8
New and Emerging Therapies
Recent scientific discoveries in human genomics and immunology combined with advanced technologies are revolutionizing the treatment of many types of cancers. Much of the drive for new therapies, not just for cancer but for other conditions as well, comes from a desire for greater precision in treatment. Personalized medicine aims to give specific therapies to patients who are most likely to benefit (targeted therapy) and to reduce the morbidity associated with conventional therapy that indiscriminately exposes malignant and normal tissues to toxicity. Improved treatment specifications, greater preservation of normal tissues, and less—or more limited—surgery with greater precision are examples of changes that are occurring in cancer treatment today. New cancer treatments with evidence of favorable outcomes are increasingly available, but there is still much to learn about these novel therapies and their impact on cancer survivorship.
The standards of cancer care, which are described in detail in other chapters of this report, can change and evolve rapidly in response to the latest research findings from clinical trials and other research. In this chapter the committee presents an overview of selected new or emerging cancer therapies, which are defined as follows: A new treatment is a therapeutic approach adopted recently in clinical practice or an established treatment for one type of cancer that is being studied for other cancers. An emerging therapy is a novel therapeutic approach under scientific investigation that has demonstrated promising results in early research but has not yet been accepted as a standard of care. Although these new and emerging therapies may or may not yet be adopted in national guidelines for cancer care or widely implemented in all health care settings, with further study and wider
adoption in clinical practice, they have the potential to become the standard of care in the near future.
The chapter begins with a brief overview of the process for developing and approving new treatments in order to provide context for the new approaches discussed in the chapter and the changing the treatment landscape. This is followed by a discussion of new and emerging therapies that are organized into three broad categories—surgery, radiation therapy, and systemic therapies—and whose use is described for the treatment of lung cancer and breast cancer in particular, but in some cases, other types of cancer as well. The final section of the chapter describes neoadjuvant and multimodal treatment approaches to treating cancer. Other types of emerging therapies—including treatments for cancer-related long-term or late-onset effects (new drugs to treat cardiotoxicity related to chemotherapy, for example) and other types of interventions such as behavior modification (diet and exercise), education programs, and counseling—are not addressed in this chapter.
DEVELOPMENT OF THERAPIES FOR CANCER
The development of cancer therapies is a complex and lengthy endeavor, which in the final stages includes testing for safety and efficacy in patients. This is a rigorous and highly regulated process. After years of testing, the U.S. Food and Drug Administration (FDA) approves a therapy or device for clinical use, and the evidence from clinical trials determines how best to use the specific therapy and under what circumstances. The committee emphasizes that its discussion of new and emerging new cancer treatments is not exhaustive, and the treatments that are mentioned, particularly drugs, are examples only and several other drugs in that category may be available for clinical use. FDA approves many cancer treatments each year. These approvals may be for novel drugs, for drugs that target treatment based on a specific characteristic of a tumor instead of its site of origin, for use of a drug to treat a new patient populations (e.g., children or adults with a particular genetic mutation), or for new formulations or manufacturers of approved drugs. FDA also approves a number of biosimilars (that is, a biological product that is highly similar to and has no clinically meaningful differences from an existing FDA-approved reference product) that may be used to treat a variety of cancers (FDA, 2020a). The development of potential therapies is based on robust basic and preclinical science involving cellular assays, in vitro studies, and studies in rodents and primates. These preliminary studies are used to inform the design of subsequent clinical trials in humans. In phase I trials a new drug is administered to a small number of people with the disease in order to identify adverse effects produced by the drug (including identifying the highest dose without
life-threatening adverse effects) and to determine how the drug is metabolized in the body. In phase II trials, which generally include a larger group of patients than phase I trials, the goals are to evaluate whether the drug works (its efficacy) and to determine more accurately its adverse effects. Phase III trials, which typically are randomized controlled trials (RCTs), provide the highest level of evidence to determine whether a new treatment is better than an existing standard treatment. In RCTs individuals with cancer are randomly assigned to either the new treatment or a standard treatment. The effects of the treatments are measured by improvement in a relevant treatment outcome, such as survival. Increasingly, RCTs also assess quality of life, based on patient-reported outcomes and other outcome measures. If these pivotal studies demonstrate that the new treatment is superior to the current standard treatment (or sometimes just not inferior, particularly if the new treatment is less toxic), FDA may grant approval of the drug for a specific cancer.
Recently, novel study designs have been used, including phase I and phase II designs where the recommended dose from phase I moves seamlessly into efficacy studies. These new designs help to reduce the time needed to perform the necessary clinical trials and to make effective drugs available more quickly.
SURGERY
Although surgical treatment for breast cancer is relatively effective and in the majority of cases results in excision of the tumor such that there are negative margins (i.e., no evidence of cancer) in the tissue, approximately 15% of patients need re-excision for inadequate margins (Chung et al., 2015; DeSnyder et al., 2018; Mamtani et al., 2019; Rosenberger et al., 2016; Tang et al., 2017). Multiple devices have been developed that help to identify residual breast cancer left behind during a lumpectomy surgery so that additional tissue can be excised. Although very low rates of positive margins can also be achieved without these additional devices by doing a pathological examination of the specimen during surgery, these new technologies rely on recognizing differences in characteristics between the normal and malignant breast tissue, including differences in their electrical properties, infrared imaging, molecular attributes, and other properties, in order to more precisely remove breast tumors (Keating et al., 2016; Schnabel et al., 2014; Smith et al., 2020).
Traditionally, a breast tumor that is non-palpable (i.e., cannot be felt on physical exam) is localized for surgical excision by a thin wire placed into the breast by radiologic guidance just prior to surgery, which can be uncomfortable for the patient and also inaccurate if the wire becomes dislodged. Newer wire-free technologies can localize a non-palpable breast
lesion using markers with various tags, including radioactivity, a magnetic signal, or a radiofrequency signal, which some studies have shown to be more accurate in localization than traditional wires (Gray et al., 2001; Hughes et al., 2008; Mayo et al., 2019).
Breast surgery for both the primary tumor (in the breast) and regional lymph nodes (in the axilla) can result in significant postoperative adverse effects, including lymphedema, deformity of the breast, pain, and other sequelae. Because surgery to remove a breast tumor and lymph nodes is often successful, greater attention has been directed toward decreasing the associated morbidity by limiting the extent of surgery performed without compromising the oncologic outcome of patients.
Surgical Approaches to Reduce Lymphedema
For many patients with cancer, lymphedema can be a significant complication of their treatment and of the disease itself which can result in considerable morbidity. Lymphedema occurs when lymphatic channels in one part of the body are disrupted, either by an intervention or by the disease, causing an accumulation of protein-rich lymphatic fluid to accumulate in a downstream area being drained by the lymphatics. For breast cancer, lymphedema of the arm is most commonly caused by surgical removal of the lymph nodes in the axilla and by radiation to the axilla. Additional details on the incidence and functional outcomes of lymphedema are discussed elsewhere in this report. In order to reduce the risk of lymphedema, several novel surgical approaches have been developed recently and are gaining acceptance as standard therapy.
Targeted Axillary Dissection
The risk of lymphedema is associated with the number of lymph nodes that are removed (Penn et al., 2019). To reduce the need for a “complete” axillary lymph node dissection (ALND) (see Chapter 5) in patients with clinically node-positive breast cancer, a new surgical approach is targeted axillary dissection with possible ALND after the completion of neoadjuvant chemotherapy (Boughey et al., 2018; Caudle et al., 2016). With this approach, only the known biopsy-proven initially metastatic lymph node (marked by a biopsy clip) is removed, along with additional sentinel lymph nodes, which are the first few lymph nodes that drain a breast cancer and are located primarily in the axilla. Sentinel lymph node dissection is not used as a standard approach for patients with clinically node-positive disease (false negative rate is >14% in the European SENTINA trial; Kuehn et al., 2013), but when combined with removal of the known biopsy-proven metastatic node with the targeted axillary dissection procedure, the false
negative rate is as low as 1.4% (Caudle et al., 2016). If the pathology examination shows that all lymph nodes removed in the targeted axillary dissection are negative for metastasis, no additional nodes are removed, but if any nodes are positive, then a complete ALND is performed. For node-positive patients with a favorable response to neoadjuvant therapy, targeted axillary dissection may allow for the omission of ALND and its associated morbidities. A similar approach called MARI (marking the axillary lymph node with a radioactive seed) developed in The Netherlands has a false negative rate of 7% (Donker et al., 2015), and, regardless of the method used to identify the biopsy-proven initial metastatic lymph node, this strategy has been incorporated into the most recent National Comprehensive Cancer Network guidelines (version 3.2020) on invasive breast cancer as a consideration for patients with clinically node-positive disease treated with neoadjuvant therapy (NCCN, 2020).
Lymphovenous bypass and lymph node transfer
For the patient who has developed lymphedema, surgical approaches to re-establish lymphatic drainage may be beneficial. With the lymphovenous bypass procedure, lymphatic vessels beyond the site of blockage are identified and connected with microsurgery (Garza and Chang, 2018; Schaverien and Coroneos, 2019). With the lymph node transfer technique, a lymph node (either single or multiple) from an unaffected region of the body is transplanted into the axilla (or other nodal basin draining the affected limb) where new lymphatic channels can then develop (Chang et al., 2018; Schaverien and Coroneos, 2019; Schaverien et al., 2018). The precise mechanism of action is unclear, but evidence suggests that the growth factors secreted from the transplanted lymph node(s) stimulate new lymphatic connections. Both procedures have been shown to result in a reduction in limb volume (reviewed in Schaverien and Coroneos, 2019), but long-term outcome data on their impact on functional improvement are needed. These procedures have primarily been evaluated as treatment options for patients with chronic lymphedema, but their role as prophylactic measures to prevent lymphedema is not well understood. Finally, these techniques have been applied for malignancies other than breast cancer that are treated with lymph node dissection, such as melanomas and head and neck cancers.
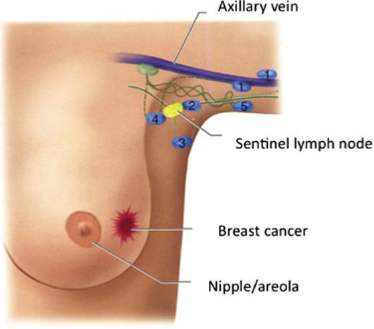
Axillary Reverse Mapping
The goal of axillary reverse mapping is to avoid the removal of the lymph nodes that drain an arm during lymph node surgery for breast cancer and thereby preserve lymphatic flow from that extremity (Thompson et al., 2007). The procedure relies on injecting a blue dye into the patient’s arm that will mark the lymph nodes in the axilla that drain the arm (Boneti et
al., 2008) (see Figure 8-1). During the sentinel lymph node or ALND surgery for a breast cancer patient, the surgeon will remove the sentinel lymph nodes or other lymph nodes for the ALND but leave the axillary reverse mapping lymph nodes (blue nodes) intact for draining.
A systematic review of eight studies with 1,142 patients undergoing axillary reverse mapping found that in up to 10% of the cases the lymph node draining the arm was also the node draining the breast cancer and had to be removed (crossover nodes) (Ahmed et al., 2016). In these cases the lymphatic vessels can be re-connected surgically during the same procedure to re-establish outflow from the arm. Results from a 5-year experience at a single institution showed that the axillary reverse mapping procedure is safe and could result in lower rates of lymphedema (Ochoa et al., 2014). A multi-institutional randomized phase III clinical trial is now under way to study how effective axillary reverse mapping is in preventing lymphedema in breast cancer patients undergoing ALND (Alliance for Clinical Trials in Oncology, 2019).
Oncoplastic Surgery for Breast Cancer
As the overall and disease-free survival rates for most types of breast cancer are excellent, increasing emphasis has been directed toward improving the quality of life for breast cancer patients, including the cosmetic result of surgery. Breast-conserving surgery (BCS, or lumpectomy) followed by adjuvant radiation can often result in a deformity of the breast with indentation, nipple deviation, and volume loss. Some women are considered to be poor candidates for BCS, given the high likelihood of an unsatisfactory cosmetic outcome. These women are typically counseled to undergo
SOURCE: Noguchi et al., 2015, with permission.
a total mastectomy, possibly with reconstruction, which is associated with increased morbidity and costs compared with BCS (Carter et al., 2016; Jagsi et al., 2016). For women who are not candidates for BCS alone, BCS with oncoplastic reconstruction is an alternative that has been shown to be oncologically safe with fewer complications compared with total mastectomy with reconstruction (Campbell and Romics, 2017; Carter et al., 2016; De La Cruz et al., 2016). With oncoplastic surgery, a lumpectomy is performed by the breast cancer surgeon, and the defect is then filled by a rearrangement of breast tissue from other parts of the breast or by adjacent soft tissue (Clough et al., 2018; Lebovic, 2010; Silverstein et al., 2014; Urban et al., 2011). The use of oncoplastic surgery has been trending upward in recent years, but more widespread adoption has been limited by the lack of available technical expertise. For optimal results many patients will need a surgical procedure on the contralateral breast to achieve symmetry, which is covered by most group insurance plans, as mandated by the Women’s Health and Cancer Rights Act of 1998.1
Minimally Invasive Surgery
For many types of cancer, surgery is associated with significant pain and dysfunction, requiring a prolonged hospital stay and outpatient recovery. Minimally invasive surgery, which can be performed laparoscopically through smaller incisions than with traditional open techniques, has been shown to be safe and feasible for a variety of cancers and to have an accelerated postoperative recovery (Chang and Rattner, 2019; Nota et al., 2019; Pawlik, 2019).
Laparoscopic surgery uses instruments, including a camera, inserted through small incisions that the surgeon and assistants manipulate at the patient’s bedside with an image of the operative field projected onto a screen. Laparoscopic surgery rapidly gained wide acceptance for cancer treatment after a clinical trial demonstrated that its safety was equivalent to that of the traditional open approach (Clinical Outcomes of Surgical Therapy Study Group et al., 2004). Importantly, patients in the minimally invasive surgery group had a decreased length of hospital stay and lower use of narcotics and other medications for postoperative pain control. Multiple studies have since confirmed the oncologic safety of laparoscopic surgery, and this approach is now commonly performed and considered standard of care for some solid tumors (e.g., colorectal, kidney, and prostate cancers) (Cohen and Kingham, 2019). For more complex cancer operations, such as liver and pancreas resections, laparoscopic surgery has only
___________________
1 Women’s Health and Cancer Rights Act of 1998, Public Law 105-277, Div. A, § 101(f) [Title IX], Oct. 21, 1998, 112 Stat. 2681–436.
recently been attempted and will need further evaluation before it is widely adopted for these malignancies.
Laparoscopic surgery is hampered by several technical limitations, including rigid instruments and poor visualization. Surgical robot platforms (e.g., the da VinciTM surgical robot from Intuitive Surgical, Inc.) overcome these limitations by using instruments that have a greater range of motion than the human wrist and a highly magnified view of the surgical field in three dimensions (Nota et al., 2019). A computer transmits a surgeon’s hand movements to activate precise movement of the robotic instruments in the operative field of the patient. The ergonomics of robotic surgery have made it possible to perform many more cancer surgeries with a minimally invasive approach. Even highly complex procedures such as pancreatic, liver, and esophageal resections have been performed with robotic assistance, although most of these surgeries are still done with a traditional open technique. While minimally invasive approaches, including the robotic platform, have revolutionized cancer surgery with reduced morbidity, questions remain regarding cost effectiveness, and therefore expansion to new indications is proceeding cautiously (Hwang and Hunt, 2020; Sheetz and Dimick, 2019).
Elimination of Surgery for Cancers Treated with Neoadjuvant Therapy
Many solid tumors are increasingly being treated with neoadjuvant therapy before surgical resection, including chemotherapy, biologic therapies (e.g., immunotherapy or targeted therapies), and possibly radiation therapy. The recent development of more effective neoadjuvant therapies has resulted in the downstaging of some cancers on the basis of preoperative imaging showing a complete disappearance of the tumor (that is, a pathologic complete response). Surgical excision can confirm a pathologic complete response, which is associated with improved long-term outcomes for various cancers, including breast cancer, rectal cancer, and lung cancer. Whether surgery can be omitted safely in patients with apparent complete response after neoadjuvant therapy depends on cancer-related and patient factors. For some patients with lung cancer, chemoradiation without surgery is considered the definitive treatment. Breast cancer patients with triple-negative or HER2-amplified tumors who have a complete response after neoadjuvant chemotherapy (“exceptional responders”) may be eligible for clinical trials to determine whether surgery can be omitted (Kuerer et al., 2018; van der Noordaa et al., 2018). Similarly, the “watch and wait” strategy for rectal cancer patients will be evaluated by the International Watch & Wait Database registry, which includes more than 1,000 patients who achieved a complete response after neoadjuvant therapy (van der Valk et al., 2018).
RADIATION
Many advances in radiation oncology in recent years have been largely due to an improved understanding of cancer biology and technological innovations in radiation delivery. Several of these advances—including three-dimensional conformal radiation therapy, intensity-modulated radiation therapy, hypofractionation, and stereotactic body radiation therapy—are discussed in Chapter 4. This section presents additional emerging treatments that are currently under active investigation.
Alternative Radiation Modalities
Most external beam radiation therapy is delivered using high-energy photons (see Chapter 4). While photons deposit the maximum dose of radiation at a certain depth of tissue, a small amount of radiation dose is absorbed by healthy tissue along the entire path of the beam before it reaches the targeted tumor (entrance radiation dose) and after it reaches the targeted tumor (exit radiation dose). On the other hand, charged particle therapy using protons and carbon ions, has very little exit radiation dose; the charged particles enter the tumor and then stop. Thus, compared with photon therapy, proton therapy and carbon ion therapy have the advantage of protecting adjacent organs or soft tissues from receiving radiation, which could lead to fewer short-term and long-term effects. Although they have been in existence for several decades, the cost of these charged-particle therapies limits their use. The cost to build a charged-particle facility ranges from $20 to $200 million, and the cost per treatment is about two to three times that of conventional photon therapy (Durante et al., 2017). Therefore, access to these types of treatments is limited to relatively few centers in high-income countries. As of September 2020, 90 proton facilities and 12 carbon-ion facilities existed worldwide, of which 37 proton facilities and no carbon ion facilities were located in the United States (PTCOG, 2020).
Proton Therapy
Currently proton therapy has a major, well-defined role in the treatment of pediatric malignancies, especially brain tumors (Greenberger and Yock, 2020; Miralbell et al., 2002; St Clair et al., 2004). Protons are also accepted as superior to photons in rare diseases where the ability to deliver high doses of radiation to the treatment target is limited by critical structures, particularly the optic nerve, the brain stem, and the spinal cord. Uveal melanoma (Courdi et al., 1999; Munzenrider, 1999) and a type of sarcoma called chordomas (Isacsson et al., 1997; Pai et al., 2001; Terahara et al., 1999) are examples of diseases where proton therapy has an advantage over photon therapy.
Outside of these contexts, the use of proton therapy in adult malignancies is a topic of much debate and controversy. For example, the majority of patients outside of the United States who receive proton therapy have prostate cancer (Martin and D’Amico, 2014) despite the lack of prospective data to support the superiority of proton therapy over photon therapy for these patients. Two large comparative effectiveness studies have shown no reductions in toxicity with protons compared with photons (Sheets et al., 2012; Yu et al., 2013). However, numerous international clinical research trials comparing these two approaches in a variety of cancers are currently under way. If these studies demonstrate that protons are superior to photons, then proton-based radiation therapy may become a standard of care in the future for specific types of cancer.
Carbon Ion Therapy
The physical properties of carbon ions have some potential advantages over protons, including providing higher doses to targets while reducing irradiation to organs at risk. The damage caused by carbon ions is clustered in the DNA, where it overwhelms the cellular repair systems (Malouff et al., 2020). There has been much less clinical experience with carbon ion radiotherapy than with proton radiation therapy, and, as such, investigators from the National Institute of Radiological Sciences in Japan concluded that randomized clinical trials are needed to determine if carbon ion radiotherapy is superior to photon or proton radiotherapy (Kamada et al., 2015). Numerous clinical trials comparing carbon ion therapy with proton or photon therapies are currently under way.
FLASH Radiotherapy
Daily radiation treatments are generally delivered in periods on the order of minutes (e.g., 2 Gy/minute). FLASH radiation therapy delivers radiation at an ultra-high dose rate, nearly instantaneously, at a rate that is on the order of 100 Gy/second (Montay-Gruel et al., 2018). In preclinical studies, FLASH has been shown to achieve the same tumor control efficacy per dose as conventional radiation therapy without damaging the surrounding normal tissue (Favaudon et al., 2014; Montay-Gruel et al., 2017), which is an important advantage. Clinical trials are currently under development to investigate FLASH radiation on a larger scale, and, if successful, this approach could revolutionize radiation treatments in the coming decades.
SYSTEMIC THERAPIES
In Chapter 4 the committee presents an overview of standard systemic therapies, which include chemotherapy, endocrine therapy, targeted therapy,
immunotherapy, and stem cell therapy. In this chapter some examples of the latest developments in targeted therapies, antibody drug conjugates, and immunotherapies are highlighted. New chemotherapy or endocrine therapy drugs are not discussed individually, as new drugs in these categories have characteristics and uses that are similar to FDA-approved medications that are already the standard of care. The committee notes that the systemic therapies discussed in this section are not exhaustive and that new cancer therapies are approved by FDA on a regular basis. Newly approved therapies can be found on the FDA website (drugs@fda) and more comprehensive lists can also be found on the National Cancer Institute websites for specific cancers (www.nci.nih.gov).
Targeted Therapies
Targeted therapies typically act on biomarkers (often genes, proteins, or enzymes) that are abnormal in a tumor. The nature and effectiveness of targeted therapies in treating cancer is briefly discussed in Chapter 4; in this section the focus is on therapies targeted to specific gene mutations or cellular pathways (see Table 8-1). Many targeted therapies are approved for one indication (and thus standard of care) while being emerging therapies for other indications. For example, multiple poly (ADP-ribose) polymerase (PARP) inhibitors are FDA approved and standard of care for the treatment of metastatic breast cancer associated with BRCA1/2 germline mutations; however, PARP inhibitors are emerging as a treatment of cancers associated with other germline mutations (such as PALB2) and as adjuvant therapy for breast cancer. The National Cancer Institute maintains a list of targeted therapies and the cancers for which they are approved by FDA (NCI, 2020).
PARP Inhibitors
PARP inhibitors have several putative mechanisms of action. Tumors in which there is a defect in homologous DNA repair, such as those associated with mutations in BRCA1 and BRCA2, appear particularly susceptible to PARP inhibitors (Domchek et al., 2020). Randomized phase III clinical trials have demonstrated a clinical benefit of PARP inhibitors in tumors associated with germline mutations in BRCA1 and BRCA2. FDA has approved the PARP inhibitors talazoparib and olaparib in BRCA1/2associated metastatic breast cancer (Litton et al., 2018; Robson et al., 2017) and olaparib in metastatic pancreatic cancer (Golan et al., 2019). Multiple PARP inhibitors (niraparib, olaparib, rucaparib) have been approved for use in ovarian cancer with and without BRCA1/2 mutations (Coleman et al., 2017; Mirza et al., 2016; Moore et al., 2018). FDA
TABLE 8-1 Selected FDA-Approved Targeted Therapies
| Drug Target | Drug | Cancer Type |
|---|---|---|
| PARPa,b | Talazoparib, olaparib niraparib, olaparib, rucaparib | Metastatic breast cancer, ovarian cancer, prostate cancer, metastatic pancreatic cancer |
| CDK4/6a,b | Palbociclib, ribociclib, abemaciclib | Breast cancer |
| Driver Gene | Drug | Cancer Type |
| EGFRa,b | Gefitinib, erlotinib, afatinib, dacomitinib, osimertinib | Lung cancer |
| HER2a,b | Trastuzumab, pertuzumab, ado-trastuzumab emtansine, lapatinib, neratinib, tucatinib | Breast cancer, gastric cancer |
| ALKa,c | Crizotinib, ceritinib, alectinib, lorlatinib | Lung cancer |
| NTRKa | Larotrectinib, entrectinib | Multiple cancers |
| BCR-ABLa | Imatinib, dasatinib, nilotinib, bosutinib, ponatinib | Chronic myelogenous leukemia, gastrointestinal stromal tumor |
| BRAFa,c | Vemurafenib, dabrafenib | Melanoma, lung cancer |
| ROS1c | Crizotinib, lorlatinib | Lung cancer |
| RETc | Selpercatinib | Thyroid cancer, lung cancer |
| METc | Capmatinib | Lung cancer |
| Flt3LGd | Gilteritinib, midostaurin | Acute myelogenous leukemia |
| PIK3CAb | Alpelisib | Breast cancer |
a Denotes targeted therapies discussed in more detail in this chapter.
b Also discussed in Chapter 5, Breast Cancer.
c Also discussed in Chapter 6, Lung Cancer.
d Also discussed in Chapter 7, Selected Topics in Other Cancers.
SOURCE: NCI, n.d.
recently approved olaparib and rucaparib for BRCA1/2-mutation-associated metastatic prostate cancer. A recent phase II study found that olaparib was effective for patients with metastatic breast cancer who had germline PALB2 mutations or somatic BRCA1/2 mutations, expanding the population of patients with breast cancer who might benefit from PARP inhibitors beyond germline BRCA1/2 mutation carriers (Tung et al., 2020). Multiple studies are ongoing to determine which other cancers (beyond ovarian cancer and those associated with BRCA1/2 mutations) might be susceptible to PARP inhibitors, either alone or in combination with other medications. PARP inhibitors are oral medications, and patients may be on them for extended periods, even years. Toxicities associated with PARP inhibitors include nausea, fatigue, decreased blood counts, and rarely, leukemia.
CDK4/6 Inhibitors
FDA has approved cyclin-dependent kinase (CDK) 4/6 inhibitors in combination with endocrine therapy for both first-line and second-line treatment of hormone-receptor-positive, HER2-negative advanced or metastatic breast cancer. Cyclin-dependent kinase is an enzyme important in cell proliferation, and these drugs work by targeting the cell-cycle machinery. CKD4/6 inhibitors such as palbociclib, ribociclib, and abemaciclib are taken orally and in combination with endocrine therapies such as aromatase inhibitors, tamoxifen, and fulvestrant (see Chapter 5). Multiple studies have demonstrated a significant progression-free survival advantage of CDK4/6 inhibitors plus endocrine therapy compared with endocrine therapy alone (Cristofanilli et al., 2016; Goetz et al., 2017; Johnston et al., 2019; Turner et al., 2015, 2018). Patients can be on CDK4/6 inhibitors for a prolonged period of time, with median progression-free survival in the range of 2 years. A recent study has demonstrated a benefit of using adjuvant abemaciclib for breast cancer, although abemaciclib is not yet FDA approved for this indication. CDK4/6 inhibitors are also under investigation for several other cancers and thus are considered to be an emerging therapy.
Human Epidermal Growth Factor Receptors
The human epidermal growth factor receptor (HER) proteins comprise a family of four members (HER1, HER2, HER3, and HER4) that regulate many functions of cells in the body in response to chemical signals in the blood. The abnormal regulation of HER signaling through gene mutation or amplification (where cells carry extra copies of the gene) drives abnormal cell growth that contributes to the development of a number of cancers, such as breast, lung, and gastric cancers. Drugs that block these abnormal signals, mainly monoclonal antibodies and oral agents, can be used to treat cancers that are driven by HER protein abnormalities.
Epidermal Growth Factor Receptor
The first two members of the HER family proteins to be discovered—HER1, more commonly known as epidermal growth factor receptor (EGFR) protein, and HER2, commonly referred to as HER2/neu—are also the most important. Mutations in EGFR are now known to drive the growth a number of cancers, particularly lung cancer, where EGFR mutations are present in approximately 10% of all advanced non-small-cell lung cancer (NSCLC) patients and in about 40% of patients who are never-smokers (Pao et al., 2004). A number of drugs that are very effective against cancers that have
EGFR mutations have been developed. The so-called first-generation inhibitors (because they were the first generation of drugs to be introduced in the clinic), gefitinib and erlotinib, shrink tumors in approximately 60% (the response rate) of patients, as compared with regular chemotherapy, which has a response rate of approximately 25%. However, resistance to first-generation EGFR inhibitors develops in a median of 10–12 months, at which point these drugs become ineffective (Rosenberger et al., 2016).
A major advance in lung cancer research has been the discovery of a common resistance mechanism to these first-generation agents. This discovery has led to the development of third-generation EGFR inhibitors with significant activity against the most common resistance mutation. In metastatic NSCLC harboring sensitive EGFR mutations, the third-generation EGFR inhibitor osimertinib improves the median survival of patients from 31.8 months with the first-generation inhibitors to 38.6 months with third-generation inhibitors (Ramalingam et al., 2020). By comparison, patients with EGFR-mutant tumors who were treated with chemotherapy alone have a median overall survival of approximately 4 months (Mok et al., 2017).
HER2/neu
The second member of the HER family to be discovered, HER2/neu, is amplified in some breast cancers and drives their growth. More than a decade ago a monoclonal antibody against HER2/neu, trastuzumab, was introduced in the clinic for breast cancer and revolutionized the care of these patients, significantly prolonging survival compared with systemic chemotherapy (McKeage and Perry, 2002). This agent blocks the growth signals that are produced by abnormal HER2/neu; it is also approved for the treatment of gastric cancers that express HER2/neu. A major problem of the targeted agents is that after continuous prolonged inhibition of the signaling protein, tumors develop resistance, and the agents lose their effectiveness. Current technology has led to the development of second- and third-generation inhibitors to overcome that resistance. In the case of HER2/neu, the second antibody to be developed was pertuzumab, which inhibits the binding of HER2 with other HER receptors and thus blocks signaling. In recent years ado-trastuzumab emtansine has been introduced into the clinic. Ado-trastuzumab emtansine is an antibody–drug conjugate consisting of trastuzumab chemically linked to the chemotherapy agent mertansine, which is so poisonous that it cannot be given directly to patients. Ado-trastuzumab emtansine is active in patients whose tumors have developed resistance to trastuzumab (Verma et al., 2012).
Anaplastic Lymphoma Kinase
The anaplastic lymphoma kinase (ALK) gene produces a protein, ALK, which is normally present in the nervous system during fetal growth but absent in adult tissues. However, in adults the ALK gene can fuse with portions of other genes (a process called gene fusion), transforming the protein product to an activated protein that can drive the growth of a number of cancers, including NSCLC and lymphoma. ALK gene fusions have been identified in about 3.4% of cases of adenocarcinoma NSCLC (Elliott et al., 2020). These cancers are very sensitive to ALK inhibitors. A meta-analysis of studies on patients with ALK-positive NSCLC found that the 12-month survival rate ranged from 70% to 86% for ALK inhibitors and 67% to 79% for chemotherapy (Elliott et al., 2020). Similar to the case with other cancers whose growth is driven by a known genetic abnormality that can be targeted with a drug, the development of resistance to ALK inhibitors is an ongoing problem. In order to combat resistant tumors, there are first-(crizotinib), second- (ceritinib, brigatinib), and third- (alectinib, lorlatinib) generation ALK inhibitors, which are all very active in these cancers. The availability of all these oral inhibitors means that patients can be treated for a number of years sequentially with targeted agents before traditional systemic chemotherapy is used. A recent study demonstrated the superiority of ALK inhibitors over chemotherapy for metastatic NSCLC, with more than 50% of patients receiving ALK inhibitors alive at 6.8 years, compared with only 2% of those receiving chemotherapy alive at 5 years (Pacheco et al., 2019). These findings highlight the significant impact of targeted therapies on the survival of NSCLC patients whose tumors have a targetable gene abnormality. As shown in Table 8-1, there are now a large number of oncogene products driving lung, breast, and other cancers that are targeted by effective agents.
Neurotrophic Tyrosine Receptor Kinase Inhibitors
The neurotrophic receptor tyrosine kinase (NTRK) genes NTRK1, NTRK2, and NTRK3 encode the tropomyosin receptor kinase (TRK) proteins TRKA, TRKB, and TRKC, respectively (Ma, 2019). These receptors play a role in the peripheral and central nervous systems, primarily to regulate pain, proprioception, appetite, and memory.
TRK-mediated cancers are rare (<1%) (Joshi et al., 2020); however, these receptor fusions have been identified in a wide variety of common adult and pediatric cancers, including cancers of the brain, breast, colon and rectum, bile ducts, lung, and pancreas as well as melanoma and sarcoma. Interestingly, NTRK fusions are highly expressed in some rare
cancer types such as infantile fibrosarcoma, mammary analog secretory carcinoma of the salivary gland, and secretory breast cancer (Ma, 2019). Alterations in these TRK receptors have recently emerged as promising therapeutic targets in solid tumors as inhibitors have been developed for them (Joshi et al., 2020).
Two kinase inhibitors of NTRK fusions, larotrectinib and entrectinib, have shown significant antitumor activity against all three kinase proteins, regardless of tumor type or patient age (Ma, 2019). In a study of larotrectinib, response rates of 80% were achieved, and 71% of all treated patients (aged 4 months to 76 years) with many different advanced cancers—lung, breast, colon, pancreas, thyroid, gall bladder, and sarcoma—were alive after 1 year (Drilon et al., 2018). The drug is well tolerated, and no patient left the trial as a result of drug-related adverse effects. These agents went into clinical trials in 2017. In 2018, FDA granted tissue-agnostic, accelerated approval for larotrectinib for adult and pediatric patients with advanced solid tumors that have an NTRK fusion without a known resistance mutation (FDA, 2020b). These gene fusions can be in the NTRK1, NTRK2, or NTRK3 genes. This drug marks a milestone in the approach to cancer therapy since this represents the first example of a targeted agent approved for the therapy of all cancers for all age groups regardless of the tissue of origin as long as the cancer has a specific genetic abnormality (Federman and McDermott, 2019). In 2019, FDA also granted accelerated approval for a second agent in this class, entrectinib (FDA, 2019a).
Other Targeted Therapies
As noted in Chapter 4, targeted therapies can act in a number of ways, including preventing the proliferation of mutant proteins in cancer cells and identifying chromosome aberrations that produce fusion genes and thus fusion proteins. Examples of a mutant protein include the altered cell growth signaling protein BRAF V600E present in many melanomas and BCR-ABL, made from pieces of two genes that get joined together in some leukemia cells and that promotes their growth. Other examples of targeted therapies are given in Table 8-1.
One of the most common cancer-causing genes is KRAS. A specific mutation in KRAS, the G12C mutation, is currently being targeted by a number of drugs in clinical trials. The KRAS inhibitors that are furthest along in development are sotorasib and MRTX849. Initial results demonstrate striking activity in NSCLC, although these agents are in trials for all solid tumors that have KRAS G12C mutations.
Antibody–Drug Conjugates
Antibody–drug conjugates represent a new class of targeted agents in which a highly toxic chemotherapy agent that cannot be infused directly into patients is linked to a monoclonal antibody that recognizes proteins on the cancer cells; in this way the chemotherapy is preferentially delivered to the cancer in high concentrations. This can potentially increase the effectiveness of the chemotherapy and reduce its toxicity. As proteins that are expressed preferentially on a large number of cancer cells are identified and antibody engineering and the design of chemicals that can safely link antibodies to chemotherapy drugs are further refined, it is expected that antibody–drug conjugates will become increasingly common cancer therapies. FDA has approved several antibody–drug conjugates for the treatment of a variety of cancers. In 2019, FDA approved ado-trastuzumab emtansine for the adjuvant treatment of HER2-positive early breast cancer (FDA, 2019b), and in 2020, it approved tafasitamab-cxix and lenalidomide to treat large B-cell lymphoma and sacituzumab govitecan-hziy to treat metastatic triple-negative breast cancer (FDA, 2020c). Several other antibody–drug conjugates have been approved by FDA over the past decade.
Immunotherapy
As discussed in Chapter 4, immunotherapy harnesses the body’s own immune system to recognize cancer cells as foreign and release chemicals called cytokines which kill these cancer cells. Cancer immunotherapy can be broadly classified into the following categories: cancer vaccines, adoptive cellular therapy, and immune checkpoint inhibitors. As a class of therapy, immunotherapy will transform how cancer is treated in the future. The main immunotherapy approaches are described below.
Vaccines
Cancer vaccines can be preventive or therapeutic. Preventive vaccines inhibit the growth of organisms that can cause cancer via infection and by so doing prevent the development of that cancer. Examples are vaccines against the human papillomavirus (HPV) and hepatitis B virus. Three vaccines are approved by FDA—human papillomavirus bivalent (types 16 and 18) vaccine, recombinant; human papillomavirus quadrivalent (types 6, 11, 16, 18) vaccine, recombinant; and human papillomavirus 9-valent vaccine, recombinant—that protect against infection from different types of HPV and that can help prevent the development of HPV-related anal, cervical, head and neck, penile, vulvar, and vaginal cancers. Hepatitis B vaccine
(recombinant), adjuvanted protects against infection with the hepatitis B virus and can prevent the development of hepatitis B-related liver cancer.
Therapeutic tumor vaccines are designed to generate a specific active immune response against a patient’s tumor. An example is the bacillus Calmette-Guérin vaccine, which uses weakened bacteria to stimulate the immune system to fight cancer. It is approved for patients with early-stage bladder cancer. A second vaccine is sipuleucel-T. Individuals have immune cells (antigen-presenting cells) that present foreign proteins to the immune system so that it can mount an attack whenever that foreign protein is encountered, thus protecting against infection. The most important antigen-presenting cell is the dendritic cell. Sipuleucel-T is approved for prostate cancer patients who have only minimal symptoms. There are a large number of therapeutic vaccines in clinical trials, and it is likely that a number of these candidate vaccine therapies will be approved for cancer treatment in the coming years.
Adoptive Cellular Therapies
In this type of immunotherapy, T cells, a type of immune cell that fights cancer, are removed from the patients’ body, grown in very large numbers in the laboratory, and then infused back into the patient to assist the immune system in killing cancer cells. Examples of adoptive cell therapy include tumor-infiltrating lymphocytes and chimeric antigen receptor (CAR) T-cell therapy.
Tumor-Infiltrating Lymphocytes Therapy
In this therapy, T cells in the tumor called tumor-infiltrating lymphocytes are removed from the patient. Those cells that are better able to attack the cancer are identified and expanded in the laboratory so that large quantities can be infused back into the patient to attack the cancer. This treatment has been more commonly used with melanoma and is still experimental. A major problem is that the isolation and expansion of T-reactive lymphocytes is not successful in all melanomas or in a number of solid tumors that do not have many tumor-infiltrating lymphocytes in them.
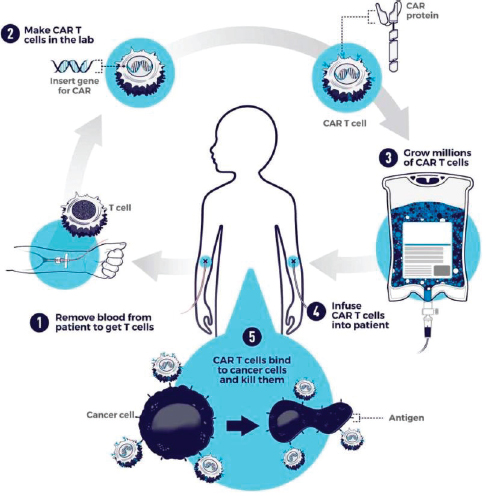
Chimeric Antigen Receptor (CAR) T-Cell Therapy
The principles behind CAR T-cell therapy2 are similar to the tumor-infiltrating therapy described above. CAR T-cell therapy is a newer
___________________
2 CAR T-cells are special receptor cells that are created in the laboratory and designed to bind to certain proteins on cancer cells. See NCI Dictionary of Cancer Terms, https://www.cancer.gov/publications/dictionaries/cancer-terms/def/car (accessed October 9, 2020).
immunotherapy that may be used for some patients with recurrent and refractory leukemia or lymphomas. Currently, CAR T-cell therapy is FDA approved for the treatment of recurrent or refractory B-cell non-Hodgkin lymphoma in adults and for acute lymphoblastic leukemia in people aged 25 years or less. For patients whose cancers recur after an autologous hematopoietic stem cell transplant or for those patients whose cancer no longer responds to traditional chemotherapies, CAR T-cell therapy offers potential long-term disease control. Its use as a treatment for solid tumors is being explored in clinical trials.
As illustrated in Figure 8-2, in this treatment a patient’s own T-cell lymphocytes are removed from the body and then genetically modified in a laboratory to express synthetic CAR proteins. This improves the ability of these T cells to identify and attack the cancer cells more efficiently. The modified T cells are then infused back into the patient as treatment for the cancer (Feins et al., 2019).
SOURCE: NCI, 2018.
This procedure may be done at specialized facilities after the patient has received chemotherapy to prepare the body for the infusion. After the infusion, patients are closely monitored, as CAR T-cell therapy is associated with potential acute life-threatening toxicities, including neurological events and cytokine release syndrome (a systemic response to CAR T cells with fever, hypotension, and change in mental status). Patients may require prolonged hospital stays, including in the intensive care unit, for treatment of these acute toxicities. At present, there are few data about the potential long-term complications and late-onset effects of this therapy.
Immune Checkpoint Inhibition
Immune checkpoint proteins are produced by the body and regulate the immune system by dampening the immune response to an immunologic stimulus, thus preventing the activated immune system from attacking and damaging normal cells. An example of an unchecked activated immune system is the development of an autoimmune disease such as rheumatoid arthritis.
Immune checkpoints are a normal protective pathway of the immune system to prevent immune cells like T cells from destroying normal cells. This normal immune suppression occurs when a programmed cell death-1 receptor (PD-1), which is a protein expressed on the cell surface of certain immune cells, binds with its ligand, programmed cell death ligand-1 (PDL1), which is present on many normal cells. When PD-1 binds to PD-L1, the T cell is “switched off” and leaves the other cell alone. A number of cancers stimulate these immune checkpoints to protect themselves from the immune system, leading to immune tolerance. Cancer cells can hijack immune checkpoints by expressing PD-L1 on their cell surface to switch T cells off, preventing T cells from recognizing and attacking the cancer cells. When this protein is blocked, the “brakes” on the immune system are released, and the ability of T cells to kill cancer cells is increased. Immune checkpoint inhibitors have emerged as an increasingly important cancer treatment. These checkpoint inhibitors target either PD-1 or PD-L1 and prevent their binding, effectively allowing the immune cells to destroy the cancer cell.
The most prominent of these checkpoints are cytotoxic T-lymphocyte associated protein 4 (CTLA4), programmed cell death protein-1 (PD-1), and programmed cell death ligand-1(PD-L1). Monoclonal antibodies3 in-
___________________
3 A monoclonal antibody is a type of protein made in the laboratory that can bind to substances in the body, including cancer cells. They can be used alone or to carry drugs, toxins, or radioactive substances directly to cancer cells. See Dictionary of Cancer Terms, https://www.cancer.gov/publications/dictionaries/cancer-terms/def/monoclonal-antibody (accessed October 9, 2020).
hibiting these proteins have been approved for use in a large variety of cancers and have revolutionized cancer therapy in the last decade. One CTLA4 inhibitor, ipilimumab, and six PD-1/PD-L1 inhibitors have been approved for treatment of a large number of cancers (see Table 8-2).
The most widely used immune checkpoint inhibitors are the PD-1 and PD-L1 inhibitors. These agents are leading to prolonged survival in some patients with advanced cancers, and there is a potential for cure in some cases. An ongoing issue is the toxicity of these compounds. Immune-related adverse effects, which occur in a minority of patients, can affect any organ in the body and can be fatal (Brahmer et al., 2018). In addition, chronic complications from the long-term use of these agents are still largely unknown. Currently, advanced cancer patients who are tolerating the PD-1/PD-L1 agents and receiving benefit are treated for 2 years, but it is unclear whether this represents the optimal duration of treatment. Ongoing and future studies will be aimed at combining the immune checkpoint inhibitors
TABLE 8-2 FDA-Approved Immune Checkpoint Inhibitors for Cancer
| Drug | Cancer Indication | |
|---|---|---|
| CTLA4 Inhibitors | ||
| Ipilimumab | Melanoma, renal cell carcinoma, microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer (combination with nivolumab) | |
| PD-1 Inhibitors | ||
| Cemiplimab | Squamous cell skin cancer | |
| Nivolumab | Melanoma, non-small-cell lung cancer, small-cell lung cancer, renal cell carcinoma, classical Hodgkin lymphoma, squamous cell carcinoma of the head and neck, urothelial carcinoma, microsatellite instability-high colorectal cancer, hepatocellular carcinoma | |
| Pembrolizumab | Melanoma, non-small-cell lung cancer, small-cell lung cancer, head and neck squamous cell carcinoma, classical Hodgkin lymphoma, primary mediastinal large B-cell lymphoma, urothelial carcinoma, microsatellite instability-high cancers, gastric cancer, esophageal cancer, cervical cancer, hepatocellular carcinoma, Merkel cell carcinoma, renal cell carcinoma, endometrial carcinoma, metastatic breast cancer | |
| PD-LI Inhibitors | ||
| Atezolizumab | Urothelial carcinoma, non-small-cell lung cancer, triple-negative breast cancer, small-cell lung cancer | |
| Avelumab | Merkel cell carcinoma, urothelial carcinoma; renal cell carcinoma (in combination with axitinib) | |
| Durvalumab | Urothelial carcinoma, non-small-cell lung cancer, small-cell lung cancer | |
SOURCE: Information from Vaddepally et al., 2020.
with therapeutic approaches that enhance their activity through different mechanisms. The areas of active investigation include making the tumor more immunogenic (increasing the likelihood that the immune system will recognize the tumor) and blocking different pathways that inhibit the body’s ability to fight the cancer (Fong et al., 2020; Whiteside et al., 2016).
LONG-TERM OUTCOMES OF NEW AND EMERGING THERAPIES
There is a wide array of new and emerging treatment approaches for cancer described in this chapter. The increasing focus on tumor-specific (oncogene/mutation) targeted therapies, as well as the increasing use of immunotherapy (which is used independent of specific tumor type), will likely lead to new combinations of therapies (multimodal therapies) yet to be defined. These will all be subjected to clinical trials which will provide information about how best to deliver treatment combinations to patients. Although patients with more advanced cancer will be the first recipients of these new combinations, successful treatments and their combinations will be rapidly incorporated into the management of patients with earlier-stage disease, many of whom may be expected to have long-term survival. In some diseases where surgical cure is rare, such as pancreatic, gastric, and esophageal cancers, neoadjuvant treatment combinations will likely be experimentally tested, and improvements in survival may occur with the earlier use of effective systemic therapies. These approaches are now standard in some cancers, such as breast cancer and osteosarcomas. Complex multimodal treatments involving combinations of systemic therapy, surgery, and radiation will also be part of these new emerging strategies and will likely only be carried out at major medical care centers. Improved survival will be the primary outcome of interest if these treatments are adopted; however, the morbidity for these types of approaches may be considerable, leading to long-term impairment even with potential cure.
There are also risks for unknown long-term toxicities when these new and emerging treatments are given for long periods (i.e., many years). For patients with advanced or widespread disease, these treatments are often given for long durations, and the short-term clinical trials that demonstrated initial efficacy seldom provide information about the persistent toxicities in long-term survivors. As an example, patients with chronic myelogenous leukemia who are on long-term oral kinase inhibitors often have fatigue and other generalized symptoms that limit their functioning as well as more serious and potentially life-threatening adverse effects (Caldemeyer et al., 2016). Although the cancer is under control, adverse effects from the long-term treatments can adversely affect an individual’s functioning. As new treatments emerge for other diseases, what are often described as “off target” effects from the treatment may occur, which means that while
the cancer target is successfully treated, secondary effects on other normal tissues can lead to chronic adverse effects.
Another consideration is that most clinical trials of new treatments recruit younger patients and those with no or few comorbid conditions. With approval based on efficacy and the lack of serious adverse effects, the assessment of long-term tolerability is absent from these studies. There are increasing calls for the testing of all new treatments in the patient population destined to receive it (Kim et al., 2017). Furthermore, because of the greater efficacy of targeted therapies, trial enrollment is often much smaller, and this means that when a drug is finally approved, little will be known about its adverse effect profile in the general population, as some adverse events may be quite rare. These limitations mean that there will be gaps in knowledge about what to expect in terms of both short- and long-term toxicity and how these may affect the patient’s ability to work and carry out usual activities.
In contrast, some of the emerging treatment approaches in surgery and radiation therapy may provide benefit in terms of reducing treatment-associated morbidities and thus allow for improved function and survival after treatment. Overall, all disciplines involved in the treatment of cancer are investigating and evaluating ways to reduce the impairments associated with treatment, which can be compounded in the multimodal setting described earlier. Avoiding unnecessary treatments is one strategy; giving courses of treatment that are likely to reduce or eliminate late toxicities (e.g., avoiding radiation treatments to the heart to protect against injury) is another. However, at the beginning of any new treatment strategy, clinical researchers may not know what to expect in the long term, and patients may have difficulties that arise many years after treatment ends.
FINDINGS AND CONCLUSIONS
Findings
- Advances in the treatment of cancer have occurred at an increasing pace in the past two decades and are leading to longer survival, particularly for patients with metastatic cancer, often using greater treatment precision and personalization of treatment plans.
- New surgical approaches such as minimally invasive techniques have been developed to reduce adverse effects related to surgery.
- New radiation techniques are focused on delivery strategies that may reduce acute and long-term adverse effects.
- The new systemic treatments that have been developed include targeted therapies, antibody-drug conjugates, and immunotherapies.
- Some of these new treatments, such as immunotherapy, are agnostic as to cancer type.
- Because cancer is a heterogeneous disease, targeted therapies may have application to only a small set of cancer types, while treatments that focus on enhancing the immune system may have more widespread application for a variety of cancer types.
- Many targeted therapies, such as PARP inhibitors, are approved for one indication (and thus standard of care) while being emerging therapies for other cancers. There are several types of targeted therapies that have different modes of action; however, many targeted agents lose their effectiveness over time, motivating the development of second- and even third-generation inhibitors.
- Antibody-drug conjugates, in which a highly toxic chemotherapeutic agent is linked to a monoclonal antibody, may improve the effectiveness of the chemotherapeutic agent and reduce its toxicity. Several such conjugates have been approved by FDA as of early 2020.
- Many new systemic therapies are harnessing the patient’s own immune system to control or eradicate cancer. Immunotherapy for cancer can take the form of preventive vaccines such as human papilloma virus and hepatitis B, both of which are associated with several cancers; therapeutic vaccines that activate the immune response against a patient’s tumor; adoptive cellular therapies such as chimeric antigen receptor T-cell therapy; and immune checkpoint inhibitors such as programmed cell death protein-1.
Conclusions
- Immunotherapies represent the most important and transformative classes of new and emerging cancer treatments, as these treatments can lead to prolonged disease-free survival for some cancers that respond poorly to conventional treatments.
- Although many new and emerging cancer therapies are improving survival, their long-term and late-onset effects are poorly understood.
- Some newer treatment approaches are focused on reducing or correcting the toxicity and morbidity of standard treatments (e.g., reduction in the extent of axillary surgery, less invasive surgical procedures, or use of proton radiation therapy). In time, these may become standard of care as their effectiveness and safety become more evident.
REFERENCES
Ahmed, M., I.T. Rubio, T. Kovacs, V.S. Klimberg, and M. Douek. 2016. Systematic review of axillary reverse mapping in breast cancer. British Journal of Surgery 103(3):170–178.
Alliance for Clinical Trials in Oncology. 2019. Axillary reverse mapping in preventing lymphedema in patients with breast cancer undergoing axillary lymph node dissection. https://clinicaltrials.gov/ct2/show/NCT03927027 (accessed September 24, 2020).
Boneti, C., S. Korourian, K. Bland, K. Cox, L.L. Adkins, R.S. Henry-Tillman, and V.S. Klimberg. 2008. Axillary reverse mapping: Mapping and preserving arm lymphatics may be important in preventing lymphedema during sentinel lymph node biopsy. Journal of the American College of Surgeons 206(5):1038–1042.
Boughey, J.C., M.D. Alvarado, R.B. Lancaster, W. Fraser Symmans, R. Mukhtar, J.M. Wong, C.A. Ewing, D.A. Potter, T.M. Tuttle, T.J. Hieken, J.M. Carter, J.W. Jakub, H.G. Kaplan, C.L. Buchanan, N.T. Jaskowiak, H.A. Sattar, J. Mueller, R. Nanda, C.J. Isaacs, P.R. Pohlmann, F. Lynce, E.A. Tousimis, J.C. Zeck, M.C. Lee, J.E. Lang, P. Mhawech-Fauceglia, R. Rao, B. Taback, M. Chen, K.M. Kalinsky, H. Hibshoosh, B. Killelea, T. Sanft, G.L. Hirst, S. Asare, J.B. Matthews, J. Perlmutter, and L.J. Esserman. 2018. Surgical standards for management of the axilla in breast cancer clinical trials with pathological complete response endpoint. NPJ Breast Cancer 4:26.
Brahmer, J.R., C. Lacchetti, B.J. Schneider, M.B. Atkins, K.J. Brassil, J.M. Caterino, I. Chau, M.S. Ernstoff, J.M. Gardner, P. Ginex, S. Hallmeyer, J. Holter Chakrabarty, N.B. Leighl, J.S. Mammen, D.F. McDermott, A. Naing, L.J. Nastoupil, T. Phillips, L.D. Porter, I. Puzanov, C.A. Reichner, B.D. Santomasso, C. Seigel, A. Spira, M.E. Suarez-Almazor, Y. Wang, J.S. Weber, J.D. Wolchok, and J.A. Thompson. 2018. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology 36(17):1714–1768.
Caldemeyer, L., M. Dugan, J. Edwards, and L. Akard. 2016. Long-term side effects of tyrosine kinase inhibitors in chronic myeloid leukemia. Current Hematologic Malignancy Reports 11(2):71–79.
Campbell, E.J., and L. Romics. 2017. Oncological safety and cosmetic outcomes in oncoplastic breast conservation surgery, a review of the best level of evidence literature. Breast Cancer 9:521–530.
Carter, S.A., G.R. Lyons, H.M. Kuerer, R.L. Bassett, Jr., S. Oates, A. Thompson, A.S. Caudle, E.A. Mittendorf, I. Bedrosian, A. Lucci, S.M. DeSnyder, G. Babiera, M. Yi, D.P. Baumann, M.W. Clemens, P.B. Garvey, K.K. Hunt, and R.F. Hwang. 2016. Operative and oncologic outcomes in 9861 patients with operable breast cancer: Single-institution analysis of breast conservation with oncoplastic reconstruction. Annals of Surgical Oncology 23(10):3190–3198.
Caudle, A.S., W.T. Yang, S. Krishnamurthy, E.A. Mittendorf, D.M. Black, M.Z. Gilcrease, I. Bedrosian, B.P. Hobbs, S.M. DeSnyder, R.F. Hwang, B.E. Adrada, S.F. Shaitelman, M. Chavez-MacGregor, B.D. Smith, R.P. Candelaria, G.V. Babiera, B.E. Dogan, L. Santiago, K.K. Hunt, and H.M. Kuerer. 2016. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: Implementation of targeted axillary dissection. Journal of Clinical Oncology 34(10):1072–1078.
Chang, E.I., M.V. Schaverien, and J.C. Selber. 2018. Lymphedema management. Seminars in Plastic Surgery 32(1):3–4.
Chang, J., and D.W. Rattner. 2019. History of minimally invasive surgical oncology. Surgical Oncology Clinics of North America 28(1):1–9.
Chung, A., A. Gangi, F. Amersi, S. Bose, X. Zhang, and A. Giuliano. 2015. Impact of consensus guidelines by the Society of Surgical Oncology and the American Society for Radiation Oncology on margins for breast-conserving surgery in stages 1 and 2 invasive breast cancer. Annals of Surgical Oncology 22(Suppl 3):S422–S427.
Clinical Outcomes of Surgical Therapy Study Group, H. Nelson, D.J. Sargent, H.S. Wieand, J. Fleshman, M. Anvari, S.J. Stryker, R.W. Beart, Jr., M. Hellinger, R. Flanagan, Jr., W. Peters, and D. Ota. 2004. A comparison of laparoscopically assisted and open colectomy for colon cancer. New England Journal of Medicine 350(20):2050–2059.
Clough, K.B., R.F.D. van la Parra, H.H. Thygesen, E. Levy, E. Russ, N.M. Halabi, I. Sarfati, and C. Nos. 2018. Long-term results after oncoplastic surgery for breast cancer: A 10-year follow-up. Annals of Surgery 268(1):165–171.
Cohen, N.A., and T.P. Kingham. 2019. Minimally invasive staging surgery for cancer. Surgical Oncology Clinics of North America 28(1):61–77.
Coleman, R.L., A.M. Oza, D. Lorusso, C. Aghajanian, A. Oaknin, A. Dean, N. Colombo, J.I. Weberpals, A. Clamp, G. Scambia, A. Leary, R.W. Holloway, M.A. Gancedo, P.C. Fong, J.C. Goh, D.M. O’Malley, D.K. Armstrong, J. Garcia-Donas, E.M. Swisher, A. Floquet, G.E. Konecny, I.A. McNeish, C.L. Scott, T. Cameron, L. Maloney, J. Isaacson, S. Goble, C. Grace, T.C. Harding, M. Raponi, J. Sun, K.K. Lin, H. Giordano, J.A. Ledermann, M. Buck, A. Dean, M.L. Friedlander, J.C. Goh, P. Harnett, G. Kichenadasse, C.L. Scott, H. Denys, L. Dirix, I. Vergote, L. Elit, P. Ghatage, A.M. Oza, M. Plante, D. Provencher, J.I. Weberpals, S. Welch, A. Floquet, L. Gladieff, F. Joly, A. Leary, A. Lortholary, J. Lotz, J. Medioni, O. Tredan, B. You, A. El-Balat, C. Hänle, P. Krabisch, T. Neunhöffer, M. Pölcher, P. Wimberger, A. Amit, S. Kovel, M. Leviov, T. Safra, R. Shapira-Frommer, S. Stemmer, A. Bologna, N. Colombo, D. Lorusso, S. Pignata, R.F. Sabbatini, G. Scambia, S. Tamberi, C. Zamagni, P.C. Fong, A. O’Donnell, M.A. Gancedo, A.C. Herraez, J. Garcia-Donas, E.M. Guerra, A. Oaknin, I. Palacio, I. Romero, A. Sanchez, S.N. Banerjee, A. Clamp, Y. Drew, H.G. Gabra, D. Jackson, J.A. Ledermann, I.A. McNeish, C. Parkinson, M. Powell, C. Aghajanian, D.K. Armstrong, M.J. Birrer, M.K. Buss, S.K. Chambers, L.M. Chen, R.L. Coleman, R.W. Holloway, G.E. Konecny, L. Ma, M.A. Morgan, R.T. Morris, D.G. Mutch, D.M. O’Malley, B.M. Slomovitz, E.M. Swisher, T. Vanderkwaak, and M. Vulfovich. 2017. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet 390(10106):1949–1961.
Courdi, A., J.P. Caujolle, J.D. Grange, L. Diallo-Rosier, J. Sahel, F. Bacin, C. Zur, P. Gastaud, N. Iborra-Brassart, J. Herault, and P. Chauvel. 1999. Results of proton therapy of uveal melanomas treated in Nice. International Journal of Radiation Oncology, Biology, Physics 45(1):5–11.
Cristofanilli, M., N.C. Turner, I. Bondarenko, J. Ro, S.A. Im, N. Masuda, M. Colleoni, A. DeMichele, S. Loi, S. Verma, H. Iwata, N. Harbeck, K. Zhang, K.P. Theall, Y. Jiang, C.H. Bartlett, M. Koehler, and D. Slamon. 2016. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. The Lancet Oncology 17(4):425–439.
De La Cruz, L., S.A. Blankenship, A. Chatterjee, R. Geha, N. Nocera, B.J. Czerniecki, J. Tchou, and C.S. Fisher. 2016. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: A systematic literature review. Annals of Surgical Oncology 23(10):3247–3258.
DeSnyder, S.M., K.K. Hunt, W. Dong, B.D. Smith, M.S. Moran, M. Chavez-MacGregor, Y. Shen, H.M. Kuerer, and A. Lucci. 2018. American Society of Breast Surgeons’ practice patterns after publication of the SSO-ASTRO-ASCO DCIS consensus guideline on margins for breast-conserving surgery with whole-breast irradiation. Annals of Surgical Oncology 25(10):2965–2974.
Domchek, S.M., E. Mardis, J.W. Carlisle, and T.K. Owonikoko. 2020. Integrating genetic and genomic testing into oncology practice. American Society of Clinical Oncology Educational Book 40:e259–e263.
Donker, M., M.E. Straver, J. Wesseling, C.E. Loo, M. Schot, C.A. Drukker, H. van Tinteren, G.S. Sonke, E.J. Rutgers, and M.J. Vrancken Peeters. 2015. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: The MARI procedure. Annals of Surgery 261(2):378–382.
Drilon, A., T.W. Laetsch, S. Kummar, S.G. DuBois, U.N. Lassen, G.D. Demetri, M. Nathenson, R.C. Doebele, A.F. Farago, A.S. Pappo, B. Turpin, A. Dowlati, M.S. Brose, L. Mascarenhas, N. Federman, J. Berlin, W.S. El-Deiry, C. Baik, J. Deeken, V. Boni, R. Nagasubramanian, M. Taylor, E.R. Rudzinski, F. Meric-Bernstam, D.P.S. Sohal, P.C. Ma, L.E. Raez, J.F. Hechtman, R. Benayed, M. Ladanyi, B.B. Tuch, K. Ebata, S. Cruickshank, N.C. Ku, M.C. Cox, D.S. Hawkins, D.S. Hong, and D.M. Hyman. 2018. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. New England Journal of Medicine 378(8):731–739.
Durante, M., R. Orecchia, and J.S. Loeffler. 2017. Charged-particle therapy in cancer: Clinical uses and future perspectives. Nature Reviews Clinical Oncology 14(8):483–495.
Elliott, J., Z. Bai, S.C. Hsieh, S.E. Kelly, L. Chen, B. Skidmore, S. Yousef, C. Zheng, D.J. Stewart, and G.A. Wells. 2020. ALK inhibitors for non-small cell lung cancer: A systematic review and network meta-analysis. PLOS ONE 15(2):e0229179.
Favaudon, V., L. Caplier, V. Monceau, F. Pouzoulet, M. Sayarath, C. Fouillade, M.F. Poupon, I. Brito, P. Hupe, J. Bourhis, J. Hall, J.J. Fontaine, and M.C. Vozenin. 2014. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Science Translational Medicine 6(245):245ra293.
FDA (U.S. Food and Drug Administration). 2019a. FDA approves entrectinib for NTRK solid tumors and ROS-1 NSCLC. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc# (accessed October 14, 2020).
FDA. 2019b. FDA approves ado-trastuzumab emtansine for early breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ado-trastuzumab-emtansine-early-breast-cancer (accessed December 4, 2020).
FDA. 2020a. Advancing health through innovation: New drug therapy approvals 2019. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019 (accessed December 8, 2020).
FDA. 2020b. FDA approves companion diagnostic to identify NTRK fusions in solid tumors for Vitrakvi. https://www.fda.gov/drugs/fda-approves-companion-diagnostic-identify-ntrk-fusions-solid-tumors-vitrakvi (accessed December 29, 2020).
FDA. 2020c. Novel drug approvals for 2020. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020 (accessed October 14, 2020).
Federman, N., and R. McDermott. 2019. Larotrectinib, a highly selective tropomyosin receptor kinase (TRK) inhibitor for the treatment of TRK fusion cancer. Expert Reviews of Clinical Pharmacology 12(10):931–939.
Feins, S., W. Kong, E.F. Williams, M.C. Milone, and J.A. Fraietta. 2019. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. American Journal of Hematology 94(S1):S3–S9.
Fong, L., A. Hotson, J.D. Powderly, M. Sznol, R.S. Heist, T.K. Choueiri, S. George, B.G.M. Hughes, M.D. Hellmann, D.R. Shepard, B.I. Rini, S. Kummar, A.M. Weise, M.J. Riese, B. Markman, L.A. Emens, D. Mahadevan, J.J. Luke, G. Laport, J.D. Brody, L. Hernandez-Aya, P. Bonomi, J.W. Goldman, L. Berim, D.J. Renouf, R.A. Goodwin, B. Munneke, P.Y. Ho, J. Hsieh, I. McCaffery, L. Kwei, S.B. Willingham, and R.A. Miller. 2020. Adenosine 2A receptor blockade as an immunotherapy for treatment-refractory renal cell cancer. Cancer Discovery 10(1):40–53.
Garza, R.M., and D.W. Chang. 2018. Lymphovenous bypass for the treatment of lymphedema. Journal of Surgical Oncology 118(5):743–749.
Goetz, M.P., M. Toi, M. Campone, J. Sohn, S. Paluch-Shimon, J. Huober, I.H. Park, O. Tredan, S.C. Chen, L. Manso, O.C. Freedman, G. Garnica Jaliffe, T. Forrester, M. Frenzel, S. Barriga, I.C. Smith, N. Bourayou, and A. Di Leo. 2017. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. Journal of Clinical Oncology 35(32):3638–3646.
Golan, T., P. Hammel, M. Reni, E. Van Cutsem, T. Macarulla, M.J. Hall, J.-O. Park, D. Hochhauser, D. Arnold, D.-Y. Oh, A. Reinacher-Schick, G. Tortora, H. Algül, E.M. O’Reilly, D. McGuinness, K.Y. Cui, K. Schlienger, G.Y. Locker, and H.L. Kindler. 2019. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. New England Journal of Medicine 381(4):317–327.
Gray, R.J., C. Salud, K. Nguyen, E. Dauway, J. Friedland, C. Berman, E. Peltz, G. Whitehead, and C.E. Cox. 2001. Randomized prospective evaluation of a novel technique for biopsy or lumpectomy of nonpalpable breast lesions: Radioactive seed versus wire localization. Annals of Surgical Oncology 8(9):711–715.
Greenberger, B.A., and T.I. Yock. 2020. The role of proton therapy in pediatric malignancies: Recent advances and future directions. Seminars in Oncology 47(1):8–22.
Hughes, J.H., M.C. Mason, R.J. Gray, S.A. McLaughlin, A.C. Degnim, J.T. Fulmer, B.A. Pockaj, P.J. Karstaedt, and M.C. Roarke. 2008. A multi-site validation trial of radioactive seed localization as an alternative to wire localization. The Breast Journal 14(2):153–157.
Hwang, R.F., and K.K. Hunt. 2020. The emergence of robotic-assisted breast surgery: Proceed with caution. Annals of Surgery 271(6):1013–1015.
Isacsson, U., H. Hagberg, K.A. Johansson, A. Montelius, B. Jung, and B. Glimelius. 1997. Potential advantages of protons over conventional radiation beams for paraspinal tumours. Radiotherapy and Oncology 45(1):63–70.
Jagsi, R., J. Jiang, A.O. Momoh, A. Alderman, S.H. Giordano, T.A. Buchholz, L.J. Pierce, S.J. Kronowitz, and B.D. Smith. 2016. Complications after mastectomy and immediate breast reconstruction for breast cancer: A claims-based analysis. Annals of Surgery 263(2):219–227.
Johnston, S., M. Martin, A. Di Leo, S.A. Im, A. Awada, T. Forrester, M. Frenzel, M.C. Hardebeck, J. Cox, S. Barriga, M. Toi, H. Iwata, and M.P. Goetz. 2019. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 5:5.
Joshi, S.K., K. Qian, W.H. Bisson, K. Watanabe-Smith, A. Huang, D. Bottomly, E. Traer, J.W. Tyner, S.K. McWeeney, M.A. Davare, B.J. Druker, and C.E. Tognon. 2020. Discovery and characterization of targetable NTRK point mutations in hematologic neoplasms. Blood 135(24):2159–2170.
Kamada, T., H. Tsujii, E.A. Blakely, J. Debus, W. De Neve, M. Durante, O. Jakel, R. Mayer, R. Orecchia, R. Potter, S. Vatnitsky, and W.T. Chu. 2015. Carbon ion radiotherapy in Japan: An assessment of 20 years of clinical experience. The Lancet Oncology 16(2):e93–e100.
Keating, J., J. Tchou, O. Okusanya, C. Fisher, R. Batiste, J. Jiang, G. Kennedy, S. Nie, and S. Singhal. 2016. Identification of breast cancer margins using intraoperative near-infrared imaging. Journal of Surgical Oncology 113(5):508–514.
Kim, E.S., S.S. Bruinooge, S. Roberts, G. Ison, N.U. Lin, L. Gore, T.S. Uldrick, S.M. Lichtman, N. Roach, J.A. Beaver, R. Sridhara, P.J. Hesketh, A.M. Denicoff, E. Garrett-Mayer, E. Rubin, P. Multani, T.M. Prowell, C. Schenkel, M. Kozak, J. Allen, E. Sigal, and R.L. Schilsky. 2017. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. Journal of Clinical Oncology 35(33):3737–3744.
Kuehn, T., I. Bauerfeind, T. Fehm, B. Fleige, M. Hausschild, G. Helms, A. Lebeau, C. Liedtke, G. von Minckwitz, V. Nekljudova, S. Schmatloch, P. Schrenk, A. Staebler, and M. Untch. 2013. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. The Lancet Oncology 14(7):609–618.
Kuerer, H.M., G.M. Rauch, S. Krishnamurthy, B.E. Adrada, A.S. Caudle, S.M. DeSnyder, D.M. Black, L. Santiago, B.P. Hobbs, A. Lucci, Jr., M. Gilcrease, R.F. Hwang, R.P. Candelaria, M. Chavez-MacGregor, B.D. Smith, E. Arribas, T. Moseley, M. Teshome, M.V. Miggins, V. Valero, K.K. Hunt, and W.T. Yang. 2018. A clinical feasibility trial for identification of exceptional responders in whom breast cancer surgery can be eliminated following neoadjuvant systemic therapy. Annals of Surgery 267(5):946–951.
Lebovic, G.S. 2010. Oncoplastic surgery: A creative approach to breast cancer management. Surgical Oncology Clinics 19(3):567–580.
Litton, J.K., H.S. Rugo, J. Ettl, S.A. Hurvitz, A. Gonçalves, K.-H. Lee, L. Fehrenbacher, R. Yerushalmi, L.A. Mina, M. Martin, H. Roché, Y.-H. Im, R.G.W. Quek, D. Markova, I.C. Tudor, A.L. Hannah, W. Eiermann, and J.L. Blum. 2018. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. New England Journal of Medicine 379(8):753–763.
Ma, P.C. 2019. “Cancer-agnostic” NTRK inhibitor approval ushers in paradigm of personalized genomics-guided therapeutics. https://www.healio.com/news/hematology-oncology/20190107/canceragnostic-ntrk-inhibitor-approval-ushers-in-paradigm-of-personalized-genomicsguided-therapeutic (accessed October 12, 2020).
Malouff, T.D., A. Mahajan, S. Krishnan, C. Beltran, D.S. Seneviratne, and D.M. Trifiletti. 2020. Carbon ion therapy: A modern review of an emerging technology. Frontiers in Oncology 10:B2.
Mamtani, A., E.C. Zabor, L.H. Rosenberger, M. Stempel, M.L. Gemignani, and M. Morrow. 2019. Was reexcision less frequent for patients with lobular breast cancer after publication of the SSO-ASTRO margin guidelines? Annals of Surgical Oncology 26(12):3856–3862.
Martin, N.E., and A.V. D’Amico. 2014. Progress and controversies: Radiation therapy for prostate cancer. CA: A Cancer Journal for Clinicians 64(6):389–407.
Mayo, R.C., 3rd, M.J. Kalambo, and J.R. Parikh. 2019. Preoperative localization of breast lesions: Current techniques. Clinical Imaging 56:1–8.
McKeage, K., and C.M. Perry. 2002. Trastuzumab: A review of its use in the treatment of metastatic breast cancer overexpressing HER2. Drugs 62(1):209–243.
Miralbell, R., A. Lomax, L. Cella, and U. Schneider. 2002. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. International Journal of Radiation Oncology, Biology, Physics 54(3):824–829.
Mirza, M.R., B.J. Monk, J. Herrstedt, A.M. Oza, S. Mahner, A. Redondo, M. Fabbro, J.A. Ledermann, D. Lorusso, I. Vergote, N.E. Ben-Baruch, C. Marth, R. Mądry, R.D. Christensen, J.S. Berek, A. Dørum, A.V. Tinker, A. du Bois, A. González-Martín, P. Follana, B. Benigno, P. Rosenberg, L. Gilbert, B.J. Rimel, J. Buscema, J.P. Balser, S. Agarwal, and U.A. Matulonis. 2016. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. New England Journal of Medicine 375(22):2154–2164.
Mok, T.S., Y.-L.Wu, M.-J. Ahn, M.C. Garassino, H.R. Kim, S.S. Ramalingam, F.A. Shepherd, Y. He, H. Akamatsu, W.S. Theelen, C.K. Lee, M. Sebastian, A. Templeton, H. Mann, M. Marotti, S. Ghiorghiu, V.A. Papadimitrakopoulou, for the AURA3 investigators. 2017. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. New England Journal of Medicine 376(7):629–640.
Montay-Gruel, P., K. Petersson, M. Jaccard, G. Boivin, J.F. Germond, B. Petit, R. Doenlen, V. Favaudon, F. Bochud, C. Bailat, J. Bourhis, and M.C. Vozenin. 2017. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiotherapy and Oncology 124(3):365–369.
Montay-Gruel, P., A. Bouchet, M. Jaccard, D. Patin, R. Serduc, W. Aim, K. Petersson, B. Petit, C. Bailat, J. Bourhis, E. Brauer-Krisch, and M. C. Vozenin. 2018. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiotherapy and Oncology 129(3):582–588.
Moore, K., N. Colombo, G. Scambia, B.-G. Kim, A. Oaknin, M. Friedlander, A. Lisyanskaya, A. Floquet, A. Leary, G. S. Sonke, C. Gourley, S. Banerjee, A. Oza, A. González-Martín, C. Aghajanian, W. Bradley, C. Mathews, J. Liu, E. S. Lowe, R. Bloomfield, and P. DiSilvestro. 2018. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. New England Journal of Medicine 379(26):2495–2505.
Munzenrider, J.E. 1999. Proton therapy for uveal melanomas and other eye lesions. Strahlentherapie und Onkologie 175:68–73.
NCCN (National Comprehensive Cancer Network). 2020. NCCN guideline with NCCN evidence blocks—Breast cancer version 3.2020. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (asccessed September 24, 2020).
NCI (National Cancer Institute). 2018. CAR T-cell therapy. https://visuals.nci.nih.gov/details.cfm?imageid=12069 (accessed September 24, 2020).
NCI. 2020. Targeted cancer therapies. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet (accessed December 30, 2020).
NCI. n.d. Drugs approved for different types of cancer. https://www.cancer.gov/about-cancer/treatment/drugs/cancer-type (accessed October 14, 2020).
Noguchi, M., S. Miura, E. Morioka, Y. Ohno, M. Yokoi-Noguchi, Y. Nakano, and T. Kosaka. 2015. Is axillary reverse mapping feasible in breast cancer patients? European Journal of Surgical Oncology 41(4):442–449.
Nota, C., F.J. Smits, Y. Woo, I.H.M. Borel Rinkes, I.Q. Molenaar, J. Hagendoorn, and Y. Fong. 2019. Robotic developments in cancer surgery. Surgical Oncology Clinics of North America 28(1):89–100.
Ochoa, D., S. Korourian, C. Boneti, L. Adkins, B. Badgwell, and V.S. Klimberg. 2014. Axillary reverse mapping: Five-year experience. Surgery 156(5):1261–1268.
Pacheco, J.M., D. Gao, D. Smith, T. Purcell, M. Hancock, P. Bunn, T. Robin, A. Liu, S. Karam, L. Gaspar, B. Kavanagh, C. Rusthoven, D. Aisner, R. Doebele, and D.R. Camidge. 2019. Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. Journal of Thoracic Oncology 14(4):691–700.
Pai, H.H., A. Thornton, L. Katznelson, D.M. Finkelstein, J.A. Adams, B.C. Fullerton, J.S. Loeffler, N.J. Leibsch, A. Klibanski, and J.E. Munzenrider. 2001. Hypothalamic/pituitary function following high-dose conformal radiotherapy to the base of skull: Demonstration of a dose-effect relationship using dose–volume histogram analysis. International Journal of Radiation Oncology, Biology, Physics 49(4):1079–1092.
Pao, W., V. Miller, M. Zakowski, J. Doherty, K. Politi, I. Sarkaria, B. Singh, R. Heelan, V. Rusch, L. Fulton, E. Mardis, D. Kupfer, R. Wilson, M. Kris, and H. Varmus. 2004. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences 101(36):13306–13311.
Pawlik, T.M. 2019. Minimally invasive oncologic surgery, part II. Surgical Oncology Clinics of North America 28(2):xiii–xiv.
Penn, I.W., Y.C. Chang, E. Chuang, C.M. Chen, C.F. Chung, C.Y. Kuo, and T.Y. Chuang. 2019. Risk factors and prediction model for persistent breast-cancer-related lymphedema: A 5-year cohort study. Supportive Care in Cancer 27(3):991–1000.
PTCOG (Particle Therapy Co-Operative Group). 2020. Particle therapy facilities in clinical operation. https://www.ptcog.ch/index.php/facilities-in-operation (accessed October 14, 2020).
Ramalingam, S.S., J. Vansteenkiste, D. Planchard, B.C. Cho, J.E. Gray, Y. Ohe, C. Zhou, T. Reungwetwattana, Y. Cheng, B. Chewaskulyong, R. Shah, M. Cobo, K.H. Lee, P. Cheema, M. Tiseo, T. John, M.C. Lin, F. Imamura, T. Kurata, A. Todd, R. Hodge, M. Saggese, Y. Rukazenkov, and J.C. Soria. 2020. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. New England Journal of Medicine 382(1):41–50.
Robson, M., S.-A. Im, E. Senkus, B. Xu, S.M. Domchek, N. Masuda, S. Delaloge, W. Li, N. Tung, A. Armstrong, W. Wu, C. Goessl, S. Runswick, and P. Conte. 2017. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. New England Journal of Medicine 377(6):523–533.
Rosenberger, L.H., A. Mamtani, S. Fuzesi, M. Stempel, A. Eaton, M. Morrow, and M.L. Gemignani. 2016. Early adoption of the SSO-ASTRO consensus guidelines on margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: Initial experience from Memorial Sloan Kettering Cancer Center. Annals of Surgical Oncology 23(10):3239–3246.
Schaverien, M.V., and C.J. Coroneos. 2019. Surgical treatment of lymphedema. Plastic and Reconstructive Surgery 144(3):738–758.
Schaverien, M.V., I. Badash, K.M. Patel, J.C. Selber, and M.H. Cheng. 2018. Vascularized lymph node transfer for lymphedema. Seminars in Plastic Surgery 32(1):28–35.
Schnabel, F., S.K. Boolbol, M. Gittleman, T. Karni, L. Tafra, S. Feldman, A. Police, N.B. Friedman, S. Karlan, D. Holmes, S.C. Willey, M. Carmon, K. Fernandez, S. Akbari, J. Harness, L. Guerra, T. Frazier, K. Lane, R.M. Simmons, A. Estabrook, and T. Allweis. 2014. A randomized prospective study of lumpectomy margin assessment with use of margin probe in patients with nonpalpable breast malignancies. Annals of Surgical Oncology 21(5):1589–1595.
Sheets, N.C., G.H. Goldin, A.M. Meyer, Y. Wu, Y. Chang, T. Sturmer, J.A. Holmes, B.B. Reeve, P.A. Godley, W.R. Carpenter, and R.C. Chen. 2012. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 307(15):1611–1620.
Sheetz, K.H., and J.B. Dimick. 2019. Is it time for safeguards in the adoption of robotic surgery? JAMA 321(20):1971–1972.
Silverstein, M.J., T. Mai, N. Savalia, F. Vaince, and L. Guerra. 2014. Oncoplastic breast conservation surgery: The new paradigm. Journal of Surgical Oncology 110(1):82–89.
Smith, B.L., C.R. Lanahan, M.C. Specht, B.N. Kelly, C. Brown, D.B. Strasfeld, J.M. Ferrer, U. Rai, R. Tang, T. Rice-Stitt, A. Biernacka, E.F. Brachtel, and M.A. Gadd. 2020. Feasibility study of a novel protease-activated fluorescent imaging system for real-time, intraoperative detection of residual breast cancer in breast conserving surgery. Annals of Surgical Oncology 27(6):1854–1861.
St Clair, W.H., J.A. Adams, M. Bues, B.C. Fullerton, S. La Shell, H.M. Kooy, J.S. Loeffler, and N.J. Tarbell. 2004. Advantage of protons compared to conventional x-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. International Journal of Radiation Oncology, Biology, Physics 58(3):727–734.
Tang, S.S., S. Kaptanis, J.B. Haddow, G. Mondani, B. Elsberger, M.K. Tasoulis, C. Obondo, N. Johns, W. Ismail, A. Syed, P. Kissias, M. Venn, S. Sundaramoorthy, G. Irwin, A.S. Sami, D. Elfadl, A. Baggaley, D.D. Remoundos, F. Langlands, P. Charalampoudis, Z. Barber, W.L.S. Hamilton-Burke, A. Khan, C. Sirianni, L.A.G. Merker, S. Saha, R.A. Lane, S. Chopra, S. Dupré, A.T. Manning, E.R. St John, A. Musbahi, N. Dlamini, C.L. McArdle, C. Wright, J.O. Murphy, R. Aggarwal, M. Dordea, K. Bosch, D. Egbeare, H. Osman, S. Tayeh, F. Razi, J. Iqbal, S.F.C. Ledwidge, V. Albert, and Y. Masannat. 2017. Current margin practice and effect on re-excision rates following the publication of the SSO-ASTRO consensus and ABS consensus guidelines: A national prospective study of 2858 women undergoing breast-conserving therapy in the UK and Ireland. European Journal of Cancer 84:315–324.
Terahara, A., A. Niemierko, M. Goitein, D. Finkelstein, E. Hug, N. Liebsch, D. O’Farrell, S. Lyons, and J. Munzenrider. 1999. Analysis of the relationship between tumor dose inhomogeneity and local control in patients with skull base chordoma. International Journal of Radiation Oncology, Biology, Physics 45(2):351–358.
Thompson, M., S. Korourian, R. Henry-Tillman, L. Adkins, S. Mumford, K.C. Westbrook, and V.S. Klimberg. 2007. Axillary reverse mapping (ARM): A new concept to identify and enhance lymphatic preservation. Annals of Surgical Oncology 14(6):1890.
Tung, N.M., M.E. Robson, S. Ventz, C.A. Santa-Maria, R. Nanda, P.K. Marcom, P.D. Shah, T.J. Ballinger, E.S. Yang, S. Vinayak, M. Melisko, A. Brufsky, M. DeMeo, C. Jenkins, S. Domchek, A. D’Andrea, N.U. Lin, M.E. Hughes, L.A. Carey, N. Wagle, G.M. Wulf, I.E. Krop, A.C. Wolff, E.P. Winer, and J.E. Garber. 2020. TBCRC 048: Phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. Journal of Clinical Oncology 38(36):4274–4282.
Turner, N.C., J. Ro, F. André, S. Loi, S. Verma, H. Iwata, N. Harbeck, S. Loibl, C. Huang Bartlett, K. Zhang, C. Giorgetti, S. Randolph, M. Koehler, and M. Cristofanilli. 2015. Palbociclib in hormone-receptor–positive advanced breast cancer. New England Journal of Medicine 373(3):209–219.
Turner, N.C., D.J. Slamon, J. Ro, I. Bondarenko, S.-A. Im, N. Masuda, M. Colleoni, A. DeMichele, S. Loi, S. Verma, H. Iwata, N. Harbeck, S. Loibl, F. André, K. Puyana Theall, X. Huang, C. Giorgetti, C. Huang Bartlett, and M. Cristofanilli. 2018. Overall survival with palbociclib and fulvestrant in advanced breast cancer. New England Journal of Medicine 379(20):1926–1936.
Urban, C., R. Lima, E. Schunemann, C. Spautz, I. Rabinovich, and K. Anselmi. 2011. Oncoplastic principles in breast conserving surgery. The Breast 20:S92–S95.
Vaddepally, R.K., P. Kharel, R. Pandey, R. Garje, and A.B. Chandra. 2020. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel) 12(3):738.
van der Noordaa, M.E.M., F.H. van Duijnhoven, C.E. Loo, E. van Werkhoven, K.K. van de Vijver, T. Wiersma, H.A.O. Winter-Warnars, G.S. Sonke, and M. Vrancken Peeters. 2018. Identifying pathologic complete response of the breast after neoadjuvant systemic therapy with ultrasound guided biopsy to eventually omit surgery: Study design and feasibility of the MICRA trial (Minimally Invasive Complete Response Assessment). Breast 40:76–81.
van der Valk, M.J.M., D.E. Hilling, E. Bastiaannet, E. Meershoek-Klein Kranenbarg, G.L. Beets, N.L. Figueiredo, A. Habr-Gama, R.O. Perez, A.G. Renehan, and C.J.H. van de Velde. 2018. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. The Lancet 391(10139):2537–2545.
Verma, S., D. Miles, L. Gianni, I.E. Krop, M. Welslau, J. Baselga, M. Pegram, D.Y. Oh, V. Dieras, E. Guardino, L. Fang, M.W. Lu, S. Olsen, and K. Blackwell. 2012. Trastuzumab emtansine for HER2-positive advanced breast cancer. New England Journal of Medicine 367(19):1783–1791.
Whiteside, T.L., S. Demaria, M.E. Rodriguez-Ruiz, H.M. Zarour, and I. Melero. 2016. Emerging opportunities and challenges in cancer immunotherapy. Clinical Cancer Research 22(8):1845–1855.
Yu, J.B., P.R. Soulos, J. Herrin, L.D. Cramer, A.L. Potosky, K.B. Roberts, and C.P. Gross. 2013. Proton versus intensity-modulated radiotherapy for prostate cancer: Patterns of care and early toxicity. Journal of the National Cancer Institute 105(1):25–32.
This page intentionally left blank.


































