A
Origin and Framework of the Development of Dietary Reference Intakes
This report is the third in a series of publications resulting from the comprehensive effort being undertaken by the Food and Nutrition Board's (FNB's) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (DRI Committee) and its panels and subcommittees.
ORIGIN
This initiative began in June 1993, when the Food and Nutrition Board (FNB) organized a symposium and public hearing entitled “Should the Recommended Dietary Allowances Be Revised?” Shortly thereafter, to continue its collaboration with the larger nutrition community on the future of the Recommended Dietary Allowances (RDAs), the FNB took two major steps: (1) it prepared, published, and disseminated the paper “How Should the Recommended Dietary Allowances Be Revised? ” (IOM, 1994), which invited comments regarding the proposed concept; and (2) it held several symposia at nutrition-focused professional meetings to discuss its tentative plans and receive responses to this initial concept. Many aspects of the conceptual framework of DRIs came from the report Dietary Reference Values for Food Energy and Nutrients for the United Kingdom (COMA, 1991).
The five general conclusions presented in the FNB's 1994 paper are as follows:
-
Sufficient new information has accumulated to support a reassessment of the RDAs.
-
Where sufficient data for efficacy and safety exist, reduction in the risk of chronic degenerative disease is a concept that should be included in the formulation of future recommendations.
-
Upper levels of intake should be established where data exist regarding risk of toxicity.
-
Components of food of possible benefit to health, although not meeting the traditional concept of a nutrient, should be reviewed, and if adequate data exist, reference intakes should be established.
-
Serious consideration must be given to developing a new format for presenting future recommendations.
Subsequent to the symposium and the release of the paper, the FNB held workshops at which invited experts discussed many issues related to the development of nutrient-based reference values, and FNB members have continued to provide updates and engage in discussions at professional meetings. In addition, the FNB gave attention to the international uses of the earlier RDAs and the expectation that the scientific review of nutrient requirements should be similar for comparable populations.
Concurrently, Health Canada and Canadian scientists were reviewing the need for revision of the Recommended Nutrient Intakes (RNIs) (Health Canada, 1990). The consensus following a symposium for Canadian scientists cosponsored by the Canadian National Institute of Nutrition and Health Canada in April 1995 was that the Canadian government should pursue the extent to which involvement with the developing FNB process would be of benefit to both Canada and the United States in terms of leading toward harmonization.
Based on extensive input and deliberations, the FNB initiated action to provide a framework for the development and possible international harmonization of nutrient-based recommendations that would serve, where warranted, for all of North America. To this end, in December 1995, the FNB began a close collaboration with the government of Canada and took action to establish the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes.
THE CHARGE TO THE COMMITTEE
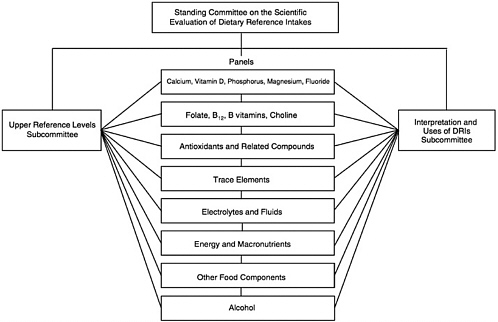
In 1995, the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (DRI Committee) was appointed to oversee and conduct this project. To accomplish this task, the DRI Committee devised a plan involving the work of seven or more expert nutrient group panels and two overarching subcommittees (Figure A-1).
The process described below is expected to be used for subsequent reports as well.
The Panel on Dietary Antioxidants and Related Compounds, composed of experts on these nutrients, has been responsible for the following: (1) propose a definition of a dietary antioxidant; (2) review the scientific literature concerning antioxidant nutrients and selected components of foods that may influence the bioavailability of these nutrients; (3) develop dietary reference levels of intake for the selected dietary antioxidants that are compatible with good nutrition throughout the life span and that may decrease the risk of developmental abnormalities and chronic disease; (4) address the safety of high intakes of these dietary antioxidants and, when appropriate, determine Tolerable Upper Intake Levels (ULs); and (5) identify a research agenda to provide a basis for public policy decisions related to recommended intakes and ways to achieve these intakes.
The panel was charged with analyzing the literature, evaluating possible criteria or indicators of adequacy, and providing substantive rationales for their choices of each criterion. Using the criterion chosen for each stage of the lifespan, the panel estimated the average requirement for each nutrient or food component reviewed, assuming that adequate data were available. As panel members reviewed data on ULs, they interacted with the Subcommittee on Upper Reference Levels of Nutrients, which assisted the panel in applying the risk assessment model to each selected nutrient. The panel also worked with the Subcommittee on Interpretation and Uses of Dietary Reference Intakes to determine appropriate examples for using the Recommended Dietary Allowances, Estimated Average Requirements, Adequate Intakes, and ULs. The Dietary Reference Intake values in this report are a product of the joint efforts of the DRI Committee, the Panel on Dietary Antioxidants and Related Compounds, the Subcommittee on Upper Reference Levels of Nutrients, and the Subcommittee on Interpretation and Uses of Dietary Reference Intakes, all under the oversight of the Food and Nutrition Board.
REFERENCES
COMA (Committee on Medical Aspects of Food Policy). 1991. Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. Report on Health and Social Subjects, No. 41. London: Her Majesty's Stationery Office.
Health Canada. 1990. Nutrition Recommendations. The Report of the Scientific Review Committee 1990. Ottawa: Canadian Government Publishing Centre.
IOM (Institute of Medicine). 1994. How Should the Recommended Dietary Allowances be Revised? Washington, DC: National Academy Press.