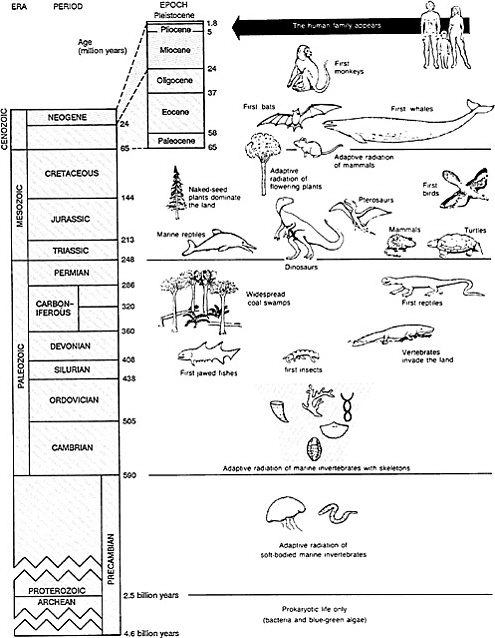
CHAPTER 2
THE LOSS OF DIVERSITY
Causes and Consequences
PAUL R.EHRLICH
Professor of Biological Sciences, Stanford University, Stanford, California
Discussions of the current extinction crisis all too often focus on the fates of prominent endangered species, and in many cases on deliberate overexploitation by human beings as the cause of the endangerment. Thus black rhinos are disappearing from Africa, because their horns are in demand for the manufacture of ceremonial daggers for Middle Eastern puberty rites; elephants are threatened by the great economic value of ivory; spotted cats are at risk because their hides are in demand by furriers; and whales are rare because, among other things, they can be converted into pet food.
Concern about such direct endangerment is valid and has been politically important, because public sympathy seems more easily aroused over the plight of furry, cuddly, or spectacular animals. The time has come, however, to focus public attention on a number of more obscure and (to most people) unpleasant truths, such as the following:
-
The primary cause of the decay of organic diversity is not direct human exploitation or malevolence, but the habitat destruction that inevitably results from the expansion of human populations and human activities.
-
Many of the less cuddly, less spectacular organisms that Homo sapiens is wiping out are more important to the human future than are most of the publicized endangered species. People need plants and insects more than they need leopards and whales (which is not to denigrate the value of the latter two).
-
Other organisms have provided humanity with the very basis of civilization in the form of crops, domestic animals, a wide variety of industrial products, and many important medicines. Nonetheless, the most important anthropocentric reason for preserving diversity is the role that microorganisms, plants, and animals
-
play in providing free ecosystem services, without which society in its present form could not persist (Ehrlich and Ehrlich, 1981; Holdren and Ehrlich, 1974).
-
The loss of genetically distinct populations within species is, at the moment, at least as important a problem as the loss of entire species. Once a species is reduced to a remnant, its ability to benefit humanity ordinarily declines greatly, and its total extinction in the relatively near future becomes much more likely. By the time an organism is recognized as endangered, it is often too late to save it.
-
Extrapolation of current trends in the reduction of diversity implies a denouement for civilization within the next 100 years comparable to a nuclear winter.
-
Arresting the loss of diversity will be extremely difficult. The traditional “just set aside a preserve” approach is almost certain to be inadequate because of factors such as runaway human population growth, acid rains, and climate change induced by human beings. A quasi-religious transformation leading to the appreciation of diversity for its own sake, apart from the obvious direct benefits to humanity, may be required to save other organisms and ourselves.
Let us examine some of these propositions more closely. While a mere handful of species is now being subjected to purposeful overexploitation, thousands are formally recognized in one way or another as threatened or endangered. The vast majority of these are on the road to extinction, because humanity is destroying habitats: paving them over, plowing them under, logging, overgrazing, flooding, draining, or transporting exotic organisms into them while subjecting them to an assault by a great variety of toxins and changing their climate.
As anyone who has raised tropical fishes knows, all organisms require appropriate habitats if they are to survive. Just as people cannot exist in an atmosphere with too little oxygen, so neon tetras (Paracheirodon innesi) cannot survive in water that is 40F (4.4C) or breed in highly alkaline water. Trout, on the other hand, cannot breed in water that is too warm or too acid. And the bacteria that produce the tetanus toxin cannot reproduce in the presence of oxygen. In order to persist, Bay checkerspot butterflies (Euphydryas editha bayensis) must have areas of serpentine grassland (to support the growth of plants that serve as food for their caterpillars and supply nectar to the adults). Whip-poor-wills, red-eyed vireos, Blackburnian warblers, scarlet tanagers, and dozens of other North American birds must have mature tropical forest in which to overwinter (see Terborgh, 1980, for example). Black-footed ferrets (Mustela nigripes) require prairie that still supports the prairie dogs on which the ferrets dine.
This utter dependence of organisms on appropriate environments (Ehrlich, 1986) is what makes ecologists so certain that today’s trends of habitat destruction and modification—especially in the high-diversity tropical forest (where at least one-half of all species are believed to dwell)—are an infallible recipe for biological impoverishment. Those politicians and social scientists who have questioned the extent of current extinctions are simply displaying their deep ignorance of ecology;
habitat modification and destruction and the extinction of populations and species go hand in hand.
The extent to which humanity has already wreaked havoc on Earth’s environments is shown indirectly by a recent study of human appropriation of the products of photosynthesis (Vitousek et al., 1986). The food resource of the animals in all major ecosystems is the energy that green plants bind into organic molecules in the process of photosynthesis, minus the energy those plants use for their own life processes—growth, maintenance, and reproduction. In the jargon of ecologists, that quantity is known as the net primary production (NPP). Globally, this amounts to a production of about 225 billion metric tons of organic matter annually, nearly 60% of it on land.
Humanity is now using directly (e.g., by eating, feeding to livestock, using lumber and firewood) more than 3% of global NPP, and about 4% of that on land. This is a minimum estimate of human impact on terrestrial systems. Since Homo sapiens is one of (conservatively) 5 million species, this may seem an excessive share of the food resource. But considering that human beings are perhaps a million times the weight of the average animal (since the overwhelming majority of animals are small insects and mites) and need on the order of a million times the energy per individual, this share might not be too unreasonable.
Yet human beings can be thought of as co-opting NPP not only by direct use but also by indirect use. Thus if we chalk up to the human account not only the NPP directly consumed, but such other categories as the amount of biomass consumed in fires used to clear land, the parts of crop plants not consumed, the NPP of pastureland (converted from natural habitat) not consumed by livestock, and so on, the human share of terrestrial NPP climbs to a staggering 30%. And if we add to that the NPP foregone when people convert more productive natural systems to less productive ones (such as forest to farm or pasture, grassland to desert, marsh to parking lot), the total potential NPP on land is reduced by 13%, and the human share of the unreduced potential NPP reaches almost 40%. There is no way that the co-option by one species of almost two-fifths of Earth’s annual terrestrial food production could be considered reasonable, in the sense of maintaining the stability of life on this planet.
These estimates alone both explain the basic causes and consequences of habitat destruction and alteration, and give reason for great concern about future trends. Most demographers project that Homo sapiens will double its population within the next century or so. This implies a belief that our species can safely commandeer upwards of 80% of terrestrial NPP, a preposterous notion to ecologists who already see the deadly impacts of today’s level of human activities. Optimists who suppose that the human population can double its size again need to contemplate where the basic food resource will be obtained.
A standard fool’s answer to that question is that indefinite expansion of the human population will be supported by the immeasurable riches of the sea. Unhappily for that notion, the riches of the sea have been quite carefully measured
and found wanting. People now use about 2% of the NPP of the sea, and the prospects even for doubling that yield are dim. The basic reason is that efficient harvesting of the sea requires the exploitation of concentrated pools of resources—schools of fishes and larger invertebrates. People cannot efficiently harvest much of the NPP that resides in tiny phytoplankton (the green plants of the sea) or in the zooplankton (animals too small to swim against the currents). Humanity appears to be already utilizing about as much of oceanic NPP as it can on a sustainable basis.
This discrepancy in the ability of Homo sapiens to exploit terrestrial and oceanic NPP is reflected in the general lack of an extinction crisis in the seas. Except for such organisms as some whales and fishes that are threatened by direct exploitation, animals that spend their entire lives in the open sea are relatively secure. Aside from some limited environments, such as certain coral reefs, the effects of habitat destruction are relatively small away from shorelines and estuaries. This situation could, of course, change rapidly if marine pollution increases—a distinct possibility.
The extirpation of populations and species of organisms exerts its primary impact on society through the impairment of ecosystem services. All plants, animals, and microorganisms exchange gases with their environments and are thus directly or indirectly involved in maintaining the mix of gases in the atmosphere. Changes in that mix (such as increases in carbon dioxide, nitrogen oxides, and methane) can lead to rapid climate change and, in turn, agricultural disaster. As physicist John Holdren put it, a carbon dioxide-induced climatic change could lead to the deaths by famine of as many as a billion people before 2020. Destroying forests deprives humanity not only of timber but also of dependable freshwater supplies and furthermore increases the danger of floods. Destruction of insects can lead to the failure of crops that depend upon insect pollination. Extermination of the enemies of insect pests (a usual result of ad lib pesticide spraying) can terminate the pest control services of an ecosystem and often leads to severe pest outbreaks. The extinction of subterranean organisms can destroy the fertility of the soil. Natural ecosystems maintain a vast genetic library that has already provided people with countless benefits and has the potential for providing many, many more.
These examples can be multiplied manyfold—the basic point is that organisms, most of which are obscure to nonbiologists, play roles in ecological systems that are essential to civilization. When a population playing a certain role is wiped out, ecosystem services suffer, even if many other populations of the same organism are still extant. If the population of Engelmann spruce trees (Picea engelmanni) in the watershed above your Colorado home is chopped down, you could be killed in a resulting flood, even though the species of spruce is not endangered. Equally, if that were the last population and it were reduced to just a dozen trees (so that, technically, the species still existed), you would not be spared the flood, and chance events would likely finish off the Engelmann spruce eventually anyway.
In most cases, numerous genetically diverse populations are necessary to ensure the persistence of a species in the face of inevitable environmental changes that occur naturally. The existence of many populations spreads the risk so that unfavorable conditions in one or a few habitats do not threaten the entire species. And the presence of abundant genetic variation within a species (virtually assured
if its populations are living in different geographic areas) increases its potential for successfully evolving in response to long-term environmental changes. Today, this genetic diversity within species is declining precipitously over much of Earth’s land surface—an unheralded loss of one of humanity’s most vital resources. That resource is largely irreplaceable. Along with fossil fuels, rich soils, ancient groundwater, and mineral deposits, genetic diversity is part of the inheritance of capital that Homo sapiens is rapidly squandering.
What then will happen if the current decimation of organic diversity continues? Crop yields will be more difficult to maintain in the face of climatic change, soil erosion, loss of dependable water supplies, decline of pollinators, and ever more serious assaults by pests. Conversion of productive land to wasteland will accelerate; deserts will continue their seemingly inexorable expansion. Air pollution will increase, and local climates will become harsher. Humanity will have to forego many of the direct economic benefits it might have withdrawn from Earth’s once well-stocked genetic library. It might, for example, miss out on a cure for cancer; but that will make little difference. As ecosystem services falter, mortality from respiratory and epidemic disease, natural disasters, and especially famine will lower life expectancies to the point where cancer (largely a disease of the elderly) will be unimportant. Humanity will bring upon itself consequences depressingly similar to those expected from a nuclear winter (Ehrlich, 1984). Barring a nuclear conflict, it appears that civilization will disappear some time before the end of the next century—not with a bang but a whimper.
Preventing such a denouement will prove extremely difficult at the very least; it may well prove to be impossible. Earth’s habitats are being nickeled and dimed to death, and human beings have great difficulty perceiving and reacting to changes that occur on a scale of decades. Our nervous systems evolved to respond to short-term crises—the potential loss of a mate to a rival, the sudden appearance of a bear in the mouth of the cave. For most of human evolutionary history there was no reason for natural selection to tune us to recognize easily more gradual trends, since there was little or nothing one could do about them. The human lineage evolved in response to changes in the ecosystems in which our ancestors lived, but individuals could not react adaptively to those changes, which usually took place slowly. The depletion of organic diversity and the potential destruction of civilization may, ironically, be an inevitable result of our evolutionary heritage.
If humanity is to avoid becoming once again a species consisting of scattered groups practicing subsistence agriculture, dramatic steps will be necessary. They can only be briefly outlined here. Simply setting aside preserves in the remaining relatively undisturbed ecosystems will no longer suffice. In most parts of the planet such areas are too scarce, and rapid climatic changes may make those preserves impossible to maintain (Peters and Darling, 1985). Areas already greatly modified by human activities must be made more hospitable for other organisms; for example, the spewing of toxins into the environment (leading to intractable problems like acid deposition) must be abated.
Above all, the growth of the human population must be halted, since it is obvious that if the scale of human activities continues to increase for even a few more decades, the extinction of much of Earth’s biota cannot be avoided. Indeed,
since Homo sapiens is now living largely on its inherited capital and in the future will have to depend increasingly on its income (NPP), one can argue persuasively that the size of the human population and the scale of human activities should be gradually reduced below present levels. Reducing that scale will be an especially difficult task, since it means that the environmental impacts of the rich must be enormously curtailed to permit the poor a chance for reasonable development.
Although improvements in the technologies used to support human life and affluence can of course help to ameliorate the extinction crisis, and to a limited extent technologies can substitute for lost ecosystem services, it would be a dangerous miscalculation to look to technology for the answer (see, for example, Ehrlich and Mooney, 1983). In my opinion, only an intensive effort to make those improvements and substitutions, combined with a revolution in attitudes toward other people, population growth, the purpose of human life, and the intrinsic values of organic diversity, is likely to prevent the worst catastrophe ever to befall the human lineage. Curiously, scientific analysis points toward the need for a quasi-religious transformation of contemporary cultures. Whether such a transformation can be achieved in time is problematic, to say the least.
We must begin this formidable effort by increasing public awareness of the urgent need for action. People everywhere should understand the importance of the loss of diversity not only in tropical forests, coastal zones, and other climatically defined regions of the world but also in demographically delineated regions such as areas of urbanization. The geological record can tell us much about catastrophic mass extinctions of the past. That, and more intensive studies of the living biota, can provide hints about what we might expect in the future. At the present time, data on the rates and direction of biodiversity loss remain sparse and often uncertain. As a result, estimates of the rate of loss, including the number and variety of species that are disappearing, vary greatly—in some cases, as pointed out by E. O.Wilson in Chapter 1, by as much as an order of magnitude. Moreover, scientists have also differed in their predictions of the eventual impact that will result from the diminishing biodiversity. Some aspects of these challenges are explored in the following five chapters comprising this section and are reflected throughout this volume.
REFERENCES
Ehrlich, A.H. 1984. Nuclear winter. A forecast of the climatic and biological effects of nuclear-war. Bull. At. Sci. 40(4):S1–S15.
Ehrlich, P.R. 1986. The Machinery of Nature. Simon and Schuster, New York. 320 pp.
Ehrlich, P.R., and A.H.Ehrlich. 1981. Extinction: The Causes and Consequences of the Disappearance of Species. Random House, New York. 305 pp.
Ehrlich, P.R., and H.A.Mooney. 1983. Extinction, substitution, and ecosystem services. BioScience 33(4):248–254.
Holdren, J.P., and P.R.Ehrlich. 1974. Human population and the global environment. Am. Sci. 62:282–292.
Peters, R.L., and J.D.S.Darling. 1985. The greenhouse effect and nature reserves. BioScience 35(11):707–717.
Terborgh, J.W. 1980. The conservation status of neotropical migrants: Present and future. Pp. 21–30 in A.Keast and E.S.Morton, eds. Migrant Birds in the Neotropics: Ecology, Behavior, Distribution, and Conservation. A symposium held at the Conservation and Research Center, National Zoological Park, Smithsonian Institution. Smithsonian Institution Press, Washington, D.C.
Vitousek, P.M., P.R.Ehrlich, A.H.Ehrlich, and P.M.Matson. 1986. Human appropriation of the products of photosynthesis. BioScience 36(6):368–373.
CHAPTER 3
TROPICAL FORESTS AND THEIR SPECIES
Going, Going…?
NORMAN MYERS
Consultant in Environment and Development, Oxford, United Kingdom
There is strong evidence that we are into the opening stages of an extinction spasm. That is, we are witnessing a mass extinction episode, in the sense of a sudden and pronounced decline worldwide in the abundance and diversity of ecologically disparate groups of organisms.
Of course extinction has been a fact of life since the emergence of species almost 4 billion years ago. Of all species that have ever existed, possibly half a billion or more, there now remain only a few million. But the natural background rate of extinction during the past 600 million years, the period of major life, has been on the order of only one species every year or so (Raup and Sepkoski, 1984). Today the rate is surely hundreds of times higher, possibly thousands of times higher (Ehrlich and Ehrlich, 1981; Myers, 1986; Raven, 1987; Soulé, 1986; Western and Pearl, in press; Wilson, 1987). Moreover, whereas past extinctions have occurred by virtue of natural processes, today the virtually exclusive cause is Homo sapiens, who eliminates entire habitats and complete communities of species in super-short order. It is all happening in the twinkling of an evolutionary eye.
To help us get a handle on the situation, let us take a lengthy look at tropical forests. These forests cover only 7% of Earth’s land surface, yet they are estimated to contain at least 50% of all species (conceivably a much higher proportion [see Erwin, Chapter 13 of this volume]). Equally important, they are being depleted faster than any other ecological zone.
TROPICAL FORESTS
There is general agreement that remaining primary forests cover rather less than 9 million square kilometers, out of the 15 million or so that may once have existed
according to bioclimatic data. There is also general agreement that between 76,000 and 92,000 square kilometers are eliminated outright each year, and that at least a further 100,000 square kilometers are grossly disrupted each year (FAO and UNEP, 1982; Hadley and Lanley, 1983; Melillo et al., 1985; Molofsky et al., 1986; Myers, 1980, 1984). These figures for deforestation rates derive from a data base of the late 1970s; the rates have increased somewhat since then. This means, roughly speaking, that 1% of the biome is being deforested each year and that more than another 1% is being significantly degraded.
The main source of information lies with remote-sensing surveys, which constitute a thoroughly objective and systematic mode of inquiry. By 1980 there were remote-sensing data for approximately 65% of the biome, a figure that has risen today to 82%. In all countries where remote-sensing information has been available in only the past few years—notably Indonesia, Burma, India, Nigeria, Cameroon, Guatemala, Honduras, and Peru—we find there is greater deforestation than had been supposed by government agencies in question.
Tropical deforestation is by no means an even process. Some areas are being affected harder than others; some will survive longer than others. By the end of the century or shortly thereafter, there could be little left of the biome in primary status with a full complement of species, except for two large remnant blocs, one in the Zaire basin and the other in the western half of Brazilian Amazonia, plus two much smaller blocs, in Papua New Guinea and in the Guyana Shield of northern South America. These relict sectors of the biome may well endure for several decades further, but they are little likely to last beyond the middle of next century, if only because of sheer expansion in the numbers of small-scale cultivators.
Rapid population growth among communities of small-scale cultivators occurs mainly through immigration rather than natural increase, i.e., through the phenomenon of the shifted cultivator. As a measure of what ultrarapid growth rates can already impose on tropical forests, consider the situation in Rondonia, a state in the southern sector of Brazilian Amazonia. Between 1975 and 1986, the population grew from 111,000 to well over 1 million, i.e., a 10-times increase in little more than 10 years. In 1975, almost 1,250 square kilometers of forest were cleared. By 1982, this amount had grown to more than 10,000 square kilometers, and by late 1985, to around 17,000 square kilometers (Fearnside, 1986).
It is this broad-scale clearing and degradation of forest habitats that is far and away the main cause of species extinctions. Regrettably, we have no way to know the actual current rate of extinction, nor can we even come close with accurate estimates. But we can make substantive assessments by looking at species numbers before deforestation and then applying the analytic techniques of island biogeography. To help us gain an insight into the scope and scale of present extinctions, let us briefly consider three particular areas: the forested tracts of western Ecuador, Atlantic-coast Brazil, and Madagascar. Each of these areas features, or rather featured, exceptional concentrations of species with high levels of endemism. Western Ecuador is reputed to have once contained between 8,000 and 10,000 plant species with an endemism rate somewhere between 40 and 60% (Gentry, 1986). If we suppose, as we reasonably can by drawing on detailed inventories in sample plots, that there are at least 10 to 30 animal species for every one plant
species, the species complement in western Ecuador must have amounted to 200,000 or more in all. Since 1960, at least 95% of the forest cover has been destroyed to make way for banana plantations, oil exploiters, and human settlements of various sorts. According to the theory of island biogeography, which is supported by abundant and diversified evidence, we can realistically expect that when a habitat has lost 90% of its extent, it will eventually lose half its species. Precisely how many species have actually been eliminated, or are on the point of extinction, in western Ecuador is impossible to say. But ultimate accuracy is surely irrelevant, insofar as the number must total tens of thousands at least, conceivably 50,000—all eliminated or at least doomed in the space of just 25 years.
Very similar baseline figures for species totals and endemism levels, and a similar story of forest depletion (albeit for different reasons and over a longer time period), apply to the Atlantic-coastal forest of Brazil, where the original 1 million square kilometers of forest cover have been reduced to less than 50,000 square kilometers (Mori et al., 1981). Parallel data apply also to Madagascar, where only 5% of the island’s primary vegetation remains undisturbed—and where the endemism levels are rather higher (Rauh, 1979).
So in these three tropical forest areas alone, with their roughly 600,000 species, the recent past must have witnessed a sizeable fallout of species. Some may not have disappeared as yet, due to the time lag in equilibration, i.e., delayed fallout effects stemming from habitat depletion. But whereas the ultimate total of extinctions in these areas in the wake of deforestation to date will presumably amount to some 150,000 species, we may realistically assume that already half, some 75,000 species, have been eliminated or doomed.
Deforestation in Brazil’s Atlantic-coastal forest and Madagascar has been going on for several centuries, but the main damage has occurred during this century, especially since 1950, i.e., since the spread of broad-scale industrialization and plantation agriculture in Brazil and since the onset of rapid population growth in Madagascar. This all means that as many as 50,000 species have been eliminated or doomed in these areas alone during the last 35 years. This works out to a crude average of almost 1,500 species per year—a figure consistent with the independent assessment of Wilson (1987), who postulates an extinction rate in all tropical forests of perhaps 10,000 species per year. Of course many reservations attend these calculations. More species than postulated may remain until a new equilibrium is established and causes their disappearance. Conversely, more species will presumably have disappeared during the later stages of the 35-year period than during the opening stage. Whatever the details of the outcome, we can judiciously use the figures and conclusions to form a working appraisal of the extent that an extinction spasm is already under way.
EXTINCTION RATES: FUTURE
The outlook for the future seems all the more adverse, though its detailed dimensions are even less clear than those of the present. Let us look again at tropical forests. We have seen what is happening to three critical areas. We can identify a good number of other sectors of the biome that feature exceptional
concentrations of species with exceptional levels of endemism and that face exceptional threat of depletion, whether quantitative or qualitative. They include the Choco forest of Colombia; the Napo center of diversity in Peruvian Amazonia, plus seven other centers (out of 20-plus centers of diversity in Amazonia) that lie around the fringes of the basin and hence are unusually threatened by settlement programs and various other forms of development; the Tai Forest of Ivory Coast; the montane forests of East Africa; the relict wet forest of Sri Lanka; the monsoon forests of the Himalayan foothills; northwestern Borneo; certain lowland areas of the Philippines; and several islands of the South Pacific (New Caledonia, for instance, is 16,100 square kilometers, almost the size of New Jersey, and contains 3,000 plant species, 80% of them endemic).
These various sectors of the tropical forest biome amount to roughly 1 million square kilometers (2.5 times the size of California), or slightly more than one-tenth of the remaining undisturbed forests. As far as we can best judge from their documented numbers of plant species, and by making substantiated assumptions about the numbers of associated animal species, we can estimate that these areas surely harbor 1 million species (could be many more)—and in many of the areas, there is marked endemism. If present land-use patterns and exploitation trends persist (and they show every sign of accelerating), there will be little left of these forest tracts, except in the form of degraded remnants, by the end of this century or shortly thereafter. Thus forest depletion in these areas alone could well eliminate large numbers of species, surely hundreds of thousands, within the next 25 years at most.
Looking at the situation another way, we can estimate, on the basis of what we know about plant numbers and distribution together with what we can surmise about their associated animal communities, that almost 20% of all species occur in forests of Latin America outside of Amazonia and that another 20% are present in forests of Asia and Africa outside the Zaire basin (Raven, 1987). That is, these forests contain some 1 million species altogether, even if we estimate that the planetary total is only 5 million. All the primary forests in which these species occur may well disappear by the end of this century or early in the next. If only half the species in these forests disappear, this will amount to several hundred thousand species.
What is the prognosis for the longer-term future? Could we eventually lose at least one-quarter, possibly one-third, or conceivably an even larger share of all extant species? Let us take a quick look at Amazonia (Simberloff, 1986). If deforestation continues at present rates until the year 2000, but then comes to a complete halt, we could anticipate an ultimate loss of about 15% of the plant species and a similar percentage of animal species. If Amazonia’s forest cover were to be ultimately reduced to those areas now set aside as parks and reserves, we could anticipate that 66% of the plant species will eventually disappear together with almost 69% of bird species and similar proportions of all other major categories of species.
Of course we may learn how to manipulate habitats to enhance survival prospects. We may learn how to propagate threatened species in captivity. We may be able to apply other emergent conservation techniques, all of which could help to relieve
the adverse repercussions of broad-scale deforestation. But in the main, the damage will have been done. For reasons of island biogeography and equilibration, some extinctions in Amazonia will not occur until well into the twenty-second century, or even further into the future. So a major extinction spasm in Amazonia is entirely possible, indeed plausible if not probable.
TROPICAL FOREST AND CLIMATIC CHANGE
Protected areas are not likely to provide a sufficient answer for reasons that reflect climatic factors. In Amazonia, for instance, it is becoming apparent that if as much as half the forest were to be safeguarded in some way or another (e.g., through multiple-use conservation units as well as protected areas), but the other half of the forest were to be developed out of existence, there could soon be at work a hydrological feedback mechanism that would allow a good part of Amazonia’s moisture to be lost to the ecosystem (Salati and Vose, 1984). The remaining forest would likely be subjected to a steady desiccatory process, until the moist forest became more like a dry forest, even a woodland—with all that would mean for the species communities that are adapted to moist forest habitats. Even with a set of forest safeguards of exemplary type and scope, Amazonia’s biotas would be more threatened than ever.
Still more widespread climatic changes with yet more marked impact are likely to occur within the foreseeable future. By the first quarter of the next century, we may well be experiencing the climatic dislocations of a planetary warming, stemming from a buildup of carbon dioxide and other so-called greenhouse gases in the global atmosphere (Bolin and Doos, 1986; DoE, 1985). The consequences for protected areas will be pervasive and profound. The present network of protected areas, grossly inadequate as it is, has been established in accord with present-day needs. Yet its ultimate viability will be severely threatened in the wake of a greenhouse effect as vegetation zones start to migrate away from the equator with all manner of disruptive repercussions for natural environments (Peters and Darling, 1985; Peters, Chapter 51 of this volume).
These, then, are some dimensions of the extinction spasm that we can reasonably assume will overtake the planet’s biotas within the next few decades (unless of course we do a massively better job of conservation). In effect we are conducting an irreversible experiment on a global scale with Earth’s stock of species.
REPERCUSSIONS FOR THE FUTURE OF EVOLUTION
The foreseeable fallout of species, together with their subunits, is far from the entire story. A longer-term and ultimately more serious repercussion could lie in a disruption of the course of evolution, insofar as speciation processes will have to work with a greatly reduced pool of species and their genetic materials. We are probably being optimistic when we call it a disruption; a more likely outcome is that certain evolutionary processes will be suspended or even terminated. In the graphic phrasing of Soulé and Wilcox (1980), “Death is one thing; an end to birth is something else.”
From what little we can discern from the geologic record, a normal recovery time may require millions of years. After the dinosaur crash, for instance, between 50,000 and 100,000 years elapsed before there started to emerge a set of diversified and specialized biotas, and another 5 to 10 million years went by before there were bats in the skies and whales in the seas (Jablonski, 1986). Following the crash during the late Permian Period, when marine invertebrates lost about half their families, as many as 20 million years elapsed before the survivors could establish even half as many families as they had lost (Raup, 1986).
The evolutionary outcome this time around could prove even more drastic. The critical factor lies with the likely loss of key environments. Not only do we appear ready to lose most if not virtually all tropical forests, but there is also progressive depletion of coral reefs, wetlands, estuaries, and other biotopes with exceptional biodiversity. These environments have served in the past as preeminent power-houses of evolution, in that they have supported the emergence of more species than have other environments. Virtually every major group of vertebrates and many other large categories of animals have originated in spacious zones with warm, equable climates, notably tropical forests. In addition, the rate of evolutionary diversification—whether through proliferation of species or through the emergence of major new adaptations—has been greatest in the tropics, again most notably in tropical forests.
Of course tropical forests have been severely depleted in the past. During drier phases of the recent Ice Ages (Pleistocene Epoch), they have been repeatedly reduced to only a small fraction, occasionally as little as one-tenth, of their former expanse. Moreover, tropical biotas seem to have been unduly prone to extinction. But the remnant forest refugia usually contained sufficient stocks of surviving species to recolonize suitable territories when moister conditions returned (Prance, 1982). Within the foreseeable future, by contrast, it seems all too possible that most tropical forests will be reduced to much less than one-tenth of their former expanse, and their pockets of holdout species will be much less stocked with potential colonizers.
Furthermore, the species depletion will surely apply across most if not all major categories of species. This is almost axiomatic, if extensive environments are eliminated wholesale. The result will contrast sharply with the end of the Cretaceous Period, when not only placental mammals survived (leading to the adaptive radiation of mammals, eventually including humans), but also birds, amphibians, and crocodiles, among other nondinosaurian reptiles. In addition, the present extinction spasm looks likely to eliminate a sizeable share of terrestrial plant species, at least one-fifth within the next half century and a good many more within the following half century. By contrast, during most mass-extinction episodes of the prehistoric past, terrestrial plants have survived with relatively few losses (Knoll, 1984). They have thus supplied a resource base on which evolutionary processes could start to generate replacement animal species forthwith. If this biotic substrate is markedly depleted within the foreseeable future, the restorative capacities of evolution will be all the more reduced.
In sum, the evolutionary impoverishment of the impending extinction spasm, plus the numbers of species involved and the telescoped time scale of the phe-
nomenon, may result in the greatest single setback to life’s abundance and diversity since the first flickerings of life almost 4 billion years ago.
REFERENCES
Bolin, B., and B.R.Doos, eds. 1986. The Greenhouse Effect: Climatic Change and Ecosystems. Wiley, New York. 541 pp.
DoE (U.S. Department of Energy). 1985. Direct Effects of Increasing Carbon Dioxide on Vegetation. U.S. Department of Energy, Washington, D.C.
Ehrlich, P.R., and A.H.Ehrlich. 1981. Extinction: The Causes and Consequences of the Disappearance of Species. Random House, New York. 305 pp.
FAO and UNEP (Food and Agriculture Organization and United Nations Environment Programme). 1982. Tropical Forest Resources. Food and Agriculture Organization of the United Nations, Rome, Italy, and United Nations Environment Programme, Nairobi, Kenya. 106 pp.
Fearnside, P.M. 1986. Human Carrying Capacity of the Brazilian Rain Forest. Columbia University Press, New York. 293 pp.
Gentry, A.H. 1986. Endemism in tropical versus temperate plant communities. Pp. 153–181 in M. E.Soul, ed. Conservation Biology: The Science of Scarcity and Diversity. Sinauer Associates, Sunderland, Mass. 584 pp.
Hadley, M., and J.P.Lanley. 1983. Tropical forest ecosystems: Identifying differences, seeing similarities. Nat. Resour. 19:2–19.
Jablonski, D. 1986. Causes and consequences of mass extinction: A comparative approach. Pp. 183–230 in D.K.Elliott, ed. Dynamics of Extinction. Wiley Interscience, New York.
Knoll, A.H. 1984. Patterns of extinction in the fossil record of vascular plants. Pp. 21–68 in M. H.Nitecki, ed. Extinctions. University of Chicago Press, Chicago.
Melillo, J.M., C.A.Palm, R.A.Houghton, G.M.Woodwell, and N.Myers. 1985. A comparison of recent estimates of disturbance in tropical forests. Environ. Conserv. 12(1):37–40.
Molofsky, J., C.A.S.Hall, and N.Myers. 1986. A Comparison of Tropical Forest Surveys. U.S. Department of Energy, Washington, D.C.
Mori, S.A., B.M.Boom, and G.T.Prance. 1981. Distribution patterns and conservation of eastern Brazilian coastal forest tree species. Brittonia 33(2):233–245.
Myers, N. 1980. Conservation of Tropical Moist Forests. A report prepared for the Committee on Research Priorities in Tropical Biology of the National Research Council. National Academy of Sciences, Washington, D.C. 205 pp.
Myers, N. 1984. The Primary Source: Tropical Forests and Our Future. W.W. Norton, New York. 399 pp.
Myers, N. 1986. Tackling Mass Extinction of Species: A Great Creative Challenge. Albright Lecture, University of California, Berkeley. 40 pp.
Peters, R.L., and J.D.S.Darling. 1985. The greenhouse effect and nature reserves. BioScience 35(11):707–717.
Prance, G.T., ed. 1982. Biological Diversification in the Tropics. Proceedings of the Fifth International Symposium of the Association for Tropical Biology, held at Macuto Beach, Caracas, Venezuela, February 8–13, 1979. Columbia University Press, New York. 714 pp.
Rauh, W. 1979. Problems of biological conservation in Madagascar. Pp. 405–421 in D.Bramwell, ed. Plants and Islands. Academic Press, London, U.K.
Raup, D.M. 1986. Biological extinction in earth history. Science 231:1528–1533.
Raup, D.M., and J.J.Sepkoski. 1984. Periodicity of extinction in the geologic past. Proc. Natl. Acad. Sci. USA 81:801–805.
Raven, P.H. 1987. We’re Killing Our World. Keynote Paper Presented to Annual Conference of the American Association for the Advancement of Science, Chicago, February 1987. Missouri Botanical Garden, St. Louis.
Salati, E., and P.B.Vose. 1984. Amazon basin: A system in equilibrium. Science 225:129–138.
Simberloff, D. 1986. Are we on the verge of a mass extinction in tropical rain forests? Pp. 165–180 in D.K.Elliott, ed. Dynamics of Extinction. Wiley, New York.
Soulé, M.E. 1986. Conservation Biology, The Science of Scarcity and Diversity. Sinauer Associates, Sunderland, Mass.
Soulé, M.E., and B.A.Wilcox, eds. 1980. Conservation Biology: An Evolutionary-Ecological Perspective. Sinauer Associates, Sunderland, Mass. 395 pp.
Western, D., and M.Pearl, eds. In press. Conservation 2100. Proceedings of International Conference on Threatened Wildlife and Species, Manhattan, October 1986, organized by the New York Zoological Society. Oxford University Press, New York.
Wilson, E.O. 1987. Biological diversity as a scientific and ethical issue. Pp. 29–48 in Papers Read at a Joint Meeting of the Royal Society and the American Philosophical Society. Volume 1. Meeting held April 24, 1986, in Philadelphia. American Philosophical Society, Philadelphia.
CHAPTER 4
ECOLOGICAL DIVERSITY IN COASTAL ZONES AND OCEANS
G.CARLETON RAY
Department of Environmental Sciences, University of Virginia, Charlottesville
Near the center of Charlottesville, Virginia, stands a heroic statue to Meriwether Lewis and William Clark, the two men Thomas Jefferson sent across our continent nearly two centuries ago. At its base, they are described as “bold and farseeing pathfinders who carried the flag of the young republic to the western ocean and revealed an unknown empire to the uses of mankind.” There soon followed an exploitative horde and a loss of landscape diversity as great as for any place on Earth during the history of mankind. How anachronistic the words on that statue sound today. Yet the seeking of empires for “the uses of mankind” is the principal factor that has led to the present marine revolution (Ray, 1970). What loss of coastal and marine biodiversity may soon result, no one can presently say. But it is my view that the coastal zone is being altered just as fast as tropical forests.
The intent of this chapter is not to describe details of the biodiversity of coasts and oceans; rather, it is to examine the challenges we face in addressing this subject. The first of these is to define diversity. Slobodkin (1986, p. 263) has pointed out, “On occasion, metaphors have replaced the empirical world as foci of discussions, while precise meanings and derivations have been forgotten in the process.” I have the impression that the word diversity is in some danger of this—that it sometimes is used to reinforce preexisting bias. In the introduction to Diversity (Patrick, 1983, p. 1), this concept is defined as a “variety or multiformity, a condition of being different in character and quality,” but the papers in that volume demonstrate that there is no single way to evaluate diversity. It surely is not merely species variety, as some of the public may be led to believe. Nor is it bound to dry land.
Those of us who practice ocean science must wonder about the oceanless world that often confronts us. Witness the cataloging of the diversity of “Realms, Biomes, and Biogeographical Provinces of the World” in the recent assessment by the World Resources Institute (1986). This “world” leaves oceanic space simply blank! This is the same biogeography that is repeated in many textbooks, conservation circles, and international aid agencies. Unfortunately, this world view is also that of the majority of society. Therefore, we first face the challenge of differentiating what sort of world-planet the Earth is against the backdrop of our bias.
COASTS AND OCEANS—A WORLD VIEW
J.E.Lovelock firmly grasped the world view when he said, “Less than a third of the Earth’s surface is land. This may be why the biosphere has been able to contend with the radical transformations wrought by agriculture and animal husbandry, and will probably continue to strike a balance as our numbers grow and farming becomes ever more intensive. We should not, however, assume that the sea, and especially the arable regions of the continental shelves, can be farmed with the same impunity. Indeed, no one knows what risks are run when we disturb this key area of the biosphere. That is why I believe that our best and most rewarding course is to sail with Gaia1 in view, to remind us throughout the voyage and in all our explorations that the sea is a vital part of her” (Lovelock, 1979, p. 106). I interpret this to say that biodiversity is the result of global as well as regional and local processes and that to conserve the biodiversity of one biogeographic realm might require the conservation of processes of others as well, both wet and dry.
Let us carry this a bit further. Our evolution as giant, terrestrial mammals causes us to draw hard lines on maps between land and sea. In fact, land maps do not usually include the sea; for that, we turn to charts, which do not include land. Despite the cartographers, from an ecological perspective there can be no sharp distinction. The coastal zone unifies the two, but it is not merely a narrow transition between dry and wet; on paleoecological, geological, and biological grounds, it is distinct in its own right (Figure 4–1). The coastal zone includes at least the extents of continental plains and continental shelves (Ketchum, 1972), that is, more than 8% of Earth, or about an Africa and a half. In volume, the wet portion alone comprises approximately 3 million cubic kilometers, just about the same volume occupied by all terrestrial life! It includes coastal forests and marshes as well as watersheds, in some cases quite far inland, and is as productive as any place on Earth—one reason for the fact that more than 50% of all humans live within it and take more than 90% of their marine-living resources from it. How species-rich it is, I cannot say, nor am I inclined to believe that species accounting should warp our view of it one way or the other. Nevertheless, the major objective is to define coastal zone ecosystems and their ecological characteristics.
|
1 |
The concept of Mother Earth, as named by the ancient Greeks. See Lovelock, Chapter 56 in this volume. |
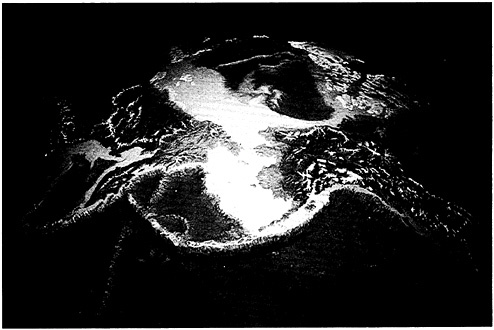
FIGURE 4–1 Coastal zones are clearly a separate but unifying region between land and sea. Photo by G.Carleton Ray.
This leads inevitably to a tripartite view of Earth in which biogeographic patterns fall within upland, open ocean, and coastal zone realms, all about equally distinct. This requires readjustments of our world view. Terrestrial realms, biomes, and provinces should not be carried to the water’s edge (e.g., Udvardy, 1975). Furthermore, our perceptions of biogeographical patterns will have to change if we are to see our planet as it really is.
LIFE ACCORDING TO THE BOOK OF TAXONOMY
Wilson (1985) wondered why there are so many species and pointed out that most are in tropical forests. There can be little doubt that tropical forests hold a major proportion of species (see also Myers, Chapter 3 in this volume). It is generally supposed that our present knowledge provides a rough approximation of the relative numbers of species in the world’s ecosystems and that about 80% of all species are terrestrial. This proportion may be seriously in error. According to the recent research of J.F.Grassle of Woods Hole Oceanographic Institution and his associates, “quantitative samples [in the deep sea] represent a fauna that rivals the tropical forests in diversity of species” (Grassle, personal communication, 1987). Only the future will tell how many species there are and which environments are most diverse.
Nevertheless, the measure of species presents but one dimension of diversity. At the other end of the taxonomic scale stand phyla. With help from Barnes (1963), Grzimek (1974), and Margulis and Schwartz (1982), we may count more
than 70 phyla of all life from bacteria to vertebrates. Those that are exclusively marine number about 20; 18 are exclusively terrestrial. Twenty-three other phyla contain marine species, whereas only 10 more contain terrestrial species. In short, diversity of the oceans is about double the land’s if it is phyla that we consider. What can we make of this? Looking further, we see that protists and invertebrates predominate in oceans and higher plants predominate on land. That is to say, these environments are vastly different in community composition, making biologically dubious any attempt to compare diversity among them simply by counting taxa. This same difficulty exists on the level of species. The tropics contain more species than do polar regions, but there are hardly any walruses in the Amazon, nor parrots in Antarctica. The species and phylum content of environments is an essential fact of ecology, but simply knowing which environments have more or fewer may be misleading and must be subject to further interpretation.
This leads to an examination of life form, that is, distinguishing species by means of verbs (describing what they do) instead of nouns (indicating what they are); this approach gathers life into functional, ecological groupings not necessarily related to their taxonomy. It is instructive to compare the aquatic and terrestrial realms from this viewpoint. I cannot think of a terrestrial life form that does not have an aquatic equivalent, but counterparts of several marine life forms are so rare on land that cartoonists have to invent them; see, for example, the sit-and-wait, deception-bait gulper-predator in Figure 4–2. The goosefish (Figure 4–3) is one example of this life form that is common in the sea. A life-style that is totally absent from land is filter feeding—an activity practiced by numerous aquatic life forms, from sponges to whales. There may be some distant terrestrial equivalents of filter feeding. I have been reminded by Dr. Eugene Morton of the Smithsonian Institution that swallows and swifts are analogs of filter feeders because they scoop high-flying “planktonic” insects from the air. But these isolated examples do not
FIGURE 4–2 A predatory life form. Cartoon by Gary Larson. This Far Side cartoon is reprinted by permission of Chronicle Features, San Francisco.
FIGURE 4–3 The goosefish (Lophius americanus)—a sit-and-wait, deception-bait gulper-predator. Photo by M.A.deCamp.
alter the fact of the predominance of some life forms in oceans that are rare or nonexistent on land.
We must therefore conclude that accounting of species alone can be highly misleading as a yardstick of diversity. It may also mislead us genetically. The genetic diversity of both land and sea species can be striking, as, for example, the variation among the hamlet fishes, Hypoplectrus (Figure 4–4). But does a family of thousands of species contain more or less genetic uniqueness than a phylum comprising one to a dozen? Some marine phyla contain very few species, but their evolutionary history is long and their species are unique; the horseshoe crab, Limulus, is an example. In sum, a major challenge in examining diversity lies in our perceptions and interpretations of it, taxonomically and functionally.
ECOLOGICAL DIVERSITY
A great diversity of life forms implies that there is an equally great diversity of food webs and trophic relationships, i.e., food supply and demand, and requirements
for nutrients. For example, filter feeders, especially zooplankton, create extra levels in aquatic food chains that do not exist on land. In the oceans, there is also much greater diversity in body sizes than on land—from picoplankton to whales—and much larger ranges of ecological time-space relationships. Consequently, aquatic food webs tend to be more complex than terrestrial ones and there are more trophic levels in food chains. Unraveling this complexity is made all the more challenging because we are almost infinitely less knowledgeable about the nature of marine systems than we are about terrestrial systems. A good many oceanographers still adhere to the concept that marine organisms are pushed around like billiard balls by the physics and chemistry of their environments. There is too little recognition that large predatory marine animals can have marked effects on the structure of their communities, and hence on nutrient cycling, and that physical and biotic processes are, no doubt, strongly linked in a cybernetic network. For terrestrial systems, this biotic influence has become obvious. For all systems, an important question is how to distinguish between biotic and physical control mechanisms. This is but one critical area where marine science lags.
Returning to the subject of biogeography, the realms, biomes, and provinces of the coastal zones and open oceans exhibit a remarkable array of environments.
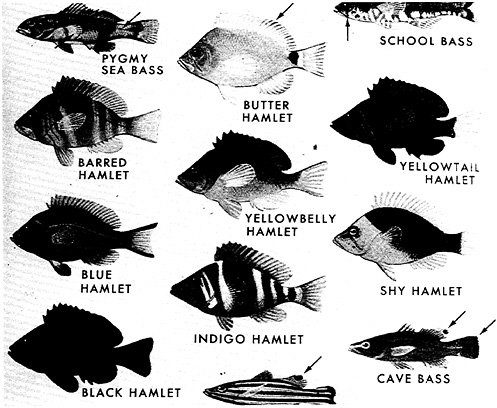
FIGURE 4–4 Hamlet fish (Hypoplectrus unicolor) occur in a variety of colors. ©John Douglass 1987. From Robins et al., 1986.
Figure 4–5 depicts our recent attempt to classify them. This is a world made even more complex by its strong three-dimensionality, which is not shown in the figure. In concordance with this classification are distinct biotic assemblages. In tropical reefs, we find many species in a wide taxonomic array, similar to the variety of tropical forests (Figure 4–6). Temperate marine communities have fewer species but generally higher productivity, well illustrated by commercial fishes, which constitute a very large biomass (Figure 4–7). There is also high productivity in polar areas where sea ice is annual, but marine birds and mammals—the largest concentrations of them on Earth—predominate there (Figure 4–8). Which ecosystems are more diverse seems almost irrelevant in this context. Rather, let us say that each has its own “characteristic” diversity. The description of characteristic diversity—including indicator and keystone species—must be our immediate focus, and the preservation of that diversity our ultimate challenge.
What does characteristic diversity imply? The study of island biogeography tells us that the geographic size of ecosystems is a factor in species richness. Of course, this does not mean that one species will be part of any community in perpetuity. Some will come and some will go, but functionally, the ecosystem processes might remain fundamentally the same. That is, the demise of Southern Ocean whales does not seem to have altered that system much, their roles being more or less assumed now by penguins and seals—or perhaps by the krill fishery. Also, we are all aware of the formidable amount of paper that has been consumed by publications discussing whether diversity somehow confers stability to ecosystems. I trust this has become a nonquestion for scientists, but perhaps it lingers on in some circles. I suspect that diversity per se has little to do with the stability of most marine systems, i.e., the nondiverse systems are just as stable as those that are diverse. More to the point is whether characteristic diversity confers some predictability to ecosystems. Behind this important question lies our definition of a system. Ecosystems are far from chance physical-biotic associations or mere heuristic creations of ecologists; they are functional units in every sense of that term. But defining them presents great challenges. Figure 4–9 shows a simplified concept of the components of coastal zone ecosystems. Following are some major factors that control coastal processes and that must be considered in defining the boundaries of these ecosystems:
-
watershed and receiving basin morphology
-
terrestrial and marine climates
-
winds, waves, currents, and tides
-
fluvial discharge, bedload, suspended load, and dissolved load
-
terrestrial and marine biota
-
human use of land or sea
Even from this simple characterization, we see that ecosystem definition requires intensive field research coupled with complex analysis. Without such an effort, one cannot reach conclusions about diversity.
We must not forget that productivity is what interests most of humanity. Is diversity a factor here? We must distinguish productivity needed to sustain ecosystems from productivity that benefits human beings (Figure 4–10). Coral reefs
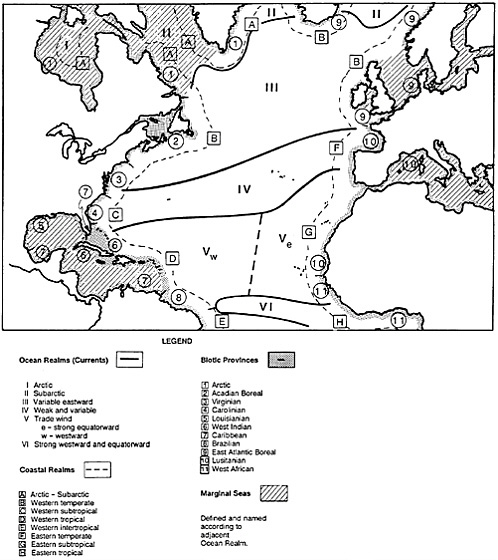
FIGURE 4–5 Classification of North Atlantic coastal and marine environments (after Hayden et al., 1984, with modifications for arctic and subarctic realms after Dunbar, 1985). This is a symbolic representation, not drawn to scale, especially for coastal realms. Ocean realms are for surface waters only. Coastal realms are highly variable, especially for temperate areas, which contain attributes of both subarctic and subtropical coastal waters.
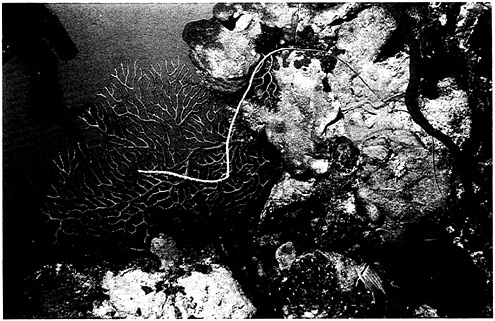
FIGURE 4–6 Coral reefs contain ecological diversity as extensive as that found in tropical forests. Photo by G.Carleton Ray.
are productive and diverse, but they are not nearly as useful for food production as are temperate seas, where schooling fishes predominate and can be easily caught over extensive banks and shelves. There is a negative correlation between diversity and productivity in these cases. By analogy, farming on land is most productive for humans when systems are simplified. One of the greatest challenges for marine science is the prediction of consequences that would result from the loss of diversity in the increasing number of coastal systems that are being farmed through aquaculture. Will this lead to the loss of the characteristic diversity of coastal systems and thus to the loss of system predictability? This is the danger of not heeding Lovelock’s warning quoted above.
Perhaps the greatest challenge of all lies in determining which characteristic species contribute most to their ecosystem, to productivity, to predictability. Are some species more essential than others from a functional, ecological point of view? In the present state of our ignorance, an attempt to answer this might lead to some nasty choices. Surely some species are more important to their ecosystems than are others, as indicators of ecological processes or as keystones that influence community structure. But which are these? We know pitifully few of them for coastal and ocean systems. So when some decision-maker asks which species might be sacrificed, we cannot say. The immense diversity of life seems simply redundant to many who are in the position of having to decide about environmental matters—and we might have to admit that some species may indeed be redundant. But when asked to identify such redundancies, we may react like the young Mozart when
told by Emperor Josef II that his sonata contained too many notes. He replied that it contained “exactly the necessary number.”
CONCLUSIONS IN PROSPECT
The ultimate challenge lies in detecting the loss of biodiversity in coastal and marine systems. The last fallen mahogany would lie perceptibly on the landscape, and the last black rhino would be obvious in its loneliness, but a marine species may disappear beneath the waves unobserved and the sea would seem to roll on the same as always. Extinction rates in the coastal zone and oceans are not known. Very few species seem to have gone. Some relicts, such as Steller’s sea cow, are gone, as are some especially vulnerable species, such as the Labrador duck and the great auk. I wonder how much effort would be spent on ensuring their survival today. Would we dare pull the plug as some would do for the California condor so that our attention and limited resources could be turned toward other equally pressing matters? Or would we use these species, like the panda is being used, to raise funds for conservation efforts?
Though the bulk of humanity lives in coastal zones, the wet portion of our planet still seems distantly remote—out of sight and out of mind to most people.

FIGURE 4–7 Temperate Atlantic Ocean school of amberjack (Seriola dumerili). Photo by M.A. deCamp.
Not so long ago in our history, the ocean was regarded primarily as a surface for commerce. Now there is more awareness that we are only beginning to know and understand the oceans. The astonishing rates at which new marine life and processes are being discovered testify to this. The phylum Loricifera was described only in 1983 as a result of the discovery of a single species, Nanaloricus mysticus, a small organism that lives in the sediment (Kristensen, 1983). The 5-meter-long mega-mouth shark Megachasma pelagios is known from but two specimens caught in only the last decade. An entirely new habitat—ocean vents, such as the sulfide chimneys called “black smokers”—contains species that were unknown until the last half decade or so. The productivity of some marine systems may have been underestimated by half due to our ignorance of the role played by bacterioplankton and to the lack of appropriate methods of measurement. Also, it has recently been revealed that wave energy creates the most productive ecosystems yet discovered, twice that of the most productive tropical forests (Leigh et al., 1987). How must we respond to all this? Clearly, we must intensify our research and communicate our findings rapidly to the public.
The goal of future efforts to address biodiversity must not be merely the compilation of lists of species. Though one must be sympathetic to intensive efforts to find out how much species diversity exists, there is no substitute for learning how systems work, the implications of their characteristic diversity, and the role individual species play. That is, I see our task not as species inventory, but more as ecological discovery. The description of species is not sufficient. Rather, we need to identify the species that are important contributors to ecosystem processes, that

FIGURE 4–8 Walruses (Odobenus rosmarus) in the Bering Sea. Birds and mammals such as these predominate in polar regions. Photo by G.Carleton Ray.
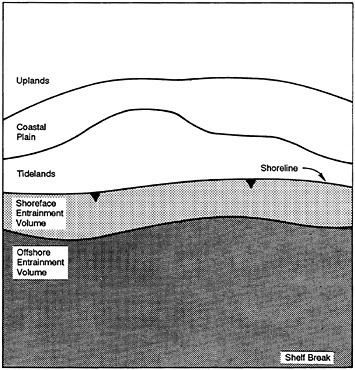
FIGURE 4–9 The ecology of the coastal zone may be influenced by the distant and nearby environments identified in the figure. Courtesy B.P.Hayden.
help structure their communities, enhance productivity, and help recycle essential nutrients. Marine scientists have been looking into such questions for some time and have decided that for some systems zooplankton is the key group. For the Southern Ocean, for example, is it the krill or the whales and seals and penguins that matter most? Or is it all of them? This is a most pragmatic question in the expensive world of marine science.
I do not wish to challenge those who would save some particular portion of this planet as a high priority simply because of its diversity. As members of the biological community, we have a common goal: the preservation of as much of this whole planet’s diversity as possible. Nevertheless, I feel compelled to emphasize that diversity often lies in the eye of the beholder. There is little question that some ecosystems have more species than others. But it does not follow that any one number of species or biomass conveys on any ecosystem more value than on any other, nor can the value of species be ranked on strictly taxonomic grounds; that is, are whales or plankton most worth saving? We are slowly growing out of our bias toward species that are most like us—the warm-blooded animals that cause our anthropocentric senses to soar and our hands to reach for our checkbooks. Furthermore, the point is often made that since the potential medical or economic value of a species cannot often be predicted, we must save them all. This is clearly impossible, and it may also be illogical.

FIGURE 4–10 Sustenance for mankind. Cartoon by Gary Larson. This Far Side cartoon is reprinted by permission of Chronicle Features, San Francisco.
Pogo once observed that he had “seen the enemy, and it is us.” Most creatures of the sea are cold-blooded and strange to us—even forbidding. Great white sharks (Carcharodon carcharias) can be crudely slaughtered with scarcely a peep from the conservation community. New hordes of people occupy the coastal zone yearly. Some hope to farm it; most just unwittingly pollute it, while increasingly drawing upon marine systems for resources and leisure. Despite dramatic advances in awareness, coastal zones and oceans continue to receive compassionate neglect. We seem to love the sea, the romance of it, the symbols (such as whales) that swim in it, and the coral reefs that we swim over. But the oceans remain foreign to most, and the concept of coastal zones as the broad systems they are continues to go largely unrecognized. Thus, the principal challenge, when addressing coastal and marine diversity, lies in recognizing its global role. If Lovelock is correct in his perception of Gaia, the coastal zone may be the single most important portion of our planet. The loss of its biodiversity may have repercussions far beyond our worst fears. Addressing this need will take an intensive research effort backed by intensive political persuasion.
We might start by giving the coastal zone and oceans equal time. The Forum on BioDiversity, whose participants have contributed to this volume, demonstrates a need in this respect. The brochure announcing the Forum’s program (and used as the jacket illustration of this book) depicts 13 insects, 6 mammals, 6 birds, 3 amphibians, a fish, and a reptile, and but three marine critters, all starfishes. Among the contributors to this publication are about 25 terrestrial scientists, overwhelmingly tropical, scatterings of economists and philosophers, about two-and-a-half classified as coastal or marine biologists, and perhaps one or two whose focus is the polar regions. A film presented during the Forum, “The Frozen Ocean,” is not merely a misnomer—there is, after all, lots of water beneath the far from continuous and mostly seasonal ice—but the program’s description of the film refers
to “the unexpected ecological riches of the Arctic.” This demonstrates that biases die hard, since such riches are “unexpected” only to those who have never been there! Meanwhile, there continues to be a benign intolerance in some conservation and development circles for supporting the basic research and concept development necessary for preservation of biodiversity. We are reminded to address problems of the “real world.” But whose real world? For want of so-called esoteric knowledge, we watch helplessly as exploited species become rare, the rare endangered, and perhaps the endangered extinct, without knowing why or what to do. Science and conservation clearly need to be joined in a much more comprehensive alliance.
Time to act to preserve the characteristic diversities of coastal and marine systems grows short. Our decision-makers and the public at large seem intent, for example, blissfully to use what is called the assimilative capacity of coastal and ocean waters as a receptacle for our wanton creation of toxic waste and garbage. We are told that this is an economic necessity, but we have few defenses that are ecological. Both the research and conservation communities must intensify their efforts to understand the relationships underlying the ecological processes that result in each ecosystem’s characteristic biodiversity. In conservation and management quarters, this requires new perceptions of research and new definitions of real worlds. This need is especially important for coastal zones and oceans, where we are so far behind. Perhaps this requires no less than a government office of biodiversity that would allocate two-thirds of its time, space, and effort to coastal and marine systems, reflecting their global proportions. This should be backed up by specific mandates for research within the National Science Foundation and elsewhere. The status quo can only result in the unwitting recording of extinction.
REFERENCES
Barnes, R.D. 1963. Invertebrate Zoology. W.B. Saunders Co., Philadelphia. 632 pp.
Dunbar, M.J. 1985. The Arctic marine ecosystem. Pp. 1–35 in F.R.Engelhardt, ed. Petroleum Effects in the Arctic Environment. Elsevier Applied Science Publishers, London and New York.
Grzimek, B., ed. 1974. Grzimek’s Animal Life Encyclopedia. 13 volumes. Van Nostrand Reinhold, New York.
Hayden, B.P., G.C.Ray, and R.Dolan. 1984. Classification of coastal and marine environments. Environ. Conserv. 11(3):199–207.
Ketchum, B.H., ed. 1972. The Water’s Edge. Critical Problems of the Coastal Zone. Coastal Zone workshop 1972, Woods Hole, Mass. The MIT Press, Cambridge, Mass. 393 pp.
Kristensen, R.M. 1983. Loricifera, a new phylum with Aschelminthes characters from the meiobenthos. Z. Zool. Syst. 21(3):163–180.
Leigh, E.G., Jr., R.T.Paine, J.F.Quinn, and T.H.Suchanek. 1987. Wave energy and intertidal productivity. Proc. Natl. Acad. Sci. USA 84:1314–1318.
Lovelock, J.E. 1979. Gaia: A New Look at Life on Earth. Oxford University Press, New York. 157 pp.
Margulis, L., and K.V.Schwartz. 1982. Five Kingdoms: An Illustrated Guide to the Phyla of Life on Earth. W.H. Freeman, San Francisco. 338 pp.
Patrick, R., ed. 1983. Diversity. Benchmark Papers in Ecology/13. Hutchinson Ross, Stroudsbourg, Pa. 413 pp.
Ray, G.C. 1970. Ecology, law, and the “Marine Revolution.” Biol. Conserv. 3(1):7–17.
Robins, C.R., G.C.Ray, and J.Douglass. 1986. A Field Guide to Atlantic Coast Fishes of North America. Houghton Mifflin, Boston. 354 pp.
Slobodkin, L.B. 1986. The role of minimalism in art and science. Am. Nat. 127(3):257–265.
Udvardy, M.D.F. 1975. A Classification of the Biogeographical Provinces of the World. IUCN Occasional Paper No. 18. International Union for the Conservation of Nature and Natural Resources, Morges, Switzerland. 49 pp.
Wilson, E.O. 1985. Time to revive systematics. Science 230(4731):1227.
World Resources Institute. 1986. World Resources 1986. An Assessment of the Resource Base That Supports the Global Economy. Basic Books, New York. 353 pp.
CHAPTER 5
DIVERSITY CRISES IN THE GEOLOGICAL PAST
DAVID M.RAUP
Sewell L.Avery Distinguished Service Professor, Department of Geophysical Sciences, University of Chicago, Chicago, Illinois
The geological record of the past several hundred million years contains a wealth of information about species extinction. With these data we can place our knowledge of present-day extinctions in the larger time context of the global evolution of life.
Are the present and projected extinctions in the moist tropics unusual in the history of life? What have been the evolutionary consequences of past extinction events, especially the mass extinctions? How resilient is the global biota when confronted with the elimination of large numbers of species within a short time?
In our attempt to tackle these and related questions, there are serious problems of scale. In most cases, the geologist is forced to work on time scales measured in millions of years. And it is rarely possible in the fossil record to do a large-scale, synoptic analysis at the population or species levels. Limited fossilization usually coarsens the analysis into higher taxonomic levels: genera, families, and even orders. Interpolation of the results back down to the level of species is possible but often difficult.
Within the overall framework of geological time, the paleontologist can operate in two rather distinct time frames. The first is so-called deep time, which includes the history of life since the emergence of complex metazoans (multicellular organisms with differentiated tissues) near the beginning of the Cambrian period, about 600 million years ago. The interval since this initial metazoan proliferation, generally called Phanerozoic time (comprising the Paleozoic, Mesozoic, and Cenozoic eras), contains most of the extinction data and yields estimates of background rates plus glimpses of the mass extinctions that so nearly ended life on Earth.
The second time frame is shallow time: the record of the past few hundred thousand years during which plants and animals were essentially modern. Data
from shallow time can be tied directly to present-day biogeography and diversity. Such data include a record of the effects of climatic change in the tropics during the Pleistocene epoch (approximately the last 2 million years), which are especially critical to the modeling of present and future changes.
THE PHANEROZOIC RECORD OF EXTINCTION
Complex life as we know it became firmly established on Earth toward the end of the Precambrian era and in the early Cambrian period. The exponential increase in diversity of multicellular organisms came after almost 3 billion years of surprisingly sluggish evolution of smaller, simpler organisms. The trigger for the diversification of higher organisms is not known for sure, but no matter what the cause, the fossil record record shows an epidemic of diversification. Most of the major phyla originated during this phase, and numbers of species increased dramatically. Ironically, the major groups of most interest to us now, including land vertebrates, insects, and higher plants, did not develop until somewhat later. But these latecomers did not profoundly affect global biology, except from our own anthropocentric viewpoint.
Following the initial diversification, species extinction was and continued to be almost as common as species origination. Average durations of species were generally less than 10 million years, and the biological composition of Earth, at least at the species level, changed completely many times. Phanerozoic time included a number of profound perturbations: the mass extinctions. The most serious of these, near the end of the Permian period (250 million years ago), eliminated an estimated 52% of the families of the marine animals then living and had significant though lesser effects on plants and terrestrial organisms. Published attempts to interpolate the 52% rate of family extinction to the level of species kill have yielded estimates ranging from 77 to 96% extinction for the marine animal species then living. If these estimates are even reasonably accurate, global biology (for higher organisms at least) had an extremely close brush with total destruction.
Another four or five Phanerozoic events are also usually classed as mass extinctions, including the Cretaceous-Tertiary event 65 million years ago. Each of these large extinctions probably eliminated at least half the animal species then living.
In the times between the big mass extinctions, there have been many smaller events, which have been used by geologists to subdivide the Phanerozoic time into periods, epochs, and smaller time units. It is not yet clear whether the smaller events are most properly lumped into a general phenomenon called background extinction, which is qualitatively different from mass extinction, or whether the smaller extinctions differ only in size from the mass extinctions. Although the biggest mass extinctions do show a qualitatively different picture of selective survival than the intervening extinctions, there is increasing evidence that even the smaller extinctions are short-lived, point events (see Raup, 1986, for review). The terms episodic and stepwise extinction have been applied to this interpretation, that is, relatively long periods of biological stability, perhaps measured in hundreds of thousands of years, punctuated by short bursts of species kill. This is rapidly becoming an important area for research, because it speaks to the problem of whether
plant and animal species are fundamentally fragile and subject to elimination throughout their existence or whether they are effectively immune to extinction except during short periods of extreme stress.
EXTINCTION RATES IN DEEP TIME
It is a simple matter to compute average rates of extinction for large portions of the Phanerozoic fossil record, but there are some serious problems of interpretation. For the entire Phanerozoic time, the average species extinction rate has been estimated to be 9% per million years (Raup, 1978). This translates into 0.000009% per year, or about one species lost every 5 years in a biosphere containing 2 million living species. This number is probably low by at least a factor of 10 because the paleontologist is generally not able to see local endemic species. But even if we increase the average extinction rate by an order of magnitude, to two species every year, the rate is trivial in comparison to the extinction presumably being caused by habitat destruction and other human activities at present.
The main problem with the average rate calculations is that they lump together times of high and low extinction. If, as is urged by the proponents of mass extinction by comet or asteroid impact, the Cretaceous-Tertiary extinctions took place over a time as short as a single year, then calculations of long-term rates become meaningless: during short intervals of extreme physical environmental stress, extinction rates were nearly infinite, whereas between these events, extinction rates may have been virtually zero. In this connection, it is interesting to note that there is no statistical correlation between the durations of the standard time units in the Phanerozoic eon and the numbers of extinctions known to occur during those units. Although this does not prove that the incidence of extinction is independent of elapsed time, it is compatible with the view that extinctions are point events rather than the result of a time-continuous process.
Until more solid research is done on the detailed timing of extinctions in the fossil record, we will not know for sure whether the extinctions now projected for the contemporary moist tropics are typical of the history of life.
EVOLUTIONARY CONSEQUENCES OF PAST EXTINCTIONS
Little is known about the condition of the biosphere immediately following mass extinctions other than the tautological inference that biodiversity must have been less than immediately before the events. For a few of the larger extinctions, however, the recovery time is sufficiently protracted that the postextinction milieu can be studied.
For at least 5 million years following the mass extinction of the Permian period, marine assemblages were clearly depauperate. Biological groups that were dominant in Permian seas are either absent altogether or are represented by just one or a few species. Often these few surviving species are surprisingly abundant. Several large class- and phylum-level groups are completely absent, even though they are known
to have survived the extinctions because of their appearance later in the Mesozoic record—a phenomenon Jablonski (1986) has called the Lazarus effect. That is, they “rose again” after apparent extinction.
The Lazarus taxa provide a special challenge for students of the fossil record, because there are two equally plausible explanations for major gaps in the fossil record: species diversity may have been so low that the organisms were not preserved as fossils, or sedimentary environments conducive to fossilization may have been absent. The choice between these two explanations is difficult to make, and no unequivocal case has yet been made for either of them. However, the presence of a few abundant species immediately following the Permian period argues in favor of the lowered diversity theory.
Other consequences of mass extinction are somewhat clearer. Many of the extinction events were followed by major shifts in dominance of biological groups and by the evolutionary radiation of new innovations. A classic example is the diversification of the mammals following the extinction of the dinosaurs. Mammals had been present in moderate numbers throughout most of the time of dinosaur dominance, but it was not until the removal of the dinosaurs during the mass extinction at the end of the Cretaceous period that mammals became truly diversified. It is presumed, though difficult to prove absolutely, that the diversification of mammals, and ultimately the evolution of Homo sapiens, was possible because of the newly available ecological space in terrestrial habitats.
Other examples of replacement resulting from extinction involve tropical reef communities. The builders of reef frameworks, now dominated by stony corals of the Scleractinia order, switched roles repeatedly during Phanerozoic time. Reefs have been built at various times by molluscs, bryozoans, calcareous algae, or coral groups only distantly related to modern corals. It is clear that the extinction-replacement phenomenon has been largely responsible for these changeovers. This is important in broader evolutionary terms, because it suggests that the evolution of communities, and the changing dominance of certain kinds of plants and animals, is not a simple progression based on species-species competition. Rather, the changes may occur simply as a result of the filling of voids left by the demise of previously dominant groups. And the extinctions of the previously dominant groups, if caused by rare conditions of extreme stress, may have little or nothing to do with adaptive level or general efficiency. Thus, there is no reason to believe that the present dominance of scleractinian corals in most tropical reefs implies anything about the fitness of these animals to that environment relative to previous occupants.
One can go further and suggest that without the perturbing effect of the extinction-replacement events, evolution as we know it would have been very different. It is easy to imagine that diversification and innovation in evolution would have come to a stop early in Phanerozoic time, the occupants of most ecological niches or adaptive zones maintaining a stable, steady state. From this viewpoint, extinction, and especially mass extinction, can be seen as a vital ingredient in the evolution of complex life as we know it. This must remain somewhat speculative, of course, because the evolution of life cannot be replayed under different conditions.
EXTINCTION IN SHALLOW TIME: THE PLEISTOCENE EXPERIENCE
Within the past million years, the Earth’s climate and biosphere have been strongly influenced by changes associated with the Pleistocene glaciations. In the context of the current concerns about extinction in the moist tropics, special attention should be paid to the effects on tropical diversity of the recent climatic fluctuations.
Many geologists and biogeographers have argued that the tropical rain forests of South America and Africa were largely replaced by dry savannas during the glacial advances. It has been postulated that the rain forests were reduced to a few small refugia, and the locations and extents of these remnant patches have been mapped in both South America and Africa (see Beven et al., 1984; Mayr and O’Hara, 1986; Simberloff, 1986).
If the refugium maps are accurate, they have profound implications for the effects of changes in tropical habitats. From theory, one would expect that total number of species would be reduced due to the greatly decreased habitable area for rain forest species and because of the elimination of the habitat of many geographically restricted species. The current estimates of present reductions in diversity caused by habitat destruction in the tropics are comparable to reductions estimated in the refugium model for the glacial intervals.
Also, if the refugium maps are accepted as reliable, it is difficult to explain the recovery of tropical diversity to present levels in the extremely short time since the last glacial advance. If present insect diversities are as great as recent estimates suggest, how did all the local endemics develop by speciation in such a short time?
There are major problems in applying the refugium model to the Pleistocene history of the tropics. The geological evidence for the climatic change comes mainly from scattered and generally inadequate data on fossil pollen. The biogeographical evidence is inferred from present-day distributions: the argument is that the refugia of the past are reflected now in concordant ranges of living species. That is, the near-coincident geographical ranges of species delineate the refuge patches from which diversification and geographical spreading occurred since the return of warm, moist conditions. There has been much argument in the recent biogeographical literature both for and against the refuge reconstructions. For both South America and Africa, strong cases have been made for opposing conclusions.
Another major problem with the refugium model is the extreme difficulty of documenting Pleistocene extinctions in the affected areas. The fossil record in present rain forest areas is notoriously poor because of the paucity of good rock exposures from which collections can be made. Furthermore, the organisms of most interest in this context—land animals, plants, and insects—have very low fossilization potentials and thus there are poor geological records, even under good circumstances. It is therefore difficult to determine from existing data whether or not the Pleistocene glaciations were accompanied by mass extinctions in the tropics. On a global scale, the Pleistocene epoch was not a time of mass extinction, but it is certainly possible that there were extensive species kills in rain forest areas.
RESEARCH FOR THE FUTURE
The fossil record has great untapped potential for contributing to our understanding of contemporary extinction. This is true for shallow as well as deep time. In deep time, considering Phanerozoic time as a whole, the most pressing and relevant priorities are closer investigation of the timing of the great mass extinctions (Did the major events take place in a matter of days, years, or millions of years?) and more analysis of the biological selectivity of extinction (Who were the survivors, who were the victims, and why?).
In shallow time, concentrating on the last few hundred thousand years, we need more direct, empirical data on the physical environmental history of the Pleistocene epoch and the biological consequences, with emphasis on species extinction, of the environmental changes. If we can substantially increase our knowledge of the Pleistocene record, we will be in a much better position to evaluate the consequences of the activities of humans in tropical regions.
Without consideration of the time perspective available from the geological record, a full evaluation of the contemporary extinction problem may prove as difficult as would be the case if a land-use planner were to attempt projections without benefit of historical experience or if an epidemiologist were to treat an infectious disease without medical records.
REFERENCES
Beven, S., E.F.Connor, and K.Beven. 1984. Avian biogeography in the Amazon basin and the biological model of diversification. J. Biogeogr. 11(5):383–399.
Jablonski, D. 1986. Causes and consequences of mass extinctions: A comparative approach. Pp. 183–229 in D.K.Elliott, ed. Dynamics of Extinction. Wiley, New York.
Mayr, E., and R.J.O’Hara. 1986. The biogeographic evidence supporting the Pleistocene refuge hypothesis. Evolution 40(1):55–67.
Raup, D.M. 1978. Cohort analysis of generic survivorship. Paleobiology 4(1):1–15.
Raup, D.M. 1986. Biological extinction in Earth history. Science 231:1528–1533.
Simberloff, D.S. 1986. Are we on the verge of a mass extinction in the tropical rain forests? Pp. 165–180 in D.K.Elliott, ed. Dynamics of Extinction. Wiley, New York.
Stanley, S.M. 1986. Earth and Life Through Time. W.H. Freeman, New York. 690 pp.
CHAPTER 6
ESTIMATING REDUCTIONS IN THE DIVERSITY OF TROPICAL FOREST SPECIES
ARIEL E.LUGO
Project Leader, Institute of Tropical Forestry, U.S. Department of Agriculture, Forest Service, Southern Forest Experiment Station, Rio Piedras, Puerto Rico
This chapter focuses on the empirical basis of estimates for species extinctions in tropical environments. The variation in estimates commonly cited (Table 6–1) points to inconsistencies that require discussion. I also call attention to examples in the tropics that suggest ecosystem resiliency in the conservation of species diversity. My intention is not to diminish in any way the sense of urgency that resource managers and government agencies should have about the progressive increment of loss and onerous consequences of a reduction in the number of species. Instead, I hope to stimulate a more critical and balanced scientific analysis of the issue.
The need for a balanced and rigorous analysis of the loss-of-species issue stems from the unquantifiable importance of species diversity to life support on a global scale. Scientists must be as precise as possible when communicating such important phenomena to the public and its governmental representatives. A loss of scientific credibility can seriously hamper continuing efforts to develop lasting popular support for the conservation of ecological diversity. Also, the time, money, and talent needed to address the ecological problems of the tropics are very limited, and their allocation is affected by public perception of the situation. Errors of perception lead to waste of resources and loss of opportunity to achieve solutions.
THE ACCEPTED VIEW
The numbers cited for species decline and used to gain public support for the conservation of species diversity are impressive. According to Myers (1979), the
TABLE 6–1 Estimates of Potential Species Extinction in the Tropics
|
Estimate |
Basis of Estimate |
Source |
|
1 species/day to 1 species/hour between 1970s and 2000 |
Unknown |
Myers, 1979 |
|
33–50% of all species between the 1970s and 2000 |
A concave relationship between percent of forest area loss and percent of species loss (see Table 6–2) |
Lovejoy, 1980 |
|
A million species or more by end of this century |
If present land-use trends continue |
National Research Council, 1980 |
|
As high as 20% of all species |
Unknown |
Lovejoy, 1981 |
|
50% of species by the year 2000 or by the beginning of next century |
Different assumptions and an exponential function (see Table 6–2) |
Ehrlich and Ehrlich, 1981 |
|
Several hundred thousand species in just a few decades |
Unknown |
Myers, 1982 |
|
25–30% of all species, or from 500,000 to several million by end of this century |
Unknown |
Myers, 1983 |
|
500,000–600,000 species by the end of this century |
Unknown |
Oldfield, 1984 |
|
0.75 million species by the end of this century |
All tropical forests will disappear and half their species will become extinct |
Raven, Missouri Botanical Gardens, personal communication to WRI and IIED, 1986 |
|
33% or more of all species in the 21st century |
Present rates of forest loss will continue |
Simberloff, 1983 |
|
20–25% of existing species by the next quarter of century |
Present trends will continue |
Norton, 1986 |
|
15% of all plant species and 2% of all plant families by the end of this century |
Forest regression will proceed as predicted until 2000 and then stop completely |
Simberloff, 1986 |
world was losing one species per day in the 1970s, and by the mid-1980s, the loss will increase to about one species per hour. By the end of this century, our planet could lose anywhere from 20 to 50% of its species (Table 6–1). Humans are the basic cause of these losses, because in the process of securing a living from the land, people modify it. The human population is growing at a faster rate in tropical latitudes than anywhere else, and this results in more habitat destruction in the tropics. In fact, the greatest losses of species are reported to occur in the tropics, which contain half of the world’s remaining forests. Some writers suggest that present tropical forests will be destroyed by the beginning of the next century and that because these forests are the world’s richest in terms of species numbers, their destruction becomes the primary source of a global loss of species.
How are these scenarios derived? What are the bases of these calculations? How firm are they? To develop such scenarios, three types of data are needed: the
relative distribution of species in each type of tropical forest, the rate of change in the area of each type of tropical forest, and the relationship between change in forest area and change in species numbers.
Most published projections of species extinctions resulting from deforestation in the tropics do not include the basis for their estimates in ways that can be examined independently (Table 6–1). Exceptions are the estimates of Love joy (1980) in the Global 2000 Report, that of Ehrlich and Ehrlich (1981) in their classic book on extinctions, and the recent paper by Simberloff (1986).
NUMBER OF SPECIES IN THE TROPICS
Estimating the total species richness of the tropical biome is probably beyond the means of scientific endeavor at this time. Total species inventory of a single tropical ecosystem does not even exist. Insufficient information handicaps any effort to estimate the number of species extinctions. Myers (1979) discussed the problems of estimating species numbers and concluded that of the 3 to 10 million species that exist globally, approximately 70% occur in the tropics. The World Resources Institute and the International Institute for Environment and Development (WRI and IIED, 1986) reported between 3.7 and 8.7 million species in the tropics (the actual number depending on whether the world has 5 or 10 million species), of which 0.6 million are known to science. Taxonomists estimate that only 1.5 to 1.7 million species are presently known to science (Raven, 1977; WRI and IIED, 1986). Clearly, scientific understanding of total numbers of species is still fragmentary. For this reason, it is best to use relative distributions of species in different forest types when making global estimates of species extinctions.
RATE OF CHANGE IN TROPICAL FOREST AREAS
The rate of change in tropical forests of all kinds has been discussed in depth only by Lanly (1982), who made an effort to document the rate of increase in the area of secondary forests (by reforestation, afforestation, and natural regeneration; see Figure 6–1) as well as the rate of forest loss. Other attempts usually emphasize conversion or modification of mature forests with little or no analysis of recovery (Myers, 1980). Lanly’s data show that of the 11.3 million hectares of mature forest land deforested annually, 5.1 million hectares are converted to secondary forest fallow. He estimated that the total area of this forest type is 409 million hectares and that almost 1 million hectares of secondary forest is created annually on unforested land through natural regeneration or human intervention. Such large forest areas cannot be dismissed as irrelevant to the conservation of species diversity because they support an extensive biota (discussed below) and because under certain conditions, they are capable of supporting more complex biota than the mature system they replace (Ewel, 1983).
Lanly’s data also show that deforestation rates are higher in closed than in open forests (Figure 6–1). Within closed forests, a large fraction of the conversion involves logged forests—forests that have previously been modified by human activ-
FIGURE 6–1 Pathways of land conversion in tropical forest lands. Data were derived from the Food and Agriculture Organization (1981) and from Lanly (1982), and are presented in millions of hectares. The numbers inside the boxes represent total area in 1980; those on the lines ending in arrowheads represent the annual rate of conversion. Closed forests have complete canopy cover; open forests do not and therefore support a grass understory.
ity. Because the dynamics of these changes in land use as well as the species richness of the forests also change according to country, region, and economic conditions, it behooves scientists to be extremely careful when projecting local experiences to global scales.
DIVERSITY OF FOREST TYPES IN THE TROPICS
The Holdridge Life Zone Classification System identifies some 120 ecological life zones in the world, 68 of which are tropical or subtropical (Holdridge, 1967). Thirty-two of the tropical and subtropical life zones are capable of supporting forests. About 19 million square kilometers of mature forests exist in the tropics and are distributed as follows: 42% in the dry forest life zones, 25% in the wet and rain forest life zones, and 33% in the moist forest life zones (Brown and Lugo, 1982). Statistically significant relationships suggest that life zone conditions relate to characteristic numbers of tree species (Holdridge et al., 1971), biomass and rate of primary productivity (Brown and Lugo, 1982), and capacity to resist and recover from disturbance (Ewel, 1977). These relationships are based on climatic data.
Some parameters increase while others decrease with water availability and temperature.
Quantitative studies of the richness of tree species and its association with environmental factors show that the total number of tree species increases linearly with rainfall (Gentry, 1982) and correlates negatively with the ratio of potential evapotranspiration to rainfall (Holdridge et al., 1971; Lugo and Brown, 1981). Gentry found a 3.5-fold difference (40–140 species per 0.1-hectare plot) in the number of tree species with a diameter at breast height (dbh) greater than 2.5 centimeters along a rainfall gradient of 1,000 to 3,000 millimeters. For every 1,000 millimeters of rainfall, the community gained about 50 tree species. Gentry indicated that species richness doubles from dry to moist forests and triples from dry to wet forests. Quantitative studies such as these are extremely important for obtaining accurate estimates of potential species extinctions resulting from forest loss. In an earlier publication, Gentry (1979) discussed such a phytogeographical approach to demonstrate that the number of species lost when forests are destroyed depends on the type of life zone environment being destroyed. Recognizing that tropical forests are diverse in terms of their ecological and species richness is critical for global estimates of species extinctions. Generalizations applied to all the tropics that are based on fragmented, qualitative studies are at best of limited utility.
An additional complicating factor is that life zones are subjected to different deforestation and regeneration rates (Tosi, 1980; Figure 6–1). In tropical America, for example, most human populations are clustered in dry and moist forest life zones that consequently suffer the greatest impacts of human activity (Tosi, 1980; Tosi and Voertman, 1964). The very wet life zones support the highest number of plant species and are subjected to the lowest rate of deforestation (particularly those in inaccessible locations; see, for example, Lugo et al., 1981). The fact that the intensities and consequent impacts of human activity vary among life zones has important implications for the reliability of species extinction estimates.
In summary, those who calculate species extinction rates must not assume that all tropical forests are subjected to equal rates of deforestation, respond uniformly to reductions in area, contain the same density of species, or turn into sterile pavement once converted. They must recognize and account for the diversity of forest types when making such calculations if estimates are to be considered reliable. Moreover, recovering secondary forests are potential foster ecosystems for endangered species, and their role in species conservation must also be considered. (This is discussed further later in this chapter.)
RELATIONSHIP BETWEEN DEFORESTATION RATE AND LOSS OF SPECIES
The nature of the relationship between deforestation rate and loss of species is not known. However, any calculation of the reduction of diversity must include this relationship. Myers (1983) suggested that islands on which 90% of the forest are “grossly disrupted” and the remaining 10% of their forests are protected stand to lose 50% of their species. Lovejoy (1980) discussed five possible functions that could be assumed in determining the relationship between forest area loss and loss
of species and used a gradually increasing rate function to arrive at the extinction estimates in the Global 2000 Report (Table 6–2). Ehrlich and Ehrlich (1981, p. 280) assumed that “the diversity of species will be lost more rapidly than the forest itself and used an exponential function to estimate depletion of species. They assigned a constant rate of increase to the rate of depletion based on human population growth (1.5% per year), human impacts in overdeveloped countries (1% per year), and forest loss (1% per year). The total rate of increase (3.5% per year) plus an assumed current rate of species extinction (1% per year in one calculation and 2% in another) were substituted in the exponential function to obtain the estimate of species depletion (Table 6–2).
The rates of deforestation used in both estimates discussed above are 3.8 to 5.5 times higher than the rates obtained by Lanly (1982). If Lanly’s values are substituted in Lovejoy’s analysis (Table 6–3), the estimate of species extinctions by the year 2000 would be almost 9% of the total biota instead of 33 to 50%. The high estimate of Ehrlich and Ehrlich would be halved simply by changing the assumed fraction of the biota presently undergoing depletion. The function used by the Ehrlichs is very sensitive to changes in assumptions because of its exponential nature and the absence of any negative feedback to stabilize its response. Therefore, any change in the value of any of the factors contributing to the rate of increase or the rate of extinctions would change the prediction significantly (Table 6–3). For example, if the estimated rate of avian extinctions in Puerto Rico (discussed below) is substituted for the total rate of extinctions, the expected species depletion by the year 2010 would be reduced to 4%. The estimates by the Ehrlichs also suffer from not taking into account the heterogeneity of destruction and regeneration among different forest types. Are these definitive estimates? Clearly not!
Correcting for differences in species richness of forests, forest recovery rates, and differential human impact by forest type will certainly lower any of the estimates that now lack consideration of mitigating factors. Furthermore, the functions used to relate forest loss to species loss are still to be established experimentally. When and if this comes about, the results may be either more or less conservative than those assumed by either Lovejoy or Ehrlich and Ehrlich.
Lower extinction rates for plants (Table 6–1) were estimated by Simberloff (1986) by using a species-area relationship, conservative assumptions about the fraction of forest area loss, a Z factor (an exponent of the forest area lost) of 0.25, and various scenarios of forest conservation. Simberloff could not derive a mass extinction of plant species by the year 2000 comparable to those of the geological past, even though his analysis does not correct for forest recovery after conversion. However, his estimates of extinction are lower than those discussed above, even though the function he used usually accounts for only 44.8% of the variation in species when area changes.
SEEKING A BETTER ESTIMATE
I believe that to estimate the reduction in the number of species in the tropics it is necessary to consider the effect of forest types on species abundance, the spatially selective (life zone) intensity of human activity, the role of secondary
TABLE 6–2 Extinction of Species in Tropical Forests as Implied by Lovejoy and by Ehrlich and Ehrlicha
|
Source of Data and Region |
Speices Present (thousands) |
Projected Deforestation (%) |
Loss of Speices (%) |
Species Extinct (thousands) |
|||
|
Low Case |
High Case |
Low Case |
High Case |
Low Case |
High Case |
||
|
Lovejoy |
|||||||
|
Latin America |
300–1,000 |
50 |
67 |
33 |
50 |
100–333 |
150–500 |
|
Africa |
150–500 |
20 |
67 |
13 |
50 |
20–65 |
75–250 |
|
South and Southeast Asia |
300–1,000 |
60 |
67 |
43 |
50 |
129–430 |
150–500 |
|
All tropics |
750–2,500 |
47 |
67 |
33 |
50 |
249–828 |
375–1,250 |
|
Ehrlich and Ehrlich (1981) |
|||||||
|
Total (Annual rate of increase, 3.5%; current rate of extinctions, 1%) |
50b |
100c |
|
|
|
||
|
Total (Annual rate of increase, 2%; current rate of extinctions, 3.5%) |
50d |
100e |
|
|
|
||
|
a bBy early part of next century cBy 2025. dBy 2000. eBy 2010. |
|||||||
TABLE 6–3 Extinction of Species in Tropical Forests When Lanly’s Data Are Substituted in the Calculations Used by Lovejoy and by Ehrlich and Ehrlich
|
Region and Author of Original Calculations |
Rates When the Data of Lanly (1982) Are Substituted in Original Calculations |
|||
|
Species Presenta (thousands) |
Projected Deforestation, 1980–2000 (%) |
Loss of Species (%) |
Extinctions (thousands) |
|
|
Lovejoy (1980) |
||||
|
Latin America |
300–1,000 |
17.1 |
10 |
30–100 |
|
Africa |
150–500 |
8.9 |
4 |
6–20 |
|
Asia |
300–1,000 |
15.1 |
10 |
30–100 |
|
All tropics |
750–2,500 |
12.3a |
8.8a |
66–220 |
|
Ehrlich and Ehrlich (1981, Table 1) |
||||
|
Total (Annual rate of increase, 3.5%; current rate of extinction, 0.62%) |
25b |
|
||
|
aWeighted average. bBy 2010. |
||||
forests as species refugia, and the role of natural disturbances in maintaining regional species richness. At a regional level, one also has to consider the importance of exotic species in the maintenance of species richness, particularly in ecosystems subjected to the impact of human activity. This approach seeks balance by considering factors that maintain species richness as well as those that decrease it. Considerable research is required to provide sound estimates based on this approach, because critical data concerning ecosystem function are not available in enough breadth to support enlightened management or policy making.
CALLING ATTENTION TO THE POSITIVE TERMS IN THE SPECIES EXTINCTION ISSUE
Most calculations of species extinction rates emphasize the negative aspects of the problem, and this can have beneficial effects in terms of public awareness of environmental issues. I call attention to the positive terms of this issue, using examples from the Caribbean. These examples must be used with caution, because natural conditions in the Caribbean (particularly the frequency of hurricanes) select for resilient ecosystem, and it could be argued that this selective force invalidates the examples given. However, human impacts have been so intense in the Caribbean that the region remains as a test case for theories that emphasize island fragility. And besides, the essence of my argument is that in the development of any prediction involving biotic phenomena (whether it is species extinction, global carbon cycle, or acid rain effects), it is necessary to include the plethora of checks and balances that typify ecosystem function. In the Caribbean example, ecosystems must cope with hurricanes and intensive human-induced disturbances, whereas elsewhere, periodic fire, earthquakes, frost, or landslides may play the natural role of ecosystem stressor.
Forest ecosystems of Caribbean islands have proven to be more resilient than one would assume on the basis of the relationships used in Table 6–2 or the idea of the fragility of island biota (Carlquist, 1974; Soulé, 1983). The Caribbean islands are densely populated (100 to 500 people per square kilometer, or about 10 times more densely populated than surrounding continental tropical lands (Lugo et al., 1981), and their lands have been intensively used and degraded for centuries. All the ills that Carlquist (1974) and Soulé (1983) described for islands (e.g., the introduction of exotic species, intensive predation, and habitat destruction) are present in this region. There are many examples of catastrophic waste of natural resources in the Caribbean islands [see, for example, Ambio 10(6) 1981, which was dedicated to environmental problems of the Caribbean], but there are also examples to give us some hope; these are the ones I am emphasizing.
In Puerto Rico, human activity reduced the area of primary forest by 99%, but because of extensive use of coffee shade trees in the coffee region and secondary forests, forest cover was never less than 10 to 15%. This massive forest conversion did not lead to a correspondingly massive species extinction, certainly nowhere near the 50% alluded to by Myers (1983). In an analysis of the bird fauna, Brash (1984) concluded that seven bird species (four of them endemic) became extinct after 500 years of human pressure (this is equivalent to an 11.6% loss of the bird fauna) and that exotic species enlarged the species pool. In the 1980s more birds are present on the island (97 species) than were present in pre-Columbian times (60 species). The resiliency of the bird fauna was attributed to its generalist survival strategy (a characteristic of island fauna) and to the location of secondary forests and coffee plantations on mountaintops along the east-west axis of the island, which acted as refugia.
Secondary forests in Puerto Rico have served as refugia for primary forest tree species as well (Wadsworth and Birdsey, 1982; R.O.Woodbury, University of Puerto Rico, personal communication, 1986). After 20 to 30 years of growth, the understory of these ecosystems is supporting species characteristic of mature forests. A random survey of 4,500 trees in secondary forests of two life zones (moist and wet forests) resulted in a tally of 189 tree species (Birdsey and Weaver, 1982). This survey excluded four of the six forested life zones in the island and the species-rich mature publicly owned forests. Yet it is important that 25% of the tree species identified on the island were recorded in this survey of secondary forests. (Puerto Rico has 750 tree species, 203 of which are naturalized; Little et al., 1974.) Dominant species in these secondary forests owe their dominance to human activity, and many of the native species that are typical of mature forests are rare in the forest canopy (142 tree species accounted for 16% of the total basal area of secondary forests) but are now beginning to appear as pole-size individual trees in these forest sites. Secondary forests in high-impact regions obviously require time to fulfill their role as foster ecosystem for endangered species, but in due time, a wide variety of tree species appear to return to forest lands.
An extreme example of the importance of species conservation and of human-dominated habitats acting as foster ecosystems for endangered species is that of the Chinese maiden hair tree (Ginkgo biloba). No one has ever seen a wild individual
of this species. This primitive tree was preserved in courtyards of temples in China and is considered to be the first species saved by humans (Stebbins, 1979).
In the United States where extensive human-caused deforestation and subsequent forest recovery have occurred, remnant secondary forest islands account for a large portion of landscape species diversity (Burgess and Sharpe, 1981). As a group, these secondary forest islands constitute a landscape with greater species richness than found in a landscape dominated only by climax forests. Clearly, secondary forests require more scientific attention before their role and value in landscapes affected by human activity can be properly assessed.
Catastrophic natural events may also be deleterious to the maintenance of species diversity, particularly to those species already at the edge of extinction. However, these catastrophic events are natural phenomena with predictable rates of recurrence to which the biota as a whole is adapted. Evidence is mounting to show that tropical forest ecosystems have endured catastrophic events for millennia, e.g., periodic fires in the moist forests of the Amazon (Sanford et al., 1985) and in Borneo (Leighton, 1984). In the Caribbean, hurricanes appear to be important in the maintenance of species diversity. Long-term studies in areas of the Luquillo Experimental Forest Biosphere Reserve have shown that there are progressive reductions in tree species between hurricane events (Crow, 1980; Weaver, 1986). The effects of periodic hurricanes maintain a diverse mix of successional and climax species on a given site. Without hurricanes, successional species would be more restricted. Sanford et al. (1985) suggest that fire performs the same function in Amazonian moist forests; Sepkoski and Raup (1986) expanded this idea to the effects of global perturbations on the history of life on the planet.
Studies of regeneration strategies for mature forests have indicated that disturbance is usually associated with the early phases of seedling germination and establishment in most forest types, including tropical forests (Pickett and White, 1985). This has led Pickett and White to propose the concept of “patch dynamics” as a focus of scientific inquiry aimed at understanding ecosystem dynamics. The relevance of this to the maintenance of species diversity is that environmental change and disturbance may be required to maintain a species-rich tropical landscape.
Because humans have facilitated immigration and created new environments, exotic (nonnative) species have successfully become established in the Caribbean islands. This has resulted in a general increase in total species inventories of birds and trees. Some of these exotic species are pests and thus are called biological pollutants (CEQ, 1980). However, many exotic species have become so well integrated into the natural landscape that most islanders consider them native.
Although conservationists and biologists have an aversion to exotic species such as predatory mammals and pests (with good reason!), this may not be totally justified if the full inventory of exotic fauna and flora and certain ecological arguments are taken into consideration. For example, the growth of exotic plant species is usually an indication of disturbed environments, and under these conditions, exotic species compete successfully (Vermeij, 1986). They accumulate and process carbon and nutrients more efficiently than do the native organisms they replace. In so doing, many exotic species improve soil and site quality and either pave the way for the
succession of native species or form stable communities themselves. There is no biological criterion on which to judge a priori the smaller or greater value of one species against that of another, and if exotic species are occupying environments that are unavailable to native species, it would probably be too costly or impossible to pursue their local extinction.
The paradox of exotic species invasions of islands with high levels of endemism is discussed by Vitousek in Chapter 20. He correctly points out that if the invasion of exotic species is at the expense of the extinction of local endemics, the total species richness of the biosphere decreases and the Earth’s biota is homogenized since most of the invading exotics are cosmopolitan.
NEED FOR BETTER LAND AND RESOURCE MANAGEMENT
In summary, strong evidence can be assembled to document the resiliency of the functional attributes of some types of tropical ecosystems (including their ability to maintain species richness) when they are subjected to intensive human use. Initial human intervention results in the loss of a few, highly vulnerable species. Massive forest destruction is probably required to remove more widely distributed species. Because massive species extinctions may be possible if human destruction of forests continues unabated, the evidence for ecosystem resiliency is not to be construed as an excuse for continued abuse of tropical environments. Rather, ecosystem resiliency is an additional tool available to managers if they choose to manage tropical resources prudently.
We cannot tell the needy of the tropical world that they must cease and desist in their struggle for survival to prevent a catastrophe whose dimensions, consequences, or mitigating conditions we cannot define with any certainty. It may turn out that the public call for conserving natural diversity is also an expression of frustration over the poor use of the natural resources of the tropics and our apparent inability to do something about it. Scientists have the responsibility of focusing the debate. Its fundamental essence, I believe, is the need for better land and resource management.
Experience in the Luquillo Experimental Forest Biosphere Reserve in Puerto Rico has demonstrated that species richness can be partially restored to lands previously used heavily for agriculture, that growing timber need not eliminate all natural species richness on site, and that tropical lands respond to sensible care through management. I know of no technical reason why sensible land management in tropical areas cannot lead to the success that is usually associated with temperate zones. The obstacles to progress are social and rooted in poor training and education programs, lack of facilities and infrastructure, weak institutions, misguided foreign aid programs, lack of commitment to forestry research and to enforcement of regulations, and the absence of a land conservation ethic. A strategy for forest and species conservation in tropical regions should focus on the restoration of forest production on former forest lands where food production is not sustainable. This, and sensible use of secondary forests and tree plantations, will reduce pressure on
forest lands with mature forests or with unique ecological characteristics and set us on a course to meet the needs of the needy while protecting species diversity.
ACKNOWLEDGMENTS
In this article, I benefited from the comments of S.Brown, P.Kangas, E.Medina, O.Solbrig, R.Waide, C.Asbury, J.Lodge, W.Lawrence, and colleagues at the Institute. I thank all of them. This work was done in cooperation with the University of Puerto Rico.
REFERENCES
Ambio 10(6) 1981. An entire issue devoted to environmental problems of the Caribbean.
Birdsey, R.A., and P.L.Weaver. 1982. The Forest Resources of Puerto Rico. USDA Forest Service Southern Forest Experiment Station. Resource Bulletin SO-85. New Orleans, U.S. Department of Agriculture. 59 pp.
Brash, A.R. 1984. Avifaunal Reflections of Historical Landscape Ecology in Puerto Rico. Tropical Resources Institute. Yale University, New Haven, Conn. 24 pp.
Brown, S., and A.E.Lugo. 1982. The storage and production of organic matter in tropical forests and their role in the global carbon cycle. Biotropica 14:161–187.
Burgess, R.L., and D.M.Sharpe, eds. 1981. Forest Island Dynamics in Man-Dominated Landscapes. Ecological Studies 41. Springer-Verlag, New York. 310 pp.
Carlquist, S.J. 1974. Island Biology. Columbia University Press, New York. 660 pp.
CEQ (Council on Environmental Quality). 1980. Environmental Quality-1980. The Eleventh Annual Report of the CEQ. U.S. Government Printing Office, Washington, D.C. 497 pp.
Crow, T. 1980. A rain forest chronicle: A 30-yr record of change in structure and composition at El Verde, Puerto Rico. Biotropica 12:42–55.
Ehrlich, P., and A.Ehrlich. 1981. Extinction. The Causes of the Disappearance of Species. Random House, New York. 305 pp.
Ewel, J.J. 1977. Differences between wet and dry successional tropical ecosystems. Geo-Eco-Trop 1:103–177.
Ewel, J.J. 1983. Succession. Pp. 217–223 in F.B.Golley, ed. Tropical Rain Forest Ecosystems, Structure and Function. Elsevier, Amsterdam.
Food and Agriculture Organization. 1981. Los Recursos Forestales de la America Tropical. Informe Tecnico 1; Forest resources of tropical Asia, Technical Report 2; Forest Resources of Tropical Africa, parts 1 and 2, Technical Report 3. UN32/6.1301–78–04, Food and Agriculture Organization, Rome. 4 volumes.
Gentry, A.H. 1979. Extinction and conservation of plant species in tropical America: A phytogeographical perspective. Pp. 110–126 in I.Hedberg, ed. Systematic Botany, Plant Utilization, and Biosphere Conservation. Proceedings of a symposium held in Uppsala in commemoration of the 500th anniversary of the university. Almquist and Wiksell International, Stockholm.
Gentry, A.H. 1982. Patterns of neotropical plant-species diversity. Evol. Biol. 15:1–85.
Holdridge, L.R. 1967. Life Zone Ecology. Tropical Science Center, San Jose, Costa Rica. 206 pp.
Holdridge, L.R., W.C.Grenke, W.H.Hatheway, T.Liang, and J.A.Tosi. 1971. Forest Environments in Tropical Life Zones, a Pilot Study. Pergamon, New York. 747 pp.
Lanly, J.P. 1982. Tropical Forest Resources. FAO Forestry Paper 30. Food and Agriculture Organization, Rome. 106 pp.
Leighton, M. 1984. Effects of drought and fire on primary rain forest in eastern Borneo. P. 48 in B.C.Klein-Helmuth and J.L.Hufnagel, compilers. Abstracts of Papers. AAAS Meeting, New York. 24–29 May, 1984. American Association for the Advancement of Science, Washington, D.C.
Little, E.L., R.O.Woodbury, and F.H.Wadsworth. 1974. Trees of Puerto Rico and the Virgin Islands. USDA Forest Service, Agricultural Handbook 449, Vol. 2. Washington, D.C. 1,024 pp.
Lovejoy, T.E. 1980. A projection of species extinctions. Pp. 328–331, Vol. 2 in G.O.Barney (study director). The Global 2000 Report to the President. Entering the Twenty-First Century. Council on Environmental Quality, U.S. Government Printing Office, Washington, D.C.
Lovejoy, T.E. 1981. Prepared statement. Pp. 175–180 in Tropical Deforestation, An Overview, the Role of International Organizations, the Role of Multinational Corporations. Hearings before the Subcommittee on International Organizations of the Committee on Foreign Affairs. House of Representatives, 96th Congress, second session, May 7, June 19, and September 19, 1980. U.S. Government Printing Office, Washington, D.C.
Lugo, A.E., and S.Brown. 1981. Tropical lands: Popular misconceptions. Mazingira 5(2):10–19.
Lugo, A.E., R.Schmidt, and S.Brown. 1981. Tropical forests in the Caribbean. Ambio 10:318–324.
Myers, N. 1979. The Sinking Ark. A New Look at the Problem of Disappearing Species. Pergamon, New York. 307 pp.
Myers, N. 1980. Conversion of Tropical Moist Forests. National Academy of Sciences, Washington, D.C. 205 pp.
Myers, N. 1982. Forest refuges and conservation in Africa with some appraisal of survival prospects for tropical moist forests throughout the biome. Pp. 658–672 in G.T.Prance, ed. Biological Diversification in the Tropics. Columbia University Press, New York.
Myers, N. 1983. Conservation of rain forests for scientific research, for wildlife conservation, and for recreation and tourism. Pp. 325–334 in F.B.Golley, ed. Tropical Rain Forest Ecosystems, Structure and Function. Elsevier, Amsterdam.
NRC (National Research Council). 1980. Research Priorities in Tropical Biology. National Academy of Sciences, Washington, D.C. 116 pp.
Norton, B.J., ed. 1986. The Preservation of Species. Princeton University Press, Princeton, N.J. 305 pp.
Oldfield, M.I. 1984. The Value of Conserving Genetic Resources. U.S. Department of the Interior, National Park Service, Washington, D.C. 360 pp.
Pickett, S.T.A., and P.S.White, eds. 1985. The Ecology of Natural Disturbance and Patch Dynamics. Academic Press, Orlando, Fla. 472 pp.
Raven, P.H. 1977. Perspectives in tropical botany: Concluding remarks. Ann. Mo. Bot. Gard. 64(4):746–748.
Sanford, R.L., Jr., J.Saldarriaga, K.E.Clark, C.Uhl, and R.Herrera. 1985. Amazon rain-forest fires. Science 227:53–55.
Sepkoski, J.J., Jr., and D.M.Raup. 1986. Periodicity in marine extinction events. Pp. 3–36 in D. K.Elliott, ed. Dynamics of Extinction. John Wiley and Sons, New York.
Simberloff, D. 1983. Are We on the Verge of Mass Extinction in Tropical Rain Forests? Unpublished monograph, July 1983.
Simberloff, D. 1986. Are we on the verge of a mass extinction in tropical rain forests? Pp. 165–180 in D.K.Elliott, ed. Dynamics of Extinction. John Wiley and Sons, New York.
Soulé, M.E. 1983. What do we really know about extinctions? Pp. 111–124 in C.M.Schonewald-Cox, S.M.Chambers, B.MacBryde, and W.L.Thomas, eds. Genetics and Conservation. Benjamin/ Cummings, London.
Stebbins, G.L. 1979. Strategies for preservation of rare plants and animals. Great Basin Naturalist Memoirs 3:87–93.
Tosi, J. 1980. Life zones, land use, and forest vegetation in the tropical and subtropical regions. Pp. 44–64 in S.Brown, A.E.Lugo, and B.Liegel, eds. The Role of Tropical Forests on the World Carbon Cycle. A Symposium held at the Institute of Tropical Forestry in Rio Piedras, Puerto Rico, on March 19, 1980. CONF-800350, U.S. Department of Energy Carbon Dioxide Program. National Technical Information Service, Springfield, Va.
Tosi, J., and R.F.Voertman. 1964. Some environmental factors in the economic development of the tropics. Econ. Geogr. 40:189–205.
Vermeij, G.J. 1986. The biology of human-caused extinction. Pp. 28–49 in B.G.Norton, ed. The Preservation of Species. Princeton University Press, Princeton, N.J.
Wadsworth, F.H., and R.A.Birdsey. 1982. Un nuevo enfogue de los bosques de Puerto Rico. Pp. 12–27 in Noveno Simposio de Recursos Naturales. Puerto Rico Department of Natural Resources, San Juan, Puerto Rico.
Weaver, P.L. 1986. Hurricane damage and recovery in the montane forests of the Luquillo Mountains of Puerto Rico. Caribb. J. Sci. 22:53–70.
WRI and IIED (World Resources Institute and International Institute for Environment and Development). 1986. World Resources 1986. Basic Books, New York. 353 pp.
CHAPTER 7
CHALLENGES TO BIOLOGICAL DIVERSITY IN URBAN AREAS
DENNIS D.MURPHY
Research Programs Director, Center for Conservation Biology, Stanford University, Stanford, California
Jaws, claws, an explosion of spray, and a grizzly emerges from the shallows, a salmon in its grasp. Mixed herds of elk, deer, and pronghorn antelope graze rolling, grassy slopes. A cougar surveys from broken chaparral and woodland above.
A scene from the shores of Yellowstone Lake? Perhaps. But it is also a scene from the shores of San Francisco Bay just 150 years ago. Now only deer and cougar remain, but well away from those shores in mountainous habitats above the sprawling metropolitan Bay Area. It seems that only the relatively recent European settlement of the West has spared those species at all. In wooded patches surrounding Milwaukee, the woodland bison, moose, wolverine, black bear, elk, and lynx have been long extinct. Now just a very few forest specialists, such as the raccoon, chipmunk, and white-footed mouse, survive in the region, and those species are gone from all but the very largest woodland patches (Matthiae and Stearns, 1981). In patches of eastern deciduous forest near Washington, D.C., migrant bird species restricted as breeders to forest interiors also survive in only the largest natural habitat remnants. A number of warbler species there show signs of imminent regional extinction (Whitcomb et al., 1981).
These are merely obvious examples of an accelerating decline in the global diversity of living things. The term biological diversity has been used to describe “the variety of life forms, the ecological roles they perform, and the genetic diversity they contain” (Wilcox, 1984, p. 640). While scientists argue about the relative enormity of tropical deforestation and its impact on biological diversity, the loss of populations, species, and entire ecological communities in human population
centers and their surrounding landscapes is well documented and inarguably immense. In urban areas of the eastern United States, only species with the most general habitat and resource requirements have remained in urban corridors. Moreover, the prospect of further erosion of biological diversity looms. In Great Britain, where the sustained assault on the environment is measured in millennia rather than in centuries, and where most vertebrate species are distant memories, a cascade of invertebrate extinctions is now being observed. For example, 80% of the resident butterfly species have declined in number in at least a major part of their British ranges during the past decade (Thomas, 1984). A number of those survive only on reserves and under rigorous management regimes. An estimated 18% of all European butterfly species are considered to be vulnerable to or imminently faced with extinction (Heath, 1981).
Unfortunately, losses of animal and plant species are restricted neither to temperate zone urban areas nor to the developed world. Urban impacts on biological diversity reach their most devastating in the Third World. Less than 2% of the Atlantic forests of coastal Brazil within the urban reach of Sao Paulo remain, and it has been estimated that thousands of species from this region of high endemism have been driven to extinction, most never having been described by taxonomists.
Although the full extent of this urban environmental degradation is virtually impossible to convey, its underlying causes are comparatively simple to identify. With few exceptions, losses of naturally occurring biological diversity are incidental to human activities. Thus, urban areas are effectively synonymous with ecosystem disruption and the erosion of biological diversity. Natural habitats are replaced directly by houses, condominiums, hotels, and malls, as well as by streets, highways, and utilities that support them. Historically, urban areas were the first regions subjected to local overkill of wildlife for food, fur, and feathers, and through misdirected predator control programs. They were also the first to experience logging and weed eradication programs. The biological diversity of urban areas has also been among the most severely affected by the introduction of animal species, which prey on native animal populations, compete for limited resources, and act as vectors for novel diseases and parasites to which native organisms can be particularly susceptible.
Great effects on biological diversity in urban areas also can result from less direct sources, including many of the air- and water-borne pollutants that imperil human health. Toxic by-products of industrial production, such as polychlorinated biphenyls (PCBs), sulfur dioxide, and oxidants as well as pesticides directed at noxious species, have been found to disrupt natural ecosystems (Ehrlich and Ehrlich, 1981). Airborne pollutants are especially insidious, since they expand the reach of urban blight far beyond city limits. More subtle impacts on biological diversity result from overdrafting local aquifers, dropping water tables, and ground subsidence. These processes are often compounded by changes in natural patterns of groundwater percolation caused by the destruction of wetlands and diversion of runoff.
This wide array of obvious and subtle factors contribute to the disruption of ecosystem function, the decoupling of interactions among species, and the disappearance of populations of organisms from urban locales. Why should that concern us? Because losses of just a few populations can result in a great destabilization
of natural ecological communities and, as a consequence, in a decrement in the ability of those communities to provide a wide array of services. Thus, many reasons for protecting diversity in urban areas are often highly utilitarian. Benefits include amelioration of climate, because foliage in cities contribute to the reduction of ambient temperatures. Large trees and shrubs reduce wind velocity and reduce evaporation of soil moisture. Plants are also useful in architecture, erosion control, watershed protection, wastewater management, noise abatement, and air pollution control (Grey and Deneke, 1986).
Nevertheless, the aesthetic reasons for preserving biological diversity are often those that most obviously affect the populace of urban areas. The great parks and natural areas of the world’s major cities, such as Central Park and the Gateway National Recreation Area in New York City and Golden Gate Park and the Golden Gate National Recreation Area in San Francisco, are regarded as prized jewels, providing opportunities for recreation and relaxation as well as habitat for a wide variety of species.
The arguments for protecting biological diversity in urban areas seem straightforward, but the implementation of conservation programs in urban areas is among the most difficult problems faced by environmentalists. Some areas are so disturbed that functioning, naturally occurring ecosystems are no longer identifiable, whereas other urban habitats remain effectively undisturbed. Open spaces in inner cities often support only species that are particularly well adapted to human impact. Such areas are nearly always small and extremely isolated, and their maintenance and enhancement demand extensive and continuous hands-on management. The conservation goals in such areas must usually aim at maximizing biological diversity to the extent possible, rather than preserving all remaining resident species.
Inner city park developers have traditionally introduced plantings of exotic species. Such settings fulfill many of the aesthetic and utilitarian roles that natural habitats offer, but their establishment and maintenance costs tend to be high, since few of the self-regenerating functions of natural ecosystems are available. Yet, although human-induced intervention such as the replacement of ecosystem components can increase the number of species locally over at least the short run, these processes nearly always upset the ecological balance of communities; hence it ultimately exerts a negative impact on naturally occurring biological diversity.
Where larger, intact ecosystems exist within cities, they are often restricted to corridors alongside steep stream canyons, such as Rock Creek Park in Washington, D.C., and Fairmont Park in Philadelphia. But the most extensive expanses of natural habitat in urban areas are those surrounding city limits. In those relatively undisturbed areas, prescriptions for the preservation of biological diversity are quite different from those for maximizing diversity in more disturbed areas. Corridors and surrounding habitats are among the most valuable urban natural areas, providing for extensive biological diversity and reducing the isolation of the largest surviving ecosystems, which may be far from urban centers.
The single greatest threat to the biological diversity of relatively intact natural communities in and around urban areas is the destruction of natural habitats and their conversion to other uses. The paving over of natural habitats as urban activities sprawl outward destroys and fragments remnant functioning ecosystems. The re-
distribution of water through channelization and impoundment of flowing waters, and the draining of some wetlands and the flooding of others, destroys undeveloped habitat areas. Activities as seemingly benign as the planting of exotic trees and shrubs in parks and along byways or the conversion of open space to golf courses disrupt the distribution of natural components of biological diversity. These activities combine to decrease, habitat area and disturb the equilibrium between extinction and immigration among remaining natural habitats, with the frequent result that some species are permanently lost.
Decreases in local biological diversity resulting from losses of habitat area and insularization of habitat remnants are compounded by the more subtle effects of fragmentation. Losses of single, specific microhabitats within an otherwise undisturbed habitat can cause the local extinction of certain species. Disruption of even narrow corridors of natural habitat between large habitat patches can lead to losses of species. The removal of understory foliage in manicured park areas and suburban housing developments can result in the loss of numerous species, most conspicuously species of birds. Vast differences in temperature, humidity, light availability, and wind exposure exist between forest edges and interiors and affect habitat suitability for some species. In addition, losses of certain species due to any one or more causes can affect closely associated species sometimes leading ultimately to secondary extinction events (Wilcox and Murphy, 1985).
In light of these basic ecological facts, conservation of the full range of urban biological diversity necessitates the protection of the largest possible expanses of natural habitat. Yet, that simple prescription is usually impossible to fill in urban areas, where the forces acting to decrease the size of remaining natural habitats are greatest. These conflicting pressures interact to determine urban conservation policy and to force biologists to justify the sizes of biological preserves.
Economic and political considerations in urban areas make preservation particularly difficult. Land costs are high because of high demand, and the vast majority of urban space is private property. The few publicly owned open spaces are subject to intensive, varied uses, many of which are incompatible with preserving biological diversity. Local political institutions usually favor development over preservation, and many agencies concerned with land and resource management, such as the U.S. Forest Service and Bureau of Land Management, have no presence in urban areas. Many conservation organizations with largely urban memberships virtually limit their concern to nonurban environments, and those involved with local issues rarely have the resources available for protracted fights over development.
The Endangered Species Act with its mandate outlawing the “take” of any endangered species is the best tool for protecting biological diversity in urban areas of this country. Although the goal of the Act is protection of individual species of concern, its “purposes…are to provide a means whereby the ecosystems upon which endangered species depend may be conserved” (USC, 1983, p. 1, §1531). Its strength resides in its ability to protect species regardless of land ownership.
Efforts to conserve the full extent of biological diversity by using the Endangered Species Act must target species that are most susceptible to habitat loss. The protection of extinction-prone species can be the key to facilitating the conservation
of biological diversity in urban areas. Species especially prone to extinction include those high on trophic pyramids, widespread species with low vagility (i.e., with poor dispersal ability), endemic and migratory species, and species with colonial nesting habits (Terbough, 1974). Many such species inhabit urban areas during all or major portions of their lives and can act as umbrellas of sorts, often conferring protection to great numbers of species in the same habitats.
The greatest erosion of extinction-prone species has usually occurred in habitat remnants that survive in those urban areas with the longest histories of settlement. Hence prescriptions for conserving remaining biological diversity differ substantially among urban areas. For example, forest patches support many more bird species than do grassland patches of similar size. All else being equal, therefore, protection of the total remaining biological diversity of oak woodlands surrounding San Francisco will demand more and larger preserves than protection of similar habitats to achieve a similar goal near less biologically diverse Washington D.C. In addition, the sizes of preserves necessary to protect biological diversity within an urban area will vary because the diversity itself varies greatly among different natural communities. Oak woodland preserves near San Francisco are likely to require more area to protect their complement of biological diversity than will native grassland preserves in the same geographic area.
In the urban United States, three groups must interact to assist the Endangered Species Act in protecting biological diversity. Field biologists must aid in the identification and survey of potential umbrella species. Conservation organizations must use that information and citizen petitions to get appropriate umbrella species protected via the endangered list. In response, the Office of Endangered Species will have to reassess listing priorities.
The San Francisco Bay Area exemplifies the challenge of preserving urban biological diversity. Without the grizzly bear, tule elk, and even the Xerces blue butterfly, San Francisco might be viewed as biologically impoverished in a sense, but the urban Bay Area remains an exceptionally rich natural region in the biologically richest state in the union. The ecological communities within a 25-kilometer radius of Berkeley include redwood, Douglas fir, and digger pine forests as well as coastal sage and inland chaparral, annual grasslands, dunes, riparian corridors, freshwater lakes, bay marshlands, and even pelagic marine communities and offshore seabird rookeries, an extraordinary array of ecological communities supporting immense biological diversity. The conservation challenge is great, especially in the shadow of a population growing at more than 3% per year; moreover, that shadow is not cast evenly. Less than 15% of San Francisco Bay marshlands remain, but much inland chaparral remains untouched.
Can this urban biological diversity be protected? In this country, the answer is a qualified yes. In many other countries the outlook is not that sanguine. In Austria, prohibitions against the collection of wildlife and plants are strictly enforced, while the conversion of natural habitats to cultivation is effectively subsidized by the government. In the Federal Republic of Germany, as the Black Forest dies from acidification, powerful lobbies thwart the implementation of speed limits on the autobahns; consequently high levels of pollution continue to prevail. Overpopu-
lation, chronic poverty, and fuel shortages in the Third World create unrelenting pressures to exploit all available local resources. These pressures certainly will become more overwhelming in the future.
Our urban centers can be viewed as bellwethers of our global environmental fate. Our success at meeting the challenges of protecting biological diversity in urban areas is a good measure of our commitment to protect functioning ecosystems worldwide. If we cannot act as responsible stewards in our own backyards, the long-term prospects for biological diversity in the rest of this planet are grim indeed.
REFERENCES
Ehrlich, P.R., and A.H.Ehrlich. 1981. Extinction. The Causes and Consequences of the Disappearance of Species. Random House, New York. 305 pp.
Grey, G.W., and F.J.Deneke. 1986. Urban Forestry. 2nd edition. John Wiley & Sons, New York. 299 pp.
Heath, J. 1981. Threatened Rhopalocera (Butterflies) in Europe. Council of Europe, Strasbourg, France. 157 pp.
Matthiae, P.E., and F.Stearns. 1981. Mammals in forest islands in southeastern Wisconsin. Pp. 55–66 in R.L.Burgess and D.M.Sharpe, eds. Forest Island Dynamics in Man-dominated Landscapes. Springer-Verlag, New York.
Terborgh, J. 1974. Preservation of natural diversity: The problem of extinction prone species. BioScience 24:715–722.
Thomas, J.A. 1984. The conservation of butterflies in temperate countries: Past efforts and lessons for the future. Pp. 333–353 in R.I.Vane-Wright and P.R.Ackery, eds. The Biology of Butterflies. Academic Press, London.
USC (United States Code). 1984. Title 16, Conservation; Section 1531 et seq. Endangered Species Act of 1973. United States Code, 1984 Lawyers Edition. Lawyers Co-operative, Rochester, N.Y.
Whitcomb, R.F., C.S.Robbins, J.F.Lynch, B.L.Whitcomb, M.K.Klimkiewicz, and D.Bystrak. 1981. Effects of forest fragmentation on avifauna of the eastern deciduous forest. Pp. 125–205 in R. L.Burgess and D.M.Sharpe, eds. Forest Island Dynamics in Man-dominated Landscapes. Springer-Verlag, New York.
Wilcox, B.A. 1984. In situ conservation of genetic resources: Determinants of minimum area requirements. Pp. 639–647 in J.A.McNeeley and K.R.Miller, eds. National Parks, Conservation, and Development: The Role of Protected Areas in Sustaining Society. Proceedings of the World Congress on National Parks, Bali, Indonesia, 11–22 October 1982. Smithsonian Institution Press, Washington, D.C.
Wilcox, B.A., and D.D.Murphy. 1985. Conservation strategy: The effects of fragmentation on extinction. Am. Nat. 125(6):879–887.