Spilled Oil Bioremediation
Lily Young
Although there are few catastrophic oil spills, there are many contaminated harbors. The sediment in these contaminated harbors typically has a very small aerobic layer where you can see the oxidized iron; however, there is no oxygen under those few centimeters in this sticky, mucky, smelly anaerobic anoxic sediment. This means that if the levels of polynuclear aromatic hydrocarbons (PAHs) are not dispersed or degraded in the water column or aerobic zone, they accumulate in this very large anoxic sediment reservoir.
Table 1 is from a publication by Huntley and others (1995), which summarizes the PAHs accumulated in the sediment of various sites in and around the New York-New Jersey harbor. Clearly, the PAHs are still in the sediment and have not disappeared. They may still be there because of limiting nutrients or, more probably, a lack of oxygen. The question is whether there is a biotechnology fix for this—perhaps.
When we look at some of the crude and refined petroleum oils and the constituents, alkanes, cycloalkanes, and aromatics, we see that all are biodegradable by a variety of microorganisms, by certain groups of mostly aerobic organisms. They use oxygen in their metabolism, which is important. For instance, there is an aerobic pathway for naphthalene that is metabolized to salicylates (salicylic acid), and this is further broken down
Biotechnology Center for Agriculture and Environment, Rutgers University, New Brunswick, NJ
TABLE 1. Selected Polynuclear Aromatic Hydrocarbons (PAHs) and Petroleum Hydrocarbons in NY/NJ Sediment (mg/kg dry wt ± s.d.)a
to constituents that are then incorporated into central metabolic pathways (Sutherland and others 1995; Figure 1).
An important point is that the key enzymes that activate this very stable bicyclic molecule—the dioxygenases—require molecular oxygen as a reactant. Oxygen therefore must participate in the reaction to activate the rings or to catalyze the ring fission that occurs here. Salicylic acid requires an oxygenase to break the ring. Hence, oxygen is a reactant.
In the typical known pathway for alkane degradation, which produces fatty acids that can then be incorporated into basic metabolic processes, monooxygenases are required. Again, oxygen is one of the reactants in the reaction that forms fatty acids.
The preceding description sets the stage. In the aerobic environment, we have organisms that require the activity of oxygenases; and in an anoxic environment, where oxygen is not present, let us consider what happens. If anything happens, it must occur through a significantly different biochemical and metabolic mechanism. That area is where I have involved my students, showing them that we can find these organisms because we know they are there. We know they can carry out certain novel degradation reactions, but we do not yet know whether this process is relevant to the environment.
The anaerobic organisms found in that large reservoir of anoxic sediment do not use oxygen but can use other inorganic electronic acceptors
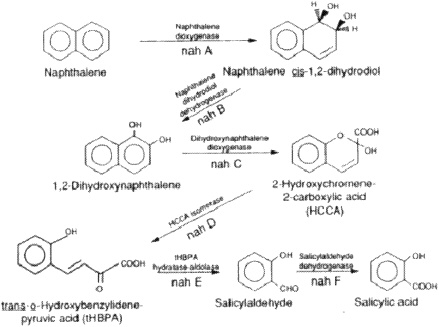
FIGURE 1. Initial steps in the metabolism of naphthalene to salicylic acid by Pseudomonas putida. The genes coding for enzymes involved in the metabolism of naphthalene are designated by nah. Reprinted with permission from Sutherland JB, Rafii F, Kaha AA, Cerniglia C. 1995. Mechanisms of polycyclic aromatic hydrocarbon degradation. In: Young LY, Cerniglia CE, eds. Microbial Transformation and Degradation of Toxic Organic Chemicals. New York: Wiley-Liss. p 269-306.
for respiration. Aerobic organisms use oxygen, and anaerobic organisms, as Dr. Costerton mentioned, can use nitrate. Certain types of microbes also use sulfate and carbonates. This ability is specific. Some organisms, like the denitrifiers, can also use nitrates if oxygen is not available. Others such as the sulfate reducers can only use sulfate and are strict anaerobes. Those that can use carbonate to form methane are called methanogens and are strict anaerobes. We know that these organisms are very important in the general carbon cycle. However, we know much less about whether these groups of organisms have a role in terms of contaminant degradation in these anoxic environments.
It may be helpful to review some of the work we have done. In one case, we looked to see whether contaminated sediment from the New York-New Jersey harbor contained anaerobic organisms that can degrade any of these polycyclic aromatic compounds. We first looked at naphthalene, 2-methylnaphthalene, phenanthrene, and pyrene, and then at some oxidized derivatives such as 1-naphthol and 1-naphthalalene. Everything was handled anaerobically; that is, there was no oxygen available. So the only electron acceptor available was either nitrate, Fe(III), sulfate, or car-
bonate. If any organisms could use these compounds (PAH) as carbon and could use these electron acceptors for respiration, then we would eventually see activity.
Among the real PAHs (not the oxidized derivatives)—naphthalene, methylnaphthalene, phenanthrene, and pyrene—the first three compounds could be metabolized using sulfate to support the metabolism. It took months for the activity to be observable —3 months were usually adequate, but 9 months were necessary in some cases. Nonetheless, we succeeded in selecting for anaerobic organisms that use PAHs in the absence of oxygen.
The time courses for naphthalene and phenanthrene can be seen in Figure 2 (Zhang and Young 1997). After the initial lag period of several
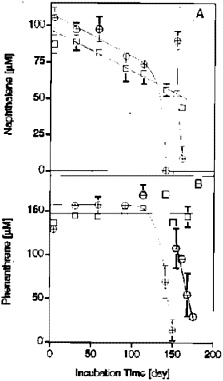
FIGURE 2. Initial degradation of NAP (A) and PHE (B) in 10% AK sedimentinoculated, sulfate-reducing enrichments. The slow decline of the NAP concentration in the autoclave controls is due to volatile loss during sampling. Data points represent the means of three replicates for active cultures () and the means of two replicates for autoclaved controls (). Reprinted with permission from Zhang X, Young LY. 1997. Carboxylation as an initial reaction in the anaerobic metabolism of naphthalene and penanthrene by sulfidogenic consortia. Appl Environ Microbiol 63:4759-4764.
months, activity was relatively rapid. One of the ways we can determine whether true metabolism has taken place is to use radiolabeled compounds (Table 2) and determine how much of the carbon in the radiolabeled naphthalene and phenanthrene can be recovered as CO2(Zhang and Young 1997). Our results indicate that the radiolabeled substrates can be converted to CO2to the extent of 89 and 92%. Hence, most of the substrate is being degraded to CO2.
We used stable isotope C13-labeled compounds and deuterated compounds to prove to ourselves that the metabolites we were seeing actually came from the substrates we gave them. Up to this point, we did not know what was happening between what we started with and when we ended up with CO2. We can now partially answer what happens in between with the following observations. In summary, we are able to show that 2-methylnaphthalene and naphthalene can both be converted to 2-naphthoic acid during degradation to carbon dioxide (Zhang and Young 1997). Did this metabolite actually come from the parent substrate or did it come from somewhere else? We can answer this question by using deuterated methylnaphthalene, and we can show by gas chromatography-mass spectrometry that deuterated naphthoic acid is produced. This evidence indicates that the microorganisms were carrying out the degradation of naphthalene through a mechanism that is very different from aerobic organisms.
We have also been able to show that the carboxylation of the naphthalene occurs through an inorganic carbonate addition to the molecule, using a stable isotope C13-carbonate in solution. After incubation and gas chromatography-mass spectrometry analyses, we showed this carboxyla-
TABLE 2. Mineralization of [14C]Polynuclear Aromatic Hydrocarbons ([14C]PAHs) in Acclimated, Sulfidogenic Consortiaa,b
|
PAH Tested |
Total added radioactivity (dpm)c |
Amount of radioactivity (dpm) Recovered as 14CO2 |
Left in slurry |
Total radioactivity recovered (%) |
|
NAP |
107,170 |
95,671 (89.3%) |
4,350 (4.1%) |
93.3 |
|
PHE |
529,076 |
487,057 (92.1%) |
27,709 (5.3%) |
97.3 |
|
a Reprinted with permission from Zhang X, Young LY. 1997. Carboxylation as an initial reaction in the anaerobic metabolism of naphthalene and penanthrene by sulfidogenic consortia. Appl Environ Microbiol 63:4759-4764. b For each PAH, six replicate samples were established and 150 µM unlabeled PAH was added. Radiolabeled PAH was then added to three of the six replicates. Samples without radioactive PAH were analyzed to monitor the progress of the PAH degradation. Incubation lasted 24 and 42 days for NAP and PHE, respectively, at room temperature (24 ± 2°C). c Standard deviations of the reported numbers were within a 2% range (n = 3). |
||||
tion occurring. The standard mass spectrum of 2-naphthoic acid has major fragments at 244, 229 and 185, 155. After using C13-carbonate, the major fragments all increase by one mass unit, namely, 245, 230, 156 (Zhang and Young 1997; Figure 3).
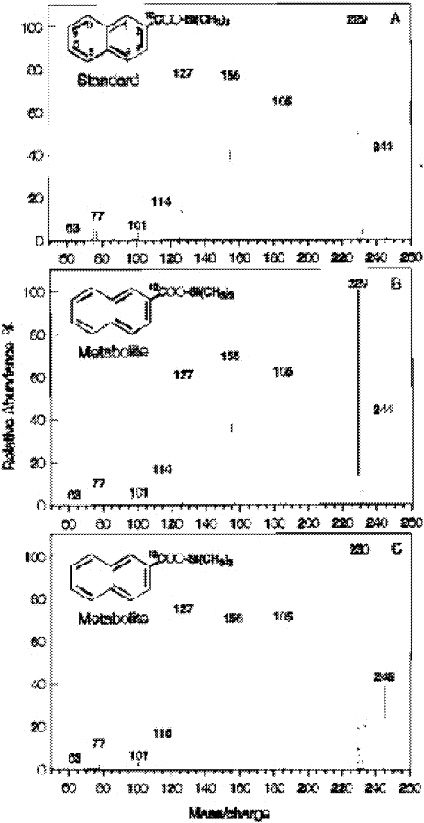
FIGURE 3. Mass spectra of trimethylsilyl derivatives of a 2-NA standard (A) and 2-NA extracted from the sample supplemented with NAP and either [12C]bicarbonate (B) or [13C]bicarbonate (C). The mass spectrum of the 2-NA standard (A) contains five major peaks: m/e 244, 229, 185, 155, and 127. The m/e 244 peak represents the molecular ion. The fragmentation ion of m/e 229 is the result of the loss of a −CH3group (244 − 15 = 229); the fragmentation ion of m/e 185 is from the loss of a −CH3group and a −COO group (244 − 59 = 185); the fragmentation ion of m/e 155 is from the loss of an −OSi(CH3)3group (244 − 89 = 155); and the fragmentation ion of m/e 127 is from the loss of a −COOSi(CH3)3 group (244 − 117 = 127). The identification of the 2-NA metabolites in panels B and C is based on comparison of the GC retention time of the derivatized standard for 2-NA (10.41 min). Reprinted with permission from Zhang X, Young LY. 1997. Carboxylation as an initial reaction in the anaerobic metabolism of naphthalene and penanthrene by sulfidogenic consortia. Appl Environ Microbiol 63:4759-4764.
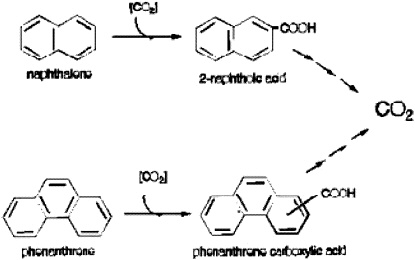
FIGURE 4. Proposed summary pathways for the anaerobic metabolism of NAP and PHE in the sulfidogenic enrichments. Reprinted with permission from Zhang X, Young LY. 1997. Carboxylation as an initial reaction in the anaerobic metabolism of naphthalene and penanthrene by sulfidogenic consortia. Appl Environ Microbiol 63:4759-4764.
From these kinds of experiments, we are able to say that naphthalene, 2-methylnaphthalene, and phenanthrene undergo an initial carboxylation. Remember that there is no oxygen, so the ring must be attacked in a different manner. An initial carboxylation activates the ring and then, through some of our other experiments, we observe a ring reduction that eventually yields ring fission (Zhang and Young 1997; Figure 4).
It may also be helpful to review some of the results we have on anaerobic alkane biodegradation. In a manner similar to the PAHs, we initially used alkane as the sole carbon source and observed whether activity occurred with any of the anaerobic electron acceptors. Again, after fairly long incubation times, we observed activity on octane, decane, and dodecane under sulfate-reducing conditions. In this set of studies, we succeeded in isolating a pure culture of an organism that carries out the reactions. We can now look at it and investigate the mechanism and biochemistry more closely.
We characterized the organism taxonomically and phylogenetically (strain AK-01) as falling within the general class of sulfate reducers. It is a different organism from what has been reported in the literature as an alkane degrader (F. Widdel's group in Bremen, Germany [Aechersberg and others 1998]). We then more closely compared our strain with that from Germany in terms of the degradation mechanisms. Interestingly, they are both sulfate reducers and they are both strict anaerobes that can
degrade alkanes, but they attack the alkanes in very different ways. If we used odd-numbered alkanes, C-15 and C-17, AK-01 formed cellular fatty acids that are odd numbered. If we used odd-numbered alkanes for the other organism, the resulting cellular fatty acids were even numbered. The opposite also occurred, i.e., even-numbered alkanes generated even-numbered fatty acids in strain AK-01, and they yielded odd-numbered fatty acids in the other strain. This pattern led us to hypothesize that these two organisms, though cousins, appear to have different mechanisms for alkane degradation. Using substrates that were unlabeled or deuterated or C13-labeled alkanes, we found that the two different strains have very different ways of attacking the alkane under anaerobic conditions.
Strain AK-01 carries out a carbon addition at the subterminal C-2 position of the alkane chain. As a consequence, the terminal carbon then swings down so it forms the methyl group of the C-2 carbon of this fatty acid. Once this occurs, the organism can carry out normal beta-oxidation. It can carry out chain elongation to form larger fatty acids as well. The other strain has a very different attack. It uses inorganic carbonate from solution as the carbon donor. This inorganic carbon is added to the C-3 position of the alkane, and the two terminal carbons are released as acetate. We then end up with two carbons removed and one carbon added to the original alkane so that the resulting fatty acid ends up as an odd-numbered fatty acid. That description is really just the tip of the iceberg because these are only two organisms that have been investigated under anaerobic conditions.
To further contemplate this area of study, consider the following questions:
-
Are microorganisms from terrestrial or freshwater systems similar to those found in marine sediments? We know too little about the diversity of these types of degradative anaerobes.
-
Are competent organisms actually present? This question is not as straightforward. When we looked for anaerobic toluene and benzene degradation in anoxic-contaminated sediments or in anoxic pristine sediments, our data indicated that the toluene loss and benzene loss occur in the contaminated sediments, but not under the same conditions as in the pristine sediments (Figure 5). This difference suggests that organisms competent for degrading these contaminants are not present in the pristine environments. If they are not there, bioaugmentation may be a viable application for adding organisms where needed.
Let me also point out that there are many pathways for aerobic degradation of PAHs. There are also many aerobic organisms able to carry this out. This kind of diversity has yet to be tapped for the anaerobic microbial community.
We also know about the aerobic degradation of alkanes in terms of
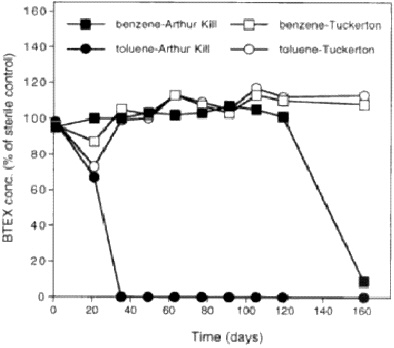
FIGURE 5. Anaerobic biodegradation of BTEX (benzene, toluene, ethylxylene, xylenes) in consortia with sediment from Arthur Kill, New York/New Jersey, harbor (contaminated) and from Tuckerton, New Jersey (uncontaminated). From Phelps CD, Young LY. Unpublished data.
the enzymes and the genes responsible for them. However, there is only one very well characterized aerobic pathway for alkanes. Considering that there are many for the PAHs, there is likely to be others for the alkanes as well; but even in the aerobic realm, our information is limited.
Another issue with respect to the ability of organisms to use different electron acceptors has been addressed by Dr. Jerry Kukor, who studied groundwater from aquifers at three different sites contaminated with benzene, toluene, and xylenes. Data from the three sites indicated that the oxygen was present in significantly lower concentrations compared with the pristine site; furthermore, the levels of oxygen in the contaminant plume is also lower than in the pristine site. In the plume, the potential for both aerobic and anaerobic activity exists. Using the organisms isolated from this site, we can see that low oxygen with nitrate supported much better degradation of toluene than oxygen without nitrate. Here is a hybrid system in which the oxygen is necessary for these organisms because all are aerobic organisms in terms of the mechanisms they use for the degradation process; however, their activity is boosted because they can use nitrate as well as oxygen for respiratory purposes.
I am not sure what mechanism is used for the degradation process, but I would like to add one possible argument for the usefulness of this hybrid. To write an equation describing toluene oxidation to carbon dioxide, 9 mol of oxygen are required for every mol of toluene. To describe it as toluene oxidation first to benzoic acid, aerobically, 1.75 moles of oxygen per mol of toluene as required. This benzoate can then be degraded to carbon dioxide using nitrates and the respiratory electron acceptor. In this case, we have spared the oxygen for the key metabolic activation step. Hence, oxygen is used exclusively for ring activation and nitrate is used for respiration. Thus, microorganisms may have many strategies for biodegradation.
Additional questions to be considered are the following:
-
Are any petroleum components inherently recalcitrant so they are not biodegradable?
-
Are any petroleum components not bioavailable because of their physical, chemical binding to soils or sediments?
-
If the answer above is affirmative, then can regulatory criteria reflect this?
-
If petroleum components are not bioavailable, then are they a risk?
-
Are anaerobes relevant in remediation of anoxic sediments?
-
If the activity of anaerobes is too slow or too minor, do they have a role in cleaning up the environment? In other words, they may not have a significant role in actively cleaning up the environment. Nonetheless, over time, their impact may still be substantial.
If we can get a handle on some of these questions, then we can determine whether certain environments do, indeed, have an intrinsic ability for biodegradation whereas other environments may not.
REFERENCES
Aechersberg F, Rainey FA, Widdel F. 1998 Growth, natural relationships, cellular fatty acids and metabolic adaptation of sulfate-reducing bacteria that utilize long-chain alkanes under anoxic conditions. Arch Microbiol 170:361-369.
Huntley SL, Bonnevie NL, Wenning RJ. 1995 Polycyclic aromatic hydrocarbons and petroleum hydrocarbon contamination in sediment from the Newark Bay estuary. Arch Environ Contamin Toxicol 28:93-107.
Sutherland JB, Rafii F, Kahn AA, Cerniglia CE. 1995 Mechanisms of polycyclic aromatic hydrocarbon degradation. In: Young LY, Cerniglia CE, eds. Microbial Transformation and Degradation of Toxic Organic Chemicals New York: Wiley-Liss. p 269-306.
Zhang X, Young LY. 1997 Carboxylation as an initial reaction in the anaerobic metabolism of naphthalene and phenanthrene by sulficogenic consortia. Appl Environ Microbiol 63:4759-4764.










