D
Gulf War Illnesses and Recognizing New Diseases
Miriam Davis, Ph.D.1
Gulf War illnesses refer to a cluster of unexplained symptoms not recognized by the medical establishment as a new syndrome or disease (Chapter 2). This appendix provides information to illuminate the general process of how a new disease gains recognition. While not a committee product (see footnote), the IOM committee has reviewed this information and offers it to describe how medical organizations make decisions about new diseases, and what types of scientific evidence they marshal. It also points to social factors, including culture and economics, which weigh into decisions about new diseases.
HOW NEW DISEASES GAIN RECOGNITION IN MEDICINE
Medicine is teeming with examples of new diseases gaining recognition. Not merely a matter for historians, the emergence of a new disease carries contemporary significance. In the past two decades alone, acquired immunodeficiency syndrome (AIDS) and several other new infectious diseases have loomed large as global threats to public health. Their emergence has galvanized public health to reckon with the possible emergence of other new infectious diseases (IOM, 1992). Outside the province of infectious diseases, conditions such as posttraumatic stress disorder and eosinophilia myalgia syndrome have gained recognition. Those with less understood etiologies include chronic fatigue syn-
drome, fibromyalgia, and irritable bowel syndrome, to name a few. Some of these conditions are, in fact, not genuinely “new:” they carry new labels for the same or a similar constellation of symptoms characterized, in some cases, up to a century ago.2
The recognition of a new disease is far from straightforward (Wegman et al., 1997). The simplest statement is that it is a process (Kety, 1974), often taking years. The purpose of the process is to demonstrate that patients are affected by a unique clinical entity distinct from all other established clinical diagnoses. The individual “steps” for gathering and interpreting evidence are not clear-cut. Evidence from biomedical research plays a prominent, but not necessarily exclusive, role in defining and classifying a new disease. Social factors, including culture and economics, influence the recognition, classification, and definition of a new disease (Rosenberg, 1988; Aronowitz, 1998; Wessely et al., 1998). The relative roles of scientific and social factors most likely vary from disease to disease, and era to era. The arbiters of what is a disease (and what is not) are health professionals, organized through public or professional organizations (Wegman et al., 1997), yet without explicit rules for decisionmaking, as explained later.
The process of disease recognition, in its most general features, is captured in Figure D.1. It must be understood at the outset that this is neither a linear nor a carefully articulated process. The process begins with detection of patients whose symptoms cannot be explained readily by existing diagnoses, hence the widely used term “unexplained” symptoms or illness. “Illness” is the term used to refer to patients’ subjective experience of morbidity that they report to their clinicians, in contradistinction to what clinicians diagnose (Eisenberg, 1977). Clinicians or epidemiologists then look for patterns or clusters of symptoms that occur together in the same patient and across many patients. When patterns of symptoms are detected, experts formulate a working “case definition” that establishes classification criteria for a potentially new syndrome. A case definition typically contains a mix of clinical, laboratory, and/or epidemiologic criteria.
The development of the first case definition is a vital milestone designed to spur research and surveillance. More like a hypothesis than a conclusion, the first case definition is an early step in the process of identifying a new clinical entity. Case definitions are protean, frequently changing over time as new evidence comes to light. They are not designed for making diagnoses, which is considered the province of medical practice (Wharton et al., 1990).
A case definition seeks to formulate criteria that effectively identify and distinguish a new patient population from patient populations with recognized
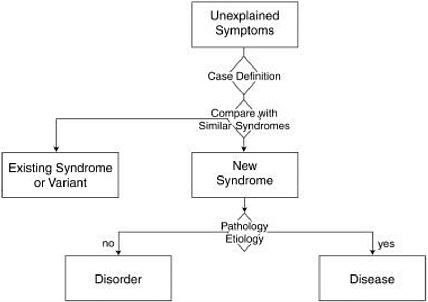
FIGURE D.1 General steps of disease recognition.
diagnoses that are often similar. Case definitions are usually the product of expert panels weighing the relevant body of research, which may include quantitative techniques such as factor analysis (Ismail et al., 1999). For example, several case definitions have been developed for Gulf War illnesses on the basis of factor analysis (Haley et al., 1997; Fukuda et al., 1998), yet none has gained strong acceptance, due either to methodological limitations or to lack of specificity (i.e., the inability to distinguish sufficiently between deployed versus nondeployed veterans). More refined case definitions are likely to emerge in light of ongoing research, and these are likely to elicit intense scrutiny. The discovery of a biological marker would likely prove decisive, as some researchers point out that a unique syndrome cannot be teased apart solely from veterans’ symptoms (Ismail et al., 1999). The point is that existing knowledge of veterans’ unexplained illnesses has not yielded a case definition that successfully specifies a new syndrome. That is why the prevailing medical convention is to resist the popular label “Gulf War syndrome.”
When evidence is presented that a case definition is successful at singling out a new patient population from comparison groups, the case definition progresses a step forward: it begins to achieve recognition by the medical establishment as a new syndrome. The term “syndrome” is by convention reserved for a reproducible set or cluster of symptoms, signs, and/or laboratory tests, without known pathology or etiology (Scadding, 1996). The identification and labeling of a new syndrome is not, according to the medical model, an end in
itself. It too is an invocation for further research on etiology, pathology, course, and treatment (Kety, 1974).
As even more knowledge unfolds about etiology and pathology, an established syndrome can rise to the level of a disease. The term “disease” is often, but not always, reserved for abnormalities in body structure or function with known etiology (e.g., virus, abnormal gene, toxin, physical or psychosocial trauma) and/or pathology (detectable lesion). Diseases are considered mutually exclusive categories (WHO, 1992). Each disease is presumed to have a unique pathophysiology, but complete knowledge rarely exists (Scadding, 1996). According to the International Nomenclature of Diseases, the name of the disease should be specific, unambiguous, and self-descriptive and, if possible, should reflect the cause (WHO, 1992). In practice, the label acts as a shorthand description of the characteristic features of a disease that confer a biological disadvantage3 and deviate from the norm (Scadding, 1996).
A syndrome can ascend the nosological hierarchy to a “disorder” even if there are only clinical manifestations (i.e., symptoms and/or signs) yet no known lesion. In contrast to a syndrome, the term “disorder” conveys that more is known about diagnostic reliability and validity, natural history, and impact on functioning (Goldman and Foreman, 1994). Impairment of functioning—social, educational, or occupational—is considered one of the quintessential criteria for a mental disorder. In its standard manual used for the classification and diagnosis of mental disorders in the United States, the American Psychiatric Association defines a mental disorder as a behavioral or psychological syndrome associated with distress and disability (i.e., impairment of functioning) (APA, 1994). Disorder is thus a more rigorous label than syndrome, but not as rigorous a label as disease, because the disorder’s pathology or etiology are, by definition, not yet known. Yet there are always exceptions in deference to current or historical usage.
The advent of AIDS offers a recent illustration of how a new disease comes to be defined and labeled. The condition was entitled a “syndrome,” according to an early case definition4 promulgated by the Centers for Disease Control and Prevention (CDC) in 1982, before the cause was found. The syndrome was defined by the unusual combination of opportunistic infections and cancer (Kaposi’s sarcoma) first detected in young male homosexuals (CDC, 1982). The rare combination of symptoms, signs, diagnoses, and young age bolstered the hypothesis that this was indeed a new syndrome. CDC’s case definition for AIDS was modified several times within the first few years of the epidemic as research uncovered other populations affected (e.g., hemophiliacs), the mode of
transmission, and the etiological agent (i.e., the human immunodeficiency virus) (Buehler et al., 1993). The most recent revision to the case definition was in 1993, a full 12 years after the first cases were recorded and 9 years after the cause was discovered. This attests to the protean nature of case definitions even, as in this example, with the advantage of objective physical findings and identified etiology. Eventually, the name of the condition was changed to “human immunodeficiency virus disease” to denote the etiological agent and to capture the full course of the disease, from primary infection, to asymptomatic stage, to late stage (WHO, 1992). Although AIDS is the term reserved for the late stage, nosologists would likely favor a name such as “late-stage HIV disease,” but common usage reigns.
Systems of Disease Classification
Formal classifications of diseases, disorders, and syndromes are found in the latest modifications of the International Classification of Diseases (ICD-10) and the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).5 These two compendia are issued about every 10 years by the World Health Organization (WHO) and the American Psychiatric Association (APA), respectively. As the most authoritative sources for public health, government agencies, and health insurers, they have a myriad of applications for medicine, health statistics, reimbursement, disability claims, and medical record keeping. Numerous health and disability statutes at the federal and state levels require a code from one of these classification manuals. Thus, from a legal and statistical perspective, a new listing in these manuals marks the official “arrival” of a new clinical entity (Wegman et al., 1997), even though it may come as little surprise to many clinicians and researchers. From their point of view, a new listing is for coding and classification purposes, not necessarily for fundamental insights into etiology, diagnosis, and treatment. A listing can be construed as the reflection of consensus of health professionals, rather than the instigator.
The formal decision to place a new clinical entity in one of these volumes is made by health professionals organized under the auspices the sponsoring agency. WHO and APA have established procedures for making revisions to ICD-9 and DSM-IV, respectively (APA, 1994; ICD-9, 1999). Yet there are no explicit criteria underlying these procedures. Neither organization publishes explicit criteria for the types of research and clinical evidence needed to revise an existing listing or to add a new listing.6 APA furnishes the most guidance about its procedures and the types of evidence that would warrant changes. It
convenes work groups to evaluate the body of clinical and research literature bearing on a potential revision and sometimes sponsors the reanalysis of existing data and the conduct of field trials. The types of evidence that APA considers important for a listing are clinical utility and diagnostic reliability and validity,7 among other factors (APA, 1994). What is not specified is how this type of evidence is amassed and what its relative importance is for decisionmaking.
How to establish the validity of a syndrome or disorder is a formidable task when there are no biological measures of pathology or etiology (Faraone and Tsuang, 1994). Since this is a problem that disproportionately, but by no means exclusively, weighs upon mental disorders, mental health researchers are actively engaged in refining innovative methods to measure validity. Drawing on techniques developed by statisticians, mental health researchers seek to define clinical entities—in the absence of a diagnostic gold standard8—through several types of reliability and validity testing, the most fundamental of which is concept validity. Concept validity seeks to answer the question, Is there a “true,” but unobservable, latent state of illness? To answer this question, Faraone and Tsuang (1994) argue for the utility of analytical procedures that are variants of latent class analysis. Latent class analysis is similar to factor analysis insofar as it attempts to identify latent (unobserved) variables from a series of correlations between observed variables. Faraone and Tsuang recommend broader use of latent class analysis, envisioning it as a supplement to other types of clinical information that help to establish the validity of a diagnosis—namely, course, outcome, response to treatment, familial aggregation, and biological measures (if available) (Robins and Guze, 1970; Kendell, 1989).
Factor analysis is widely used in the social sciences but has had more limited application in medical research. It attempts to infer the existence of something that cannot be observed directly or verified empirically. In the application of factor analysis to Gulf War illnesses, researchers strive to infer, and thereby “define,” a potentially new Gulf War syndrome on the basis of the cooccurrence (or associations) of certain self-reported symptoms. However, other syndromal “definitions” may be equally consistent with the observed associations. A drawback of factor analysis is that the findings can become reified, or treated as if they are true states of nature (Gould, 1981), rather than, as in this case, a means of grouping symptoms that cluster together.
Much of the effort in classifying disorders in the mental health field is directed at confirming the validity of previously recognized disorders, not establishing the existence of new disorders (APA, 1994). Awareness of the lack of guidance for defining new conditions prompted Wegman and colleagues (1997) to
pose this provocative question as the title of a recent publication: “How Would We Know a Gulf War Syndrome If We Saw One?” The authors propose five steps for defining a new disease: (1) establish that the complex of symptoms and other findings are sufficiently different from recognized disease entities;9 (2) ensure that the boundaries of the definition are not too narrow or too broad to exclude a common etiology; (3) ensure that the condition can be observed and confirmed by a broad range of clinicians drawn from different fields of medicine;10 (4) attempt to include considerations of cause and effect, such as identifying common exposures, susceptibilities, or demographics; and (5) recognize the social, financial, and political pressures that promote or discourage acceptance of a new category. The authors proffer these criteria to encourage more systematic approaches to defining a new condition from Gulf War symptoms (Wegman et al., 1997).
Implications of Disease Classification
The classification of disease facilitates further study of a defined patient population. This is the very first step in the path to progress in diagnosis, treatment, and ultimately prevention. Classification also stimulates further understanding of etiology, risk factors, and natural history of the disease, all of which are critical for the public health goals of primary prevention or prevention of disease-related disability. Classification fosters communication among health professionals and patients, enhances surveillance, and supports patients’ claims for reimbursement and disability.
The diagnosis of disease by clinicians, as noted earlier, is distinct from, but often dependent upon, classification criteria. Clinicians have the liberty to make diagnoses according to their clinical judgment in the practice of medicine. With the advent of a new disease, they generally rely on classification criteria promulgated by specialty organizations of medical professionals (e.g., the APA). In the absence of such criteria, clinicians still have the prerogative to make diagnoses, however unorthodox.
For patients, the diagnosis of a newly defined disease appears to carry both benefits and risks (Wegman et al., 1997; Wessely et al., 1998). Although empirical research is limited, the greatest benefits are patient acceptance of treatment and possible compensation for treatment and disability. Other benefits of diagnosis are patients’ feelings of legitimation and feelings of greater mastery over their symptoms (Woodward et al., 1995). Some research suggests that patients are even likely to improve because of what they perceive as an explanation for their illness (Wessely et al., 1998). Finally, a diagnosis may dissuade patients from seeking alternative or complementary treatments of unproven value and possibly high expense. However, staving off patients’ pursuit of alter-
natives may succeed only as long as their ongoing relationships with medical professionals continue to be satisfactory for them (Ax et al., 1997).
The diagnosis also may carry disadvantages for patients, especially if the condition is stigmatized. Stigma is most pronounced for conditions falling under the categories of mental disorders and addictive disorders (Link et al., 1999). Stigma refers to negative attitudes and beliefs that motivate others to stereotype patients as malingerers, blameworthy, weak, or flawed, and/or to avoid socializing and working with them. Stigma can trigger low self-esteem, isolation, and hopelessness in sufferers (Penn and Martin, 1998). While most of the research on stigma is devoted to severe mental disorders, there is evidence of stigma being perceived by chronic pain patients (Marbach et al., 1990) and those with fatiguing disorders (Wessely et al., 1998). Patients with multisystem organic complaints such as headache and fatigue are especially resistant to receiving a diagnosis of a mental disorder for a host of reasons—the fear of not being taken seriously, stigma, and concerns about lower financial reimbursement (Sparks et al., 1994; Sharpe, 1998; Wessely et al., 1998). Lastly, a diagnosis of a new, yet poorly understood, disorder possibly may result in affirmation of patients’ expectations of disability (Sparks et al., 1994; Wessely et al., 1998; Barsky and Borus, 1999). These concerns figure prominently in physicians’ reluctance to communicate with patients a diagnosis of an unexplained illness (Woodward et al., 1995).
GULF WAR ILLNESSES AND RELATED HEALTH CONDITIONS
There are unmistakable parallels between unexplained illnesses in Gulf War veterans and several other unexplained illnesses. The most striking similarity is in symptom presentation. The symptom cluster of headache, fatigue, cognitive dysfunction, and muscle and joint pain—along with lack of objective laboratory findings—also applies to several unexplained illnesses in civilian and military populations. Other similarities are poor functional status, disability, and apparently chronic course (Table D.1). The fundamental question propelling research is to determine whether unexplained illnesses in Gulf War veterans are variants of, or distinct from, similar conditions.
This section first describes medically unexplained illnesses and then presents the evidence for overlap between unexplained illnesses in Gulf War veterans and civilian populations. By closely examining resemblances between illnesses in Gulf War veterans and civilian populations, researchers hope to acquire insights into etiology and risk factors, prevention, course, and treatment. In fact, the knowledge gained with other populations already has generated experimental treatments for veterans. Several treatments found effective in civilians with unexplained illnesses are being tested in Gulf War veterans, as this section explains.
Medically Unexplained Illnesses
There are numerous illnesses with a symptom profile resembling that seen in Gulf War veterans. The broad category often used to label these syndromes is variously called “medically unexplained illnesses,” “medically unexplained symptom syndromes,” “functional somatic syndromes,” “chronic multisystem illness,” or “symptom-based conditions.” These labels refer to conditions marked by somatic complaints unaccompanied by objective laboratory findings or established causation. For simplicity, the remainder of this appendix refers to these conditions as medically unexplained illnesses, but any one of these labels could apply. That the nomenclature is still so variable is but one indication of the uncertainty enveloping these conditions.
In ongoing research, the medically unexplained illnesses most frequently compared with illnesses in Gulf War veterans are fibromyalgia, chronic fatigue syndrome, and multiple chemical sensitivity (for summary descriptions, see Boxes D.1–D.3). All three are characterized by multisystem somatic complaints, usually pain, headache, and fatigue (Table D.1, Table D.2). For this reason, patients diagnosed with one of these conditions frequently meet case criteria for one or more of the others (Buchwald and Garrity, 1994; Slotkoff et al, 1997; Donnay and Ziem, 1999). Chronic fatigue syndrome and fibromyalgia have such overlapping presentations that the two conditions may possibly be different presentations of the same underlying condition (Buchwald and Garrity, 1994; Buchwald, 1996). On the other hand, a factor analysis study has offered evidence that chronic fatigue syndrome and fibromyalgia are distinct clinical entities (Robbins et al., 1997). The research question of whether the three conditions are separate conditions or variants of the same underlying condition is likely to remain unresolved until more is known about the etiology and pathogenesis11 of each. For the present, they are considered discrete conditions, as reflected by separate case criteria and/or separate listings in ICD-10 (WHO, 1992; see Table D.1).
Other medically unexplained illnesses, documented in military populations from past conflicts tracing back to the U.S. Civil War, include DaCosta syndrome, effort syndrome, and combat stress reaction (Hyams et al., 1996). Several other medically unexplained illnesses, investigated mostly in civilian populations, have been labeled sick building syndrome, irritable bowel syndrome, and silicone-associated atypical rheumatic disease (Hyams, 1998; Wessely et al., 1999). The names of some of these conditions are often disputed, as is their very existence as distinct clinical entities (AMA, 1992; AAAAI, 1999). Some regard unexplained illnesses as manifestations of depression, anxiety, or somatization
TABLE D.1 Gulf War Illnesses and Related Conditions
|
|
Unexplained Illnesses in Gulf War Veterans |
Multiple Chemical Sensitivity (MCS) |
Fibromyalgia (FB) |
Chronic Fatigue Syndrome (CFS) |
|
Most common symptoms |
Fatigue Headache Muscle and joint pain Skin rash Impaired memorya |
Fatigue, low energy Inability to concentrate Memory problems Nasal congestion Headache Throat soreness Joint discomfortb |
Widespread muscle pain and stiffness Tenderness at specified soft tissues sites Fatigue Sleep disturbance Impaired cognitionc |
Severe fatigue Headaches Postexertional fatigue Impaired cognition Muscle pain Multijoint pain Sore throat Unrefreshing sleep Sudden onset of symptoms with a flulike illnessd |
|
Classification criteria |
No widely accepted criteriae |
No widely accepted criteriaf |
1990 American College of Rheumatologyg |
1988 CDC case definition, revised 1994h |
|
ICD-10 listing |
No |
No |
YesI |
Yesj |
|
Causes |
Unknown |
Unknown |
Unknown |
Unknown |
|
Pathology or laboratory test |
None |
None |
None |
None |
|
Animal model |
None |
Neurobehavioral sensitization and/or limbic kindlingk |
None |
None |
|
Biological correlates |
None yet identified |
None yet identifiedl |
HPA dysregulation Alterations in pain mediators (substance P, dynorphin) Growth hormone deficiencym |
HPA dysregulation and other CNS abnormalities Immune activation Physical and cardiovascular de-conditioningn |
|
|
Unexplained Illnesses in Gulf War Veterans |
Multiple Chemical Sensitivity (MCS) |
Fibromyalgia (FB) |
Chronic Fatigue Syndrome (CFS) |
|
Patients with significant disability (%) |
12% of Gulf War veterans receive disability from VAu |
43% of MCS patients report disabilityv |
26.5 % of FB patients report receiving disability paymentsw |
Striking disability in role and social functioning and vitality 37% unemployedx |
|
NOTE: CNS = central nervous system; NA = not available. aJoseph, 1997; Murphy et al., 1999. bMore than 50% of 90 subjects reported these symptoms in an uncontrolled study (Ziem and McTamney, 1997). cWolfe et al., 1990; Buchwald and Garrity, 1994. dBuchwald and Garrity, 1994. eSee Chapter 2. fResearch and clinical criteria are reported in Cullen (1987); Nethercott et al. (1993); and Archives of Environmental Health (1999). gWolfe et al., 1990. hHolmes et al., 1988; Fukuda et al., 1994. Other criteria are used in the United Kingdom and Australia (Wessely et al., 1998). iListed as synonym under M79.0 “Rheumatism, Unspecified,” under Diseases of the Musculoskeletal System and Connective Tissue (WHO, 1992). jListed under G93.3 “Post-Viral Fatigue” under Diseases of the Nervous System, as well as under F48.0 “Neurasthenia” under Mental and Behavioral Disorders (WHO, 1992). |
||||
|
BOX D.1 Chronic fatigue syndrome (CFS), true to its name, is marked by severe and persistent fatigue, along with a cluster of other symptoms. Fatiguing syndromes, given names such as neurasthenia and DaCosta’s syndrome, were chronicled 100 years ago and greeted thereafter with considerable dissent by the medical establishment (Straus, 1991; Wessely et al., 1998). The recognition and classification of CFS was transformed only in the past decade with the development of a case definition sponsored by the Centers for Disease Control and Prevention. The CDC’s case definition, first published in 1988 and revised in 1994, requires fatigue, dysfunction, and four other defining symptoms at least 6 months’ duration (Holmes et al., 1988; Fukuda et al., 1994). The latter symptoms most commonly include headaches, postexertional malaise, impaired cognition, and muscle pain (Buchwald and Garrity 1994). Established for research and surveillance purposes, the case definition also requires exclusion of several other disorders known to cause fatigue. Less than 1 percent of the population meets the case definition for CFS, although many more patients report chronic fatigue (Komaroff and Buchwald, 1998; Wessely et al., 1998). The etiology of CFS is unknown, and there are no accepted laboratory tests or pathological hallmarks (Epstein, 1995). Several biological correlates of the syndrome have emerged recently, including dysregulation of the hypothalamic–pituitary–adrenal axis, immune activation, and other measures (Goshorn, 1998). While infectious agents may trigger some cases of CFS, a complex, multifactoral etiology is proposed, incorporating biological, psychological, and social factors (Wessely et al., 1998). The degree of disability associated with chronic fatigue syndrome is striking, leaving high rates of unemployment (Bombardier and Buchwald, 1996; Buchwald et al., 1996). |
(Hudson and Pope, 1989; Black et al., 1990).12 These views are fueled by the well-documented coexistence of diagnosable mental disorders in a sizable subset of patients with several medically unexplained illnesses. However, many observers have pointed out the difficulty of disentangling cause and effect. Mental disorders such as depression and anxiety may be causes, risk factors, covariates, or consequences of medically unexplained illnesses (Abbey and Garfinkel, 1991; Hyams, 1998). Further, there is some evidence that patients with unexplained illnesses do not satisfy criteria for somatization (Buchwald and Garrity, 1994; Kipen and Fiedler, 1999).
Attempts to systematically study medically unexplained illnesses have been thwarted by problems in case definition and classification of patients (Hyams, 1998). The problems stem not only from the absence of abnormal physical signs
or laboratory tests, but also from the nature of the symptoms themselves. The symptoms are nonspecific and common, both in the community (Kroenke and Price, 1993) and in primary care (Kroenke et al., 1994). About 33 percent of patients in primary care, for instance, complain of four or more common symptoms (Kroenke et al., 1994). With nonspecific, common symptoms and no objective abnormalities, researchers have few guarantees that they are studying a homogeneous patient population. If the population is heterogeneous, this obscures researchers’ ability to detect differences between those with the index condition and those without. This is referred to as the problem of specificity, and it plagues research on medically unexplained illnesses (Hyams, 1998). What is now grouped together as “unexplained illnesses” in Gulf War veterans, for example, might comprise heterogeneous illnesses with different etiologies, pathogenesis, and risk factors.
One potential solution to the problem of specificity is to take a dimensional, rather than a categorical, approach to identifying cases (Wessely et al., 1999). These and other investigators argue that the symptom overlap across medically unexplained illnesses13 is so great that the differences represent an artifact of medical specialization or semantics (Clauw and Chrousos, 1997; Wessely et al.,
|
BOX D.2 The hallmarks of fibromyalgia are widespread muscle pain and tenderness upon palpation at numerous preestablished soft tissue sites on the body, according to classification criteria promulgated by the American College of Rheumatology (Wolfe et al., 1990). The formulation of criteria was a watershed event in the evolution of a condition that had been described for more than a century and given various labels, the most recent of which was fibrositis. Other common symptoms entail fatigue, sleep disturbance, morning stiffness, and cognitive impairment, but these are not sensitive and specific enough to use for classification (Wolfe et al., 1990). Early characterizations of the condition as an inflammation of muscle (hence the label fibrositis) have not been borne out through research (Goldenberg, 1999). There is no pathological or laboratory test with which to confirm the diagnosis. Nor is there any widely accepted etiology. Fibromyalgia’s prevalence is about 2 percent of the population, making it one of the more common rheumatological disorders (Wolfe et al., 1995). Fibromyalgia is 10 times more common in females, and its occurrence increases with age (Wolfe et al., 1995). On the basis of longitudinal studies, the course is chronic, yet variable in intensity (Wolfe et al., 1997). Several types of treatment have been found to be effective in controlled trials, including the tricyclic antidepressant amitriptyline, alone or in combination with fluoxetine (Prozac), as well as cognitive behavioral therapy and exercise. Anti-inflammatory and analgesic medications are no more effective than placebo (Goldenberg, 1999). |
|
BOX D.3 Multiple chemical sensitivity (MCS) is a controversial condition marked by heightened sensitivity to low levels of chemical exposures. Patients report disabling symptoms of fatigue, cognitive impairments, respiratory inflammation, headaches, among other symptoms, in uncontrolled studies (Ziem and McTamney, 1997). Although described by physicians since the 1950s, major medical associations have questioned the very existence of MCS (American College of Physicians, 1989; AMA, 1992; AAAAI, 1999). However, a recent evaluation of the biomedical literature, commissioned at the behest of the United Kingdom Health and Safety Executive, found “suggestive” evidence that MCS exists. Still, there are no pathological or laboratory tests. There are no widely used case criteria (Sparks et al., 1994). Most frequently, patients report that their symptoms are triggered or exacerbated by air pollution, cigarette smoke, solvent fumes, or perfumes (Buchwald and Garrity, 1994). No treatments for MCS have been studied in controlled clinical trials. On the basis of case studies and anecdotal reports, current treatments include avoidance (of the chemical[s] associated with symptoms), cognitive behavioral therapy, environmental control, diet, and sauna therapy (to mobilize and excrete toxins). The etiology of MCS is unknown, although several models are being studied. The UK review cited above identified neuronal sensitization of the mesolimbic pathway of the brain as the etiological model with the best empirical support (Graveling et al.,1999). Sensitization refers to the progressive amplification of a given response after repeated exposures to the same stimulus. A battery of environmental chemicals (e.g., formaldehyde), endogenous substances (e.g., interleukin-2, corticotropin-releasing hormone), drugs (e.g., ethanol), and stressors (physical and psychosocial) can initiate neurobehavioral sensitization in animals (Bell et al., 1998a). |
1999). A dimensional approach assumes that the defining features of unexplained illnesses (pain, fatigue, headache, etc.) occur as a continuum in the-population, and that there are no distinct boundaries between people with different types of unexplained illnesses and those without them. Wessely and colleagues (1999) recommend a dimensional approach that divides patients with unexplained illnesses, not along categorical lines, but according to the number and chronicity of symptoms, associated mood disturbance, patients’ attributions for symptoms, and identifiable physiological processes.
Evidence for Overlap
In past and ongoing research, Gulf War illnesses have been compared with fibromyalgia, chronic fatigue syndrome, and multiple chemical sensitivity (Boxes D.1–D.3). Fibromyalgia and chronic fatigue syndrome are more rigorously studied and more accepted diagnoses, as reflected by their inclusion
TABLE D.2 Overlap of Symptoms
|
|
Fibromyalgia |
Chronic Fatigue Syndrome |
Multiple Chemical Sensitivity |
Gulf War Illnesses |
|
Symptoms: |
|
|||
|
Back pain |
X |
|
|
|
|
Joint pain |
X |
X |
X |
X |
|
Extremity pain |
X |
X |
X |
X |
|
Headache |
X |
X |
X |
X |
|
Weakness |
X |
X |
X |
X |
|
Fatigue |
X |
X |
X |
X |
|
Sleep disturbance |
X |
X |
X |
|
|
Difficulty concentrating |
X |
X |
X |
X |
|
Nasal congestion |
|
|
X |
|
|
Throat soreness |
|
|
X |
|
|
Etiology |
Unknown |
Unknown |
Unknown |
Unknown |
|
Pathology biomarkers |
• HPA dysregulation • Growth hormone deficiency |
• HPA dysregulation • Immune activation |
Unknown |
Unknown |
in ICD-10 (WHO, 1992). Multiple chemical sensitivity (MCS) continues to be poorly characterized and more controversial among clinicians. One major reason is that chemical sensitivity at extremely low doses appears to defy principles of toxicology (Sparks et al., 1994; Reid, 1999). A recent review, commissioned by the United Kingdom Health and Safety Executive, identified “suggestive” evidence that MCS exists as a clinical entity (Graveling et al., 1999), yet this review is unlikely to quell skepticism about MCS.
The first indication of Gulf War veterans reporting symptoms consistent with these conditions emerged from the DoD and VA registries (Joseph, 1997; Murphy et al., 1999).14 The large, well-designed epidemiologic studies summa-
rized in Chapter 2 also furnished evidence of overlapping symptomatology. In the Iowa study, symptoms of fibromyalgia were reported by 19.2 percent of Gulf War veterans versus 9.6 percent of nondeployed controls. Symptoms of chronic fatigue were reported by 1.3 percent of veterans versus 0.3 percent of controls (Table 2.3).15 Both findings were statistically significant (Iowa Persian Gulf Study Group, 1997). Similarly, in the study of Canadian forces sent to the Persian Gulf, symptoms of multiple chemical sensitivity, fibromyalgia, and chronic fatigue were significantly elevated in veterans relative to controls (Goss Gilroy, 1998). Finally, the study of U.K. veterans revealed symptoms of chronic fatigue syndrome to have been significantly heightened in Gulf War veterans in relation to Bosnia and Gulf era controls (3.3 percent in veterans versus 0.8 percent in both control groups) (Unwin et al., 1999). These three population-based studies were methodologically strong, based on either a random sample of the Gulf War veteran population (Iowa,16 United Kingdom) or the entire Gulf War veteran population (Canada). Each study used veterans not deployed to the Persian Gulf for comparison purposes.
A questionnaire study of 1,935 veterans randomly sampled from the VA registry found 15.7 percent to qualify for chronic fatigue syndrome and 13.1 percent to qualify for multiple chemical sensitivity (Kipen et al., 1999). Smaller studies have focused on symptoms of chemical sensitivity in Gulf War veterans. In a pilot study of 48 veterans, Bell and colleagues (1998b) found that 12 out of 14 (86 percent) veterans reporting poorer global health status described themselves in a questionnaire as currently being chemically sensitive (in contrast to healthy Gulf War veterans and two other control groups of Gulf era veterans). Using a newly validated questionnaire to measure chemical sensitivity, Miller and Prihoda (1999) compared Gulf War veterans (n = 72), implant recipients (n = 89), individuals with multiple chemical sensitivity (n = 186) and healthy controls (n = 76). Chemical intolerance and symptom severity scores were significantly greater for all three groups than for controls.
The only published study thus far to have examined Gulf War veterans expressly for a diagnosis of multiple chemical sensitivity, as well as for chronic fatigue syndrome and fibromyalgia, was undertaken by Pollet and coworkers (1998). They performed physical examinations on 53 veterans who had volunteered for the VA registry with complaints of fatigue or chemical sensitivity. Of this group, 33 (62 percent) met diagnostic criteria for chronic fatigue syndrome, 20 for multiple chemical sensitivity, and 3 for fibromyalgia.17 When compared
|
|
March 1995, the VA registry program provided clinicians with separate codes (non-ICD codes) for chronic fatigue syndrome and fibromyalgia, but no diagnostic criteria were provided (IOM, 1998). |
with civilians diagnosed with chronic fatigue syndrome, the veterans in this group were judged to have a milder form of this condition on the basis of symptom severity, reduction in activity, and ability to work. Although this study did not compare the prevalence of these unexplained illnesses in deployed versus nondeployed veterans, it provides evidence that some veterans with fatigue and chemical sensitivity fulfill case definitions for chronic fatigue syndrome, multiple chemical sensitivity, and fibromyalgia. Further studies are in progress searching for similarities and differences between Gulf War illnesses and these related conditions (Research Working Group, 1999).
Benefits to Veterans
There are several immediate and potential advantages to veterans from investigating relationships between their illnesses and fibromyalgia, chronic fatigue syndrome, and multiple chemical sensitivity. These advantages may still hold even if research eventually demonstrates that Gulf War illnesses constitute a new clinical entity.
One benefit is that the body of research and clinical practice, especially for chronic fatigue syndrome and fibromyalgia, is more advanced than that for Gulf War illnesses. If even a subgroup of veterans with unexplained illnesses fulfill diagnostic criteria for any of these conditions, they form a more homogeneous population for study. The classification of patients, as discussed earlier, is a crucial first step, ushering in advances in treatment, etiology, and prevention, among other benefits. Another benefit is the possibility of studying long-term course. Since there have been no large prospective studies of Gulf War veterans from the time of their return, a key opportunity has been missed to elucidate the course of unexplained illnesses in this population (IOM, 1999). Prospective studies can be performed, however, in newly diagnosed civilian populations with chronic fatigue syndrome, fibromyalgia, and/or multiple chemical sensitivity. Such studies might indirectly benefit veterans by yielding novel insights about course of illness, determinants of severity, and approaches to prevention of disability.
Veterans also may benefit from treatments found effective in these other illnesses. This already has occurred with the initiation of a new treatment trial for veterans combining exercise and cognitive behavioral therapy (Engel et al., 1998), two mainstays of treatment for fibromyalgia and chronic fatigue syndrome (Table D.1). Cognitive behavioral therapy is a long-standing type of psychotherapy designed to alter faulty cognitions and replace them with thoughts and self-statements that promote adaptive behavior (Kazdin, 1996). This form of therapy seeks to replace self-defeatist expectations (e.g., “I’m going to be sick forever”) with positive expectations (e.g., “I can get better”). Cognitive behavioral therapy also has been found effective in a randomized clinical trial for patients with many medically unexplained physical symptoms (Speckens et al., 1995).
Finally, new hypotheses about the etiology of Gulf War unexplained illnesses may be forged in light of findings from other unexplained illnesses. Their
etiology and pathogenesis are not well established, but there is no dearth of hypotheses guiding research. With fibromyalgia and chronic fatigue syndrome, several biological abnormalities have been detected in both sets of patients, including dysfunction of the HPA (hypothalamic–pituitary–adrenal) axis, leading to hypocortisolism (Demitrack and Crofford, 1998). Some observers postulate that the chain of causation begins with dysfunction of the central nervous system, which in turn triggers changes in immune function and changes in pain processing pathways (Clauw and Chrousos, 1997). Other biological correlates of chronic fatigue syndrome include anatomical abnormalities in subcortical white matter of the brain (via neuroimaging), chronic activation of the immune system, and reactivation of several latent viruses (Komaroff and Buchwald, 1998). With multiple chemical sensitivity, the etiology and pathogenesis are unclear. The model considered to have the strongest empirical support focuses on dysfunction of the mesolimbic pathway of the central nervous system (Graveling et al., 1999). Proposed by Bell and colleagues (1998a), the model posits that exposure to a host of exogenous and/or endogenous agents can elicit sensitization of neurons in the mesolimbic pathway. Because this pathway mediates autonomic, endocrine, and cognitive function, perturbations could lead to a broad array of seemingly unrelated symptoms. The model relates to chemical intolerance,18 an unpleasant subjective sensation evoked by low-level exposures, rather than to multiple chemical sensitivity per se, which is a severe form of chemical intolerance. Since chemical intolerance also is common to chronic fatigue syndrome and fibromyalgia, Bell and colleagues (1998a) postulate that their model may also apply to these conditions.
SUMMARY
This appendix highlights the process of how a new disease comes to be recognized in medicine. The objective of this usually protracted process is to demonstrate through research that patients are affected by a unique clinical entity, one that is distinct from all other established clinical entities. The strength and coherence of research findings are not the only determinants of whether a new disease gains recognition by the medical establishment. Social factors, including culture and economics, also contribute to decisions, which are made by medical professionals. Professionals are convened under the auspices of the World Health Organization and the American Psychiatric Association, which publish their listings in the International Classification of Diseases and the Diagnostic and Statistical Manual of Mental Disorders, respectively.
|
18 |
The advantage of studying chemical intolerance is that it can be measured in animal models. Other attributes of medically unexplained illnesses, such as fatigue, pain, and headache, are far more difficult to measure in animal behavior. Multiple chemical sensitivity is one of the few medically unexplained conditions for which an animal model has been developed (Table D.1). |
Gulf War illnesses refer to a cluster of symptoms that remain unexplained. Thus far, there is insufficient evidence to classify veterans’ symptoms as a new syndrome (Chapter 2). Still to be answered through more research is whether the symptoms do constitute a syndrome and, if so, whether the syndrome is genuinely new or is a variant form of other conditions, such as chronic fatigue syndrome, fibromyalgia, or multiple chemical sensitivity. This appendix has provided some evidence for overlap between Gulf War illnesses and these three conditions. New insights will be garnered from better understanding of the still-elusive etiology and pathogenesis of Gulf War illnesses.
REFERENCES
AAAAI (American Academy of Allergy, Asthma and Immunology), Board of Directors. 1999. Idiopathic environmental intolerances. J Allergy Clin Immunol 103(1 Pt 1): 36–40.
Abbey SE, Garfinkel PE. 1991. Chronic fatigue syndrome and depression: Cause, effect, or covariate. Rev Infect Dis 13(Suppl 1):S73–83.
AMA (American Medical Association), Council on Scientific Affairs. 1992. Clinical ecology. JAMA 268(24):3465–3467.
APA (American Psychiatric Association). 1994. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4th edition. Washington, DC: APA.
American College of Physicians. 1989. Position paper on clinical ecology. Ann Intern Med 111(2):168–178.
Archives of Environmental Health. 1999. Multiple chemical sensitivity: A 1999 consensus. Archives of Environmental Health 54(3):147-149.
Aronowitz RA. 1991. Lyme disease: The social construction of a new disease and its social consequences. Milbank Q 69(1):79–112.
Aronowitz RA. 1998. Making Sense of Illness: Science, Society, and Disease. New York: Cambridge University Press.
Ax S, Gregg VH, Jones D. 1997. Chronic fatigue syndrome: Sufferers’ evaluation of medical support. J R Soc Med 90(5):250–254.
Barsky AJ, Borus JF. 1999. Functional somatic syndromes. Ann Intern Med 130(11): 910–921.
Bates DW, Buchwald D, Lee J, Kith P, Doolittle T, Rutherford C, Churchill WH, Schur PH, Wener M, Wybenga D, et al. 1995. Clinical laboratory test findings in patients with chronic fatigue syndrome. Arch Intern Med 155(1):97–103.
Bell IR, Baldwin CM, Schwartz GE. 1998a. Illness from low levels of environmental chemicals: Relevance to chronic fatigue syndrome and fibromyalgia. Am J Med 105(3A):74S–82S.
Bell IR, Warg-Damiani L, Baldwin CM, Walsh ME, Schwartz GE. 1998b. Self-reported chemical sensitivity and wartime chemical exposures in Gulf War veterans with and without decreased global health ratings. Mil Med 163(11):725–732.
Bennett RM. 1998. Disordered growth hormone secretion in fibromyalgia: A review of recent findings and a hypothesized etiology. Z Rheumatol 57(suppl 2):72–76.
Black DW, Rathe A, Goldstein RB. 1990. Environmental illness. A controlled study of 26 subjects with “20th century disease.” JAMA 264(24):3166–3170.
Bombardier CH, Buchwald D. 1996. Chronic fatigue, chronic fatigue syndrome, and fibromyalgia. Disability and health-care use. Med Care 34(9):924–930.
Buchwald D. 1996. Fibromyalgia and chronic fatigue syndrome: Similarities and differences. Rheum Dis Clin North Am 22(2):219–243.
Buchwald D, Garrity D. 1994. Comparison of patients with chronic fatigue syndrome, fibromyalgia, and multiple chemical sensitivities. Arch Intern Med 154(18):2049–2053.
Buchwald D, Pearlman T, Umali J, Schmaling K, Katon W. 1996. Functional status in patients with chronic fatigue syndrome, other fatiguing illnesses, and healthy individuals. Am J Med 101(4):364–370.
Buehler JW, Ward JW, Berkelman RL. 1993. The surveillance definition for AIDS in the United States. AIDS 7(4):585–587.
CDC (Centers for Disease Control and Prevention). 1982.Update on acquired immune deficiency syndrome (AIDS)—United States. MMWR 31(37):507–508, 513–514.
Clauw DJ, Chrousos GP. 1997. Chronic pain and fatigue syndromes: Overlapping clinical and neuroendocrine features and potential pathogenic mechanisms. Neuroimmuno-modulation 4(3):134–153.
Cullen MR. 1987. Multiple chemical sensitivities: Summary and directions for future investigators. Occup Med 2(4):801–804.
De Lorenzo F, Xiao H, Mukherjee M, Harcup J, Suleiman S, Kadziola Z, Kakkar VV. 1998. Chronic fatigue syndrome: Physical and cardiovascular deconditioning. QJM 91(7):475–481.
Demitrack MA, Crofford LJ. 1998. Evidence for and pathophysiologic implications of hypothalamic–pituitary–adrenal axis dysregulation in fibromyalgia and chronic fatigue syndrome. Ann N Y Acad Sci 840:684–697.
Donnay A, Ziem G. 1999. Prevalence and overlap of chronic fatigue syndrome and fibromyalgia syndrome among 100 new patients with multiple chemical sensitivity syndrome. J Chronic Fatigue Syndrome 5(3/4):71–80.
Eisenberg L. 1977. Disease and illness. Distinctions between professional and popular ideas of sickness. Cult Med Psychiatry 1(1):9–23.
Engel CC, Roy M, Kayanan D, Ursano R. 1998. Multidisciplinary treatment of persistent symptoms after Gulf war service. Mil Med 163(4):202–208.
Epstein KR. 1995. The chronically fatigued patient. Med Clin North Am 79(2):315–327.
Erickson AR, Enzenauer RJ, Bray VJ, West SG. 1998. Musculoskeletal complaints in Persian Gulf War veterans. J Clin Rheumatol 4(4):181–185.
Faraone SV, Tsuang MT. 1994. Measuring diagnostic accuracy in the absence of a “gold standard.” Am J Psychiatry 151(5):650–657.
Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. 1994. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 121(12):953–959.
Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, Noah DL, Barrett DH, Randall B, Herwaldt BL, Mawle AC, Reeves WC. 1998. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA 280(11):981–988.
Garrett L. 1994. The Coming Plague: Newly Emerging Diseases in a World Out of Balance. New York: Farrar, Straus and Giroux.
Goldenberg DL. 1999. Fibromyalgia syndrome a decade later: What have we learned? Arch Intern Med 159(8):777–785.
Goldman HH, Foreman SA. 1994. Psychopathology: Psychiatric diagnosis and psychosocial formulation. In: Goldman HH, ed. Review of General Psychiatry. Norwalk: Appleton and Lange. Pp. 99–105.
Goshorn RK. 1998. Chronic fatigue syndrome: A review for clinicians. Semin Neurol 18(2):237–242.
Goss Gilroy Inc. 1998. Health Study of Canadian Forces Personnel Involved in the 1991 Conflict in the Persian Gulf, Vol. 1. Ottawa, Canada: Department of National Defence.
Gould SJ. 1981. The Mismeasure of Man. New York: Norton.
Graveling RA, Pilkington A, George JP, Butler MP, Tannahill SN. 1999. A review of multiple chemical sensitivity. Occup Environ Med 56(2):73–85.
Haley RW, Kurt TL, Hom J. 1997. Is there a Gulf War syndrome? Searching for syndromes by factor analysis of symptoms. JAMA 277(3):215–222.
Hodgson MJ, Kipen HM. 1999. Gulf war illnesses: Causation and treatment. J Occup Environ Med 41(6):443–452.
Holmes GP, Kaplan JE, Gantz NM, Komaroff AL, Schonberger LB, Straus SE, Jones JF, Dubois RE, Cunningham-Rundles C, Pahwa S. 1988. Chronic fatigue syndrome: A working case definition. Ann Intern Med 108(3):387–389.
Hudson JI, Pope HG Jr. 1989. Fibromyalgia and psychotherapy: Is fibromyalgia a form of “affective spectrum disorder”? J Rheumatol Suppl 19:15–22.
Hyams KC. 1998. Developing case definitions for symptom-based conditions: The problem of specificity. Epidemiol Rev 20(2):148–156.
Hyams KC, Wignall FS, Roswell R. 1996. War syndromes and their evaluation: From the U.S. Civil War to the Persian Gulf War. Ann Intern Med 125(5):398–405.
ICD-9. 1999. ICD-9: International Classification of Diseases, 9th Revision, Clinical Modification, 5th ed. Los Angeles, CA: Practice Management Information Corporation.
IOM (Institute of Medicine). 1992. Emerging Infections: Microbial Threats to Health in the United States. Washington, DC: National Academy Press.
IOM (Institute of Medicine). 1998. Adequacy of the VA Persian Gulf Registry and Uniform Case Assessment Protocol. Washington, DC: National Academy Press.
IOM (Institute of Medicine). 1999. Gulf War Veterans: Measuring Health. Washington, DC: National Academy Press.
Iowa Persian Gulf Study Group. 1997. Self-reported illness and health status among Gulf War veterans: A population-based study. JAMA 277(3):238–245.
Ismail K, Everitt B, Blatchley N, Hull L, Unwin C, David A, Wessely S. 1999. Is there a Gulf War syndrome? Lancet 353(9148):179–182.
Joseph SC. 1997. A comprehensive clinical evaluation of 20,000 Persian Gulf War veterans. Mil Med 162(3):149–155.
Kazdin AE. 1996. Cognitive behavioral approaches. In: Lewis M, ed. Child and Adolescent Psychiatry: A Comprehensive Textbook, 2nd edition. Baltimore: Williams and Wilkins. Pp. 115–126.
Kendell RE. 1989. Clinical validity. Psychol Med 19(1):45–55.
Kety SS. 1974. From rationalization to reason. Am J Psychiatry 131(9):957–963.
Kipen HM, Fiedler N. 1999. Invited commentary: Sensitivities to chemicals—Context and implications. Am J Epi 150(1):13–16.
Kipen HM, Hallman W, Kang H, Fiedler N, Natelson BH. 1999. Prevalence of chronic fatigue and chemical sensitivities in Gulf registry veterans. Arch Environ Health 54(5):313–318.
Komaroff AL, Buchwald DS. 1998. Chronic fatigue syndrome: An update. Ann Rev Med 49:1–13.
Kreutzer R, Neutra RR, Lashuay N. 1999. Prevalence of people reporting sensitivities to chemicals in a population-based survey. Am J Epidemiol 150(1):1–12.
Kroenke K, Price RK. 1993. Symptoms in the community. Arch Intern Med 153(21): 2474–2480.
Kroenke K, Spitzer RL, Williams JB, Linzer M, Hahn SR, deGruy FV III, Brody D. 1994. Physical symptoms in primary care. Predictors of psychiatric disorders and functional impairment. Arch Fam Med 3(9):774–779.
Link BG, Phelan JC, Bresnahan M, Stueve A, Pescosolido BA. 1999. Public conceptions of mental illness: Labels, causes, dangerousness, and social distance. Am J Public Health 89(9):1328–1333.
Marbach JJ, Lennon MC, Link BG, Dohrenwend BP. 1990. Losing face: Sources of stigma as perceived by chronic facial pain patients. J Behav Med 13(6):583–604.
Miller CS, Prihoda TJ. 1999. A controlled comparison of symptoms and chemical intolerances reported by Gulf War veterans, implant recipients and persons with multiple chemical sensitivity. Toxicol Ind Health 15(3–4):386–397.
Murphy FM, Kang H, Dalager NA, Lee KY, Allen RE, Mather SH, Kizer KW. 1999. The health status of Gulf War veterans: Lessons learned from the Department of Veterans Affairs Health Registry. Mil Med 164(5):327–331.
Nethercott JR, Davidoff LL, Curbow B, Abbey H. 1993. Multiple chemical sensitivities syndrome: Toward a working case definition. Arch Environ Health 48(1):19–26.
Penn DL, Martin J. 1998. The stigma of severe mental illness: Some potential solutions for a recalcitrant problem. Psychiatr Q 69(3):235–247.
Pollet C, Natelson BH, Lange G, Tiersky L, DeLuca J, Policastro T, Desai P, Ottenweller JE, Korn L, Fiedler N, Kipen H. 1998. Medical evaluation of Persian Gulf veterans with fatigue and/or chemical sensitivity. J Med 29(3/4):101–113.
Reid S. 1999. Multiple chemical sensitivity—Is the environment really to blame? J R Soc Med 92(12):616–619.
Research Working Group of the Persian Gulf Veterans Coordinating Group. 1999. Annual Report to Congress: Federally Sponsored Research on Gulf War Veterans’ Illnesses for 1998. Washington, DC: Department of Veterans Affairs.
Robbins JM, Kirmayer LJ, Hemami H. 1997. Latent variable models of functional somatic distress. J Nerv Ment Dis 185(10):606–615.
Robins E, Guze SB. 1970. Establishment of diagnostic validity in psychiatric illness: Its application to schizophrenia. Am J Psychiatry 126(7):983–987.
Rosenberg CE. 1988. Disease and social order in America: Perceptions and expectations. In: Fee E, Fox DM, eds. AIDS: The Burden of History. Berkeley: University of California Press. Pp. 12–33.
Russell IJ. 1998. Advances in fibromyalgia: Possible role for central neurochemicals. Am J Med Sci 315(6):377–384.
Scadding JG. 1996. Essentialism and nominalism in medicine: Logic of diagnosis in disease terminology. Lancet 348(9027):594–596.
Sharpe M. 1998. Doctors’ diagnoses and patients’ perceptions. Lessons from chronic fatigue syndrome. Gen Hosp Psychiatry 20(6):335–338.
Sharpe M, Hawton K, Simkin S, Surawy C, Hackmann A, Klimes I, Peto T, Warrell D, Seagroatt V. 1996. Cognitive behaviour therapy for the chronic fatigue syndrome: A randomized controlled trial. BMJ 312(7022):22–26.
Simon GE, Daniell W, Stockbridge H, Claypoole K, Rosenstock L. 1993. Immunologic, psychological, and neuropsychological factors in multiple chemical sensitivity. A controlled study. Ann Intern Med 119(2):97–103.
Slotkoff AT, Radulovic DA, Clauw DJ. 1997. The relationship between fibromyalgia and the multiple chemical sensitivity syndrome. Scand J Rheumatol 1997 26(5):364–367.
Sparks PJ, Daniell W, Black DW, Kipen HM, Altman LC, Simon GE, Terr AI. 1994. Multiple chemical sensitivity syndrome: A clinical perspective. J Occup Med 36(7): 718–737.
Speckens AE, van Hemert AM, Spinhoven P, Hawton KE, Bolk JH, Rooijmans HG. 1995. Cognitive behavioural therapy for medically unexplained physical symptoms: A randomised controlled trial. BMJ 311(7016):1328–1332.
Straus SE. 1991. History of chronic fatigue syndrome. Rev Infect Dis 13(Suppl 1):S2–7.
Unwin C, Blatchley N, Coker W, Ferry S, Hotopf M, Hull L, Ismail K, Palmer I, David A, Wessely S. 1999. Health of UK servicemen who served in the Persian Gulf War. Lancet 353(9148):169–178.
Wakefield JC. 1992. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychol 47(3):373–388.
Wearden AJ, Morriss RK, Mullis R, Strickland PL, Pearson DJ, Appleby L, Campbell IT, Morris JA. 1998. Randomised, double-blind, placebo-controlled treatment trial of fluoxetine and graded exercise for chronic fatigue syndrome. Br J Psychiatry 172: 485–490.
Wegman DH, Woods NF, Bailar JC. 1997. Invited commentary: How would we know a Gulf War syndrome if we saw one? Am J Epidemiol 146(9):704–711; discussion 712.
Wessely S, Hotopf M, Sharpe M. 1998. Chronic Fatigue and Its Syndromes. New York: Oxford University Press.
Wessely S, Nimnuan C, Sharpe M. 1999. Functional somatic syndromes: One or many? Lancet 354(9182):936–939.
Wharton M, Chorba TL, Vogt RL, Morse DL, Buehler JW. 1990. Case definitions for public health surveillance. MMWR 39(RR-13):1–43.
WHO (World Health Organization). 1992. International Statistical Classification of Diseases and Related Health Problems: Tenth Revision (ICD-10). Geneva: WHO.
Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. 1990. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33(2):160–172.
Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. 1995. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 38(1):19–28.
Wolfe F, Anderson J, Harkness D, Bennett RM, Caro XJ, Goldenberg DL, Russell IJ, Yunus MB. 1997. Health status and disease severity in fibromyalgia: Results of a six-center longitudinal study. Arthritis Rheum 40(9):1571–1579.
Woodward RV, Broom DH, Legge DG. 1995. Diagnosis in chronic illness: Disabling or enabling—The case of chronic fatigue syndrome. J R Soc Med 88(6):325–329.
Ziem G, McTamney J. 1997. Profile of patients with chemical injury and sensitivity. Environ Health Perspect 105(Suppl 2):417–436.
























