopherol, and selenium. The smoking prevalence, including individuals who had ever smoked cigarettes for 6 or more months, was 30 percent.
At the present time, the data pertaining to a possible adverse ef-
fect of β-carotene in smokers are somewhat conflicting. The results of ongoing studies may help resolve this issue. There also appears to be a relationship between the adverse effects of β-carotene and both smoking and alcohol consumption in the ATBC and CARET trials. In the ATBC trial, only those men who consumed more than 11 g/day of alcohol (approximately one drink per day) showed an adverse response to β-carotene supplementation (Albanes et al., 1996). In the CARET study, adverse effects were associated with the individuals in the highest quartile of alcohol intake (Omenn et al., 1996a).
Carotenodermia. Carotenodermia is characterized by yellowish discoloration of the skin that results from an elevation of plasma carotene concentrations. This condition has been reported in adults taking supplements containing 30 mg/day or more of β-carotene for long periods of time or consuming high levels of carotenoid-rich foods such as carrots (Bendich, 1988) and is the primary effect of excess carotenoid intake noted in infants, toddlers, and young children (Lascari, 1981). Carotenodermia is distinguished from jaundice in that the ocular sclera are yellowed in jaundiced subjects but not in those with carotenodermia. Carotenodermia is considered harmless and is readily reversible when carotene ingestion is discontinued.
Lycopenodermia. Lycopenodermia results from high intakes of lycopene-rich foods such as tomatoes and is characterized by a deep orange discoloration of the skin. Lycopene is a more intensely colored pigment than carotene and may cause discoloration at lower concentrations than other carotenoids (Lascari, 1981).
Other Adverse Effects. Allergic reactions, increased incidence of prostate cancer, retinopathy, leukopenia, and reproductive disorders have been associated anecdotally with high carotene consumption (Bendich, 1988; Kobza et al., 1973; Shoenfeld et al., 1982). None of these effects has been confirmed by clinical trials. There is no evidence of hypervitaminosis A in individuals consuming high levels of β-carotene or other carotenoids (up to 180 mg/day) (Lewis, 1972; Mathews-Roth, 1986; Mathews-Roth et al., 1972, 1974).
Dose-Response Assessment
The data on the potential for β-carotene to produce increased lung cancer rates in smokers are conflicting and not sufficient for a
dose-response assessment and derivation of a Tolerable Upper Intake Level (UL) for this endpoint. Supplements of 30 mg/day or more of β-carotene for long periods of time may be associated with carotenodermia, but this effect is more cosmetic than adverse and can be considered harmless and readily reversible. Because of the inconsistent data on adverse effects of β-carotene, a UL cannot be established at this time. ULs are not established for other carotenoids due to a lack of suitable data.
Intake Assessment
Data for intakes of carotenoids (β-carotene, α-carotene, β-cryptoxanthin, lutein and zeaxanthin, and lycopene) from the 1988 –1992 Third National Health and Nutrition Examination Survey (NHANES III) based on an expanded food composition database for carotenoids are presently being analyzed and are not available to be included in this report. Thus, they will be included in the appendix of the next DRI report that will include vitamin A.
Risk Characterization
A possible increase in lung cancer incidence has been noted only in smokers taking high-dose supplements of β-carotene (20 mg/day or greater). As discussed earlier, supplemental forms of β-carotene have markedly greater bioavailability than β-carotene from foods. The bioavailability of β-carotene from supplements can also be variable depending on the formulation, nutritional status of the person or population, and dietary intake pattern (e.g., fat intake). Given these substantial differences in bioavailability, it is perhaps logical to characterize the risk as a function of plasma β-carotene concentration (see Figure 8-4). Median serum β-carotene concentrations in the participants receiving 20 mg/day of β-carotene in the Finnish trial rose from 0.32 µmol/L (17 µg/dL) at baseline to 5.66 µmol/L (300 µg/dL) at 3 years; this blood concentration was associated with an adverse effect (ATBC Cancer Prevention Study Group, 1994). In CARET, the median postintervention plasma concentration of β-carotene was 3.96 µmol/L (210 µg/dL); this blood concentration also was reported to be associated with an adverse effect (Omenn et al., 1996a). The first to ninety-ninth percentile for plasma β-carotene from NHANES III is also indicated in Appendix Table F-4. These data suggest that the concentrations associated with possible adverse effects on lung cancer are well beyond the concentrations achieved via dietary intake.
Thus, while 20 mg/day of β-carotene in the form of a supplement is sufficient to raise blood concentrations to a range reported to be associated with an increase in lung cancer risk, the same amount of β-carotene in foods is not. Micozzi et al. (1992) demonstrated that 30 mg/day of supplemental β-carotene produced more than a five-fold increase in plasma β-carotene compared to 29 mg/day of β-carotene from carrots.
Based on these considerations, the existing recommendation for consumption of five or more servings of fruits and vegetables per day is supported because this would provide 3 to 6 mg/day of β-carotene. A UL has not been set for β-carotene or carotenoids. Instead, it is concluded that β-carotene supplements are not advisable for the general population. This conclusion is based on a totality of evidence that includes several large-scale randomized trials of supplemental β-carotene. These trials indicate a lack of evidence of overall benefit on total cancer or cardiovascular disease and possible harm in certain subgroups such as current smokers or asbestosexposed subjects. This advisement does not pertain to the possible use of supplemental β-carotene as a provitamin A source or for the prevention of vitamin A deficiency in populations with inadequate vitamin A nutriture or in patients suffering from erythropoietic protoporphyria.
RESEARCH RECOMMENDATIONS FOR β-CAROTENE AND OTHER CAROTENOIDS
-
As described earlier, β-carotene and other carotenoids have been shown to modulate a variety of intermediate endpoints. However, studies validating that changes in an intermediate endpoint are predictive of changes in a health outcome are critically needed. As an example, macular pigment optical density (MPOD) is a promising intermediate marker for age-related macular degeneration (AMD), but human studies validating this endpoint prospectively are needed, as are studies demonstrating that changes in MPOD are predictive of changes in risk of macular degeneration.
-
As a corollary, studies are needed on the effects of long-term depletion of β-carotene and subsequent repletion, with an evaluation of validated intermediate endpoints.
-
Significantly more research is needed on health effects of dietary carotenoids other than β-carotene. Possible associations between lycopene and decreased prostate cancer risk, between lutein and zeaxanthin and lowered risk of AMD, and between α-carotene or lutein and various cancers have to be evaluated in additional
-
observational studies, in animal models, and in human intervention trials, if justified. Studies should consider not only the other carotenoids, but also the cis-versus trans-configuration of the carotenoid.
-
Since the data from the human intervention trials of β-carotene are contradictory, additional data are needed from intervention trials involving β-carotene, several of which are ongoing. An examination is needed of health effects in populations with varying baseline risk profiles and, in particular, of studies evaluating interventions in populations with poor baseline nutritional status. Post-trial follow-up of completed β-carotene trials is also needed.
-
Studies aimed at the identification of correlates of higher β-carotene intake and plasma concentrations, which might help to explain the lower risks of cancer associated with carotene-rich diets, are needed.
-
Additional research is needed that targets putative mechanisms to explain a possible increase in lung cancer risk in heavy smokers taking high-dose β-carotene supplements (animal studies, biochemical studies, and molecular studies). In particular, confirmation and extension of findings such as those of recent reports regarding lung metaplasia (Wang et al., 1999) and carotenoid oxidation products (Salgo et al., 1999), and their relevance to cancer development in humans, are needed.
-
Surveys are needed that routinely assess and report dietary intakes of individual food carotenoids from large, representative population samples. Intakes from both foods and dietary supplements must be considered.
-
Efforts should be directed toward evaluating equivalency and demonstrating efficacy of carotenoids in foods to meet vitamin A needs in vitamin A-deficient populations, in order to develop sustainable strategies to eradicate this worldwide public health problem.
REFERENCES
Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa ER, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK. 1996. α-Tocopherol and β-carotene supplements and lung cancer incidence in the Alpha-Tocopherol Beta-Carotene Prevention Study: Effects of base-line characteristics and study compliance. J Natl Cancer Inst 88:1560–1570.
Albanes D, Virtamo J, Taylor PR, Rautalahti M, Pietinen P, Heinonen OP. 1997. Effects of supplemental beta-carotene, cigarette smoking, and alcohol co sumption on serum carotenoids in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr 66:366–372.
Allard JP, Royall D, Kurian R, Muggli R, Jeejeebhoy KN. 1994. Effects of beta carotene supplementation on lipid peroxidation in humans. Am J Clin Nutr 59:884–890.
ATBC (Alpha-Tocopherol, Beta Carotene) Cancer Prevention Study Group . 1994. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 330:1029–1035.
Baker DL, Krol ES, Jacobsen N, Liebler DC. 1999. Reactions of beta-carotene with cigarette smoke oxidants. Identification of carotenoid oxidation products and evaluation of the prooxidant/antioxidant effect. Chem Res Toxicol 12:535–543.
Batieha AM, Armenian HK, Norkus EP, Morris JS, Spate VE, Comstock GW. 1993. Serum micronutrients and the subsequent risk of cervical cancer in a popul tion-based nested case-control study. Cancer Epidemiol Biomarkers Prev 2:335–339.
Bendich A. 1988. The safety of beta-carotene. Nutr Cancer 11:207–214.
Block G, Patterson B, Subar A. 1992. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr Cancer 18:1–29.
Blot WJ, Li J-Y, Taylor PR, Guo W, Dawsey S, Wang G-Q, Yang CS, Zheng S-F, Gail M, Li G-Y, Yu Y, Liu B-Q, Tangrea J, Sun Y-H, Liu F, Fraumeni JF Jr, Zhang Y-H, Li B. 1993. Nutrition intervention trials in Linxian, China: Supplement tion with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst 85:1483–1492.
Blot WJ, Li J-Y, Taylor PR, Li B. 1994. Lung cancer and vitamin supplementation. N Engl J Med 331:614.
Boileau TW, Moore AC, Erdman JW Jr. 1999. Carotenoids and vitamin A. In: Papas AM, ed. Antioxidant Status, Diet, Nutrition, and Health. Boca Raton, FL: CRC Press. Pp. 133–158.
Bone RA, Landrum JT, Tarsis SL. 1985. Preliminary identification of the human macular pigment. Vision Res 25:1531–1535.
Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. 1993. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci 34:2033–2040.
Bonithon-Kopp C, Coudray C, Berr C, Touboul P-J, Feve JM, Favier A, Ducimetiere P. 1997. Combined effects of lipid peroxidation and antioxidant status on carotid atherosclerosis in a population aged 59–71 y: The EVA Study . Am J Clin Nutr 65:121–127.
Brady WE, Mares-Perlman JA, Bowen P, Stacewicz-Sapuntzakis M. 1996. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr 126:129–137.
Brown L, Rimm EB, Seddon JM, Giovannucci EL, Chasan-Taber L, Spiegelman D, Willett WC, Hankinson SE. 1999. A prospective study of carotenoid intake and risk of cataract extraction in US men. Am J Clin Nutr 70:517–524.
Burri BJ, Dixon ZR, Fong AK, Kretsch MJ, Clifford AJ, Erdman JW Jr. 1993. Possible association of skin lesions with a low-carotene diet in premenopausal women. Ann NY Acad Sci 691:279–280.
Butte NF, Calloway DH. 1981. Evaluation of lactational performance of Navajo women. Am J Clin Nutr 34:2210–2215.
Calzada C, Bizzotto M, Paganga G, Miller NJ, Bruckdorfer KR, Diplock AT, Rice-Evans CA. 1995. Levels of antioxidant nutrients in plasma and low density lipoproteins: A human volunteer supplementation study. Free Radic Res 23:489–503.
Canfield LM, Giuliano AR, Neilson EM, Yap HH, Graver EJ, Cui HA, Blashill BM. 1997. Beta-carotene in breast milk and serum is increased after a single beta carotene dose. Am J Clin Nutr 66:52–61.
Canfield LM, Giuliano AR, Neilson EM, Blashil BM, Graver EJ, Yap HH. 1998. Kinetics of the response of milk and serum beta-carotene to daily beta-car tene supplementation in healthy, lactating women. Am J Clin Nutr 67:276–283.
Castenmiller JJ, West CE. 1998. Bioavailability and bioconversion of carotenoids. Annu Rev Nutr 18:19–38.
Chandler LA, Schwartz SJ. 1987. HPLC separation of cis-trans carotene isomers in fresh and processed fruits and vegetables. J Food Sci 52:669–672.
Chappell JE, Francis T, Clandinin MT. 1985. Vitamin A and E content of human milk at early stages of lactation . Early Hum Devel 11:157–167.
Chasan-Taber L, Willett WC, Seddon JM, Stampfer M, Rosner B, Colditz GA, Speizer FE, Hankinson SE. 1999. A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in US women. Am J Clin Nutr 70:509–516.
Chow CK, Thacker RR, Changchit C, Bridges RB, Rehm SR, Humble J, Turbek J. 1986. Lower levels of vitamin C and carotenes in plasma of cigarette smokers . J Am Coll Nutr 5:305–312.
Chug-Ahuja JK, Holden JM, Forman MR, Mangels AR, Beecher GR, Lanza E. 1993. The development and application of a carotenoid database for fruits, veget bles, and selected multicomponent foods. J Am Diet Assoc 93:318–323.
Clevidence BA, Khachik F, Brown ED, Nair PP, Wiley ER, Prior RL, Cao G, Morel DW, Stone W, Gross M, Kramer TR. 1997. Human consumption of carotenoid rich vegetables. In: Aruoma OI, Cuppett SL, eds. Antioxidant Methodology: In Vivo and In Vitro Concepts. Champaign, IL: AOCS Press. Pp 53–63.
Clinton SK. 1998. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr Rev 56:35–51.
Clinton SK, Emenhiser C, Schwartz S, Bostwick DG, Williams AW, Moore BJ, Erdman JW Jr. 1996. Cis-trans lycopene isomers, carotenoids, and retinol in the human prostate . Cancer Epidemiol Biomarkers Prev 5:823–833.
Comstock GW, Menkes MS, Schober SE, Vuilleumier J-P, Helsing KJ. 1988. Serum levels of retinol, beta-carotene, and alpha-tocopherol in older adults. Am J Epidemiol 127:114–123.
Connett JE, Kuller LH, Kjelsberg MO, Polk BF, Collins G, Rider A, Hulley SB. 1989. Relationship between carotenoids and cancer. The Multiple Risk Factor Intervention Trial (MRFIT) Study. Cancer 64:126–134.
Coodley GO, Nelson HD, Loveless MO, Folk C. 1993. Beta-carotene in HIV infection. J Acquir Immune Defic Syndr 6:272–276.
de Pee S, West CE, Muhilal, Karyadi D, Hautvast J. 1995. Lack of improvement in vitamin A status with increased consumption of dark-green leafy vegetables. Lancet 346:75–81.
de Pee S, West CE, Permaesih D, Martuti S, Muhilal, Hautvast J. 1998. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and beta-carotene in schoolchildren in Indonesia. Am J Clin Nutr 68:1058–1067.
Dietz JM, Kantha SS, Erdman JW Jr. 1988. Reversed phase HPLC analysis of alpha and beta-carotene from selected raw and cooked vegetables. Plant Food Hum Nutr 38:333–341.
Dixon ZR, Burri BJ, Clifford A, Frankel EN, Schneeman BO, Parks E, Keim NL, Barbieri T, Wu M-M, Fong AK, Kretsch MJ, Sowell AL, Erdman JW Jr. 1994. Effects of a carotene-deficient diet on measures of oxidative susceptibility and superoxide dismutase activity in adult women. Free Radic Biol Med 17:537–544.
Dixon ZR, Shie F-S, Warden BA, Burri BJ, Neidlinger TR. 1998. The effect of a low carotenoid diet on malondialdehyde-thiobarbituric acid (MDA-TBA) concentrations in women: A placebo-controlled double-blind study. J Am Coll Nutr 17:54–58.
EDCCSG (Eye Disease Case-Control Study Group). 1993. Antioxidant status and neovascular age-related macular degeneration . Arch Ophthalmol 111:104–109.
Eichholzer M, Stahelin HB, Gey KF. 1992. Inverse correlation between essential antioxidants in plasma and subsequent risk to develop cancer, ischemic heart disease and stroke respectively: 12-year follow-up of the Prospective Basel Study. Exp Suppl 62:398–410.
Elinder LS, Hadell K, Johansson J, Molgaard J, Holme I, Olsson AG, Walldius G. 1995. Probucol treatment decreases serum concentrations of diet-derived ant oxidants. Arterioscler Thromb Vasc Biol 15:1057–1063.
Erdman JW Jr, Bierer TL, Gugger ET. 1993. Absorption and transport of car tenoids. Ann NY Acad Sci 691:76–85.
Fukao A, Tsubono Y, Kawamura M, Ido T, Akazawa N, Tsuji I, Komatsu S, Minami Y, Hisamichi S. 1996. The independent association of smoking and drinking with serum beta-carotene levels among males in Miyagi, Japan. Int J Epidemiol 25:300–306.
Gartner C, Stahl W, Sies H. 1997. Lycopene is more bioavailable from tomato paste than from fresh tomatoes . Am J Clin Nutr 66:116–122.
Gaziano JM, Hennekens CH. 1993. The role of beta-carotene in the prevention of cardiovascular disease . Ann NY Acad Sci 691:148–155.
Gaziano JM, Hatta A, Flynn M, Johnson EJ, Krinsky NI, Ridker PM, Hennekens CH, Frei B. 1995. Supplementation with beta-carotene in vivo and in vitro does not inhibit low density lipoprotein oxidation. Atherosclerosis 112:187–195.
Gebre-Medhin M, Vahlquist A, Hofvander Y, Uppsall L, Vahlquist B. 1976. Breast milk composition in Ethiopian and Swedish mothers. I. Vitamin A and beta-carotene. Am J Clin Nutr 29:441–451.
Gey KF, Moser UK, Jordan P, Stahelin HB, Eichholzer M, Ludin E. 1993a. Increased risk of cardiovascular disease at suboptimal plasma concentrations of essential antioxidants: An epidemiological update with special attention to carotene and vitamin C. Am J Clin Nutr 57:787S–797S.
Gey KF, Stähelin HB, Eichholzer M. 1993b. Poor plasma status of carotene and vitamin C is associated with higher morbidity from ischemic heart disease and stroke: Basel Prospective Study. Clin Invest 71:3–6.
Giovannucci E. 1999. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J Natl Cancer Inst 91:317–331.
Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. 1995. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst 87:1767–1776.
Giuliano AR, Neilson EM, Kelly BE, Canfield LM. 1992. Simultaneous quantitation and separation of carotenoids and retinol in human milk by high-performance liquid chromatography. Methods Enzymol 213:391–399.
Giuliano AR, Neilson SM, Yap H-H, Baier M, Canfield LM. 1994. Quantitation of and inter/intra-individual variability in major carotenoids of mature human milk. J Nutr Biochem 5:551–556.
Goodman DS, Blomstrand R, Werner B, Huang HS, Shiratori T. 1966. The intestinal absorption and metabolism of vitamin A and beta-carotene in man. J Clin Invest 45:1615–1623.
Gottlieb K, Zarling EJ, Mobarhan S, Bowen P, Sugerman S. 1993. Beta-carotene decreases markers of lipid peroxidation in healthy volunteers. Nutr Cancer 19:207–212.
Greenberg ER, Baron JA, Karagas MR, Stukel TA, Nierenberg DW, Stevens MM, Mandel JS, Haile RW. 1996. Mortality associated with low plasma concentr tion of beta carotene and the effect of oral supplementation. J Am Med Assoc 275:699–703.
Hammond BR Jr, Fuld K. 1992. Interocular differences in macular pigment density. Invest Ophthalmol Vis Sci 33:350–355.
Hammond BR Jr, Curran-Celentano J, Judd S, Fuld K, Krinsky NI, Wooten BR, Snodderly DM. 1996. Sex differences in macular pigment optical density: R lation to plasma carotenoid concentrations and dietary patterns. Vision Res 36:2001–2012.
Hammond BR Jr, Johnson EJ, Russell RM, Krinsky NI, Yeum K-J, Edwards RB, Snodderly DM. 1997. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci 38:1795–1801.
Handelman GJ, Dratz EA, Reay CC, Van Kuijk JG. 1988. Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci 29:850–855.
Handelman GJ, Packer L, Cross CE. 1996. Destruction of tocopherols, carotenoids, and retinol in human plasma by cigarette smoke. Am J Clin Nutr 63:559–565.
Hanusch M, Stahl W, Schulz WA, Sies H. 1995. Induction of gap junctional co munication by 4-oxoretinoic acid generated from its precursor canthaxanthin. Arch Biochem Biophys 317:423–428.
Health Canada. 1997. Canada's Food Guide to Healthy Eating. Minister of Public Works and Government Services Canada.
Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. 1996. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 334:1145–1149.
Herbeth B, Didelot-Barthelemy L, Lemoine A, Le Devehat C. 1988. Plasma fat soluble vitamins and alcohol consumption. Am J Clin Nutr 47:343–344.
Herbeth B, Chavance M, Musse N, Mejean L, Vernhes G. 1990. Determinants of plasma retinol, beta-carotene, and alpha-tocopherol . Am J Epidemiol 132:394–396.
Heywood R, Palmer AK, Gregson RL, Hummler H. 1985. The toxicity of beta-carotene. Toxicology 36:91–100.
Hininger I, Chopra M, Thurnham DI, Laporte F, Richard M-J, Favier A, Roussel A-M. 1997. Effect of increased fruit and vegetable intake on the susceptibility of lipoprotein to oxidation in smokers. Eur J Clin Nutr 51:601–606.
Holden JM, Eldridge AL, Beecher GR, Buzzard M, Bhagwat S, Davis CS, Douglass LW, Gebhardt S, Haytowitz D, Schakel S. 1999. Carotenoid content of U.S. foods: an update of the database. Food Comp Anal 12:169–196.
Hollander D, Ruble RE. 1978. Beta-carotene intestinal absorption: bile, fatty acid, pH, and flow rate effects on transport. Am J Physiology. 235:e686–e691.
Hughes DA, Wright AJ, Finglas PM, Peerless AC, Bailey AL, Astley SB, Pinder AC, Southon S. 1997. The effect of beta-carotene supplementation on the i mune function of blood monocytes from healthy male nonsmokers. J Lab Clin Med 129:309–317.
Jacques PF, Chylack LT Jr. 1991. Epidemiologic evidence of a role for the antiox dant vitamins and carotenoids in cataract prevention. Am J Clin Nutr 53:352S–355S.
Jalal F, Nesheim MC, Agus Z, Sanjur D, Habicht JP. 1998. Serum retinol concentr tions in children are affected by food sources of beta-carotene, fat intake, and anthelmintic drug treatment. Am J Clin Nutr 68:623–629.
Johnson EJ, Russell RM. 1992. Distribution of orally administered beta-carotene among lipoproteins in healthy men. Am J Clin Nutr 56:128–135.
Johnson EJ, Qin J, Krinsky NI, Russell RM. 1997. Beta-carotene isomers in human serum, breast milk and buccal mucosa cells after continuous oral doses of all-trans and 9-cis beta-carotene. J Nutr 127:1993–1999.
Kaplan LA, Lau JM, Stein EA. 1990. Carotenoid composition, concentrations, and relationships in various human organs. Clin Physiol Biochem 8:1–10.
Khachik F, Bernstein PS, Garland DL. 1997a. Identification of lutein and zeaxa thin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci 38:1802–1811.
Khachik F, Spangler CJ, Smith JC, Canfield LM, Steck A, Pfander H. 1997b. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal Chem 69:1873–1881.
Kobza A, Ramsay CA, Magnus IA. 1973. Oral carotene therapy in actinic reticuloid and solar urticaria. Failure to demonstrate a photoprotective effect against long wave ultraviolet and visible radiation. Br J Dermatol 88:157–166.
Kohlmeier L, Hastings SB. 1995. Epidemiologic evidence of a role of carotenoids in cardiovascular disease prevention. Am J Clin Nutr 62:1370S–1376S.
Kohlmeier L, Kark JD, Gomez-Gracia E, Martin BC, Steck SE, Kardinaal AF, Ringstad J, Thamm M, Masaev V, Riemersma R, Martin-Moreno JM, Huttunen JK, Kok FJ. 1997. Lycopene and myocardial infarction risk in the EURAMIC Study. Am J Epidemiol 146:618–626.
Koonsvitsky BP, Berry DA, Jones MB, Lin PY, Cooper DA, Jones DY, Jackson JE. 1997. Olestra affects serum concentrations of alpha-tocopherol and car tenoids but not vitamin D or vitamin K status in free-living subjects . J Nutr 127:1636S–1645S.
Kostic D, White WS, Olson JA. 1995. Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr 62:604–610.
Kramer TR, Burri BJ. 1997. Modulated mitogenic proliferative responsiveness of lymphocytes in whole-blood cultures after a low-carotene diet and mixed-carotenoid supplementation in women. Am J Clin Nutr 65:871–875.
Krinsky NI. 1993. Actions of carotenoids in biological systems. Annu Rev Nutr 13:561–587.
Kuhnlein HV, Soueida R, Receveur O. 1996. Dietary nutrient profiles of Canadian Baffin Island Inuit differ by food source, season, and age. J Am Diet Assoc 96:155–162.
Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. 1996. Dietary antio idant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med 334:1156–1162.
Lachance PA. 1997. Nutrient addition to foods: The public health impact in cou tries with rapidly westernizing diets. In: Bendich A, Deckelbaum RJ, eds. Preventive Nutrition: The Comprehensive Guide for Health Professionals . Totowa, NJ: Humana Press. Pp. 441–454.
Lascari AD. 1981. Carotenemia. A review. Clin Pediatr 20:25–29.
Le Marchand L, Hankin JH, Kolonel LN, Beecher GR, Wilkens LR, Zhao LP. 1993. Intake of specific carotenoids and lung cancer risk. Cancer Epidemiol Biomarkers Prev 2:183–187.
Lewis MB. 1972. The effect of beta-carotene on serum vitamin A levels in erythr poietic protoporphyria. Australas J Dermatol 13:75–78.
Lin Y, Burri BJ, Neidlinger TR, Muller HG, Dueker SR, Clifford A. 1998. Estimating the concentration of beta-carotene required for maximal protection of low-density lipoproteins in women. Am J Clin Nutr 67:837–845.
LSRO/FASEB (Life Sciences Research Office/Federation of American Societies for Experimental Biology). 1995. Third Report on Nutrition Monitoring in the United States. Washington, DC: US Government Printing Office.
Mahapatra S, Manorama R. 1997. The protective effect of red palm oil in compa ison with massive vitamin A dose in combating vitamin A deficiency in Orissa, India . Asia Pacific J Clin Nutr 6:246–250.
Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E. 1993. Carotenoid content of fruits and vegetables: An evaluation of analytic data. J Am Diet Assoc 93: 284–296.
Manson JE, Gaziano JM, Jonas MA, Hennekens CH. 1993. Antioxidants and cardi vascular disease: A review. J Am Coll Nutr 12:426–432.
Mares-Perlman JA, Brady WE, Klein R, Klein BE, Palta M, Bowen P, Stacewicz-Sapuntzakis M. 1994. Serum levels of carotenoids and tocopherols in people with age-related maculopathy. Invest Ophthalmol Vis Sci 35:2004.
Mares-Perlman JA, Brady WE, Klein BE, Klein R, Palta M, Bowen P, Stacewicz-Sapuntzakis M. 1995. Serum carotenoids and tocopherols and severity of n clear and cortical opacities. Invest Ophthalmol Vis Sci 36:276–288.
Margetts BM, Jackson AA. 1996. The determinants of plasma beta-carotene: Inte action between smoking and other lifestyle factors. Eur J Clin Nutr 50:236–238.
Martini MC, Campbell DR, Gross MD, Grandits GA, Potter JD, Slavin JL. 1995. Plasma carotenoids as biomarkers of vegetable intake: The University of Mi nesota Cancer Prevention Research Unit Feeding Studies. Cancer Epidemiol Biomarkers Prev 4:491–496.
Mathews-Roth MM. 1986. Beta-carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Biochimie 68:875–884.
Mathews-Roth MM, Pathak MA, Parrish J, Fitzpatrick TB, Kass EH, Toda K, Clemens W. 1972. A clinical trial of the effects of oral beta-carotene on the respon es of human skin to solar radiation. J Invest Dermatol 59:349–353.
Mathews-Roth MM, Pathak MA, Fitzpatrick TB, Harber LC, Kass EH. 1974. Beta-carotene as an oral photoprotective agent in erythropoietic protoporphyria. J Am Med Assoc 228:1004–1008.
Mayne ST. 1996. Beta-carotene, carotenoids, and disease prevention in humans. FASEB J 10:690–701.
Mayne ST. 1998. Beta-carotene, Carotenoids and Cancer Prevention. In: DeVita VT Jr, Hellman S, Rosenberg SA, eds. Principles and Practice of Oncology (PPO), 5th Edition Updates. Philadelphia, PA: Lippincott-Raven Publishers. Pp. 12:1–15.
Mayne ST, Goodwin WJ Jr. 1993. Chemoprevention of head and neck cancer. Current Opinion Otolaryn Head Neck Surg 1:126–132.
Mayne ST, Cartmel B, Silva F, Kim CS, Fallon BG, Briskin K, Zheng T, Baum M, Shor-Posner G, Goodwin WJ Jr. 1998. Effect of supplemental beta-carotene on plasma concentrations of carotenoids, retinol, and alpha-tocopherol in humans. Am J Clin Nutr 68:642–647.
Menkes MS, Comstock GW, Vuilleumier JP, Helsing KJ, Rider AA, Brookmeyer R. 1986. Serum beta-carotene, vitamins A and E, selenium, and the risk of lung cancer. N Engl J Med 315:1250–1254.
Micozzi MS, Brown ED, Edwards BK, Bieri JG, Taylor PR, Khachik F, Beecher GR, Smith JC. 1992. Plasma carotenoid response to chronic intake of selected foods and beta-carotene supplements in men. Am J Clin Nutr 55:1120–1125.
Mobarhan S, Bowen P, Andersen B, Evans M, Stacewicz-Sapuntzakis M, Sugerman S, Simms P, Lucchesi D, Friedman H. 1990. Effects of beta-carotene repletion on beta-carotene absorption, lipid peroxidation, and neutrophil superoxide formation in young men. Nutr Cancer 14:195–206.
Morris DL, Kritchevsky SB, Davis CE. 1994. Serum carotenoids and coronary heart disease: The Lipid Research Clinics Coronary Primary Prevention Trial and Follow-up Study. J Am Med Assoc 272:1439–1441.
Mosca L, Rubenfire M, Mandel C, Rock C, Tarshis T, Tsai A, Pearson T. 1997. Antioxidant nutrient supplementation reduces the susceptibility of low dens ty lipoprotein to oxidation in patients with coronary artery disease. J Am Coll Cardiol 30:392–399.
Nebeling LC, Forman MR, Graubard BI, Snyder RA. 1997. Changes in carotenoid intake in the United States: The 1987 and 1992 National Health Interview Surveys. J Am Diet Assoc 97:991–996.
Nierenberg DW, Dain BJ, Mott LA, Baron JA, Greenberg ER. 1997. Effects of 4 y of oral supplementation with beta-carotene on serum concentrations of retinol, tocopherol, and five carotenoids. Am J Clin Nutr 66:315–319.
Nomura AM, Stemmermann GN, Heilbrun LK, Salkeld RM, Vuilleumier JP. 1985. Serum vitamin levels and the risk of cancer of specific sites in men of Japanese ancestry in Hawaii. Cancer Res 45:2369–2372.
Nomura AM, Stemmermann GN, Lee J, Craft NE. 1997. Serum micronutrients and prostate cancer in Japanese Americans in Hawaii. Cancer Epidemiol Biomarkers Prev 6:487–491.
Olson JA. 1989. Biological actions of carotenoids. J Nutr 119:94–95.
Olson JA. 1994. Absorption, transport, and metabolism of carotenoids in humans. Pure Appl Chem 66:1011–1016.
Olson JA. 1999. Carotenoids. In: Shils ME, Olson JA, Shike M, Ross AC, eds. Modern Nutrition in Health and Disease, 9th edition. Baltimore, MD: Williams & Wilkins. Pp. 525–541.
Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL Jr, Valanis B, Williams JH Jr, Barnhart S, Cherniack MG, Brodkin CA, Hammar S. 1996a. Risk factors for lung cancer and for interve tion effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst 88:1550–1559.
Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL Jr, Valanis B, Williams JH Jr, Barnhart S, Hammar S. 1996b. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 334:1150–1155.
Ostrea EM Jr, Balun JE, Winkler R, Porter T. 1986. Influence of breast-feeding on the restoration of the low serum concentration of vitamin E and beta-carotene in the newborn infant. Am J Obstet Gynecol 154:1014–1017.
Pamuk ER, Byers T, Coates RJ, Vann JW, Sowell AL, Gunter EW, Glass D. 1994. Effect of smoking on serum nutrient concentrations in African-American women. Am J Clin Nutr 59:891–895.
Pandey DK, Shekelle R, Selwyn BJ, Tangney C, Stamler J. 1995. Dietary vitamin C and beta-carotene and risk of death in middle-aged men. The Western Electric Study. Am J Epidemiol 142:1269–1278.
Parker RS. 1988. Carotenoid and tocopherol composition of human adipose tissue. Am J Clin Nutr 47:33–36.
Parker RS. 1996. Absorption, metabolism, and transport of carotenoids. FASEB J 10:542–551.
Patton S, Canfield LM, Huston GE, Ferris AM, Jensen RG. 1990. Carotenoids of human colostrum. Lipids 25:159–165.
Pool-Zobel BL, Bub A, Muller H, Wollowski I, Rechkemmer G. 1997. Consumption of vegetables reduces genetic damage in humans: First results of a human intervention trial with carotenoid-rich foods . Carcinogenesis 18:1847–1850.
Richards GA, Theron AJ, Van Rensburg CE, Van Rensburg AJ, Van der Merwe CA, Kuyl JM, Anderson R. 1990. Investigation of the effects of oral administration of vitamin E and beta-carotene on the chemiluminescence responses and the frequency of sister chromatid exchanges in circulating leukocytes from cigarette smokers. Am Rev Respir Dis 142:648–654.
Riemersma RA, Wood DA, Macintyre CC, Elton RA, Gey KF, Oliver MF. 1991. Risk of angina pectoris and plasma concentrations of vitamins A, C, and E and carotene. Lancet 337:1–5.
Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. 1993. Vitamin E consumption and the risk of coronary heart disease in men . N Engl J Med 328:1450–1456.
Rock CL, Swendseid ME. 1992. Plasma beta-carotene response in humans after meals supplemented with dietary pectin. Am J Clin Nutr 55:96–99.
Rodriguez MS, Irwin MI. 1972. A conspectus of research on vitamin A requir ments of man. J Nutr 102:909–968.
Roels OA, Trout M, Dujacquier R. 1958. Carotene balances on boys in Ruanda where vitamin A deficiency is prevalent. J Nutr 65:115–127.
Rust P, Eichler I, Renner S, Elmadfa I. 1998. Effects of long-term oral beta-car tene supplementation on lipid peroxidation in patients with cystic fibrosis. Int J Vitam Nutr Res 68:83–87.
Sahyoun NR, Jacques PF, Russell RM. 1996. Carotenoids, vitamins C and E, and mortality in an elderly population . Am J Epidemiol 144:501–511.
Salgo MG, Cueto R, Winston GW, Pryor WA. 1999. Beta carotene and its oxidation products have different effects on microsome mediated binding of benzo[a]pyrene to DNA. Free Radic Biol Med 26:162–173.
Salonen JT, Nyyssonen K, Parviainen M, Kantola M, Korpela H, Salonen R. 1993. Low plasma beta-carotene, vitamin E and selenium levels associate with acce erated carotid atherogenesis in hypercholesterolemic eastern Finnish men. Circulation 87:678.
Santos MS, Meydani SN, Leka L, Wu D, Fotouhi N, Meydani M, Hennekens CH, Gaziano JM. 1996. Natural killer cell activity in elderly men is enhanced by beta-carotene supplementation. Am J Clin Nutr 64:772–777.
Santos MS, Gaziano JM, Leka LS, Beharka AA, Hennekens CH, Meydani SN. 1998. Beta-carotene-induced enhancement of natural killer cell activity in elderly men: An investigation of the role of cytokines. Am J Clin Nutr 68:164–170.
Schmitz HH, Poor CL, Wellman RB, Erdman JW Jr. 1991. Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue. J Nutr 121:1613–1621.
Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, Yannuzzi LA, Willett W. 1994. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. J Am Med Assoc 272:1413–1420.
Shoenfeld Y, Shaklai M, Ben-Baruch N, Hirschorn M, Pinkhaus J. 1982. Neutropenia induced by hypercarotenaemia. Lancet 1:1245.
Sies H, Stahl W. 1997. Carotenoids and intercellular communication via gap junctions. Int J Vitam Nutr Res 67:364–367.
Snodderly DM. 1995. Evidence for protection against age-related macular degene ation by carotenoids and antioxidant vitamins. Am J Clin Nutr 62:1448S–1461S.
Stahelin HB, Gey KF, Eichholzer M, Ludin E, Bernasconi F, Thurneysen J, Brubacher G. 1991. Plasma antioxidant vitamins and subsequent cancer mortality in the 12-year follow-up of the Prospective Basel Study. Am J Epidemiol 133:766–775.
Stahl W, Sies H. 1992. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J Nutr 122:2161–2166.
Stahl W, Schwarz W, Sundquist AR, Sies H. 1992. Cis-trans isomers of lycopene and beta-carotene in human serum and tissues . Arch Biochem Biophys 294:173–177.
Steinberg FM, Chait A. 1998. Antioxidant vitamin supplementation and lipid pe oxidation in smokers . Am J Clin Nutr 68:319–327.
Stryker WS, Kaplan LA, Stein EA, Stampfer MJ, Sober A, Willett WC. 1988. The relation of diet, cigarette smoking, and alcohol consumption to plasma beta-carotene and alpha-tocopherol levels. Am J Epidemiol 127:283–296.
Taylor A. 1993. Cataract: Relationship between nutrition and oxidation. J Am Coll Nutr 12:138–146.
Taylor A, Jacques PF, Epstein EM. 1995. Relations among aging, antioxidant status, and cataract. Am J Clin Nutr 62:1439S–1447S.
Traber MG, Diamond SR, Lane JC, Brody RI, Kayden HJ. 1994. Beta-carotene transport in human lipoproteins. Comparisons with alpha-tocopherol . Lipids 29:665–669.
VandenLangenberg GM, Brady WE, Nebeling LC, Block G, Forman M, Bowen PE, Stacewicz-Sapuntzakis M, Mares-Perlman JA. 1996. Influence of using diffe ent sources of carotenoid data in epidemiologic studies. J Am Diet Assoc 96:1271–1275.
van het Hof KH, Brouwer IA, West CE, Haddeman E, Steegers-Theunissen RP, van Dusseldorp M, Weststrate JA, Eskes TK, Hautvast JG. 1999. Bioavailability of lutein from vegetables is 5 times higher than that of beta-carotene. Am J Clin Nutr 70:261–268.
van Poppel G, Kok FJ, Duijzings P, de Vogel N. 1992a. No influence of beta-car tene on smoking-induced DNA damage as reflected by sister chromatid exchanges. Int J Cancer 51:355–358.
van Poppel G, Kok FJ, Hermus RJ. 1992b. Beta-carotene supplementation in smo ers reduces the frequency of micronuclei in sputum. Br J Cancer 66:1164–1168.
van Poppel G, Poulsen H, Loft S, Verhagen H. 1995. No influence of beta carotene on oxidative DNA damage in male smokers . J Natl Cancer Inst 87:310–311.
van Vliet T, Schreurs WH, van Den Berg H. 1995. Intestinal beta-carotene absor tion and cleavage in men: Response of beta-carotene and retinyl esters in the triglyceride-rich lipoprotein fraction after a single oral dose of beta-carotene. Am J Clin Nutr 62:110–116.
Wahlqvist ML, Wattanapenpaiboon N, Macrae FA, Lambert JR, MacLennan R, Hsu-Hage BH. 1994. Changes in serum carotenoids in subjects with colorectal adenomas after 24 mo of beta-carotene supplementation. Australian Polyp Prevention Project Investigators. Am J Clin Nutr 60:936–943.
Wang X-D. 1994. Absorption and metabolism of beta-carotene. J Am Coll Nutr 13:314–325.
Wang X-D, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell RM. 1999. Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst 91:60–66.
Wang Y, Ichiba M, Oishi H, Iyadomi M, Shono N, Tomokuni K. 1997. Relationship between plasma concentrations of beta-carotene and alpha-tocopherol and life-style factors and levels of DNA adducts in lymphocytes. Nutr Cancer 27:69–73.
West S, Vitale S, Hallfrisch J, Munoz B, Muller D, Bressler S, Bressler NM. 1994. Are antioxidants or supplements protective for age-related macular degeneration? Arch Ophthalmol 112:222–227.
Weststrate JA, Meijer GW. 1998. Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr 52:334–343.
Weststrate JA, van her Hof KH. 1995. Sucrose polyester and plasma carotenoid concentrations in healthy subjects. Am J Clin Nutr 62:591–597.
White WS, Stacewicz-Sapuntzakis M, Erdman JW Jr, Bowen PE. 1994. Pharmacok netics of beta-carotene and canthaxanthin after ingestion of individual and combined doses by human subjects. J Am Coll Nutr 13:665–671.
Winklhofer-Roob BM, Puhl H, Khoschsorur G, van't Hof MA, Esterbauer H, Shmerling DH. 1995. Enhanced resistance to oxidation of low density lipopr teins and decreased lipid peroxide formation during beta-carotene supplementation in cystic fibrosis. Free Radic Biol Med 18:849–859.
Witter FR, Blake DA, Baumgardner R, Mellits ED, Niebyl JR. 1982. Folate, carotene, and smoking. Am J Obstet Gynecol 144:857.
WCRF/AICR (World Cancer Research Fund/American Institute for Cancer Research). 1997. Food, Nutrition and the Prevention of Cancer: A Global Perspective . Menasha, WI: BANTA Book Group.
Yong LC, Forman MR, Beecher GR, Graubard BI, Campbell WS, Reichman ME, Taylor PR, Lanza E, Holden JM, Judd JT. 1994. Relationship between dietary intake and plasma concentrations of carotenoids in premenopausal women: Application of the USDA-NCI carotenoid food-composition database. Am J Clin Nutr 60:223–230.
Zhang L-X, Cooney RV, Bertram JS. 1991. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: Relationship to their cancer chemopreventive action. Carcinogenesis 12:2109–2114.
Zheng W, Blot WJ, Diamond EL, Norkus EP, Spate V, Morris JS, Comstock GW. 1993. Serum micronutrients and the subsequent risk of oral and pharyngeal cancer. Cancer Res 53:795–798.
Ziegler RG, Colavito EA, Hartge P, McAdams MJ, Schoenberg JB, Mason TJ, Fraumeni JF Jr. 1996a. Importance of alpha-carotene, beta-carotene, and other phytochemicals in the etiology of lung cancer. J Natl Cancer Inst 88:612–615.
Ziegler RG, Mayne ST, Swanson CA. 1996b. Nutrition and lung cancer. Cancer Causes Control 7:157–177.
Zino S, Skeaff M, Williams S, Mann J. 1997. Randomised controlled trial of effect of fruit and vegetable consumption on plasma concentrations of lipids and antioxidants. Br Med J 314:1787–1791.