4
Diagnosis, Staging, and Treatment of Cancer
Each person’s experience of cancer is unique from the time of diagnosis, through treatment, to survivorship, and for some patients to end of life. A patient’s cancer care trajectory, shown in Figure 4-1, rests on many factors including the type of cancer the patient has, how far the cancer has progressed in the body, what treatments are available and tolerated by the patient, and the outcomes they experience, including mental and physical health. Figure 4-1 illustrates that once a patient is diagnosed and a treatment plan is developed, several care pathways are possible. Many cancer patients, particularly those with early-stage disease, are treated with the goal of being cured, respond to treatment, and may expect to have long-term, cancer-free survival. Others may not experience a successful treatment response and/or chronic, stable, or intermittent disease, and will experience a progression of their cancer. Some patients will be diagnosed with advanced or metastatic cancer and be treated with palliative intent, rather than curative intent, with the goal of improving their symptoms, quality of life, or length of life. Those who are cancer free with no evidence of disease and those with chronic, stable, or intermittent disease will typically undergo routine surveillance for recurrent and new cancers. If, months to years later, a cancer survivor experiences a recurrence or new primary cancer, she or he may re-enter the trajectory with a new diagnosis and treatment plan. Survivorship care promotes a patient’s general health and wellness by addressing the physical and mental health symptoms and adverse effects of the cancer and its treatments, as well as the emotional, spiritual, social, and financial impacts of cancer throughout the survivor’s lifetime (see Chapter 10). Regardless of the patient’s cancer care trajectory or where she or he is
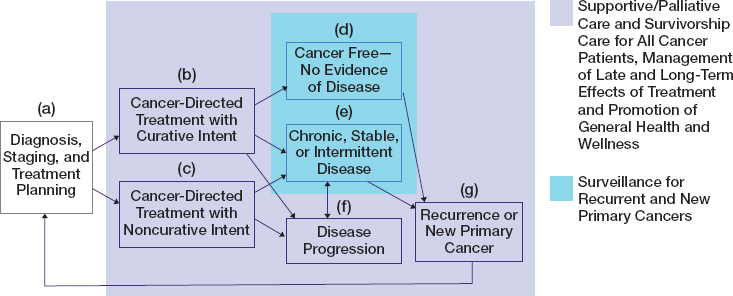
SOURCE: Adapted from IOM, 2006, p. 190.
along it, palliative or supportive care and survivorship care are relevant to all patients and should be initiated as soon as a patient is diagnosed. Palliative or supportive care (distinct from end-of-life care) encompass treatments and services designed to prevent and alleviate suffering and support the best possible quality of life for patients. Death can occur at any point in the trajectory and may be the result of cancer for those with disease progression or incomplete response to treatment, of long-term or late-onset effects of treatment (such as cardiac effects), or from causes other than cancer.
This chapter presents an overview of the screening, diagnosis, staging, treatment, and prognosis of cancer. At the time of diagnosis, the clinical features of the cancer and its extent throughout the body are assessed. This information will be used to identify treatment options and develop the patient’s treatment plan. In the following chapters, the committee delves more deeply into these topics, specifically for breast cancer (see Chapter 5) and lung cancer (see Chapter 6). In Chapter 7 the committee provides a brief overview of these topics with respect to other cancers that are among the most common diagnoses received by the U.S. Social Security Administration in disability claims.
This chapter emphasizes current strategies and technologies that constitute a standard of care that addresses the specific features of an individual’s
cancer (its microscopic pathology and its molecular and genomic characteristics) as well as how far and where cancer has spread within the body. Staging is the process used to determine whether a cancer has affected tissues or organs beyond a localized place. The biology of the specific tumor (e.g., identification of biomarkers) and the tumor’s anatomic characteristics are what determine the treatment plan.
Ideally, although not always in practice, the clinical cancer care team works with the patient—and caregivers if appropriate—to decide the best treatment strategy. This multidisciplinary care team is composed of clinicians with expertise in radiology, pathology, surgery, medical oncology, and radiation oncology who will review and synthesize the diagnostic findings and recommend treatment options. Members of the medical team will then discuss these options with the patient and caregivers to determine which treatments are most likely to meet the patient’s needs and provide the best long-term outcomes and quality of life. Patient outcomes are dependent in part on the ability of the cancer care team to provide the patient with appropriate treatments from surgery to the newest immunotherapies. The care team may recommend that a patient enroll in a clinical trial to help establish the effectiveness of new treatments or expand the use of existing treatments. Participation in clinical trials is considered a best management practice by organizations such as the National Comprehensive Cancer Network. However, the committee notes that not all patients may be eligible for or can access a clinical trial and that an appropriate trial may not be enrolling new patients. Treatment options may include surgery, radiation, and systemic therapies. In some cases close follow-up with periodic laboratory and imaging surveillance is also an accepted approach to the management of certain cancers; this approach may be described as “active surveillance” or “watchful waiting,” depending on the circumstance. Survivorship care (the purple area in Figure 4-1) may also include rehabilitation to restore function or to reduce or prevent impairments and treatment for adverse effects resulting from the cancer treatments themselves.
The committee emphasizes that both cancer and its treatments can affect a survivor’s quality of life. The cancer disease can result in impairments, such as fatigue, chronic pain, or limited range of motion, that prevent a person from conducting normal activities, including gainful employment. In addition, cancer treatments, whether surgery, radiation, or systemic treatments (e.g., chemotherapy, endocrine therapy, or targeted therapy), can also result in impairments, some of which may be similar to those produced by the cancer itself. These treatment-related impairments may be transient and resolve when treatment is complete, or they may be long term and last for months or years. In some cases the treatments may cause impairments that do not become evident until long after the treatment is complete (i.e., late-onset effects). As women and men are living increasingly longer lives
after their cancer diagnosis and treatment, whether disease free or with metastatic cancer, the importance of identifying and managing these impairments also increases since the impairments may have lifelong impacts on a cancer survivor’s quality of life.
SCREENING FOR CANCER
Cancers are typically diagnosed in one of two ways: screening patients without symptoms for the purpose of identifying cancers early or carrying out a clinical evaluation of symptoms. A small proportion of patients may also be diagnosed with asymptomatic cancers because of an incidental finding during an evaluation conducted for other reasons (e.g., a lung nodule identified on a computed tomography [CT] scan evaluating shortness of breath).
Although screening tests are improving, no screening test provides a perfectly accurate cancer diagnosis. For a screening test to be considered valid and reliable it should demonstrate few false negative results (i.e., not finding cancer when it is present) and few false positives (i.e., finding cancer when it is not present). Having accurate screening tests is important for achieving decreases in cancer mortality, rather than just increases in survival time. Screening tests are subject to several types of biases that may affect conclusions about the benefits of screening for a particular cancer. For example, overdiagnosis is a bias that occurs when screening finds subclinical cancers that, in the absence of screening, would never become evident before the patient dies of another cause. Overdiagnosis is a problem because these patients may be treated for cancer without receiving any real benefits from the treatment.
Screening Recommendations
The goal of cancer screening is to detect cancer before overt symptoms occur, when there is the greatest likelihood that the cancer can be effectively treated and cured. Effective screening tests are available for several cancers, including colorectal cancer (e.g., colonoscopy or stool testing), cervical cancer (e.g., Pap smear), and breast cancer (e.g., mammography).
Several organizations, including the U.S. Preventive Services Task Force (USPSTF) and the American Cancer Society, have developed screening recommendations for cancers. USPSTF evidence-based recommendations for screening of asymptomatic individuals who are not at increased risk of cancer are listed in Table 4-1 and include recommendations for cancers of the breast, lung, colon, and rectum among others. For most other cancers, there is little or no clinical evidence to support screening for the average-risk person, and for several cancers there is high-quality evidence from
TABLE 4-1 Selected U.S. Preventive Services Task Force Recommendations for Cancer Screening of Adults at Average Risk for Cancer
| Cancer Type | Age (yrs) | Screening Recommendations |
|---|---|---|
| Breast cancer* | 40–49 |
|
| 50–74 |
|
|
| 75+ |
|
|
| Colorectal cancer† | 45–75 |
|
| 76–85 |
|
|
| ≥86 |
|
|
| Lung cancer | 50–80 |
|
| 80+ |
|
|
NOTES: CT = computed tomography; DNA = deoxyribonucleic acid; FIT = fecal immunohistochemical testing.
* The American Cancer Society recommends mammograms every year for women aged 45–54 and then mammograms every 2 years until a woman’s life expectancy is less than 10 years (Smith et al., 2019).
† The American Cancer Society recommends initiating colorectal cancer screening at age 45 (Smith et al., 2019).
randomized clinical trials demonstrating that existing screening approaches (e.g., for cancers of the ovary, pancreas, testicle, and thyroid) provide no benefit at all (USPSTF, 2020c).
USPSTF recommendations address screening for individuals who are at average risk for cancer, although exceptions are noted for some individuals based on a personal or family history associated with increased cancer risk. A thorough family history and review of prior medical and nonmedical exposures can help to identify individuals who may have a genetic predisposition for cancer (e.g., carriers of BRCA gene variants or patients with Lynch syndrome) or who have prior exposures that increase cancer risk (e.g., workplace exposure to carcinogenic substances or a history of radiation exposure). For these higher-risk individuals, cancer screening may be started at a younger age and conducted more frequently than is recommended for the general population. In some cases individuals may benefit from additional tests that are not typically used for screening in average-risk populations, such as breast magnetic resonance imaging (MRI) for women with BRCA mutations.
Using breast cancer as an example, the committee notes that although there is substantial evidence to suggest that mammography reduces breast cancer mortality in women, particularly those in their 60s, the benefit is more modest for women in their 40s (Nelson et al., 2016). Increasing evidence about the harms of cancer screening, including false positives, unnecessary biopsies, and overdiagnosis, as well as the modest benefits of such screening led USPSTF to conclude that the benefits of breast cancer screening may not outweigh the harms for most women in their 40s, although it may be appropriate for some of these women. Thus, engaging all women aged 40 and older in shared decisions about breast cancer screening may be the best course of action (Keating and Pace, 2018).
How Cancer Screening Is Changing
There are increasing efforts to minimize the potential harms of cancer screening through the use of less invasive test procedures (e.g., human papillomavirus [HPV] testing instead of Pap smears for cervical cancer and fecal DNA testing instead of colonoscopy for colorectal cancer screening). In addition, there is increasing interest in the use of molecular assays, such as those that are used to detect early molecular changes in the blood (Liu et al., 2020). Research is being conducted to determine if such blood-based testing (sometimes called “liquid biopsy”) could serve as effective screening tests for some cancers. It is also possible that improvements in cancer prevention (e.g., the HPV vaccine to prevent cervical cancer) and advances in cancer treatment (e.g., targeted therapies) may result in screening being a less important aspect of cancer control. Along with these developments, there is a growing awareness of the need for shared decision making among clinicians and their patients to ensure that patients understand both the benefits and the potential harms of screening.
DIAGNOSING CANCER
Cancer is usually diagnosed as part of a medical evaluation of persistent or unexplained symptoms (e.g., coughing up blood, back pain, change in bowel pattern, or weight loss). As cancer can occur in any part of the body, and because it can spread (metastasize) to sites distant from the primary tumor (e.g., to the brain, liver, or lungs), the presenting symptoms that bring a person to medical attention may be the beginning of a diagnostic journey. A patient’s presenting symptoms generally reflect the cancer type and any cancer-specific patterns of metastases. Table 4-2 includes common symptoms associated with frequently diagnosed cancers.
TABLE 4-2 Common Symptoms of the Cancers Considered in This Report
| Cancer Type | Common Symptoms |
|---|---|
| Breast | Breast lump, swelling/thickening/dimpling/irritation of skin, nipple retraction |
| Lung and bronchus | Cough, blood in sputum, shortness of breath, chest pain, unexplained weight loss, loss of appetite, wheezing, hoarseness |
| Colon and rectum (colorectal) | Change in bowel habits, rectal bleeding, abdominal discomfort, unexplained weight loss |
| Melanoma | Change in existing mole, new pigmented or unusual growth on skin |
| Non-Hodgkin lymphoma | Painless swelling of lymph nodes, fatigue, fever, night sweats, shortness of breath, unexplained weight loss |
| Leukemia | Fever/chills, fatigue/weakness, recurrent infections, unexplained weight loss, swollen lymph nodes, easy bleeding/bruising, night sweats |
| Pancreas | Abdominal pain radiating to back, back pain, loss of appetite, unexplained weight loss, yellowing of skin, light colored stools, dark urine, itchy skin |
| Oral cavity and pharynx (head and neck) | Mass in mouth/throat/neck, pain or difficulty with swallowing, blood in saliva or nasal secretions, nonhealing sores in mouth, unexplained weight loss |
| Ovary | Abdominal bloating or swelling, weight loss, feeling full when eating, pelvic discomfort, constipation |
SOURCE: Mayo Clinic, 2018a,b, 2019a,b,c,d,e, 2020a,b,c.
Evaluating Symptoms
Individuals with symptoms suggestive of cancer should undergo further evaluation to identify the cause of their symptoms. This evaluation may include diagnostic tests, such as laboratory testing and imaging studies (x-rays, CT scans, ultrasound imaging, and MRIs). For certain cancers, direct visualization may also be useful (e.g., colonoscopy for suspected colon or rectal cancer or bronchoscopy for suspected lung cancer). For some cancers, such as pancreatic cancer and ovarian cancer, the common symptoms are nonspecific, making diagnosis challenging. While new onset of fatigue, low back pain, or abdominal bloating may be the first symptoms of many cancers, most individuals with these symptoms do not have cancer. Persistent symptoms that do not resolve or that are associated with more concerning findings, such as unexplained weight loss, should lead to further assessment to exclude cancer as the cause.
Establishing a Cancer Diagnosis
When cancer is suspected, confirming the diagnosis typically requires a biopsy procedure to collect a tissue sample for microscopic examination by a pathologist. For many cancers this may involve a needle biopsy through the skin, often conducted with ultrasound or CT guidance. For other cancers a biopsy may be done as part of a diagnostic procedure (e.g., endoscopic biopsy of a colon mass found on colonoscopy). For hematologic cancers such as lymphoma, this may involve examining a smear of a patient’s blood, followed by a bone marrow biopsy.
Although the diagnosis of cancer can often be made with a needle biopsy, this may not be feasible for tumors in anatomic locations that are difficult to access or in cases where the biopsy does not yield a sufficient amount of tissue for an accurate diagnosis. In these situations, a surgical excisional biopsy (i.e., removal of the entire tumor) or incisional biopsy (i.e., removal of part of the tumor) may be needed. The surgical biopsy is planned carefully to take into account the possible need for future surgery and to avoid contaminating surrounding tissue.
Examination of the biopsy specimen allows the pathologist to identify cancer cells based on the cells’ appearance (morphology) and histology. Lung cancers are classified by the microscopic appearance of the cancer cells, typically adenocarcinoma, squamous cell carcinoma, or small-cell carcinoma. Pathologists may also use biomarker tests and special stains, known as immunohistochemical tests, to confirm or support conclusions about the cancer’s suspected tissue of origin.
STAGING AND CHARACTERIZATION OF CANCER
Once a person is diagnosed with cancer, determining its stage is a critical next step on the path to developing a treatment plan. Cancer staging refers to gathering the diagnostic information needed to identify the extent of cancer; staging may occur simultaneously with diagnosis. A localized or early-stage cancer is generally a single tumor without evidence of spread beyond the initial affected site, while an advanced-stage cancer shows evidence of a primary tumor with metastasis to one or more distant sites. For example, a patient with cancer diagnosed as a single lump in the breast without spread to the local lymph nodes or distant organ sites would be classified as early-stage. An advanced-stage lung cancer might present as a primary tumor of the lung with evidence that it has spread to the liver, bones, brain, or other sites. In general, early-stage cancers are treated with local therapies, such as surgery and radiation. Intermediate-stage (or regional) cancers, such as cancers that have spread to regional lymph nodes, may be treated with systemic therapies (e.g., chemotherapy) in addition to
local therapies. Advanced-stage cancers are treated primarily with systemic therapies.
There are many systems for categorizing and describing the stage of a cancer. The most commonly used cancer staging system was developed by the American Joint Committee on Cancer (AJCC) and is currently in its eighth edition (AJCC, 2017). The AJCC staging system is defined differently for each cancer type and assigns ranges from stage I (localized/early stage) to stage IV (metastatic/advanced stage). Stage 0 denotes carcinoma in situ (noninvasive cancer, with no current metastatic potential) and is almost always determined by pathologic examination.
The AJCC staging system is often referred to as TNM staging, because the overall stage is determined by a combination of the size or extent of the primary tumor (T stage), the extent of regional lymph node spread (N stage), and whether distant metastases are present (M stage). For example, a colon cancer that invades the muscular wall of the colon, involves five regional lymph nodes, and has spread to the liver would be described as T3N2M1; this corresponds to an overall classification of stage IV.
AJCC staging is used for many cancer types, including breast, lung, and colorectal cancer and is periodically updated as new information becomes available to help stage various cancers more accurately. For other cancers different staging systems may be used. For example, lymphomas are staged with the Lugano classification system (Cheson et al., 2014), ovarian cancer is usually staged with the International Federation of Gynecology and Obstetrics staging system (Javadi et al., 2016), and pancreatic cancer is commonly staged as resectable, borderline resectable, locally advanced, or metastatic.
The clinical workup required to complete cancer staging depends on the information available at the time of cancer diagnosis. Imaging studies are used to determine the extent of the disease and the presence of metastasis. The type of imaging studies used for staging depends on the suspected cancer and on the concern for its spread in the body, as informed by the patient’s history and physical examination findings. Examples of the imaging studies used to determine lymph node or other organs involvement include CT scans of the chest, abdomen, and pelvis, sometimes used in conjunction with a bone scan. Alternatively, 18-fluorodeoxyglucose positron emission tomography (FDG-PET) is a functional imaging test used for the diagnosis and staging of lung cancer, head and neck cancers, and certain other cancers.
When it is important to determine the local extent of the disease for surgical decisions, high-resolution CT scans or MRI scans may be used. MRIs are commonly used to identify the presence of brain metastases for cancers that frequently spread to the brain (e.g., lung cancers). If a cancer is suspected to have spread to the lymph nodes or to a distant site, tissue
confirmation with a biopsy is often indicated since these findings would affect the cancer’s assigned stage, treatment, and prognosis. For example, a patient may present with a cough and bloody sputum production, have a CT chest image showing a lung mass with enlarged lymph nodes, and have a FDG-PET scan showing tracer uptake in the lung mass, the lymph nodes, and in a nodule of the right adrenal gland. Initially this patient would be considered to have a potentially curable stage III lung cancer; however, the patient would ultimately be diagnosed with stage IV lung cancer should an adrenal gland biopsy confirm metastasis from a primary lung adenocarcinoma. In this case the adrenal gland biopsy completes the cancer staging, which confirms both the cancer diagnosis (adenocarcinoma arising from the lung) and the stage (stage IV, based on the finding of distant metastatic disease). In most cases, completion of the staging workup happens subsequent to the initial diagnostic biopsy of the primary tumor site.
Characterizing Cancer with Biomarkers
While staging is necessary for evaluating cancer treatment options and prognosis, the new precision or personalized treatment approaches increasingly require additional diagnostic information that goes beyond the anatomic information included in cancer staging systems and histology. For example, breast cancers are further classified based on the presence (expression) of specific receptor proteins on the surface of cancer cells, including estrogen receptors (ERs), progesterone receptors (PRs), and human epidermal growth factor receptor 2 (HER2, also called HER2/neu). Lung cancers may be further classified by whether the cancer cells exhibit specific abnormalities in cancer driver genes,1 such as mutations of the epidermal growth factor receptor gene or a chromosomal translocation of the ALK gene, or by their expression of the programmed death ligand-1 (PD-L1) cell-surface receptor protein. These additional diagnostic characteristics are often referred to as biomarkers, and such biomarkers are used together with the cancer stage to determine a cancer treatment plan. In addition to contributing to treatment decisions, molecular markers can also provide information about prognosis (i.e., the likely clinical outcome).
Testing for cancer biomarkers has evolved rapidly in recent years, and this evolution is likely to continue. Cancer biomarker testing increasingly includes an analysis of DNA from cancer cells, either from a tissue sample (biopsy) or from tumor DNA fragments circulating in the peripheral blood (circulating tumor DNA). In certain patients a wide array of genomic and
___________________
1 A cancer driver gene is defined as “one whose mutations increase net cell growth under the specific microenvironmental conditions that exist in the cell in vivo” (Tokheim et al., 2016).
molecular biomarker tests, often referred to as next-generation sequencing (NGS), can be evaluated simultaneously. NGS and other emerging biomarkers are generally more useful in patients with advanced, metastatic cancer, where these tests can help to identify a relatively small number of patients who may benefit from targeted systemic therapies. At present, NGS and other emerging biomarker tests are of limited use in most patients with early- or intermediate-stage disease, who are often best treated with local therapies (i.e., surgery and radiation therapy). However, there is increasing interest in using biomarker testing to identify patients with early-stage disease who may or may not benefit from additional cancer treatment. For example, the molecular assays Oncotype DX® and Mammaprint® are used to identify which patients with early-stage breast cancer are likely to benefit from chemotherapy (see Chapter 5).
CURRENT TREATMENTS
Cancer staging and additional characterization serve the related but distinct purposes of establishing a prognosis and defining potential treatment approaches. Ideally, prognosis and treatment are closely aligned. For example, a patient with an early-stage kidney cancer should have an excellent prognosis (very low risk of death or cancer recurrence) if he or she receives an effective local therapy such as surgery. If the same patient develops widespread metastases, then surgery would be unable to fully remove all the cancer sites, and the long-term prognosis would be poor (risk of cancer-related death would be high), even if the cancer initially responded to systemic therapy.
Many factors predict whether a patient with cancer is likely to do well or poorly following a cancer diagnosis. In addition to the stage of the cancer and the individual’s age and comorbid conditions, performance status is another important parameter used to estimate prognosis and select the best treatment for a patient. Performance status is a numerical rating of a patient’s ability to perform normal activities, including the ability to care for oneself (getting dressed, eating, and bathing) and to engage in more vigorous activity (cleaning a house, working at a job). Performance status scales are used to assess a patient’s expected ability to tolerate cancer treatment and are often used in clinical trials to help keep study populations consistent. In general, patients who have poor performance status tend to have more trouble tolerating rigorous cancer treatments and have less favorable outcomes than patients with better performance status, regardless of the treatments given.
Two widely-used performance status scales are the Eastern Cooperative Oncology Group scale (0–4) (also called the Zubrod scale) and the Karnofsky scale (100–0) (see Table 4-3). The patient population, disease,
TABLE 4-3 Performance Status Scoring for the Eastern Cooperative Oncology Group (ECOG) Scale and the Karnofsky Scale
| ECOG Scalea,b | Karnofsky Scalec |
|---|---|
|
0—Fully active, able to carry on all predisease performance without restriction 1—Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature (e.g., light house work, office work) 2—Ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hours 3—Capable of only limited self-care; confined to bed or chair more than 50% of waking hours 4—Completely disabled; cannot carry on any self-care; totally confined to bed or chair 5—Dead |
100—Normal, no complaints; no evidence of disease 90—Able to carry on normal activity; minor signs or symptoms of disease 80—Normal activity with effort, some signs or symptoms of disease 70—Cares for self but unable to carry on normal activity or to do active work 60—Requires occasional assistance but is able to care for most of personal needs 50—Requires considerable assistance and frequent medical care 40—Disabled; requires special care and assistance 30—Severely disabled; hospitalization is indicated although death not imminent 20—Very ill; hospitalization and active supportive care necessary 10—Moribund 0—Dead |
c Karnofsky and Burchenal, 1949.
SOURCE: Adapted from ECOG-ACRIN Research Group, 2020, with permission.
study goals, and other criteria influence which scale is used. Performance status often changes over time—worsening or improving—depending on a patient’s response to treatment and changes in the patient’s cancer-related symptoms.
In this section the common kinds of active cancer therapies are described, beginning with local therapies, that is, therapies that are directed at one or more specific anatomic sites, such as a localized lung or breast tumor. Local therapies include surgery, radiation, and other, less common therapies such as ablation and embolization. Systemic therapies are considered next. These therapies are drugs or drug combinations that are administered orally, intravenously, or by injection. Systemic treatments are carried by the circulatory system and can travel throughout the body to treat even widely metastatic cancers. Some systemic therapies, such as endocrine therapies, may be used for months or years after a patient is diagnosed with cancer.
Active treatment of cancer is not the only cancer management option, however. There are certain cancers that are monitored by active surveillance.
For example, localized, low-risk prostate cancers are generally slow growing, and cancer surveillance is a reasonable management approach in lieu of active treatment with surgery or radiation—either of which may cause significant complications such as impotency and incontinence. Ideally, the patient’s specific medical situation and preferences—along with a thorough discussion among the patient’s cancer care team and shared decision making with the patient about the potential risks and benefits of the treatment options—determine the choice of treatment.
Therapeutic Goals
Local and systemic therapies can be used for different patient objectives. The term “therapeutic intent” describes the goal of a cancer treatment plan, and therapeutic intent can be generally categorized as either “curative” or “palliative.” However, these descriptors can be misleading, as not all patients receiving curative-intent therapies will be cured, and not all patients receiving palliative-intent therapies have symptoms in need of palliation. Generally speaking, curative-intent therapies are treatments with a significant likelihood of resulting in a cure (i.e., a resolution of the cancer without the need for ongoing treatments). Palliative-intent therapies are used in situations where potentially curative treatments are infeasible or unacceptable and where a noncurative therapy can either prolong life or reduce the severity of cancer-related symptoms. Specific medications, including many chemotherapy agents, are often used in both curative and palliative situations, such as endocrine therapy for breast cancer.
For most cancers, local treatments—especially surgery and radiation—are essential components of curative-intent therapy. Systemic therapies may also be used in curative-intent treatment approaches; for example, chemotherapy can be used as a curative-intent therapy for certain types of leukemia, lymphoma, or testicular cancer. Chemotherapy may also be used before or after surgery as part of curative-intent treatment for many solid organ cancers. In these situations chemotherapy is considered an “adjuvant” therapy, meaning that it is used in addition to surgery, as part of a sequence of treatments with the intent to cure the cancer. By convention, cancer treatments that are used before a curative-intent surgery (i.e., preoperative) are described as “neoadjuvant” treatments, while “adjuvant” is reserved for postoperative treatments.
Treatment Planning
Ideally, treatment planning should incorporate all aspects of a patient and his or her cancer diagnosis. Patient characteristics include age, sex, preferences, performance status, socioeconomic status, social support, and
various other factors that may affect treatment tolerance in the short or long term. Key cancer characteristics include the primary cancer site, cancer stage, and cancer biomarkers. By weighing these factors, considering the associated prognosis, and establishing realistic therapeutic goals, patients and clinicians can collaborate to develop an individualized treatment plan designed to maximize expected health outcomes in the context of the patient’s preferences. Although a patient’s impairments and functional limitations may affect treatment tolerance, most cancer clinicians lack the expertise to comprehensively assess them, which may result in a suboptimal consideration of the potential risks and harms of some treatments for particular patients.
Standards of Care
Treatment guidelines for both clinicians and patients have been developed by several professional organizations, including the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN). These guidelines are usually specific for a cancer site (e.g., breast, hepatobiliary) or a specific subtype (e.g., Hodgkin lymphoma, non-small-cell lung cancer). NCCN has also developed guidelines for cancer detection, prevention, and risk reduction, and for supportive care, including distress management, fatigue, cancer pain, survivorship, and palliative care. Many of these guidelines encourage patients to participate in clinical trials as the standard of care, although only about 1 in 20 patients are enrolled in such trials (Unger et al., 2016). In addition, the National Coalition for Cancer Survivorship has developed guidance for survivorship care (see Chapter 10 for information on survivorship care).
The best cancer guidelines are evidence based, have been developed by an interdisciplinary group of experts, and have undergone a thorough review and had periodic updates as new evidence has become available (IOM, 2011). Table 4-4 lists some of the organizations that generate and regularly update cancer treatment guidelines. The committee notes that despite the availability of these guidelines, numerous studies have shown that cancer care often does not adhere to existing recommendations, including recommendations to engage patients in shared decision making with a multidisciplinary care team to determine their treatment plan and monitor adverse effects. Adherence to guidelines varies across the types of cancer, patient characteristics such as race/ethnicity and socioeconomic status, geography, insurance coverage, patient preferences, and physician recommendations (Bierbaum et al., 2020; Bristow et al., 2015; Chagpar et al., 2012; Fang et al., 2018; Jazieh et al., 2019; Shah et al., 2016; Visser et al., 2012). In some cases guidelines-recommended care may not be offered because the treatment needs to be individualized for a patient (e.g.,
TABLE 4-4 Selected Organizations That Have Developed Clinical Practice Guidelines (CPGs) for Cancer Treatment
| Organization | Summary | Website/Citation |
|---|---|---|
| American Society for Radiation Oncology | Provides guidance in five forms—CPGs, consensus guidance, white papers, practice parameters, and model policies—depending on the topic and evidence available. CPGs for several cancers and palliative/supportive/survivorship care. | https://www.astro.org/Patient-Care-andResearch/Clinical-Practice-Statements/Clinical-Practice-Guidelines |
| American Society of Clinical Oncology | Develops CPGs, provisional clinical opinions, and guidelines endorsements; generally disease- or modality-oriented. Clinical areas include assays and predictive markers, numerous specific cancers, neuro-oncology, patient and survivor care, and resource-stratified supportive care and treatment-related issues. | https://www.asco.org/research-guidelines/quality-guidelines/guidelines |
| National Comprehensive Cancer Network | CPGs for treatment of cancer by site; detection, prevention, and risk reduction; supportive care; specific populations; and patients. | https://www.nccn.org/professionals/physician_gls/default.aspx |
| Society for Integrative Oncology | CPGs on incorportating complementary and integrative therapies into conventional oncology clinical practice. | https://integrativeonc.org/integrative-oncology-guidelines |
| Society of Surgical Oncology | Collaborates with other organizations to develop CPGs for several cancer types and diagnostic and treatment techniques. | https://www.surgonc.org/resources/guidelines |
comorbid illness may make the patient a poor candidate for guideline-recommended treatments). In other cases clinicians may not be aware of guidelines, may disagree with guidelines, or may not have processes in place to ensure that patients receive guidelines-recommended care. For example, an oncologist may recommend radiation therapy and refer a patient to a radiation oncologist but may not track whether the patient makes the appointment, initiates radiation treatment, or completes the recommended number of treatments.
Local Therapies
Surgery and radiation therapy are the two most commonly used local treatments for cancer. Additional types of local therapy, such as ablation, may also be used. Surgery, radiation therapy, and other local treatments are described below.
Surgery
Surgery is often a first-line, local therapy intended to control cancer at the site of origin and thereby reduce the risk of metastases. It can also be used to treat metastatic lesions in order to improve the outcome in some situations such as a single focus of metastatic cancer. Surgery can also have nontherapeutic goals including the palliation of symptoms, cancer staging, cancer risk reduction (prophylaxis), and reconstruction.
All surgical procedures, whether they are performed for a therapeutic or nontherapeutic objective, can have adverse effects that result in impairments and functional limitations. Although cancer surgery is associated with several acute and chronic adverse effects, many of these effects will typically resolve as the patient heals and, if necessary, receives additional treatment for them. The severity of the effects depends on the site and extent of surgery and on the patient’s health status at the time of surgery. The possible adverse effects include acute and chronic pain, limitations in the patient’s range of motion, wound bleeding, infection, and disfigurement, which may be cosmetic (e.g., from a mastectomy) or functional (e.g., amputation).
Local Disease
Cancer surgery is typically defined as the excision of the primary area of cancer and sometimes also the surrounding tissue or potential sites of regional metastasis, such as lymph nodes, blood vessels, nerves, and other adjacent organs. The usual goal of cancer surgery is to provide a definitive treatment to improve clinical outcomes and reduce the risk of recurrence. Thus, surgical resection (i.e., surgically removing part or all of a tissue, structure, or organ) is designed to remove the cancer tumor such that the edges (margins) of the excised tissue show no signs of cancer (i.e., a sufficient amount of tissue, including the tumor, has been removed such that the tissue margins are negative or uninvolved). Traditionally, surgical resection tissue margins have been classified as: R0, no residual tumor; R1, microscopic residual tumor; or R2, macroscopic residual tumor at the primary cancer site or regional nodal sites (AJCC, 2017). However, the definition of adequate margins is specific to each disease site.
Metastatic Disease
Historically, surgery has not been recommended for patients with metastatic disease. However, for some cancers surgical resection of metastatic lesions can improve survival, such as the resection of pulmonary metastases from sarcomas or resection of hepatic and pulmonary metastases from
colorectal cancer. Even a surgery to remove the primary tumor for patients with limited metastatic disease may be beneficial in select cases.
Other Goals of Surgery
Diagnosis and staging
Cancers are often diagnosed by needle biopsies, and staging is determined by imaging studies. However, tumors that are in a location that is difficult to access (e.g., in the brain or lung) may need surgical removal for an accurate diagnosis. The extent of the disease, such as the degree of lymph node involvement, may not be assessed accurately by imaging or needle biopsy, and many solid tumors may require the surgical biopsy of the nodes (lymph node biopsy) for definitive staging. In these situations the lymphadenectomy guides treatment options and informs clinicians on the overall prognosis, but it may not be directly therapeutic.
Palliation
The goal of palliation surgery is to improve the patient’s quality of life rather than to extend life. Patients with advanced cancer may have debilitating symptoms such as severe pain, bleeding, infection, and other symptoms that may be a direct result of the cancer itself or cancer treatment. For example, a patient with extensive metastatic breast cancer with a very large, painful breast tumor may benefit from a mastectomy to relieve her or his symptoms even though it may not improve her or his chances of survival.
Cancer prevention
Patients at increased risk of developing cancer due to factors such as genetic abnormalities or a family history of cancer may undergo surgery to remove an organ before cancer develops in it (prophylactic surgery). For example, women with a BRCA1/2 gene mutation who are at increased risk of developing breast and ovarian cancer may undergo a prophylactic mastectomy or oophorectomy.
Reconstruction
Cancer patients may require surgery to reconstruct anatomic defects or to address functional loss or disfigurement resulting from tumor resection or complications from cancer treatment. The most common reconstructive procedures are for breast cancer, head and neck cancer, extremity sarcomas, and skin cancers.
Oncologic emergencies
Surgery may occasionally be performed for cancer patients experiencing severe effects of their disease or treatment. Patients with gastrointestinal cancers or those who have had previous abdominal surgery may develop intestinal blockage (obstruction) either from the cancer itself or from scar tissue (adhesions). If the obstruction does not resolve with conservative nonsurgical measures, surgery may be needed to remove
or bypass the site of intestinal obstruction. Brain tumors that cause excessive pressure from bleeding or cancer growth may require craniotomy surgery to open the skull and relieve the pressure on the brain.
Radiation Therapy
Radiation therapy is the use of high-energy x-rays (photons) or charged particles (i.e., electrons, protons) to eradicate cancer cells. Radiation therapy acts directly or indirectly to damage the DNA of the cancer cells. Radiation can damage the DNA in many ways, but it is double-strand breaks that are lethal to the cancer cell (Hall and Giaccia, 2012). Radiation therapy is a first-line therapy as part of a curative approach for many cancer types and is also used to help palliate symptoms such as pain in patients with metastatic cancer.
Table 4-5 summarizes the various methods used to deliver radiation therapy, including external beam radiation therapy (EBRT), brachytherapy, intraoperative radiation therapy, and systemic radiation therapy.
Nearly all patients that receive adjuvant or neoadjuvant EBRT for curative or palliative purposes will undergo a CT simulation a few days to a couple of weeks prior to starting radiation. The CT simulation involves immobilizing the patient in a reproducible position and conducting a CT scan
TABLE 4-5 Different Types of Radiation Therapy Delivery Methods
| Delivery Method | Key Points |
|---|---|
| External beam radiation therapy (EBRT) | Most common form of radiation delivery, delivered externally while the patient is in a secured, immobilized position Given on an outpatient basis with no anesthesia or hospital stay Nearly all types of cancer are treated with this form of radiation |
| Brachytherapy | Radioactive sources are placed in a cavity near the tumor or directly into the tissue Patient is generally sedated and may have a hospital stay Commonly used with EBRT or as the sole treatment for cancers of the uterus, cervix, prostate, and breast |
| Intraoperative radiation therapy | Delivery of a single, high dose of radiation directly to the tumor bed in the operating room Requires specialized, mobile linear accelerators that use electrons or very low energy photons to deliver the radiation Most commonly used for breast cancer and for recurrent tumors in patients who have had prior EBRT |
| Systemic radiation therapy | Delivery of radioactive isotopes either by injection or infusion (e.g., radium-223 injections to treat bone metastases in prostate cancer) Isotopes may be linked to monoclonal antibodies to help target the radiation to tumor cells |
in that position. EBRT may be further classified by the radiation therapy techniques listed in Table 4-6.
The unit used to quantify the absorbed radiation dose is the gray (Gy). Most cancers are treated with radiation doses on the order of 45–60 Gy. This total dose is generally divided into a series of small doses (fractions) that are typically delivered once per day over a period of several weeks. This fractionation allows for reoxygenation of the tumor cells between treatments (oxygen is critical for making the DNA damage permanent) and helps time the radiation to when the tumor cells are most susceptible to it. Fractionation also allows normal cells to repair DNA damage and
TABLE 4-6 Common EBRT Radiation Therapy Techniques
| Delivery Method | Key Points | |
|---|---|---|
| 3-D conformal radiation therapy (3DCRT) |
|
|
| Intensity-modulated radiation therapy (IMRT)/volumetric-modulated arc radiotherapy (VMAT) |
|
|
| Stereotactic body radiation therapy (SBRT) |
|
|
| Stereotactic radiosurgery (SRS) and stereotactic radiotherapy (SRT) |
|
|
NOTE: Gy = gray, a unit denoting the joules of radiative energy deposited per kilogram of body mass.
permits the stem cells of normal tissues to repopulate the damaged tissues, both of which help to reduce toxicity (Hall and Giaccia, 2012). Different fractionation schedules include
- Conventional fractionation: uses daily fraction sizes of 1.8–2 Gy resulting in radiation courses that are approximately 5–6 weeks in length on average (25–30 treatments to achieve a total dose of 45–60 Gy). It is the most commonly used schedule (Ahmed et al., 2014).
- Hypofractionation: uses daily fraction sizes (usually >2.5 Gy/fraction) delivered over a smaller number of treatments to achieve the same biological effect as conventional fractionation over a shorter period of time. Most commonly used in breast cancer (see Chapter 5).
- Hyperfractionation: uses small fraction sizes of 1.2–1.5 Gy given two or three times per day (separated by at least 4–6 hours) over several weeks; it is the least commonly used schedule. The rationale for this schedule is that the lower radiation doses used to deliver each fraction should minimize the damage to surrounding tissues, which can then repair the radiation damage; tumor cells are less capable of repairing their damaged DNA. Hyperfractionation is used for cancers that are known to proliferate rapidly, such as small-cell lung cancer, and for patients who need to go undergo re-irradiation to an area that had been irradiated in the past.
The adverse effects of radiation therapy are generally classified as acute (those that happen during the course of radiation and last for several weeks to a few months) and late-onset. Fatigue is a common acute, systemic effect that can occur when any region of the body is treated with radiation therapy. Skin irritation is a local, acute adverse effect that occurs with most courses of EBRT. The severity of the irritation depends on the total dose of radiation. Aside from fatigue and skin irritation, all other acute adverse effects of radiation depend on the site that is treated. Acute, long-term, and late-onset effects of radiation are described in more detail for breast cancer in Chapter 5 and for lung cancer in Chapter 6.
Other Local Therapies
While surgery and radiation therapy are the standard local therapies for cancer, other local therapy approaches are used in selected situations. In this section, three of them are briefly discussed: ablation, embolization, and local administration of drugs or immunotherapies.
Ablation—a technique that uses thermal energy (heat) to destroy tumor tissue—is usually performed as an outpatient procedure without general anesthesia by an interventional radiologist.
Using real-time imaging (e.g., ultrasound, CT scan, MRI, or endoscopy) a needle is advanced through the skin into a tumor. Once it is positioned, thermal energy in the form of radio waves (i.e., radiofrequency ablation), microwaves (i.e., microwave ablation), or laser or ultrasound energy is applied at the tip of the needle to destroy adjacent tissues by increasing the tissue temperature to approximately 50°C (Ahmed et al., 2011). Other ablation techniques use freezing (cryoablation) to destroy tumor cells. Ablation is most effective for relatively small tumors (i.e., 3 cm or less) in anatomically favorable locations, such as the liver or kidney. Thermal ablation is operator-dependent; expertise in this procedure may not be available at all institutions, and is an important consideration when recommending nonsurgical approaches to patients.
Minimally invasive tumor ablation should completely eradicate all the targeted tumor cells with minimal injury to surrounding tissues. Ablative techniques have been used to treat precancerous lesions or early-stage cancers with low risk of lymph node metastases since regional lymph nodes, which are a potential site of metastasis for more advanced cancers, are typically not treated. Ablation has also been used to treat metastatic lesions when a patient’s prognosis may be poor and the morbidity that would accompany more extensive surgery should be avoided. Many of the patients who are poor candidates for surgery are elderly patients or patients with medical conditions such as pulmonary, cardiac, or liver disorders that increase their risk of complications from open surgery and general anesthesia. Among the cancers typically treated with ablation are urologic cancers (prostate, kidney), liver metastases and primary tumors, esophageal metaplasia (Barrett’s esophagus) and early-stage esophageal cancer, lung metastases and primary cancers, and small breast cancers (Capitanio and Montorsi, 2016; Fernando, 2008; Hansen et al., 2015; Harris et al., 2017; Luo et al., 2017; McClure et al., 2017; Rajaram and Hofstetter, 2018; Schwartzberg et al., 2018; Simmons et al., 2016).
Embolization is another local therapy used by interventional radiologists, most commonly for the treatment of hepatocellular cancer. Embolization of a tumor involves the injection of small particles into the blood vessels supplying the tumor. Embolization particles are directed selectively to the tumor using a catheter that is advanced through the arterial system. The types of embolization include “bland” embolization (i.e., transarterial embolization) using uncoated microspheres; transarterial chemoembolization, using a chemotherapy emulsion or chemotherapy-eluting beads; and radioembolization, using beads containing a radioactive isotope. Embolization kills cancer cells by disrupting the blood and nutrient supply to a tumor.
Embolization is sometimes used in combination with ablation, radiation, or surgery. Patients may receive multiple embolization procedures over time.
Another local therapy is the localized (rather than systemic) administration of a drug or immune therapy. For example, heated intraperitoneal chemotherapy involves the application of drugs in the abdominal cavity to treat cancer cells growing on peritoneal surfaces. In intravesicular therapy a transurethral catheter is placed into the bladder to allow the application of immunotherapy or chemotherapy for the local treatment of a bladder tumor.
Systemic Therapies
Systemic therapies are drug or cellular therapies that are given intravenously, orally, or by injection into the muscle or subcutaneous tissue. The categories of systemic therapies included in this section are: chemotherapy, endocrine therapy, targeted therapy, immunotherapy, and stem cell therapy. The choice to use any of these therapies depends on the type of cancer, its staging, and patient characteristics such as genetics. Most systemic therapies are administered repeatedly over time; for example, endocrine therapy agents for breast cancer are taken on a daily basis, usually for 1 year or longer.
The U.S. Food and Drug Administration (FDA) approves the use of new systemic therapies for specific cancers based on the results of clinical trials. However, systemic therapies for cancer change rapidly, and the distinctions between the categories of therapies can be fluid. The descriptions below provide a general overview of the different types of systemic therapies. Chapter 8 provides more information on new and emerging systemic therapies for cancer such as immunotherapy. The use of these therapies specifically for breast cancer, lung cancer, and selected other cancers is discussed in Chapters 5, 6, and 7, respectively.
Chemotherapy
Chemotherapy stops the growth of cancer cells, either by killing the cells or by stopping them from dividing; it is given in many different phases of cancer treatment. Chemotherapy is nonselective, that is, it has effects on rapidly growing cells regardless of whether they are cancer cells or normal cells. In this way chemotherapy differs from “targeted” therapies that preferentially affect cancer cells. Different chemotherapy agents are used for different tumor types (e.g., solid tumors, blood cancers, localized, and metastatic), and they are frequently used in combination with one another (see Table 4-7). Chemotherapy regimens and schedules are determined based on the results of clinical trials.
Chemotherapy can be the sole or primary curative intent treatment (e.g., for acute myelogenous leukemia), or for some cancers it may be used
TABLE 4-7 Examples of Chemotherapy Regimens
| Chemotherapy Regimen | Disease Type | Phase of Care |
|---|---|---|
| Doxorubicin and cyclophosphamide in combination followed by paclitaxel (also called AC-T: Adriamycin® and Cytoxan® followed by Taxol®) | Breast cancer | Adjuvant |
| Docetaxel and cyclophosphamide (also called TC: Taxotere and Cytoxan®) | Breast cancer | Adjuvant |
| Folinic acid (also called leucovorin) with fluorouracil, and oxaliplatin (also called FOLFOX) | Colon cancer | Metastatic |
| Carboplatin and paclitaxel | Ovarian cancer | Adjuvant and recurrent |
| Cisplatin and pemetrexed | Nonsquamous non-small-cell lung cancer | Adjuvant |
with radiation (e.g., lymphoma or small-cell lung cancer). Adjuvant chemotherapy attempts to decrease the risk of systemic cancer recurrence and death. Neoadjuvant chemotherapy is used to decrease the size of the tumor and improve the outcomes of surgical resection. Chemotherapy is also used in recurrent or metastatic cancer for the palliation of symptoms and prolonged survival. New chemotherapy drugs and combinations of drugs are constantly being developed and tested, and the standards of care continue to evolve in response to results from clinical trials.
Endocrine Therapy
Endocrine therapies are primarily used in breast and prostate cancer in both the adjuvant and metastatic settings. Approximately 75% of breast cancers express hormone receptors (HRs) and are termed HR-positive (either ER- or PR-positive, or both) (Yersal and Barutca, 2014). Endocrine therapy plays a large role in the treatment of HR-positive breast cancer (see Chapter 5) through two mechanisms: the first mechanism involves blocking the ER through the use of selective ER modulators such as tamoxifen or selective ER degraders such as fulvestrant; the second mechanism decreases estrogen levels via ovarian suppression or through the use of aromatase inhibitors (aromatase converts other hormones into estrogen).
Targeted Therapy
Advances in molecular biology and genomics have identified a number of genetic aberrations that lead to the synthesis of abnormal proteins that
drive tumor formation and growth. Anticancer agents have been developed to bind these specific proteins or receptors (i.e., targets) in the tumor, inhibiting their growth and leading to cell death. A critical requirement for these agents to be effective is that the target is expressed in the tumor and thus is a biomarker that predicts the efficacy of the drug. Targeted therapy is a form of precision or personalized medicine—that is, treatment that is based on specific features of a patient’s tumor. Most targeted therapies are either small-molecule drugs or monoclonal antibodies. Examples include drugs such as trastuzumab, ado-trastuzumab emtansine, and pertuzumab, which target the HER2 oncogene; and gefitinib, erlotinib, afatinib, dacomitinib and osimertinib, which target the epidermal growth factor receptor (EGFR).
For targeted therapies, FDA typically approves the drug along with a companion diagnostic test. The tests are used to determine if the biomarker is present in the patient’s biopsy tissue and therefore if the cancer is likely to respond to the targeted therapy. Examples include vemurafenib and BRAF V600 mutation testing, crizotinib and ALK rearrangement testing, and osimertinib and EGFR mutation testing.
Targeted therapies are much more effective than classic chemotherapeutic agents and have been shown to result in significantly improved survival for many patients with advanced cancers. Unlike classic chemotherapy where a drug is administered for a short duration because of its toxicity to normal tissues, most targeted agents are used until the tumor progresses, indicating they are no longer effective. Such long-term administration may result in a variety of chronic toxicities, including diarrhea, abnormal liver function, and fatigue, that may affect a patient’s quality of life. The majority of targeted therapies are oral agents taken daily, which may result in continuous adverse effects, unlike the cyclic toxicity of many chemotherapy agents. Daily administration combined with the effectiveness of these agents is gradually making the management of certain cancers similar to the management of other chronic diseases such as diabetes, chronic obstructive pulmonary disease, and heart disease.
Immunotherapy
Immunotherapy uses the body’s immune system to fight cancer. Different immunotherapies have different therapeutic goals, including the regulation of osteoclast function, the delivery of cytotoxic drugs into tumor cells, the blockade of oncogenic pathways and neo-angiogenesis, and the modulation of the immune response (Chiavenna et al., 2017). Clinicians have known for many years that the use of nonspecific immune system enhancers, such as interleukin-2 and interferon, can result in improved clinical responses for some patients with kidney cancer or melanoma, but unfortunately these approaches proved to be effective for only a few of
these patients. Other immunotherapy approaches, such as the use of cancer vaccines and adoptive T-cell therapy, have also had limited to modest success. In recent years an advanced form of adoptive T-cell therapy, called chimeric antigen receptor (CAR) T-cell therapy, in which a patient’s immune cells are collected through apheresis, programmed to fight cancer in the laboratory, and re-infused into the patient, has had remarkable success in treating leukemia and lymphoma (see Chapter 8).
Immunologic approaches that rely on designing antibodies that bind the proteins that drive cancer growth have had moderate success. Recent advances in understanding the mechanisms of host immune tolerance to tumor-specific antigens have led to the development of immune checkpoint proteins, such as monoclonal antibodies that bind to receptors on the tumor cell surface, against the checkpoint proteins anti-programmed cell death (PD)-1 and anti-PD ligand-1 (PDL-1). These immune checkpoint proteins are significantly improving survival and have been approved to treat a number of different cancers, including some types of breast cancer, non-small-cell lung cancer, and several other cancers; they are discussed in more detail in Chapter 8.
These agents, however, can cause immune-related adverse effects if the patient’s activated immune system attacks normal organs, potentially resulting in diabetes, hypothyroidism, adrenal failure, or chronic diarrhea from bowel inflammation. These adverse effects can affect every organ in the body and can be fatal. Such effects need to be recognized and managed aggressively by oncologists.
Hematopoietic Stem Cell Transplant
Hematopoietic stem cell transplant (HSCT), also called bone marrow transplant, is frequently used for patients with hematologic cancers. HSCT is indicated when the initial chemotherapy for high-risk leukemias or plasma cell blood cancers (myelomas) is complete in order to reduce the risk of relapse and also after additional chemotherapy for recurrent or persistent lymphomas.
There are two broad categories of HSCT. The first is autologous HSCT (using the patient’s own stem cells), which is often used for patients with lymphomas or myeloma to help their bone marrow recover from high doses of chemotherapy. For example, patients with lymphoma may achieve remission with chemotherapy, but the high doses kill their bone marrow cells. These patients can then be given their own stem cells to help the bone marrow recover, with hospitalization lasting approximately 2–3 weeks.
The second category of HSCT is allogeneic HSCT, which entails receiving cells from a donor such as a sibling or even an unrelated individual. Allogeneic HSCT is often used for patients with acute leukemias, with the
donor’s cells replacing the patient’s bone marrow and killing any remaining malignant cells. This process is lengthier and has significantly more risk than autologous HSCT. For example, patients with high-risk acute leukemia who are in remission may receive 5–7 days of chemotherapy or radiation, or both, to destroy their bone marrow prior to receiving a donor’s stem cells. The donor’s stem cells are then infused into the patient, with the patient typically hospitalized for 4–5 weeks. There are risks of significant morbidity and mortality that may persist for several months or even a couple of years after transplant. Morbidity and mortality are due to the toxicity of the transplant and any recurrent disease that may develop. Research is under way to develop tools to assess patients’ risks for these adverse outcomes (Sorror et al., 2005).
Allogenic HSCT toxicity may be influenced by pretransplant chemotherapy, by the use of radiation therapy, and by the chronic immunosuppression that patients receive to prevent graft-versus-host disease (see Chapter 9); all of these increase a patient’s risk for infection and organ dysfunction. Patients’ pre-existing comorbidities may also substantially increase their risk of death after this high-risk transplant procedure. Patients who survive several years after transplant continue to be at risk for significant late-onset effects in multiple organs, including bone disorders, cognitive dysfunction, new primary cancers, cardiac disease, pulmonary disease, chronic fatigue, and pain (Bhatia et al., 2017; Inamoto and Lee, 2017).
PROGNOSIS
The term “prognosis” is commonly used to indicate what patients may expect with respect to the trajectory of their cancer and how long they might live. But as can be seen from Figure 4-1, prognosis is a difficult and complex term to define for a specific patient because of the many factors that may influence a patient’s choice of and response to treatment in both the short term and long term. Furthermore, the prognoses for different cases of a single type and even a single subtype of cancer are also highly variable and also depend on many factors, including the actual cancer diagnosis (tumor staging, grade, and biomarkers), the expertise of the care team, and the characteristics of the patients themselves including the presence of comorbidities and the particular patient’s ability to tolerate and participate in treatments. As shown in Figure 3-8 (see Chapter 3), the 5-year survival estimates for the cancers considered in this report differ considerably by type of cancer and by whether the disease is localized (typically stage I/II) or has metastasized regionally (stage III) or to distant parts of the body (stage IV).
Prognosis can encompass a variety of cancer outcomes, including survival (typically estimated for 1, 5, or occasionally 10 years after diagnosis),
recurrence, remission, the likelihood of developing new primary cancers, and the occurrence and persistence of cancer-related impairments that may lead to functional limitations in normal activities, such as walking, lifting, and cognition, as well as reduced quality of life (see Chapter 9). The committee emphasizes that characterizing survival after a cancer diagnosis entails more than simply providing a number for how long a person may live. Some cancer survivors may experience few long-term effects from their disease and treatment, for instance, while others may live for years but with debilitating effects that make their lives extremely difficult. These effects may require further treatment or result in impairments that are not captured by a number indicating how long the patient has lived.
Patient self-reported outcomes have been shown to have a strong prognostic value for overall survival across varying cancer diagnoses and populations over and above those predictions associated with standard clinical and sociodemographic factors (Atherton et al., 2015; Husson et al., 2020; Quinten et al., 2020; Sim et al., 2020). In one study of patients with non-small-cell lung cancer, patient self-reported outcomes on validated quality-of-life questionnaires did a better job of predicting the risk of death in these patients than the standard prognostic factors (Movsas et al., 2009).
Recurrence
In the best-case scenario, a patient who is diagnosed with cancer will achieve a state in which there is no evidence of disease—that is, no cancer can be detected by the usual clinical tests—and will apparently be cured. For certain cancers, such as leukemia, this state is called “remission.” Unfortunately, many patients who have an initial period with no evidence of cancer will later experience recurrence (sometimes described as “relapse”). Recurrence means that the cancer has returned after a period in which the cancer seemed to have been resolved. In the case of solid tumor (non-hematologic) cancers, recurrence can be either a local or a distant phenomenon. Local recurrence means that the cancer has returned in the same location in which it was previously diagnosed—for example, a breast cancer recurring after surgery within the residual breast tissue. Distant recurrence means that the cancer has returned in a new, distant location in the body, such as a brain cancer that occurs several months after the surgical removal of a primary lung cancer.
Conventions concerning the staging of recurrent cancer can be confusing. In the AJCC staging system discussed earlier, the cancer stage determined at the time of diagnosis does not change with recurrence, even if the cancer is more widespread at recurrence (AJCC, 2017). Thus, it can be important to distinguish cancer stage (assigned at time of diagnosis) from
the current clinical status of cancer. For example, a patient with early-stage colon cancer that has recurred in the liver after initial colectomy may be described as having “stage I cancer, with distant recurrence in the liver.”
In the majority of cases, recurrent cancers are more difficult to treat than the initial cancer. This is because recurrent cancers occur despite initial attempts at treatment, and often the extent of cancer is greater at the time of recurrence than at the time of the initial diagnosis. Nevertheless, the treatment of recurrent cancers follows a similar approach to that used for treating a new cancer. Patients with recurrent cancer usually have a biopsy to confirm the cancer recurrence and imaging tests to determine the extent of the recurrent cancer (sometimes called “restaging”). The treatment of recurrent cancers may involve surgery, radiation, chemotherapy, or other interventions, as with the initial cancer. The term “salvage therapy” is sometimes used to describe curative-intent treatment of locally recurrent cancers.
New Primary Cancers
One reason for closely monitoring cancer patients who have been successfully treated is the possibility of the development of new primary cancers (sometimes called “second primary cancers” or more recently “subsequent primary cancers”). These new primary cancers should be differentiated from metastases or relapse of the original, index cancer. There are a number of potential etiologies for the development of new primary cancers among those with a prior history of cancer (see Figure 4-2). Patients may develop cancers because of exposure to carcinogens, such as tobacco products, or as a result of lifestyle behaviors or their environmental exposures. For example, a patient who has been successfully treated for lung cancer may subsequently develop a new lung cancer or a head and neck cancer. Exposure to viruses, such as the human papilloma virus, may predispose a patient to develop more than one head and neck cancer. Some patients may have a genetic predisposition to cancer and develop new cancers after successful treatment for an initial cancer. For example, mutations in genes such as BRCA1/2 increase the risk of several cancers, including breast cancer, ovarian cancer, colorectal cancer, and prostate cancer.
Additionally, some cancer treatments can themselves cause cancer. Radiation therapy to the chest for patients with certain kinds of lymphoma can lead to the later development of cancers such as breast cancer (Dracham et al., 2018) and thyroid cancers. Chemotherapy for testicular and ovarian cancer has been associated with the development of leukemia (Travis et al., 1999, 2000), while radiation therapy has been associated with early-onset colon cancer among childhood cancer survivors (Daniel
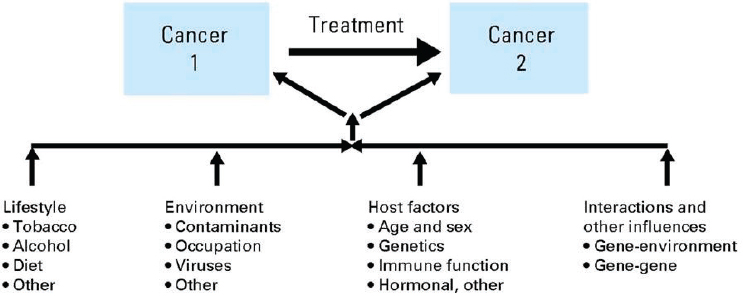
SOURCE: Wood et al., 2012, with permission.
et al., 2015). Stem cell transplantation has also been associated with a number of cancers (Bhatia et al., 1996), mainly due to chemotherapy or radiation therapy. Finally, a patient may develop a new primary cancer that is unrelated to the first cancer. New primary cancers may occur anywhere from months to years after the original cancer has been treated. A recent analysis of SEER data from 1992–2017 of more than 1,537,101 adult cancer survivors who survived at least 5 years after their primary diagnosis found that, compared with the general population, men had an 11% higher risk and women a 10% higher risk of developing a new primary cancer and that men had a 45% higher risk and women a 33% higher risk of dying from the new primary cancer; 58.8% of the survivors lived longer than 10 years after the diagnosis of their first cancer (mean age of survivors was 60.8 years). The most common new primary cancers in men were lung (19.1%), prostate (13.7%), urinary bladder (11.1%), and colorectal cancers (10.1%) and in women the new primary cancers with the greatest incidence were lung (19.3%), breast (17.3%), colorectal (11.0%), and uterine corpus cancers (7.4%) (Sung et al., 2020). Given the significant percentage of new primary cancers associated with lifestyle risk factors (e.g., obesity and smoking), primary care clinicians have an important role in counseling survivors on risk mitigation and preventive care approaches including the need for behavioral changes (e.g., weight loss, tobacco cessation), screenings, and immunizations (Ganz and Casillas, 2020; see Chapter 10 for more information on the cancer care team). Targeted cancer screening for early detection of new primary cancers and additional risk counseling for young adult survivors (ages 20 to 39 years) is critical as this population has lower survival rates compared with older individuals when diagnosed with the same new primary cancer (Ganz and Casillas, 2020).
FINDINGS AND CONCLUSIONS
Findings
- There are screening recommendations for a few cancers, such as breast and lung cancer, but most cancers do not have any reliable screening tests. As a result many people without known risk factors, particularly younger people, may present with more advanced cancers when detected.
- While presenting symptoms may suggest cancer, additional procedures, including biopsies, imaging, and laboratory tests, are necessary to definitively diagnose cancer.
- Cancer staging helps determine the extent and prognosis of the cancer and treatment options. There is well-established guidance for staging different cancers. The widely used AJCC staging system is defined differently for each cancer type and ranges from stage I (localized/early stage) to stage IV (metastatic/advanced stage).
- Characterization of a cancer typically requires biopsies, including an assessment of immunohistochemical markers, if appropriate, and molecular assays for biomarkers such as genetic mutations in cancer cells or the presence of specific receptor proteins on the surface of cancer cells.
- While cancers continue to be classified on the basis of the primary cancer site, the characterization of cancer biomarkers is of increasing importance in defining cancer prognosis and identifying appropriate cancer treatments that are specific for an individual’s cancer.
- Clinical practice guidelines developed by professional organizations such as ASCO and NCCN provide recommendations on standards of care for many kinds of cancer, including breast cancer and lung cancer. However, there is not universal adherence to the guidelines for a number of reasons, including the need to individualize treatment for a specific patient, clinicians’ awareness of and agreement with the guidelines, patient preferences, and the lack of sufficient processes to ensure that patients receive guidelines-recommended care after such care is prescribed.
- Surgery is a first-line treatment for many cancers, particularly for localized disease. The adverse effects of cancer surgery may include loss of range of motion, chronic pain, disfigurement, and infection.
- Radiation treatment is also a first-line treatment for many cancers and several treatment protocols may be used to improve its effectiveness and reduce adverse effects. Radiation therapy can be used as part of the curative approach to cancer as well as to palliate the symptoms of metastatic disease. The adverse effects of radiation
- therapy can be acute and chronic and are dependent on the part of the body exposed to radiation as well as the total dose, daily dose, and volume of normal tissue exposed during the course of treatment.
- Other local therapies such as ablation and embolization may be used for some cancers, but are less common.
- Systemic therapies include a number of standard-of-care agents such as chemotherapy, endocrine therapy, targeted therapy, immunotherapy, and stem cell therapy.
- Chemotherapy agents are often given in combination and can have both acute and long-term adverse effects.
- Endocrine therapy may be used for hormone-receptor-positive cancers, such as some breast cancers. It may be given for years after localized treatments and also is associated with acute and long-term adverse effects.
- Newer systemic therapies that are increasing the arsenal of potential anticancer agents include targeted therapies and immunotherapies; however, little is known about the long-term and late-onset effects of these therapies.
- Although prognosis often focuses on the likelihood of cure, progression, or death, it is increasingly recognized that prognosis also should consider the likelihood of persistent cancer- or treatment-related impairments and functional limitations.
- As the arsenal of effective treatments for cancer has grown, the prognosis of many cancers has improved in terms of survival, but prognosis is complex and does not typically include an assessment of the long-term adverse effects and impairments that cancer survivors may experience as a result of their cancer or its treatment.
- Patients who are considered to be “cancer free” after treatment are subject to cancer recurrence or new primary cancers even years after they were first diagnosed with cancer.
Conclusions
- The cancer care trajectory for each patient and survivor is unique and depends on the characteristics of his or her cancer, its treatment, and factors such as age and comorbidities.
- Screening can help to detect cancers early, when they are most likely to be cured; however, screening tests are not always used and are not available for many cancers.
- Many systemic therapies beyond chemotherapy, such as immunotherapy and targeted therapy, are now used to treat localized and advanced cancer.
- The increasingly common use of new molecular and genomic assays to refine the diagnosis of many cancers can help to identify patients who may benefit from targeted treatments.
REFERENCES
Ahmed, M., C.L. Brace, J.F.T. Lee, and S.N. Goldberg. 2011. Principles of and advances in percutaneous ablation. Radiology 258(2):351–369.
Ahmed, K.A., C.R. Correa, T.J. Dilling, N.G. Rao, R. Shridhar, A.M. Trotti, R.B. Wilder, and J.J. Caudell. 2014. Altered fractionation schedules in radiation treatment: A review. Seminars in Oncology 41(6):730–750.
AJCC (American Joint Committeee on Cancer). 2017. AJCC cancer staging manual, 8th edition. New York: Springer International Publishing.
Atherton, P., B. Stutchbury, D.-Y. Wang, D. Jethwa, R. Tsang, E. Meiler-Rodriguez, P. Wang, N. Bate, R. Zent, I.L. Barsukov, B.T. Goult, D.R. Critchley, and C. Ballestrem. 2015. Vinculin controls talin engagement with the actomyosin machinery. Nature Communications 6(1):10038.
Bhatia, S., L.L. Robison, O. Oberlin, M. Greenberg, G. Bunin, F. Fossati-Bellani, and A.T. Meadows. 1996. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. New England Journal of Medicine 334(12):745–751.
Bhatia, S., S.H. Armenian, and W. Landier. 2017. How I monitor long-term and late effects after blood or marrow transplantation. Blood 130(11):1302–1314.
Bierbaum, M., F. Rapport, G. Arnolda, B. Nic Giolla Easpaig, K. Lamprell, K. Hutchinson, G. P. Delaney, W. Liauw, R. Kefford, I. Olver, and J. Braithwaite. 2020. Clinicians’ attitudes and perceived barriers and facilitators to cancer treatment clinical practice guideline adherence: A systematic review of qualitative and quantitative literature. Implementation Science 15(1):39.
Bristow, R.E., J. Chang, A. Ziogas, B. Campos, L.R. Chavez, and H. Anton-Culver. 2015. Impact of National Cancer Institute comprehensive cancer centers on ovarian cancer treatment and survival. Journal of the American College of Surgeons 220(5):940–950.
Capitanio, U., and F. Montorsi. 2016. Renal cancer. Lancet 387(10021):894–906.
Chagpar, R., Y. Xing, Y.-J. Chiang, B.W. Feig, G.J. Chang, Y.N. You, and J.N. Cormier. 2012. Adherence to stage-specific treatment guidelines for patients with colon cancer. Journal of Clinical Oncology 30(9):972–979.
Cheson, B.D., R.I. Fisher, S.F. Barrington, F. Cavalli, L.H. Schwartz, E. Zucca, T.A. Lister, Australasian Leukaemia and Lymphoma Group Alliance, Eastern Cooperative Oncology Group, European Mantle Cell Lymphoma Consortium, Italian Lymphoma Foundation, European Organisation for Research, Treatment of Cancer/Dutch Hemato-Oncology Group, Grupo Español de Médula Ósea, German High-Grade Lymphoma Study Group, German Hodgkin’s Study Group, Japanese Lymphorra Study Group, Lymphoma Study Association, NCIC Clinical Trials Group, Nordic Lymphoma Study Group, and United Kingdom National Cancer Research Institute. 2014. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. Journal of Clinical Oncology 32(27):3059–3068.
Chiavenna, S.M., J.P. Jaworski, and A. Vendrell. 2017. State of the art in anti-cancer mAbs. Journal of Biomedical Science 24(1):15.
Daniel, C.L., C.L. Kohler, K.L. Stratton, K.C. Oeffinger, W.M. Leisenring, J.W. Waterbor, K.F. Whelan, G.T. Armstrong, T.O. Henderson, K.R. Krull, L.L. Robison, and P.C. Nathan. 2015. Predictors of colorectal cancer surveillance among survivors of childhood cancer treated with radiation: A report from the Childhood Cancer Survivor Study. Cancer 121(11):1856–1863.
Dracham, C.B., A. Shankar, and R. Madan. 2018. Radiation induced secondary malignancies: A review article. Radiation Oncology Journal 36(2):85–94.
ECOG-ACRIN (Eastern Cooperative Oncology Group-American College of Radiology Imaging Network) Research Group. 2020. ECOG Performance Status. https://ecog-acrin.org/resources/ecog-performance-status (accessed November 19, 2020).
Fang, P., W. He, D. Gomez, K.E. Hoffman, B.D. Smith, S.H. Giordano, R. Jagsi, and G.L. Smith. 2018. Racial disparities in guideline-concordant cancer care and mortality in the United States. Advances in Radiation Oncology 3(3):221–229.
Fernando, H.C. 2008. Radiofrequency ablation to treat non-small cell lung cancer and pulmonary metastases. Annals of Thoracic Surgery 85(2):S780–S784.
Ganz, P.A., and J.N. Casillas. 2020. Incorporating the risk for subsequent primary cancers into the care of adult cancer survivors moving beyond 5-year survival. JAMA 324(24):2493–2495.
Hall, E.J., and A.J. Giaccia. 2012. Radiobiology for the radiologist, seventh ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins.
Hansen, P.D., M.A. Cassera, and R.F. Wolf. 2015. Ablative technologies for hepatocellular, cholangiocarcinoma, and metastatic colorectal cancer of the liver. Surgical Oncology Clinics 24(1):97–119.
Harris, K., J. Puchalski, and D. Sterman. 2017. Recent advances in bronchoscopic treatment of peripheral lung cancers. Chest 151(3):674–685.
Husson, O., B.H. de Rooij, J. Kieffer, S. Oerlemans, F. Mols, N.K. Aaronson, W.T.A. van der Graaf, and L.V. van de Poll-Franse. 2020. The EORTC QLQ-c30 summary score as prognostic factor for survival of patients with cancer in the “real-world”: Results from the population-based profiles registry. The Oncologist 25(4):e722–e732.
Inamoto, Y., and S.J. Lee. 2017. Late effects of blood and marrow transplantation. Haematologica 102(4):614–625.
IOM (Institute of Medicine). 2006. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press.
IOM. 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
Javadi, S., D.M. Ganeshan, A. Qayyum, R.B. Iyer, and P. Bhosale. 2016. Ovarian cancer, the revised FIGO staging system, and the role of imaging. American Journal of Roentgenology 206(6):1351–1360.
Jazieh, A., M.O. Alkaiyat, Y. Ali, M.A. Hashim, N. Abdelhafiz, and A. Al Olayan. 2019. Improving adherence to lung cancer guidelines: A quality improvement project that uses chart review, audit and feedback approach. BMJ Open Quality 8(3):e000436.
Karnofsky, D., and J. Burchenal. 1949. The clinical evaluation of chemotherapeutic agents in cancer. In Evaluation of chemotherapeutic agents. Edited by C. MacLeod. New York: Columbia University Press. Pp. 191–205.
Keating, N.L., and L.E. Pace. 2018. Breast cancer screening in 2018: Time for shared decision making. JAMA 319(17):1814–1815.
Liu, M.C., G.R. Oxnard, E.A. Klein, D. Smith, D. Richards, T.J. Yeatman, A.L. Cohn, R. Lapham, J. Clement, A.S. Parker, M.K. Tummala, K. McIntyre, M.A. Sekeres, A.H. Bryce, R. Siegel, X. Wang, D.P. Cosgrove, N.R. Abu-Rustum, J. Trent, D.D. Thiel, C. Becerra, M. Agrawal, L.E. Garbo, J.K. Giguere, R.M. Michels, R.P. Harris, S.L. Richey, T.A. McCarthy, D.M. Waterhouse, F.J. Couch, S.T. Wilks, A.K. Krie, R. Balaraman, A. Restrepo, M.W. Meshad, K. Rieger-Christ, T. Sullivan, C.M. Lee, D.R. Greenwald, W. Oh, C.-K. Tsao, N. Fleshner, H.F. Kennecke, M.F. Khalil, D.R. Spigel, A.P. Manhas, B.K. Ulrich, P.A. Kovoor, C. Stokoe, J.G. Courtright, H.A. Yimer, T.G. Larson, C. Swanton, M.V. Seiden, S.R. Cummings, F. Absalan, G. Alexander, B. Allen, H. Amini, A.M. Aravanis, S. Bagaria, L. Bazargan, J.F. Beau-sang, J. Berman, C. Betts, A. Blocker, J. Bredno, R. Calef, G. Cann, J. Carter, C. Chang,
H. Chawla, X. Chen, T.C. Chien, D. Civello, K. Davydov, V. Demas, M. Desai, Z. Dong, S. Fayzullina, A. P. Fields, D. Filippova, P. Freese, E.T. Fung, S. Gnerre, S. Gross, M. Halks-Miller, M.P. Hall, A.-R. Hartman, C. Hou, E. Hubbell, N. Hunkapiller, K. Jagadeesh, A. Jamshidi, R. Jiang, B. Jung, T. Kim, R.D. Klausner, K.N. Kurtzman, M. Lee, W. Lin, J. Lipson, H. Liu, Q. Liu, M. Lopatin, T. Maddala, M.C. Maher, C. Melton, A. Mich, S. Nautiyal, J. Newman, J. Newman, V. Nicula, C. Nicolaou, O. Nikolic, W. Pan, S. Patel, S.A. Prins, R. Rava, N. Ronaghi, O. Sakarya, R.V. Satya, J. Schellenberger, E. Scott, A.J. Sehnert, R. Shaknovich, A. Shanmugam, K.C. Shashidhar, L. Shen, A. Shenoy, S. Shojaee, P. Singh, K.K. Steffen, S. Tang, J.M. Toung, A. Valouev, O. Venn, R.T. Williams, T. Wu, H.H. Xu, C. Yakym, X. Yang, J. Yecies, A.S. Yip, J. Youngren, J. Yue, J. Zhang, L. Zhang, L. Zhang, N. Zhang, C. Curtis, and D.A. Berry. 2020. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Annals of Oncology 31(6):745–759.
Luo, W., Y. Zhang, G. He, M. Yu, M. Zheng, L. Liu, and X. Zhou. 2017. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: A systematic review and meta-analysis. World Journal of Surgical Oncology 15(1):126.
Mayo Clinic. 2018a. Leukemia: Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/leukemia/symptoms-causes/syc-20374373 (accessed September 9, 2020).
Mayo Clinic. 2018b. Non-Hodgkin’s lymphoma: Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/non-hodgkins-lymphoma/symptoms-causes/syc-20375680 (accessed September 16, 2020).
Mayo Clinic. 2019a. Breast cancer: Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/breast-cancer/symptoms-causes/syc-20352470 (accessed September 16, 2020).
Mayo Clinic. 2019b. Colon cancer: Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/colon-cancer/symptoms-causes/syc-20353669 (accessed September 16, 2020).
Mayo Clinic. 2019c. Mouth cancer: Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/mouth-cancer/symptoms-causes/syc-20350997 (accessed September 16, 2020).
Mayo Clinic. 2019d. Ovarian cancer: Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/ovarian-cancer/symptoms-causes/syc-20375941 (accessed September 16, 2020).
Mayo Clinic. 2019e. Skin cancer: Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/skin-cancer/symptoms-causes/syc-20377605 (accessed September 16, 2020).
Mayo Clinic. 2020a. Lung cancer: Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/lung-cancer/symptoms-causes/syc-20374620 (accessed September 16, 2020).
Mayo Clinic. 2020b. Pancreatic cancer: Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/pancreatic-cancer/symptoms-causes/syc-20355421 (accessed September 16, 2020).
Mayo Clinic. 2020c. Rectal cancer: Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/rectal-cancer/symptoms-causes/syc-20352884 (accessed September 16, 2020).
McClure, T.D., D.J.A. Margolis, and J.C. Hu. 2017. Partial gland ablation in the management of prostate cancer: A review. Current Opinion in Urology 27(2):156–160.
Movsas, B., J. Moughan, L. Sarna, C. Langer, M. Werner-Wasik, N. Nicolaou, R. Komaki, M. Machtay, T. Wasserman, and D.W. Bruner. 2009. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: An analysis of RTOG 9801. Journal of Clinical Oncology 27(34):5816–5822.
Nelson, H.D., R. Fu, A. Cantor, M. Pappas, M. Daeges, and L. Humphrey. 2016. Effectiveness of breast cancer screening: Systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Annals of Internal Medicine 164(4):244–255.
Oken, M.M., R.H. Creech, D.C. Tormey, J. Horton, T.E. Davis, E.T. McFadden, and P.P. Carbone. 1982. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 5(6):649–655.
Quinten, C., C. Kenis, M. Hamaker, A. Coolbrandt, B. Brouwers, L. Dal Lago, P. Neven, P. Vuylsteke, G. Debrock, H. Van Den Bulck, A. Smeets, P. Schöffski, U. Wedding, and H. Wildiers. 2020. The added value of geriatric assessment in evaluating a patient’s health-related quality-of-life: A study in ≥70-year-old early-stage invasive breast cancer patients. European Journal of Cancer Care 29(5):e13278.
Rajaram, R., and W.L. Hofstetter. 2018. Mucosal ablation techniques for Barrett’s esophagus and early esophageal cancer. Thoracic Surgery Clinics 28(4):473–480.
Schwartzberg, B., J. Lewin, O. Abdelatif, J. Bernard, H. Bu-Ali, S. Cawthorn, M. ChenSeetoo, S. Feldman, S. Govindarajulu, L. Jones, A. Juette, S. Kavia, R. Maganini, S. Pain, M. Shere, C. Shriver, S. Smith, A. Valencia, E. Whitacre, and R. Whitney. 2018. Phase 2 open-label trial investigating percutaneous laser ablation for treatment of early-stage breast cancer: MRI, pathology, and outcome correlations. Annals of Surgical Oncology 25(10):2958–2964.
Shah, B.A., M.M. Qureshi, S. Jalisi, G. Grillone, A. Salama, T. Cooley, K. Zaner, O. Sakai, and M.T. Truong. 2016. Analysis of decision making at a multidisciplinary head and neck tumor board incorporating evidence-based National Cancer Comprehensive Network (NCCN) guidelines. Practical Radiation Oncology 6(4):248–254.
Sim, J.-A., G. Hyun, T.M. Gibson, Y. Yasui, W. Leisenring, M.M. Hudson, L.L. Robison, G.T. Armstrong, K.R. Krull, and I.C. Huang. 2020. Negligible effects of the survey modes for patient-reported outcomes: A report from the Childhood Cancer Survivor Study. JCO Clinical Cancer Informatics 4:10–24.
Simmons, R.M., K.V. Ballman, C. Cox, N. Carp, J. Sabol, R.F. Hwang, D. Attai, M. Sabel, D. Nathanson, A. Kenler, L. Gold, C. Kaufman, L. Han, A. Bleznak, J. Stanley Smith, D. Holmes, B. Fornage, C. Le-Petross, S. Hoda, L. McCall, and K.K. Hunt. 2016. A phase II trial exploring the success of cryoablation therapy in the treatment of invasive breast carcinoma: Results from ACOSOG (Alliance) z1072. Annals of Surgical Oncology 23(8):2438–2445.
Smith, R.A., K.S. Andrews, D. Brooks, S.A. Fedewa, D. Manassaram-Baptiste, D. Saslow, and R.C. Wender. 2019. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA: A Cancer Journal for Clinicians 69(3):184–210.
Sorror, M.L., M.B. Maris, R. Storb, F. Baron, B.M. Sandmaier, D.G. Maloney, and B. Storer. 2005. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 106(8):2912–2919.
Sung, H., N. Hyun, C.R. Leach, K.R. Yabroff, and A. Jemal. 2020. Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. JAMA 324(24):2521–2535.
Tokheim, C.J., N. Papadopoulos, K.W. Kinzler, B. Vogelstein, and R. Karchin. 2016. Evaluating the evaluation of cancer driver genes. Proceedings of the National Academy of Sciences 113(50):14330–14335.
Travis, L.B., E.J. Holowaty, K. Bergfeldt, C.F. Lynch, B.A. Kohler, T. Wiklund, R.E. Curtis, P. Hall, M. Andersson, E. Pukkala, J. Sturgeon, and M. Stovall. 1999. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. New England Journal of Medicine 340(5):351–357.
Travis, L.B., M. Andersson, M. Gospodarowicz, F.E. van Leeuwen, K. Bergfeldt, C.F. Lynch, R.E. Curtis, B.A. Kohler, T. Wiklund, H. Storm, E. Holowaty, P. Hall, E. Pukkala, D.T. Sleijfer, E.A. Clarke, J.D. Boice, Jr., M. Stovall, and E. Gilbert. 2000. Treatment-associated leukemia following testicular cancer. Journal of the National Cancer Institute 92(14):1165–1171.
Unger, J.M., E. Cook, E. Tai, and A. Bleyer A. 2016. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. American Society of Clinical Oncology Education Book 35:185–198.
USPSTF (U.S. Preventive Services Task Force). 2016. Final recommendation statement: Breast cancer: Screening. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening (accessed September 17, 2020).
USPSTF. 2020a. Draft recommendation statement: Colorectal cancer: Screening. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/colorectal-cancer-screening3#tab1 (accessed December 1, 2020).
USPSTF. 2020b. Draft recommendation statement: Lung cancer: Screening. https://www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/lung-cancer-screening-2020 (accessed September 17, 2020).
USPSTF. 2020c. Recommendation topics, Grade D. https://www.uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=All&grades%5B%5D=D&searchterm= (accessed September 9, 2020).
Visser, B.C., Y. Ma, Y. Zak, G.A. Poultsides, J.A. Norton, and K.F. Rhoads. 2012. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. HPB 14(8):539–547.
Wood, M.E., V. Vogel, A. Ng, L. Foxhall, P. Goodwin, and L.B. Travis. 2012. Second malignant neoplasms: Assessment and strategies for risk reduction. Journal of Clinical Oncology 30(30):3734–3745.
Yersal, O., and S. Barutca. 2014. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World Journal of Clinical Oncology 5(3):412–424.
Zubrod, C.G., M. Schneiderman, E. Frei, C. Brindley, G.L. Gold, B. Shnider, R. Oviedo, J. Gorman, R. Jones, U. Jonsson, J. Colsky, T. Chalmers, B. Ferguson, M. Dederick, J. Holland, O. Selawry, W. Regelson, L. Lasagna, and A.H. Owens. 1960. Appraisal of methods for the study of chemotherapy of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. Journal of Chronic Diseases 11(1):7–33.




































