CHAPTER 12
OUR DIMINISHING TROPICAL FORESTS
PETER H.RAVEN
Director, Missouri Botanical Garden, St. Louis, Missouri
In any discussion of biological diversity, tropical forests must occupy center stage. Broadly defined, these forests are home to at least two-thirds of the world’s organisms, a number that amounts to no fewer than 3 million species, and could be 10 or more times greater than that amount. Striking, however, is the fact that only about 500,000 species from the tropical and subtropical regions of the world have been given names and been cataloged in the scientific literature. This means, very simply, that where one might expect to identify the great majority of any collection of insects or other arthropods made within the boundaries of Europe or temperate North America, only a very few of those in any reasonably diverse sample of tropical organisms—at least among relatively small and inconspicuous groups—could be located in the world’s collections, or are mentioned in the world’s literature.
Even among those very few, only a tiny fraction would be known from more than one or several specimens, a few short lines of technical description, and a locality. In short, identifying them would not provide much help concerning their ecology, their evolutionary relationships, their behavior—or any of the components that might have been involved in their history, or that might contribute to their chances of survival. Such matters must be considered seriously as we learn more about the diversity of organisms itself.
Regardless of whether there are 2.5 million more tropical organisms to be named or 25 million, the task facing us is enormous. All the activities of all those concerned with cataloging organisms over the past centuries in all types of ecosystems throughout the world have resulted in the naming of only about 1.5 million of them, and a task at least twice, and perhaps many times, that large confronts us now. All the scientific and societal gains that depend on an increased knowledge of these
organisms (we must know that they exist before we can understand or use them) depend on the degree to which that task can be completed. Since all of human society depends directly or indirectly on our ability to manage plants, animals, and microorganisms effectively, the task is one of enormous importance.
In light of the rapid destruction of tropical forests, it is an especially urgent matter to catalog the organisms in those regions and to establish well-considered priorities for this undertaking. It is clear that most tropical forests will have been destroyed or severely damaged within the next 25 years, because of the size of the human population in the tropics and subtropics, already constituting a majority of the world’s people and growing explosively; the extensive poverty there, which afflicts well over a third of the people; and our collective ignorance of effective ways to manage tropical ecosystems so that they will be productive on a sustainable basis. By 2010, the only large blocks of undamaged forest remaining will be those in the western and northern Brazilian Amazon, the interior of the Guyanas, and the central Zaire (Congo) basin in Africa. All the forests in other parts of the tropics and subtropics (those in Mexico, Central America, the West Indies, Andean South America, the eastern and southern portions of the Amazon), all the forests of Africa outside the central Zaire basin, and all the forests of tropical and subtropical Asia will have been devastated by that time.
There will of course be exceptional preserved areas within these regions, their sizes depending on the effectiveness of local conservation programs and on the nature of the soils underlying particular pockets of vegetation. Some areas will simply be too steep to cultivate, others too rocky, and still others too wet. In these pockets of vegetation, populations of organisms will survive; however, they will be reduced to relatively few individuals in most cases, subjected to the effects of light and heat penetrating from the edges of the fragmented patches of forest in which they are surviving, and assaulted by human activities related to their greater accessibility. For example, the hunting of primates and other animals (discussed by Mittermeier in Chapter 16) is often greatly intensified when the surviving patches of vegetation are small. Because of the nature of small populations—they are unlikely to persist long owing to chance alone—and the increasing strains on these pockets of vegetation, many of the species that initially survive locally are likely to become extinct within a very few years.
The question arises as to whether large, important preserves such as Manu Park in Peru or the Tai Forest in the Ivory Coast can survive until the projected stabilization of the human population in the second half of the next century. As in other tropical and subtropical countries, human pressures in Peru and the Ivory Coast are incredible, and resources tend to be consumed in meeting the needs of rapidly growing populations with high proportions of poor, often malnourished people. Over the next few years, the confrontation between human needs and forest preservation, already evident in many areas, will become more acute. The protection of such major reserves is conceivable, however, if there is a genuine willingness to share resources on a global level—to provide major support from industrialized countries not merely for the protection of parks and reserves but for the creation of conditions in which all people can live with a measure of human
dignity. The decisive factors will be social, political, and economic; they will not be limited simply to a willingness to conserve.
Putting these relationships in another context, and assuming that two-thirds of the world’s 4 to 5 million species are located in the tropics and subtropics, nearly half the world’s species of plants, animals, and microorganisms will be destroyed or severely threatened over the next quarter century—well within the expected life span of most people living today. If half these organisms become extinct during the next several decades—surely a conservative estimate—the world will experience a major episode of extinction. This episode could amount to the loss of perhaps 10% of the world’s species by the end of the century and to more than a 25% loss within the next couple of decades. These estimates are compatible with the predictions of extinction rates for primates and other relatively well-studied groups of organisms and with the closely coupled nature of the biological relationships involved. To find a comparable rate of extinction, one needs to go back more than 65 million years to the end of the Cretaceous period, when the dinosaurs disappeared along with a major loss of other life on Earth. In fact, the rate of extinction that will be characteristic of most of the remaining lifetimes of those now living is estimated to be at least 1,000 times the normal rate. Since there are now many more species than there were 65 million years ago, the absolute loss in number of species will be much greater.
For a more concrete example, consider the flowering plants. We obtain 85% of our food directly or indirectly from just 20 kinds of plants, and about two-thirds from just three: maize (corn), wheat, and rice. The 20 species were brought into cultivation thousands of years ago, largely because they were easy to grow; they were not selected because of their ability to contribute to the needs of a modern industrial civilization. Despite that, they are precious. Widespread starvation in the tropics and subtropics, however, reminds us that temperate-zone agriculture is not suitable everywhere, and suggests that an enhanced ability to cultivate some of the other 250,000 species of flowering plants might offer rewards by providing food crops that can be cultivated successfully in areas where the cultivation of present food plants is now difficult or impossible.
In evaluating our future opportunities to use the lesser known plants, however, consider the significance of the extinction projections reviewed above. Some 25,000 species of plants—about 5 species a day—are expected to disappear between now and the end of the century, and then perhaps 10 species a day will become extinct over the following couple of decades. Clearly, many of the 50,000 species of plants expected to vanish forever during our lives hold exceptional promise for producing food, fodder, wood, medicine—all the factors that increase the quality and stability of human existence on Earth. Given our record numbers, and the extreme pressure with which we are assaulting the global ecosystem, it seems absolutely mandatory that we redouble our efforts to survey, classify, preserve, and understand these plants, as well as members of other groups of organisms, while they still exist.
The consequences of the destruction of tropical and subtropical forests are grave; basically, our collective actions are denying to our children and grandchildren the ability to play the game of survival with the tools that we have at our disposal
today. In effect, we are, by our passivity, making the effort to survive through the creation and maintenance of stable, productive ecosystems more difficult for them than it is for us. The kind of restoration ecology described so eloquently by Janzen for the dry forest in Chapter 14 will undoubtedly come to be practiced widely as the human population stabilizes and our relationships with the global ecosystem become more realistic. The preservation of individual species of plants, animals, and microorganisms now offers the best chance of achieving the most complete success in this complex area during the twenty-first century. Analogous is the need to preserve genes for future use in the developing field of genetic engineering. It will be decades or centuries before it is possible to synthesize genes that can confer desirable traits on recipient organisms in any but the most simplistic ways; yet living organisms contain an enormous library of such genes, already tested by nature and available for use until the organisms themselves become extinct. Through our endless preoccupation with immediate, seemingly pressing domestic problems, we are seriously damaging our prospects for the very near future by losing scientific, societally relevant, and aesthetic possibilities beyond imagining.
Nonetheless, as we confront this grim spectacle, we must remember that the opportunities for studying and preserving biological diversity are greater today than they will ever be in the future. Of critical importance will be our ability to abandon our passivity and face the situation as it is, devoting increased resources to the exploration of diversity and using the information that we gain for our common benefit. In this effort, the importance of the kinds of studies described in this section is evident; they provide models of the variety of activities that should be intensified and multiplied while the opportunities are as great as those we enjoy now.
CHAPTER 13
THE TROPICAL FOREST CANOPY
The Heart of Biotic Diversity
TERRY L.ERWIN
Curator, Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, D.C.
A few years ago in a short paper in the Coleopterists Bulletin, I hypothesized that instead of the current estimate of 1.5 million species on Earth, there were 30 million species of insects alone (Erwin, 1982). This hypothesis was based on collections of beetles from tropical forest canopy samples in Panama (Erwin and Scott, 1980), rather than on the catalog counts of taxonomic names used in all the earlier estimates. I used simple arithmetic based on actual numbers of beetle species in my samples, estimated numbers of tropical forest tree species given me by the leading botanists, and a conservative estimate of the host specificity of tropical forest canopy insects. Host specificity in this sense means that a species in some way is tied to the host tree species and cannot exist without it.
This reestimation of the magnitude of life on Earth got a lot more coverage than I anticipated and began the usual controversy of right or wrong. Those engaged in the controversy, most of whom never read this obscure paper in the Coleopterists Bulletin, in a way actually missed the point of the paper. Consequently, I now want to take the opportunity to clarify the situation.
Science, at least in natural history, proceeds from casual observations, usually in the field or on museum specimens, to the erecting of hypotheses and finally to the testing of those hypotheses. Repeated failure to prove a hypothesis false lends support to the possibility that it may be true. For the 30 million species of insects hypothesis, which was based on a brand new set of observations never before available to scientists, I suggested that testing must begin by refining of our knowledge about host specificity of insects in tropical forests.
In a subsequent paper, analyzing data from the canopies of four different forests
in the central Amazon around Manaus, Brazil, I showed that 83% of the beetle species in the samples were found in only the samples of one of the types of forest, 14% of the species were shared between two, and only 1% of the species of beetles was found in all four forest types (Erwin, 1983a). This added fuel to the “numbers” controversy, because of the numerous types of forest known to exist in the Amazon Basin alone and the fact that the analysis was based on more than 1,000 species of beetles, a fairly substantial data base. At this point, I turned my attention to the now well-refined sampling techniques of insecticidal fogging of forest canopies at the Tambopata Reserved Zone in the southeast corner of Amazonian Peru. I developed these techniques for the purpose of testing the main hypothesis regarding biological diversity in tropical forests and the subhypothesis that host specificity is a main feature of the lifestyle of tropical canopy insects. The following paragraphs provide some glimpses of the Tambopata Canopy Project, some preliminary observations on the fauna itself, and what I believe to be the status of the 30 million species hypothesis. With a data base of a million specimens (we’ll get to the number of species later), it will take a long time to complete the data analysis from just 1 year of collecting.
THE PROBLEM
It has been predicted that in 25 to 30 years, much of the humid tropical forest could be gone or severely converted (see Raven, this volume, Chapter 12). Between 25 and 40% has already been lost to misguided human exploitation. The best estimate is that an area the size of Honduras is being lost or converted each year, and by the year 2000 some popular accounts have predicted that a million species will become extinct. Although I regard such guesses as a bit low, a point discussed later in this chapter, they mean that in our generation we, the only species on Earth with the mental capacity to reason, will see the virtual disappearance of contiguous tropical forests and probably the extermination of more than 20% of the diversity of life on Earth, and we humans will have caused it.
THE HISTORY
The Amazon basin (Figure 13–1) has the richest biota on Earth. There are several factors involved, not the least of which is the sheer size of the basin. We must start the historical analysis with the Amazon basin as it was on the western portion of the megacontinent Gonwanaland some 100 million years ago. The biota of today is a result of many events that occurred after two supercontinents, South America and Africa, rifted, and South America drifted in a westerly direction. As this occurred, the uplift of the Andes began. This wonderful mountain chain, extending from Venezuela down into Chile, became a dike that reversed the western flow of all the rivers of Gonwana, turning them around and beginning their flow to the east. In the last 40 million years, this event has caused a mosaic of habitats, the fine-grained resolution of which we have no comprehension at this time. As I am discovering in some of my work in Peru, the fine-grainness of habitats is far, far greater than what the botanical classifications have led us to believe. We need
from the botanists a better picture of tree species distribution and habitats, and of the small communities made up by these tree species microdistributions.
During this 40 million years of Andean orogeny, there were three uplifts of crystalline rock across the Amazon, represented by the red arches in Figure 13–1. The two gray areas in the north and south are bedrock, the Guyana and Brazilian Shields. From this perspective, we now see the development of this mosaic of habitats, defined by the meandering river systems of the Amazon basin itself. The study of these rivers and the areas between them offers an interpretation of the events of the past (Erwin and Adis, 1982).
A mosaic component that extends throughout the Amazon basin is the oxbow lake, a lake formed when a loop of a river becomes isolated from the river as a result of sedimentation. The formation of an oxbow lake is the first stage in succession that culminates in forest. This small “island” of aquatic life will soon become an island of grassy life, which will then become an island of palm tree life and so on until it returns to climax inundation or upland forest of some type. During succession, it may be crosscut by another twist of the river or another small river, which will then subdivide it into four successional stages each with a different time differential. This kind of successional evolution on a massive 6-million-square-kilometer area is but one of the features that has provided the evolutionary pathway for Amazonia’s fantastic diversity.
What we see today from the air is a forest canopy that extends more or less unbroken across those 6-million-square kilometers, except for the rivers, the hy-
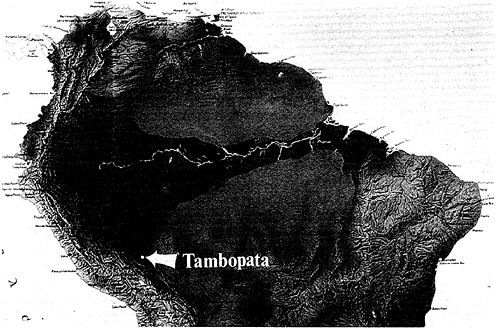
FIGURE 13–1 The South American land mass. The Guyana and Brazilian Shields are shown in gray; the hatched areas represent the three arches of crystalline rock.
droelectric projects, the Rondonia project, and various other development projects that are starting to break up that vast expanse of forest. From the air, one can detect even finer and finer mosaics. It is very easy to pick out the trees in blossom, the trees with tough dark-green leaves, trees that lost their leaves during the dry season and are now getting a new flush of very light pale leaves (the ones the insects like to eat the most), and vines that reach up into the canopy to spread their leaves over the tree leaves or intermingle them with the leaves of the canopy trees. All 150 or more species of canopy trees or vines per hectare contribute to the mosaic. There is an intermingling of leaves between two species of trees, between the vines and the trees, and between one tree overshadowing the other, resulting in the creation of microenvironments for the little creatures that are so important in providing the richness of the world’s biotic diversity.
Depending on forest type, the tops of the trees range from 15 meters to as high as 55 meters. Tambopata was chosen for my preliminary studies because logistically it is very difficult to get equipment and people into a virgin rain forest, keep them there for long periods, and get the material back to the museum to study it under the microscope. The average length of the beetles in the canopy is about 2 to 3 millimeters, so one needs pretty good facilities to make detailed studies. Tambopata served the logistic purposes as well as another purpose—approximately 11 different types of forests are found within walking distance. That seemed like too much to handle during 1 year, so only five were selected for intense collecting. In each of these five forests, we selected three 12-meter-square plots (Erwin, 1983b).
All 15 plots were sampled in the early rainy, late rainy, early dry, and late dry seasons. The data collected included tree canopy sizes, species of trees, and exact location of the collecting trays. All this information has been computerized and allows museum specimens to be traced back to the actual square meter of rain forest where they were collected. This gives us the opportunity to return in subsequent years and resample in order to see what the canopy, or what the forest in general, is doing over long periods. Long-term cycles have been largely overlooked, except by a few researchers for only a few species. My research team is now beginning to computerize the canopy in three dimensions so that we can describe exactly where these insect species reside in the canopy.
Beyond this data set, we also have the branching patterns, the leaf structure, and other details of microhabitats. It has taken a long time to develop our data collections, because we have paid attention to the finest details. I am trying to look at the canopy habitat through the eyes of these 2- to 3-millimeter-long beetles.
To date, we have analyzed about 3,000 species of beetles from only five plots. When we complete our analysis, we will have a large data set. A comparison of the tree composition of the different kinds of forest has shown that the forest in Manaus and two of our upland terra firma forests contain entirely different tree families. There are more big trees in the Peruvian sites than in the Manaus sites. Perhaps that accounts in part for the larger size of the insects in the canopy in Peru than in Manaus.
Only 2.6% of the species are shared between Manaus and Tambopata (Figure 13–2). This seems reasonable, because the two sites are 1,500 kilometers apart.
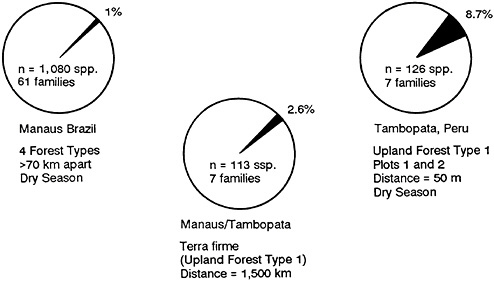
FIGURE 13–2 Pie diagrams of shared beetle species among forests in Peru and Brazil in percent of fauna.
But we found that of the 1,080 species analyzed, there was only a 1% overlap of species in all four forest types in Manaus (Erwin, 1983a).
Data collected during three seasons for two forest plots in the same type of forest 50 meters apart in Tambopata indicate that only 8.7% species are shared. When we add the fourth season data (which will come in shortly), we predict that the percentage of shared species will drop.
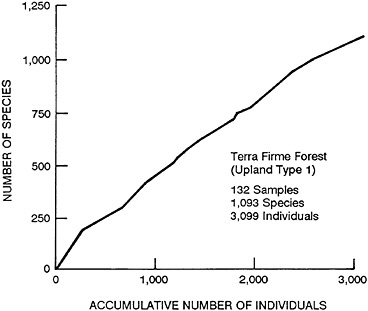
Figure 13–3 is a cumulation species curve, which shows the increase in the number of species as we increase the samples. After this figure was made, some more samples were analyzed and the curve became much steeper. These data are just from Plot 1 in Upland Forest Type 1 (Erwin, 1985). The 3,000 species already analyzed amount to more than all the samples from Brazil.
A canopy beetle is shown in Figure 13–4. In fully describing the distribution of these insects in time and space in the tropics, we should think in terms of more than 30 million, or perhaps 50 million or more, species of insects on Earth. A large number of species are tied only to certain forest types that are found on very small patches of soil deposited differentially through time by the vast and meandering Amazon River system. The extermination of 50% or more of the fauna and flora would mean that our generation will participate in an extinction process involving perhaps 20 to 30 million species. We are not talking about a few endangered species listed in the Red Data books, or the few forbish louseworts and snail darters that garner so much media attention. No matter what the number we are talking about, whether 1 million or 20 million, it is massive destruction of the biological richness of Earth.
We are rapidly acquiring a new picture of Earth, and it is crammed with millions upon millions of nature’s species on the verge of being replaced by billions upon billions of hungry people, asphalt, brick, glass, and useless eroded red clay baked by a harsh tropical sun. Many driving forces of evolution have affected carabid beetles and much of the other life on this planet. Very late in the scale of geologic time, a new driving force, humans, appeared. There is little question in my mind that Isaac Asimov (1974) in his wonderful Foundation Trilogy may have been particularly visionary when he described the planet Trantor, a sphere of steel and concrete; a hollow joke of its former self. Could Trantor be future Earth? Perhaps; perhaps not. Perhaps the biocrisis can be avoided. Human beings are starting to pay attention to the problem, and we’re a very resilient species and have a lot of good ideas. But do we have the resolve to rise above profit and greed?
REFERENCES
Asimov, I. 1974. Foundation Trilogy. Avon, New York. 684 pp.
Erwin, T.L. 1982. Tropical forests: Their richness in Coleoptera and other Arthropod species. Coleopt. Bull. 36(1):74–75.
Erwin, T.L. 1983a. Beetles and other Arthropods of the tropical forest canopies at Manaus, Brasil, sampled with insecticidal fogging techniques. Pp. 59–75 in S.L.Sutton, T.C.Whitmore, and A.C. Chadwick, eds. Tropical Rain Forests: Ecology and Management. Blackwell Scientific Publications, Oxford.
Erwin, T.L. 1983b. Tropical forest canopies, the last biotic frontier. Bull. Entomol. Soc. Am. 29(1):14–19.
Erwin, T.L. 1985. Tambopata Reserved Zone, Madre de Dios, Peru: History and description of the Reserve. Rev. Peru. Entomol. 27:1–8.
Erwin, T.L., and J.Adis. 1982. Amazon inundation forests: Their role as short-term refuges and generators of species richness and taxon pulses. Pp. 358–371 in G.Prance, ed. Biological Diversification in the Tropics. Columbia University Press, New York.
Erwin, T.L., and J.C.Scott. 1980. Seasonal and size patterns, trophic structure, and richness of Coleoptera in the tropical arboreal ecosystem: The fauna of the tree Luehea seemannii Triana and Planch in the Canal Zone of Panama. Coleopt. Bull. 34(3):305–322.
CHAPTER 14
TROPICAL DRY FORESTS
The Most Endangered Major Tropical Ecosystem
DANIEL H.JANZEN
Professor of Biology, University of Pennsylvania, Philadelphia, Pennsylvania
The rain forest is not the most threatened of the major tropical forest types. The tropical dry forests hold this honor. When the Spaniards arrived in the Western Hemisphere, there were 550,000 square kilometers of dry forest (approximately five times the size of Guatemala, or the size of France) on the Pacific coast of Mesoamerica (an area extending north from Panama to western Mexico). Today, only 0.09% of that region (approximately 480 square kilometers) has official conservation status, and less than 2% is sufficiently intact to attract the attention of the traditional conservationist. If there is to be a conserved neotropical (i.e., Western Hemisphere) dry forest wildland large enough to maintain the organisms and the habitats that were present when the Spaniards arrived, and if it is to be large enough to be easily maintained and thus a project willingly undertaken and managed into the indefinite future by the society in which it is imbedded, then we will have to grow it (Janzen, 1986a).
The story is the same for the dry tropical regions of Australia, Southeast Asia, Africa, and major parts of South America. The cause of the severe habitat loss is straightforward. Dry forest is easily cleared with fire, and woody regeneration in fields or pastures is easily suppressed with fire. Furthermore, fire does not stay where you put it; many areas are unintentionally cleared. The farmer is also aided by the severe dry season; it suppresses pest and weed populations, facilitates the use of fire as a tool to clean up pastures and fields, and slows soil degradation caused by continuous rain and farming. Many tropical dry forest regions even have good soils; they are downwind of volcanic mountain ranges or are situated on alluvia.
What are the conditions in a tropical dry forest (cf. Hartshorn, 1983; Janzen, 1986a; Murphy and Lugo, 1986)? Its 4- to 7-month rain-free dry season is sufficiently
harsh that many species of trees, vines, and herbs are deciduous for 2 to 6 months. Its rainy season, during which 1 to 3 meters of rain can fall, is as wet, if not wetter, than that of a rain forest. In the dry season, the sun penetrates to the forest floor, the leaf litter becomes very dry (and virtually ceases to decompose), watercourses dry up or greatly diminish in flow, and daytime relative humidity ranges from 20 to 60%. The dry forest may appear uniformly green during the rainy season, but during the dry season this homogeneity changes into a complex mosaic of tens of habitat types distinguished by the differential drying rates of different soils and exposures, different ages of succession, and different vegetation types. Many animals migrate to moist refugia (hollow logs and caves, moist riparian sites, north-facing slopes protected from the wind, and sites close to rain forests). During the dry season, most plants cease their vegetative activities, but many species of woody plants flower, mature their fruits, and disperse their seeds. Some species of animals feed on dry season fruits, seeds, and flowers; for them, the dry season is the bountiful time of year and the rainy season, inimical.
DIVERSITY IN THE DRY FOREST
What is the level of diversity in tropical dry forests? A lowland dry forest adjacent to a lowland rain forest (such as a portion of the Pacific dry and Atlantic wet sides of northern Costa Rica) sustains a fauna and flora about 50 to 100% as species-rich as does the neighboring rain forest (Janzen, 1986a). Floras are the least similar in richness of species, largely because the dry forest epiphytes and trees are substantially less rich in species. The greatest similarity in species richness is represented by mammals and major insect groups such as butterflies and moths (Lepidoptera) and Hymenoptera such as bees, wasps, and ants. Species overlap between the two areas, ranging from less than 5% (e.g., epiphytes, amphibians) to as high as 80% (e.g., sphingid moths, mammals). In the 11,000-hectare dry forest of Santa Rosa National Park in Costa Rica, I estimate that there are 13,000 species of insects, and fairly accurate counts indicate that there are 175 breeding species of birds (Stiles, 1983). There are also 115 species of nonmarine mammals (Wilson, 1983) and about 75 species of reptiles and amphibians (Savage and Villa, 1986). All the species of small herbs and grasses have not yet been collected, but the final list of angiosperms (which include vascular plants such as orchids and trees) will probably not exceed 700 species (Janzen and Liesner, 1980). When such a dry forest habitat is replaced by fencerows, ditchsides, unkempt pastures, and woodlots, the species richness of the breeding fauna and flora is reduced by 90 to 95%.
It is true that long lists of species have been used as criteria for identifying tropical habitats worthy of conservation. However, an approach that merely considers the number of species present is incomplete. This can be seen by examining the tropical dry forest, which is less rich than the rain forest in total species but is much richer in its variety of species’ activities. It contains many species that remain dormant in inclement (wet or dry) weather, and species that magically find enough water to develop flowers, fruits, and leaves at the height of the dry season (e.g., Janzen, 1967, 1982a,b). Its parasitoids and grazers range from absolutely monophagous to extremely polyphagous; that is, some subsist only on one type of
food, whereas others feed on a variety of organisms. Where else can you find white-tailed deer eating fruits dropped by spider monkeys and coyotes foraging side by side with jaguars? Moreover, the Santa Rosa dry forest has the only wind-pollinated legume (Janzen, in press a), the fiercest ant-plant mutualism (Janzen, 1966), and an enormous seasonal pulse of caterpillars that changes to a nearly total absence of caterpillars while the host plants are still in full leaf (Janzen, in press b). If you want a plantation timber tree that will grow throughout the year in a tropical rain forest, yet withstand the droughts produced by agricultural clearing, look to dry forest trees rather than to rain forest trees. The dry forest is also the parental climate for many major tropical crops and food animals such as cebu cattle, chickens, cotton, rice, corn, beans, sweet potatoes, sorghum, and pasture grasses (Janzen, 1986b).
Rather than focusing just on lists of species, tropical conservationists should also concentrate on saving interactions among species. What good are such interactions? Interactions make wildlands interesting, and they provide the raw materials used by the natural historian to construct the stories and the visions that are the real value of the natural world to humanity (Janzen, 1986b).
The conservation world has by and large failed to exploit the real enticements of the areas it conserves. Tropical forests can be likened to libraries and books. The value of a book is not measured by the number of words it contains or even by the number of kinds of words it contains. Put most simply, how long will the public continue to support a library whose goal is to have enormous holdings but no card catalog, no librarians, and thus no readers. Such a library is doomed to fail during the next paper shortage or governmental budget cut. Books are also comparable to the species in the dry forest in that they have little meaning except in context. The context of the dry forest is unique because of its species; it was once widespread and certainly has as much potential for biocultural development as does any type of tropical vegetation. It is this context that we must save.
The likelihood of long-term survival of a conserved wildland area is directly and strongly proportional to the economic health and stability of the society in which that wildland is imbedded. The farm- and ranchland once occupied by dry forest often sustains economically strong regional subcultures, which can thrive without the need to exploit the conserved area. Land blessed with conservation status in such a subculture has a much higher chance of survival than do the more abundant wildlands in frontierlike subcultures, many of which are also based on marginal farmland.
Overemphasis on the length of species lists is also potentially misleading, because the extraordinary peaks in species richness encountered at certain sites can be highly atypical. They are very interesting ecologically but are not representative of the tropics as a whole. They may not even be representative of the site itself, since many of the species-rich sites bear accumulations of strays at the overlap between several less species-rich habitats. Furthermore, the remaining sites of very great endemism and extraordinarily extensive species richness are often sites with peculiar physical characteristics that render them less likely to be occupied by humans at present and therefore easier to conserve. The apparently successful conservation of such areas gives a feeling of accomplishment that makes it easier
to accept conservation failures or inactivity with less species-rich, and therefore more ordinary, tropical sites. In the headlong rush to conserve diversity, we risk leaving the next generation with a handful of pretty baubles rather than the substance of the tropics. Saving a habitat with 300 species of endemic orchids on an Andean mountain top may not have the same long-term geological, intellectual, or economic value as does saving remnants of once widespread and less species-rich lowland forest.
A MANY-FACED THREAT
The threats to the tropical dry forest are multiple and complex. The concerned observer is correct to be no longer stirred to action by the simplistic chant, “The beef cow is responsible for the demise of the tropics”; the music is more daunting, more complicated, and more site-specific.
There are almost no large blocks of dry forest still standing that can be destroyed and thus cause concern among the public and academic world. Equally important, there are few opportunities to recognize the biocultural deprivation of the ranching and farming cultures that have been sustained for hundreds to thousands of years by the soils that once supported dry forest. As tropical conservation has swung into high gear during the past three decades, it has become comfortable to focus largely on the remaining rain forest and not to worry about scraps of other scattered vegetation types such as the dry forest. A traditional conservation battle for tropical dry forests would have to have been fought in 1900. Today, restoration ecology and habitat management (e.g., Janzen, in press c) are the only answers.
The acquisition and restoration of dry forest wildlands conflict with traditional conservationist protocol in numerous ways:
-
Land apparently used for agricultural production, or land that has produced something, is being set aside. This is expensive land, and its acquisition is often accompanied by a last-minute harvest of the few remaining trees and other resources by sellers who can do very serious damage to the anticipated restoration of the site.
-
The sellers—poor to very wealthy—are likely to be involved in neighborhood functions and politics far into the future; they cannot be bought out and left as resentful recipients of a bad deal.
-
The frontier is gone. The audience is local. The power is local. Within a few decades, if it hasn’t happened already, almost all members of tropical societies situated on dry forest soils will be settled on firmly titled land and will be leading a real or vicarious urban life with amenities such as good roads and schools. Survival of a wildland will depend on regional policy decisions by government institutions and planning commissions, and those decisions will be made by or in conjunction with the local community.
-
Many dry forest species are relatively robust, largely due to their evolutionary history of exposure to seasonal changes. Thus even tiny population fragments and severely altered populations can be ecologically reworked into viable, interacting populations and complex habitats that are replicas or facsimiles of what once was
-
on the site. Habitats that appear well beyond recovery can be restored if the seed sources are present.
-
Intensive cleaning up of the Mesoamerican dry forest agroscape during the last two to three decades is leading to the final extirpation of species and habitats that survived the first wave of megafaunal extinction by hunters 9,000 years ago, the extensive agriculture that began 5,000 years ago, and the ever more intensive agriculture that began 500 years ago. Either we act very soon or we will witness the elimination of many species that have persisted through many seemingly more severe perturbations than the contemporary, innocuous-appearing clearing of the last fencerows.
-
Conserved areas of dry forest wildlands will be rich in plant and animal opportunistic species and may well be the only places where most of today’s weeds survive. Weeds may be the most information-rich carriers of the genetic information for environmental toughness—information of obvious value in genetic engineering for crop species in harsh habitats.
-
Fires and invasion by grasses are the most serious contemporary ecological threats to the restoration and maintenance of dry forest wildlands. Properly manipulated, domestic animals may be the best tools for managing these threats, and they may even pay for their own maintenance: they mow the competing grass, they eat the fuel for the next dry season’s grass fires, and they disperse tree seeds far into pastures.
-
Dry forest conservation requires not only restoration but also explicit efforts to eliminate the various species initially introduced for agricultural or ranching purposes.
-
The biggest and a perpetual problem in dry forest management lies in deciding which areas of the wildland will be managed in what manner and to what end. Yes, it can be returned to a natural state, given the availability of seed sources. But which natural state do you want? On a time scale of at least thousands of years, the state to which it returns or in which it remains depends on many factors, such as the initial condition of the site, the species that arrived, and the order in which they arrived.
What means can be used to restore a tropical dry forest habitat?
-
Initiate and maintain a heavy flow of biological information, both biocultural and economic, from the site into the neighboring social system. The process of restoration, and the biology and interactions of the organisms being restored, must become as familiar to the region as are its irrigation projects, school development, and health programs. This task is both more difficult and more sustainable if the conservation effort is focused on habitats, interactions, and caterpillars rather than on redwoods, lions, and condors.
-
Stop man-made fires, hunting, cattle ranching, and other free-ranging perturbations. That is to say, give the site back to the remaining or adjacent dry forest organisms to recolonize by their own means. However, while this multihectare regeneration appears to be natural, such megacolonization of pastures and fields does not occur anywhere in nature. It is also not risk-free; neighboring blocks of
-
pristine vegetation are likely to be severely altered by invasions of secondary successional organisms from the large and ever-growing areas under restoration.
-
If some organisms are to be reintroduced from elsewhere, how far back in time shall we reach? Do we return the Pleistocene horse to Central American dry forest wildlands? How do we do that without adding its predators? If we put the tapir and white-lipped peccary back into El Salvador, do we also bring back their food plants? There are no correct choices per se, but it is clear that certain major dry forest areas must be set aside purely for agriculture and that no effort must be spared to maintain certain other (much smaller) areas as wildlands.
-
If the goal of restoration ecology is to conserve a maximum number of species, the management plan would be quite different than if the goal is to conserve habitats and interactions with whatever species they normally contain. Management directed at the conservation of a maximum number of species leads at least to the fragmentation of the wildland into a mosaic of successional types and ages and the introduction of species from other areas. If the goal is to conserve interactions as well as species, then the wildland manager must predict rather than simply react. The interactions that are saved will depend on the management steps taken, which depend on the interactions that are desired (and there will be errors and surprise outcomes). Conservation abruptly graduates from the art of patrolling a boundary against poachers to a variety of technical activities, such as the research-based studies of ecological succession, evolutionary biology, species packing, competitive exclusion, and epidemiology.
-
Rain forest wildlands must be conserved within migratory reach of the dry forest areas that are subject to restoration. A dry forest does not exist unto itself and neither does a rain forest. In Central America, the rain forest and the dry forest are the mutual recipients of each other’s migrants—migrants that are important parts of the interactive structure that holds tropical habitats together. Birds migrating from Wisconsin to Costa Rica are not the only ecological link over large agroscapes.
-
The dry forest is not only a collection of many kinds of habitats, each rich in unique species, but the members of a given habitat can be important interactants in adjacent habitats. If only certain (usually species-rich) dry forest habitats are slated for conservation, one quickly discovers that a substantial portion of the species in those habitats spend critical parts of their lives in other nearby habitats. To put it another way, the conversion of highly deciduous forest on dry ridges to pasture may have a severely depressing effect on the species-richness of organisms in the very species-rich adjacent alluvial bottomlands.
The dry tropics contain adult remnants of a once thriving forest, juveniles from gradually dwindling seed reservoirs, and waifs from as yet intact wildlands. These organisms now stand on a trashed agroscape and will die without replacement. They are the living dead—all physiologically alive but can be regarded as dead if they were already lying in the litter (Janzen, 1986b). If they flower, they fail to set seed (lack of pollinators). If they set seed, the seeds do not disperse (lack of seed dispersers). If seeds disperse, they do not develop as new members of their population (lack of adequate conditions for growth and development). If they
develop, they do not thrive in a sustained population (the caprice of agroecosystem development eradicates conditions needed for population maintenance within a generation or two). These organisms are usually included in the lists of species in a region and are often used to demonstrate that a species is not threatened with extinction—even though it is (Janzen, 1972). Agroscapes, seemingly still supporting long lists of widespread species, are primed for massive extinction as individuals of these species senesce or are killed through intensification of contemporary agriculture. If all these species were to be physically removed as soon as they have no future, the catastrophe would be much more noticeable and would therefore arouse the sentiments normally associated with massive extinction.
The dry forest is more prone to these less visible catastrophes than is the rain forest, in which scraps of vegetation left when the forest is cleared die more quickly than do those of the dry forest. Thus the threatened plant species in dry forest are available for a longer time and can be used as basic stock in restoration projects (though the price paid is that they dilute the visual impact of a largely demolished forest).
A Central American tropical dry forest wildland that is large enough to be visited and used by humans is substantially larger than a wildland that is to exist without human intrusion. Tropical habitats are very rich in behaviorally sensitive species and species that exist in low-density populations. Moreover, dry forests contain many small habitats (e.g., springs, dry ridge tops, marshes, edaphic outcrops, temporary streams, and pickets of forest sheltered from the wind). Visitors (tourists researchers, seed collectors, and habitat managers) will perturb ecological interactions substantially more and effect them more permanently in dry forests than in most extra tropical or rain forest habitats. This calls for strict zoning for habitat use and replicate habitats, both of which can be compatible with conservation management only if a large acreage is set aside.
FUTURE PROSPECTS
Ignore the voice that demands that a monetary value be placed on a wildland or a species before it can be conserved. Is that what you would do to determine the need for a public library, a public hospital, a public school? Can you tell me the dollar value of these institutions to your children? Are we to continue to be led by commercial interests to sanctify the production of material goods? The great majority of tropical humans live as draft animals; they are sold to the highest bidders along with the habitats that maintain them, and the purchasers are not generally benevolent. Through the swirl of changing market values, there will eventually come a day when the living organisms in a tropical wildland would be as doomed as would be libraries, if books were valued only for their paper pulp and the price of paper pulp were to rise.
Many organisms we believe to be safe are really endangered, and those we call endangered are in reality extinct. Guards will not save tropical wildlands. The world’s dry tropics are already way beyond their capacity for accommodating human activity. Thus a contract between managers of wildlands and society is mandatory. And the scientific community must aggressively participate in writing and executing
the contract. Without this participation, tropical biology will be nothing but low-grade and gradually diminishing restoration ecology. The conservation community has valiantly propped up the fortress walls, but they are too few. The future lies in the children, but we cannot wait for a well-educated cohort to replace its parents. The tropical dry forest is a living classroom, and its students are its neighbors. The collective power to turn the game around resides with policy makers. We cannot force the world to conserve tropical nature; we must seduce it, and the bait is intellectual mutualism—not the dollar value of a caterpillar.
ACKNOWLEDGMENTS
The people of Costa Rica have inspired me to believe there is still a chance. U.S. tax dollars through the National Science Foundation have financed the acquisition of the knowledge to see the chance, and the academic community has given the peer approval to know that this is the right direction. I thank P.Raven, W.Hallwachs, P.May, and A.Ugalde for help with the manuscript.
REFERENCES
Hartshorn, G.S. 1983. Plants. Pp. 118–157 in D.H.Janzen, ed. Costa Rican Natural History. University of Chicago Press, Chicago.
Janzen, D.H. 1966. Coevolution of mutualism between ants and acacias in Central America. Evolution 20:249–275.
Janzen, D.H. 1967. Synchronization of sexual reproduction of trees with the dry season in Central America. Evolution 21:620–637.
Janzen, D.H. 1972. The uncertain future of the tropics. Nat. Hist. 81:80–89.
Janzen, D.H. 1982a. Cenizero tree (Leguminosae: Pithecellobium saman) delayed fruit development in Costa Rican deciduous forests. Amer. J. Bot. 69:1269–1276.
Janzen, D.H. 1982b. Variation in average seed size and fruit seediness in a fruit crop of a guanacaste tree (Leguminosae: Enterolobium cyclocarpum). Amer. J. Bot. 69:1169–1178.
Janzen, D.H. 1986a. Guanacaste National Park: Tropical Ecological and Cultural Restoration. Editorial Universidad Estatal a Distancia, San Jose, Costa Rica. 103 pp.
Janzen, D.H. 1986b. The future of tropical ecology. Ann. Rev. Ecol. Syst. 17:305–324.
Janzen, D.H. In press a. Natural history of a wind-pollinated Central American dry forest legume tree (Ateleia herbert-smithii Pittier). In C.H.Stirton and J.L.Zarucchi, eds. Advances in Legume Biology. Mo. Bot. Gard. Monogr. Syst. Bot.
Janzen, D.H. In press b. Ecological characterization of a Costa Rican dry forest caterpillar fauna. Biotropica.
Janzen, D.H. In press c. Management of habitat fragments in a tropical dry forest: Growth. Ann. Mo. Bot. Gard.
Janzen, D.H., and R.Liesner. 1980. Annotated check-list of plants of lowland Guanacaste Province, Costa Rica, exclusive of grasses and non-vascular cryptogams. Brenesia 18:15–90.
Murphy, P.G., and A.E.Lugo. 1986. Ecology of tropical dry forest. Ann. Rev. Ecol. Syst. 17:67–88.
Savage, J.M., and J.Villa. 1986. Introduction to the Herpetofauna of Costa Rica. Society for the Study of Amphibians and Reptiles, Athens, Ohio. 207 pp.
Stiles, F.G. 1983. Checklist of birds. Pp. 530–544 in D.H.Janzen, ed. Costa Rican Natural History. University of Chicago Press, Chicago.
Wilson, D.E. 1983. Checklist of mammals. Pp. 443–447 in D.H.Janzen, ed. Costa Rican Natural History. University of Chicago Press, Chicago.
CHAPTER 15
DEFORESTATION AND INDIANS IN BRAZILIAN AMAZONIA
KENNETH I.TAYLOR
Executive Director, Survival International (USA), Washington, D.C.
Deforestation of tropical forests affects not only the plants and animals of these regions but also their human inhabitants. The Indian populations of Amazonia are successful managers of the forest. Long ago, they discovered the secrets of sustainable use of its resources. In this chapter I discuss the knowledge and management of the forest environment exhibited by the Yanomami and Kayapo Indians of Brazilian Amazonia and the importance that their knowledge and their presence as part of the forest ecosystem has for us all. Not only is this forest ecosystem now being destroyed at a rapid rate, but we (the non-Indians) do not yet know how to care for and make use of whatever areas of forest will be left when this process of destruction is brought to a halt.
THE YANOMAMI OF NORTHERN BRAZIL
The use and management of natural resources by the Yanomami include hunting, fishing, and collecting faunal resources, gathering and collecting floral resources, and shifting cultivation of bananas, plantains, manioc, several varieties of potatolike tubers, and a number of lesser crops. Their population is small and widely dispersed, resulting in an extremely low population density of 777 hectares per person. For the standard of living to which they are adapted, the forest provides them with an abundance of everything they need for a well-fed, healthy, and gratifying life. To date, there is no satisfactory evidence that they ever overused their resources or in any way degraded their environment. In fact, there are a number of indications that they vitalize and rejuvenate the forest, adding to its diversity and the size of its faunal and floral populations.
I have lived for more than 2 years among the Yanomami. The following account of Yanomami life in the forest is, for the most part, based on my own observations from that period (Taylor, 1974, 1983).
A Yanomami settlement is a clearing in the forest containing one or more of the several types of houses used by the different subgroups of the Yanomami. Directly associated with the site is a year-round source of water at a nearby stream or river. Radiating out from the settlement are numerous trails leading to the fields currently in use, to abandoned fields, to hunting, fishing, and gathering locations, to campsites in the forest, and to other settlements. The several fields actively cultivated by the families of the community are generally cut in primary forest, though occasionally in secondary forest, and usually no more than a 2-hour walk from the settlement. In more distant fields, a second family house is built for temporary stays of 1 to 2 weeks during the dry season. Several hours away from the settlement are a number of campsites used during dry-season fishing expeditions, long-term hunting trips, and journeys to other communities. The forest around the settlement is also criss-crossed by a number of minor trails used on hunting or gathering trips for food and raw materials of all kinds. These trails link together a series of regularly used locations, such as stands of fruit trees where game birds feed; streams where fish, crabs, or frogs can be found at certain times of the year; and places where different species of terrestrial game feed at times. And in all directions a number of major trails extend into the territories and lead to the settlement sites of other communities. These trails are more or less frequently and regularly used as friendships and alliances between communities come and go over the years.
This complex and ever-changing network of trails and the sites that they link together are not, of course, evenly spread out over a uniform and homogeneous circle of forest. In the Yanomami area, when you travel through the forest for more than even a few minutes, one of the most striking things you notice is its extraordinary diversity.
The use of the various hunting zones, and therefore the various biotopes around a Yanomami settlement, varies according to the type of hunting practiced: dawn/ dusk, day, or festival hunting. In some parts of Yanomami territory there may be totally unused areas that for years at a time function as game preserves (Gross, 1975; Harner, 1972; Hickerson, 1970).
A Yanomami community certainly needs access to a relatively large, ecologically heterogeneous territory that is contiguous with the territories of a number of neighboring communities, but we may wonder if so much land as 777 hectares per person is really needed. To the best of my knowledge, however, land use in Amazonia with non-Indian techniques, which involve clearing large areas of their protective forest cover for introduced, and inappropriate, crops and livestock, is leading to an ultimate degradation of the environment and is not self-sustaining on a permanent basis. The apparent exceptions of the riverine caboclos (forest-dwellers) (Frechione et al., 1985) and the rubber tappers of, for example, the State of Acre (Allegretti, 1985) are, in fact, land uses by settlers of long standing who have learned from the Indians a number of the basic requirements of a self-sustaining life-style in Amazonia. Of primary importance among these, and first to be ignored
by government plans for colonization of the region, is the essential feature of low—extremely low—population density.
THE KAYAPO OF CENTRAL BRAZIL
The Kayapo Indians of central Brazil live far to the south of the Yanomami in the watershed of the Xingu River, which is one of the major right-bank tributaries of the Amazon. Their territory is near the southern limit of the tropical forests of Amazonia and includes terra firme and gallery forests interspersed with areas of more or less open cerrado (similar to savannah). Their knowledge, management, and use of the floral and faunal resources of the forests in their territory are astonishingly subtle and complex. It is unlikely that the Kayapo are unique—they are simply, and by far, the best studied of the many Indian groups of Amazonia, with regard to this aspect of their way of life.
Like almost all the Indian groups in Amazonia, the Kayapo hunt, fish, and gather a great many species of the fauna and flora of the forests and practice shifting cultivation. They also concentrate native plants by growing them in resource islands, forest fields, forest openings, tuber gardens, agricultural plots, and old fields, and beside their trails through the forest. They select and transplant a number of semidomesticated native plants and manipulate some species of animals (birds, fish, bees, and mammals) used as food or food sources. The nests of two species of bees, for example, are brought from the forest and mounted on housetops until the honey is ready to be harvested. Forest patches (apete) are created from open cerrado in areas prepared with crumbled termite and ant nests and mulch (Posey, 1983, 1985; Posey et al., 1984).
The Kayapo Indians are probably not unique. More likely they are typical of indigenous societies in tropical forests. They not only live a healthy and well-fed life as the human component of a thriving tropical forest ecosystem but they also beneficially manage, manipulate, and modify the flora and fauna of their territory. As a result of their presence and remarkable way of life, the plant and animal resources of their area are more diverse, more locally concentrated, of greater population size and density, and more youthful and vigorous than would be found in a forest empty of these Indian resource managers.
Perhaps the most surprising and significant of their many resource management techniques is the creation of the apete forest patches. Posey became aware that these isolated patches of forest were man-made only in the seventh year of his research among the Kayapo (D.A.Posey, Museu Emilio Goeldi, ![]() , Brazil, personal communication, 1986). As he pointed out, “Perhaps the most exciting aspect of these new data is the implication for reforestation. The Indian example not only provides new ideas about how to build forests ‘from scratch,’ but also how to successfully manage what has been considered to be infertile campo/cerrado” (Posey, 1985, p. 144).
, Brazil, personal communication, 1986). As he pointed out, “Perhaps the most exciting aspect of these new data is the implication for reforestation. The Indian example not only provides new ideas about how to build forests ‘from scratch,’ but also how to successfully manage what has been considered to be infertile campo/cerrado” (Posey, 1985, p. 144).
THE RIGHTS AND WRONGS OF SHIFTING CULTIVATION
Shifting cultivation involves the felling or cutting of the vegetation in an area selected for a field or garden and the burning of the felled trees, bushes, underbrush, or grasses. It is a widely used technique that has been around for a long, long time. Conklin (1961) gives a definitive overview of the long history (since the Neolithic period) and distribution (worldwide, especially in the tropics) of this form of agriculture and discusses the various forms it can take, in terms of whether primary or secondary forest or grasslands are being used, crop-fallow time ratios, types of crop, dispersal relative to human settlements, concomitant presence of livestock, and tools and techniques used. It is unlikely that a form of agriculture so time-honored and widespread would be inefficient or destructive of the environment; yet many people regard it as just that—as one way in which the remaining tropical forests are being destroyed.
Between 1968 and 1976, I had the opportunity to fly in light aircraft and helicopter over most of Yanomami territory in the Ajarani, Catrimani, Mucajai, Parima, and Auaris river basins in the territory of Roraima, Brazil. The vitality, the exuberance, and the seeming endlessness of the dense carpet of forest cover made a lasting impression on me. Yet this is where most of the Yanomami lead their lives. Whatever else the Yanomami may or may not be doing, they are most certainly not destroying the forest. As discussed above, Posey and collaborators describe the Kayapo as Indians who are greatly enhancing the vitality of the forests of their region.
But aren’t the Yanomami and the Kayapo what some people call slash-and-burn agriculturalists? And isn’t it by cutting and burning that all those thousands of acres of tropical forest are being destroyed in Amazonia and around the world? The answer to both these questions is yes, but obviously there is a difference. The difference, of course, is one of scale.
The Yanomami and the Kayapo Indians live in the forest and are part of the forest. If they destroy it, they destroy themselves. They therefore make their modest-size fields and plant crops sufficient only for their needs. It is the non-Indian agriculturalists (or investors) who order the destruction of a forest they may never have seen in order to install quite inappropriate plantations or cattle ranches. This, of course, they must do on as large a scale as they can afford to ensure that their profit margin is to their liking. The enormous clearings that result are far beyond the ability of even the most healthy forest to regenerate. One example of such extensive destruction is the notorious case of the Volkswagen ranch whose burning became public knowledge only when seen from space by the Skylab satellite (Bourne, 1978).
In contrast, the forest itself begins reclaiming the relatively tiny Indian fields cut in its midst by supporting the growth of pioneer species (weeds, some would call them) even before the Indians have taken the two or three harvests they find practical before returning the field to the forest and its regenerative process. In many cases, in fact, the Indians stop using a field not so much because the pro-
ductiveness of the tropical soils decline so rapidly but because clearing the field of weeds is just more trouble than it is worth.
Done the right way, the Indian way, shifting cultivation rejuvenates the forest. It is the use of the technique on too large a scale by the non-Indian that is destructive.
INDIAN PRODUCTIVITY IN THE TROPICAL FOREST
The Indians of Amazonia have what we would consider an extremely low standard of living. Living in relative isolation from the national societies of the countries within whose boundaries they live, their economic production, whether from their agricultural practices or their use of game, fish, and natural forest resources, is strictly for their own subsistence. As a result, they are commonly stereotyped as poor and lazy with no potential as producers of anything for the regional, national, or international markets. Quite the opposite is true. A now-famous example is the successful production and marketing of highly marketable Brazil nuts by the Gavioes of Para State, Brazil—an activity they began on their own initiative in the mid-1970s (Ferraz, 1982; Ramos, 1980). Almost overnight, the Gavioes became not only quite well-to-do by local (non-Indian) standards but also transformed themselves, in the eyes of their non-Indian neighbors, from lazy good-for-nothings to productive members of society. In 1975 I knew of one Yanomami community that after exhausting the supply of bananas in its own fields, began a new, additional plantation so that it could continue to sell bananas to the tin miners who worked in Yanomami territory for a time in 1975 and 1976. As long ago as 1930, Curt Nimuendaju (1974) spoke of how the Ramkokamekra Canela Indians could have produced a marketable surplus of manioc flour but explained that they never did develop this potential because they had no way to transport the flour to market. Another example is the production and marketing of natural rubber in a recent community development project undertaken by one subgroup of the Nambiquara.
These are only a few examples. There has been considerable discussion of the possibly marketable products that can be grown in a properly and sustainably managed tropical forest (see, for example, Goodland, 1980). It is not yet known quite how productive the tropical forest can be or how large (or small) a population of resident producers it can support. The point here is simply that the Indians who live in the forest and know its ecology so well have long ago demonstrated their ability to function as valuable and effective producers of its marketable resources.
THE IMPACT OF DEFORESTATION ON INDIAN LIFE
The destruction of the tropical forests has both a direct and an indirect impact on the resources and livelihood of Indian populations. In some cases, deforestation is occurring inside recognized Indian areas. Even when the deforestation occurs outside these areas, however, the impact on the animal and plant resources, the water supply, and the rivers, which serve as avenues of transportation, in and near Indian areas can be devastating.
Deforestation takes place along with traditional frontier expansion and with the implementation of large-scale development projects. In either case, there is an influx of outsiders into regions previously but sparsely inhabited. Among the inhabitants of these regions there are often relatively isolated Indian populations. This isolation is broken overnight as land-hungry settlers begin invading Indian lands. In Brazil, colonists are aware of this well-known technique even before they move to Amazonia. If a settler can establish an illegal smallholding on Indian lands (among the least protected by the authorities of all land categories in Brazil’s interior) then, either as a matter of squatter’s rights or in compensation for being evicted, he will have improved his chances of acquiring a plot of land through the government colonization program.
In all such cases, the Indians’ control of their own natural resources is eroded and the supply of these resources declines. One of the first results of these processes is the impoverishment, if not the dispossession, of the Indian populations, leading to their migration to towns and cities where the best they can hope for is to swell the ranks of the urban under- and unemployed and a life of disease, prostitution, homelessness, and begging.
INDIAN MANAGERS OF THE RAIN FORESTS
The indigenous inhabitants of the tropical rain forests are valuable participants in these ecosystems. Their relationship and interaction with their forest environment not only affords them a sustainable and nondestructive livelihood but also enhances the vigor, diversity, and population size of the forest’s flora and fauna. Deforestation, both within and outside Indian lands, can so devastate the natural resources on which the Indian way of life depends that it becomes impossible for the Indian population to remain in the area and lead any semblance of its traditional way of life. But given the opportunity, Indian groups can rapidly adapt to a productive relationship with national and international society. They can produce a wide range of natural and cultivated products for the marketplace while still pursuing their way of life in and as part of the forest.
When we speak of the preservation of the tropical forests we must make clear, explicitly and emphatically, that we mean the preservation of the forests’ flora and their fauna and their indigenous human inhabitants. These indigenous peoples are our representatives of choice who can bring the forests, little by little, to their full productive potential and keep them healthy and well and with their magnificent biological diversity intact for the benefit of us all.
REFERENCES
Alegretti, M.H. 1985. The Rubber Tappers and Environmental Issues in the Amazon Region. Message sent to the UN World Commission on Environment and Development. Instituto de Estudos Socio-Economicos (INESC), Brasilia.
Bourne, R. 1978. Assault on the Amazon. Victor Gollancz, London. 320 pp.
Conklin, H.C. 1961. The study of shifting cultivation. Curr. Anthropol. 2(1):27–61.
Ferraz, I. 1982. Os Indios Gavioes: Observacoes Sobre uma Situacao Critica. Unpublished manuscript. Rio de Janeiro. 31 pp.
Frechione, J., D.A.Posey, and L.Francelino da Silva. 1985. The Perception of Ecological Zones and Natural Resources in the Brazilian Amazon: An Ethnoecology of Lake Coari. Paper presented at the annual meeting of the American Anthropological Association, Washington, D.C.
Goodland, R.J.A. 1980. Environmental Ranking of Amazonian Development. Pp. 1–20 in F. Barbira-Scazzocchio, ed. Land, People and Planning in Contemporary Amazonia. Centre of Latin American Studies, Occasional Publication No. 3. Cambridge University, Cambridge.
Gross, D.R. 1975. Protein capture and cultural development in the Amazon Basin. Am. Anthropol. 77(3):526–549.
Harner, M.J. 1972. The Jivaro: People of the Sacred Waterfalls. Doubleday/Natural History Press, Garden City, N.Y. 233 pp.
Hickerson, H. 1970. The Chippewa and Their Neighbors: A Study in Ethnohistory. Holt, Rinehart and Winston, New York. 133 pp.
Nimuendaju, C. 1974. Farming among the Eastern Timbira. Pp. 111–119 in P.J.Lyons, ed. Native South Americans. Little, Brown, Boston.
Posey, D.A. 1983. Indigenous knowledge and development: An ideological bridge to the future. Cienc. Cult. 35(7):877–894.
Posey, D.A. 1985. Indigenous management of tropical forest ecosystems: The case of the Kayapo Indians of the Brazilian Amazon. Agroforestry Sys. 3:139–158.
Posey, D.A., J.Frechione, J.Eddins, and L.F.da Silva. 1984. Ethnoecology as applied anthropology in Amazonian development. Hum. Org. 43(2):95–107.
Ramos, A.R. 1980. Development, integration and the ethnic integrity of Brazilian Indians. Pp. 222–229 in F.Barbira-Scazzocchio, ed. Land, People and Planning in Contemporary Amazonia. Centre of Latin American Studies, Occasional Publication No. 3. Cambridge University, Cambridge.
Taylor, K.I. 1974. Sanuma Fauna, Prohibitions and Classifications. Monograph No. 18. Fundacion La Salle de Ciencias Naturales, Instituto Caribe de Antropologia y Sociologia, Caracas, Venezuela. 138 pp.
Taylor, K.I. 1983. Las necesidades de tierra de los Yanomami. (Abstract in English) America Indigena XLIII(3):629–654.
CHAPTER 16
PRIMATE DIVERSITY AND THE TROPICAL FOREST
Case Studies from Brazil and Madagascar and the Importance of the Megadiversity Countries
RUSSELL A.MITTERMEIER
Vice-President for Science, World Wildlife Fund/The Conservation Foundation, Washington, D.C.
Much of the early interest in wildlife conservation grew out of a desire to save some of the world’s most spectacular mammals, and to some extent, these so-called charismatic megavertebrates are still the best vehicles for conveying the entire issue of conservation to the public. They are really our flagship species, both here in the United States and in the developing countries, and primates in particular are perhaps the best flagships for tropical forest conservation. Nonhuman primates are of particular interest in this context for three basic reasons: they are of great importance to our own species; they are largely a tropical order, roughly 90% of all primate species being restricted to the tropical forest regions of Asia, Africa, and the Neotropics; and they are members of the elite group called the charismatic megavertebrates.
The threats to primates and their tropical forest habitats can be seen by examining two tropical forest regions: Brazil, particularly the Atlantic forest region of eastern Brazil, and the island of Madagascar. These are clearly two of the most important countries for primate conservation, and they are among the world’s richest countries for living organisms in general—countries that I call the megadiversity countries and that are critical to the survival of the majority of the world’s biological diversity.
Most people are aware of the importance of the Order Primates, which of course includes our own species, Homo sapiens. However, few realize how diverse the Order of Primates actually is, including as it does some 200 species that range from the tiny mouse lemur (Microcebus murinus) of Madagascar and the tarsiers (Tarsius spp.) of Southeast Asia to the great apes, which include our closest living relatives, the chimpanzee (Pan troglodytes) and the pygmy chimpanzee (Pan paniscus). Our
nonhuman primate relatives are valuable to us in many ways, and the rapid growth of the science of primatology over the past 25 years has reflected this. Studies of these animals have taught us a great deal about the intricacies of our own behavior, they have clarified questions about our evolution and our origins, and they have played a significant role in biomedical research. Furthermore, the importance of primates as key elements of the tropical forest (e.g., seed dispersers) is only starting to be understood.
Unfortunately, wild populations of most nonhuman primates are decreasing all over the world. Many spectacular species like the mountain gorilla (Gorilla gorilla beringei) from Rwanda, Uganda, and Zaire, the golden lion tamarin (Leontopithecus rosalia) and the muriqui (Brachyteles arachnoides) from Brazil, and the indri (Indri indri) and the aye-aye (Daubentonia madagascariensis) from Madagascar are already endangered, and many others are headed in the same direction.
Without a doubt, the major cause of the decline of primate populations is destruction of their tropical forest habitat, which is occurring at a rate of some 10 to 20 million hectares per year (OTA, 1984), the latter figure being equivalent to a loss of an area the size of California every 2 years.
Another very important factor in the decline of these populations is hunting of primates, mainly as a source of food, but these animals are also hunted for their supposed medicinal value, for the ornamental value of their skins and other body parts, and for their use as bait for other animals, or to eliminate them from agricultural areas where they have become crop raiders. The effects of hunting vary greatly from region to region and from species to species, but hunting of primates as food is known to be a very serious threat in at least three parts of the world—the Amazonian region of South America, West Africa, and Central Africa. Many thousands of primates are killed every year in these regions for culinary purposes, and such overhunting has already resulted in the elimination of certain species from large areas of otherwise suitable forest habitat (e.g., the elimination of woolly monkeys and spider monkeys in Amazonia) (Mittermeier, 1987; Mittermeier et al., 1986).
Live trapping of primates, either for export or for local use, plays an important role as well. Live primates are used in biomedical research and testing, or they may be sold as pets or exhibits, both internationally and within their countries of origin. For the most part, this is a less important factor than habitat destruction or hunting, but for certain endangered and vulnerable species that happen to be in heavy demand, it can be quite serious. Species that have been hurt by the trade in live primates include the chimpanzee and the cotton-top tamarin (Saguinus oedipus), both of which were important biomedical research models, and the woolly monkeys (Lagothrix spp.), which were and still are very popular as pets for local people in Amazonia.
All these factors have combined to bring about a worldwide decline in primate populations. According to the International Union for Conservation of Nature (IUCN), one out of every three of the world’s 200 primate species is already in some danger and one in seven is highly endangered and could be extinct by the turn of the century or even sooner if something isn’t done quickly. These are minimum estimates. Very often when specialists go into the field to investigate
the status of poorly known species, they find it necessary to add to the endangered list.
To prevent the extinction of the world’s nonhuman primates, the Primate Specialist Group of IUCN’s Species Survival Commission put together a Global Strategy for Primate Conservation in 1977. This document (Mittermeier, 1977) was the first effort to take a worldwide view of primate conservation problems, and its purpose was to make the Primate Specialist Group’s goal of maintaining the current diversity of the Order of Primates a reality. It placed dual emphasis on ensuring the survival of endangered species wherever they occur and on providing effective protection for large numbers of primates in areas of high primate diversity or abundance. This original Global Strategy, which is now out of date, is being updated by a series of new regional plans for Africa (Oates, 1986), Asia (Eudey, 1987), Madagascar, and the Neotropical region, which will guide primate conservation activities for the remainder of this decade.
To find the financial support for the activities identified in the original Global Strategy, the World Wildlife Fund established a special Primate Program in 1979. Since that time, the program has funded and helped implement more than 150 projects, large and small, in 31 different countries, and it continues to grow. In addition, the program produces a wide variety of educational materials and publishes Primate Conservation, the newsletter and journal of the IUCN/SSC Primate Specialist Group, which is the major means of communication among the world’s primate conservationists.
A number of other organizations have also helped to support projects identified by the Primate Specialist Group, among them the New York Zoological Society, the Wildlife Preservation Trust International, the African Wildlife Foundation, the Fauna and Flora Preservation Society, the Brookfield Zoo, and the Frankfurt Zoological Society, to name just a few. Although the combined efforts of the World Wildlife Fund Primate Program and the other organizations have achieved a great deal on behalf of primates over the past decade, it is clear that much more will have to be done over the next few years to ensure that all of the world’s 200 primate species are still with us as we enter the next century.
Two tropical countries, Brazil and Madagascar, are particularly important in efforts to conserve primate diversity, since they alone are home to 40% of the world’s living primate species. Brazil, with 357 million hectares of tropical forest, is by far the richest country in the world for this biome, containing more than three times more forest than the next country on the list, which is Indonesia, and 30% of all the tropical forest on our planet (Table 16–1). Not surprisingly, Brazil is also home to far more primates than any other country; its 53 species account for about 27%, or one in every four, primates in the world (Table 16–2).
Although one usually hears much more about Amazonia, the highest priority area within Brazil is its Atlantic forest region, which is the most developed and most devastated part of the country. The Atlantic forest is a unique series of ecosystems quite distinct from the much more extensive Amazonian forests to the northwest. At one time, it stretched pretty much continuously from the state of Rio Grande do Norte at the easternmost tip of South America out as far as Rio Grande do Sul, the southernmost state in Brazil, and it included some of the
TABLE 16–1 Countries of the World Containing the Largest Areas of Closed Tropical Foresta
richest, tallest, and most beautiful forest on Earth. In its primeval state, the Atlantic forest complex covered over 1 million square kilometers in 14 states or about 12% of Brazil, and its length from north to south extended a greater distance than the entire Atlantic seaboard of the United States from northern Maine to the Florida Keys. However, this region was the first part of Brazil to be colonized, it has developed into the agricultural and industrial center of the country, and it has within its borders two of the three largest cities in all of South America—Rio de Janeiro and São Paulo, which is now one of the largest cities on Earth.
The result has been large-scale forest destruction, especially in the last two decades of rapid economic development, to obtain lumber and charcoal and to make way for plantations, cattle pasture, and industry—to the point that only 1 to 5% of the original forest remains in this region. As might be expected, the animals and plants native to the Atlantic forest are not doing very well under such circumstances. Many of these are endemic (including 40% of all the small, non-volant, i.e., nonflying, mammals, 54% of the trees, and 64% of the palms), and increasing numbers are being added to the endangered species list. The best example is probably the effect on the primates, 80% of which are endemic to the Atlantic forest. Twenty-one species and subspecies of monkeys are found in this region, and the studies that have been carried out with World Wildlife Fund support since 1979 indicate that fully 14 of these are endangered and that several are literally on the verge of extinction. Of these 14 endangered species, 13 are found nowhere else in the world (Mittermeier et al., 1986).
Two of the Atlantic forest primate species stand out among the rest: the muriqui (Brachyteles arachnoides), which is the largest and most apelike of the South American monkeys, and the golden lion tamarin (Leontopithecus rosalia), which is surely one of the most beautiful of all mammals. These animals are representatives of the two highly endangered genera that are endemic to the Atlantic forest, and they have been subjects of two major public awareness campaigns that have been under way for the past 5 years (Dietz, 1985; Mittermeier et al., 1985). They have really become the flagship species for the entire region, and the campaigns using them as symbols are excellent examples of the way in which key groups of animals can be used to sell the whole issue of conservation, both in the tropical countries and in the developed world.
The campaigns for the muriqui and the golden lion tamarin have been multifaceted, including ecological research, survey work, development of museum exhibits, production of films, and distribution of a wide variety of educational and promotional materials, including posters, stickers, T-shirts, and various publications. The result is that these two species, which were virtually unknown to the general public in Brazil 5 years ago, are now so popular that they appear on the cover of phone books, on postage stamps, as themes of parades and theater presentations, and as subjects of numerous magazine and newspaper articles. All this, and of course a broad spectrum of some 50 other conservation projects being supported by the World Wildlife Fund in this region, has led to a general increase in conservation awareness, which we hope will be instrumental in helping to save what remains of the Atlantic forest and its spectacular fauna and flora.
The situation in Madagascar is even more critical than in the Atlantic forest region of eastern Brazil. Madagascar is a unique evolutionary experiment and a living laboratory that is unlike anyplace else on Earth. The island has been separated
TABLE 16–2 Countries of the World Containing the Greatest Primate Diversitya
TABLE 16–3 Primate Endemism in the 15 Countries With the Greatest Primate Diversity
|
Country |
Endemic Species (%) |
Endemic Genera (%) |
|
Madagascar |
93 |
92 |
|
Indonesia |
44–50 |
12.5 |
|
Brazil |
35 |
12.5 |
|
Colombia |
11 |
0 |
|
Peru |
7 |
0 |
|
Zaire |
6–7 |
0 |
|
Nigeria |
4 |
0 |
|
Cameroon |
0 |
0 |
|
Congo |
0 |
0 |
|
Equatorial Guinea |
0 |
0 |
|
Central African Republic |
0 |
0 |
|
Gabon |
0 |
0 |
|
Uganda |
0 |
0 |
|
Bolivia |
0 |
0 |
|
Angola |
0 |
0 |
from the African mainland for perhaps as long as 200 million years, if in fact it was ever connected, and most of the plant and animal species found there have evolved in isolation and are unique to the island.
The most striking and conspicuous animals on Madagascar are the primates, which consist entirely of lemurs. Among these lemurs are some of the most unusual primates on Earth, ranging from the mouse lemur, which is the smallest living primate, to the indri, which is the largest living prosimian, and the aye-aye, which is the strangest of all primates and the only representative of an entire primate family, the Daubentoniidae.
This lemur radiation on Madagascar is one of the most diverse primate faunas anywhere, its 29 species placing it fourth on the world list of primate diversity behind Brazil, Zaire, and Indonesia (even though it is only 7% the size of Brazil, Table 16–2). When endemism is considered, Madagascar’s primate fauna seems even more impressive, since 93% of all its species are restricted to that country—a figure not even approached by any other country (Table 16–3). Furthermore, the two lemur species found outside Madagascar reside only on the nearby Comoros Islands and are probably recent introductions by humans.
The situation is much the same for most other groups of organisms in Madagascar. Seven of the eight species of carnivores found there are endemic, as are 29 of the 30 tenrecs, 106 of the 250 birds, 233 of the 245 reptiles, 142 of the 144 frogs, 110 of the 112 species of palms, and 80% of its nearly 8,000 angiosperm plants. It is not just endemism that is impressive on Madagascar, however, but total diversity as well. Although Madagascar is only about 40% again as large as the state of California and accounts for less than 2% of the African region, its 8,000 angiosperm plants represent 25% of all angiosperms in Africa (P.Lowry, personal communication, 1987), it has more orchids than the entire African mainland, and its 13 living primate genera approach the 14 to 15 mainland genera in total diversity.
Unfortunately, most of Madagascar’s spectacular fauna and flora is endangered, mainly, once again, because of forest destruction. Although human beings arrived on Madagascar only some 1,500 to 2,000 years ago, human activity has resulted in the loss of some 80% of Madagascar’s forests, and the major remaining forest formations are being chipped away for firewood and charcoal and for slash-and-burn agriculture. Hunting is a problem as well, especially with the breakdown of local cultures, which formerly included many taboos against the hunting of primates and other wildlife.
Lest anyone believe that extinctions are a figment of the conservationist’s imagination, he or she need only look at what has already been lost on Madagascar over the past 2,000 years. Among the species that have disappeared are the elephant birds (Aepyornis spp.), which were the largest birds that ever lived, a pygmy hippopotamus, an aardvark, and fully six genera of lemurs, representing one-third of all known Malagasy lemur species. Included among the species lost are animals like Megaladapis (Figure 16–1), which moved like a huge koala and grew to be as large as a female gorilla (Sussman et al., 1985).
Almost all the species that have already disappeared were diurnal and larger than the surviving species. If this trend continues, the next in line would be the indri, which is the largest, and the sifakas (Propithecus spp.), which are next in size. In fact, several of these are already endangered. One, the black sifaka (Propithecus diadema perrieri) from northeastern Madagascar, is now down to only about 100 individuals and must be considered on the verge of extinction.
FIGURE 16–1 Above: The extinct giant lemur Megaladapis from Madagascar, as reconstructed by Stephen D.Nash. Left: An extant ring-tailed lemur in Madagascar.
At present, about 40% of Malagasy lemurs are considered endangered and many more are likely to enter the endangered category as we learn more about them. And what is happening to lemurs is happening to the rest of Madagascar’s fauna and flora as well.
Despite the many problems, there is cause for optimism in Madagascar. In November 1985, a special National Conservation Strategy Conference held there attracted representatives from many international organizations, including IUCN, the World Wildlife Fund, the United Nations Environment Program, the Food and Agriculture Organization, the World Bank, and a number of bilateral aid organizations, including the U.S. Agency for International Development. This conference generated a great deal of enthusiasm for conservation among the Malagasy themselves and should serve as an important take-off point for future conservation activities. Several projects supported by the World Wildlife Fund are also serving as models for community involvement in conservation, and are attracting international attention to the need for conservation in this all-important country. Of particular importance in this respect is the Beza-Mahafaly project in southwestern Madagascar, which is being conducted by researchers from the University of Madagascar, Yale University, Washington University, and the Missouri Botanical Garden (Sussman et al., 1985).
To be sure, a great deal still needs to be done in Madagascar to ensure that the country’s amazing biological diversity is maintained for future generations. Nevertheless, the time appears to be ripe to accomplish something of major proportions there and in effect to change the course of conservation history in this unique country.
As indicated in Table 16–2, there is a very disproportionate distribution of primate diversity in the world. Just four countries, Brazil, Madagascar, Zaire, and Indonesia, by themselves account for approximately 75% of all the world’s primate species. If we are going to maintain global primate diversity, we must pay special attention to these countries over the next few decades, not to the exclusion of others but certainly more than we have in the past.
Needless to say, these megadiversity countries are not just important for primates. Although we are still in the process of compiling data, it appears that approximately 50 to 80% of the world’s total biological diversity will be found in some 6 to 12 tropical countries. The first 6 of these to have emerged from the preliminary analysis are Brazil, Colombia, Mexico, Zaire, Madagascar, and Indonesia (see Figure 16–2). Not only do these countries have a major portion of the world’s biological diversity, they have an even higher percentage of the world’s diversity at risk—the very diversity that is in danger of disappearing over the next decade and that is of so much concern to conservation biologists. All these countries are undergoing rapid environmental change, are facing severe economic problems, and in general, lack the resources to develop the broad-based conservation programs needed to conserve biological diversity on their own. This means that people of the developed world are going to have to work in much closer collaboration with colleagues in these countries in the years to come and that the developed countries will have to provide far more resources for conservation than ever before.
FIGURE 16–2 Megadiversity countries identified by the World Wildlife Fund.
I do not believe in a gloom-and-doom approach to conservation, which can be quite detrimental to our efforts. On a more upbeat note, I believe that much of our planet’s biological diversity can be maintained and that conservation in general has to be considered the art of the possible. The example of Brazil, which may be the single most diverse country in the world, is most encouraging. One hears a great deal about destruction and the many environmental problems faced by Brazil but very little about the successes. Nonetheless, the successes are there, and for those of us who have been working in Brazil for two decades, the advances in conservation in that country seem little short of phenomenal. They lead me to believe that a very large proportion of Brazil’s biological diversity can be maintained. With the proper input of resources from both the developed world and the developing countries themselves, there is no reason why these successes cannot be repeated on a global basis.
REFERENCES
Dietz, L.A. 1985. Captive-born lion tamarins released into the wild: A report from the field. Primate Conserv. 6:21–27.
Eudey, A.A. 1987. Action Plan for Asian Primate Conservation. International Union for the Conservation of Nature and Natural Resources/Species Survival Commission Primate Specialist Group, World Wildlife Fund, and United Nations Environment Programme, Washington, D.C. 70 pp.
Mittermeier, R.A. 1977. A Global Strategy for Primate Conservation. International Union for the Conservation of Nature and Natural Resources/Species Survival Commission Primate Specialist Group, Cambridge, Mass. 325 pp.
Mittermeier, R.A. 1987. The effects of hunting on rain forest primates. Pp. 109–146 in C.Marsh and R.A.Mittermeier, eds. Primate Conservation in the Tropical Rain Forest. Alan R.Liss, New York.
Mittermeier, R.A., and J.F.Oates. 1985. Primate diversity: The world’s top countries. Primate Conserv. 5:41–48.
Mittermeier, R.A., C.Valle, I.B.Santos, C.Alves, C.A.Machado Pinto, and A.F.Coimbra-Filho. 1985. Update on the muriqui. Primate Conserv. 5:28–30.
Mittermeier, R.A., J.F.Oates, A.A.Eudey, and J.Thornback. 1986. Primate conservation. Pp. 3–72 in G.Mitchell and J.Erwin, eds. Comparative Primate Biology, Vol. 2A, Behavior, Conservation and Ecology. Alan R. Liss, New York.
Oates, J.F. 1986. Action Plan for African Primate Conservation. International Union for the Conservation of Nature and Natural Resources/Species Survival Commission Primate Specialist Group, World Wildlife Fund and United Nations Environment Programme, Washington, D.C. 41 pp.
OTA (Office of Technology Assessment). 1984. Technologies to Sustain Tropical Forest Resources. Congress of the United States, Office of Technology Assessment, Washington, D.C. 344 pp.
Sussman, R.W., A.F.Richard, and G.Ravelojaona. 1985. Madagascar: Current projects and problems in conservation. Primate Conserv. 5:53–59.