
































Below is the uncorrected machine-read text of this chapter, intended to provide our own search engines and external engines with highly rich, chapter-representative searchable text of each book. Because it is UNCORRECTED material, please consider the following text as a useful but insufficient proxy for the authoritative book pages.
17 This chapter describes the sampling and testing program implemented during Phase II. The research team used data from the laboratory test program to study the precision and bias of measurements from different test procedures. The team also compared the measurements from electrochemical properties with observations of corrosion rates with respect to the same sources to evaluate the veracity of the corresponding characterizations of corrosion potential. This information was used to develop recommendations and propose protocols for sampling earthen materials, proper testing, and characterizations of corrosion potentials. 3.1 Introduction The objectives of Phase II were to determine when test results from the current AASHTO test procedures were most applicable to characterizing the steel corrosion potential of earthen materials and when alternative test methods for measurements of geochemical and electro- chemical properties should be applied. During Phase II, the research team studied alternative laboratory test procedures for measuring the electrochemical properties of soils applied to a sampling domain incorporating a broad range of materials (mostly those commonly used in MSE wall constructions). The data included characterization of different sample sources (e.g., maximum particle size and gradation) along with measurements of geochemical and electro- chemical properties of the samples, including resistivity, pH, and chloride and sulfate contents. This chapter summarizes the laboratory data obtained from 27 different samples of earthen materials. Performance data (i.e., corrosion rates) of plain and galvanized steel specimens embedded in 19 of these sources were documented. While electrochemical test results were used to characterize the corrosion potential of each source, the performance data were used to correlate these characterizations with the corrosion rates. The results from applying different test standards were compared with those obtained from equivalent AASHTO tests, and the reasons for the observed differences were identified (i.e., the AASHTO tests were used as a reference). The data set used in this chapter (the 27 different material samples) is briefly described in the next section. Then, the key results obtained from different test methods in the form of resistivity/conductivity, pH, and chloride and sulfate content are presented. Finally, the trends observed within the data sets are discussed, and the results from the alternatives are compared with the results from AASHTO test methods. Salient details about the test procedures, precision, and bias are presented. 3.2 Description of Data Set Tables 3-1 to 3-3 summarize the materials that were included in the laboratory test program for this project and their sources. The research team collected samples for the laboratory test program from various sources (n) throughout North America, as follows: New York (n = 5), C H A P T E R 3 Laboratory Measurements
18 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials North Carolina (n = 3), South Carolina (n = 2), Florida (n = 1), Louisiana (n = 1), Arkansas (n = 1), Texas (n = 10), British Columbia (n = 1), and Calgary (n = 1). Overall, 27 samples were obtained from these 25 sites. The mineralogy of aggregate sources included limestone (13), granite (2), sandstone (1), natural sands/silica (6), glacial till (1), and expanded clay lightweight fill (LWF) (2). In addition, three separate samples were collected from different depths at the Palisades Interstate Parkway (PIP) site in Orangeburg, New York. Details of each sample in are presented in Figure 3-1 in terms of (a) composition, (b) grading number, and (c) percentage passing the No. 10 sieve. The samples represent a broad range of gradations and compositions ranging from fine sand to coarse, clean, and open-graded gravel. The composition is described in terms of the percentages of gravel (retained on the ¼-in. sieve), coarse to medium sand (passing the ¼-in. sieve and retained on the No. 40 sieve), fine sand (passing the No. 40 sieve and retained on the No. 200 sieve), and fines (passing the No. 200 sieve). Sieve analyses were conducted in accordance with Tex-110-E, whereby the percentage passing the No. 200 sieve was determined by dry sieving. The following list summarizes the composition of the materials: ⢠Three samples were predominately (i.e., more than 50%) fine sand; ⢠Six samples were predominantly coarse to medium sands; ⢠Two samples were mixtures of fine and coarse particles, where none of the components were equal to or greater than 50% of the total; ⢠Sixteen samples were predominately gravel, varying from sandy gravels to clean and open- graded gravels with no sand content; and ⢠None of the samples had more than 5% passing the No. 200 sieve. Site (sample type/USCS designation) Characteristic Florida (sand/SP) El Paso, TX, MSE (sand/SP) M-U-D, NY (sand/SP) Latitude 29°10'44.60"N 31°44'30.5"N 43°8'12.86"N Longitude 82°8'40.85"W 106°22'26.6"W 75°16'14.39"W Under asphalt/concrete Samples taken from within median, near corrosion- monitoring station established by Florida DOT in 1997. Sample taken from within an existing MSE wall. Samples retrieved from beneath a paved median, near a corrosion-monitoring station installed during construction (2000) by the New York State DOT. Drainage inlet nearby No subdrain behind the wall. UTEP installed corrosion- monitoring stations at this site. Pavement edge drains and drainage inlets are located within MSE fill. Water runoff Surface runoff from pavement is directed to side slopes of approach embankment. Viaduct spans across a railway and there are no surface waters nearby. Surrounding area is relatively flat. Paved median was originally constructed to be sloped away from MSE wall face, but ponded water was observed. Superelevation of highway pavement also directs water toward median in some locations. Location on wall MSE wall is an in-line abutment spanning between two bridge approaches and a median. MSE walls serve as abutment facing and as a grade separation along the approach. In-line abutment spans the median. Chloride/sulfate levels Extremely low chloride and sulfate content. Low chloride and sulfate content. Extremely low chloride and sulfate content. Note: USCS = Unified Soil Classification System; M-U-D = Marcy-Utica-Deerfield; SP = poorly graded sand; UTEP = University of Texas at El Paso. Table 3-1. Summary of sample sources: Fine.
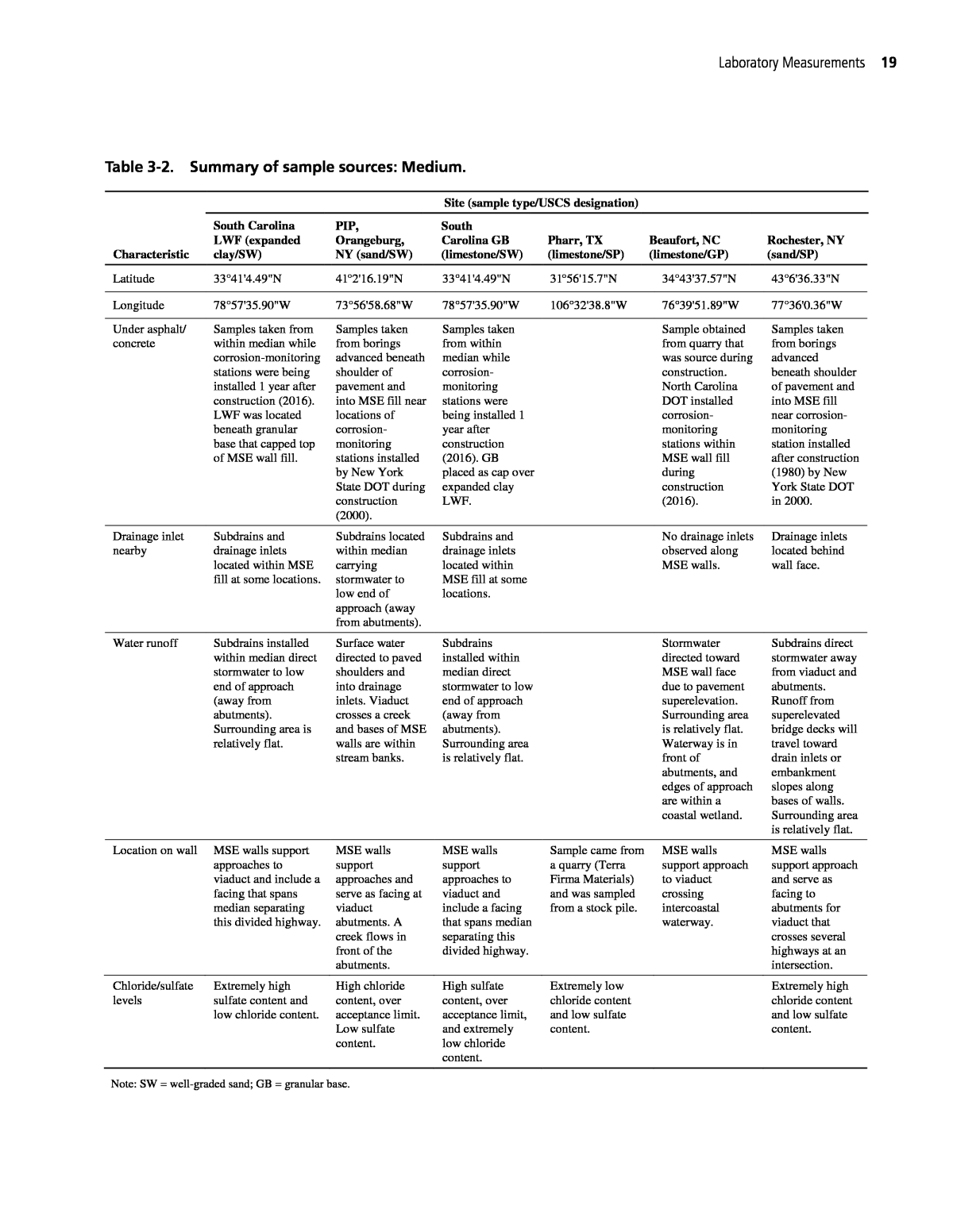
Laboratory Measurements 19 Characteristic Site (sample type/USCS designation) South Carolina LWF (expanded clay/SW) PIP, Orangeburg, NY (sand/SW) South Carolina GB (limestone/SW) Pharr, TX (limestone/SP) Beaufort, NC (limestone/GP) Rochester, NY (sand/SP) Latitude 33°41'4.49"N 41°2'16.19"N 33°41'4.49"N 31°56'15.7"N 34°43'37.57"N 43°6'36.33"N Longitude 78°57'35.90"W 73°56'58.68"W 78°57'35.90"W 106°32'38.8"W 76°39'51.89"W 77°36'0.36"W Under asphalt/ concrete Samples taken from within median while corrosion-monitoring stations were being installed 1 year after construction (2016). LWF was located beneath granular base that capped top of MSE wall fill. Samples taken from borings advanced beneath shoulder of pavement and into MSE fill near locations of corrosion- monitoring stations installed by New York State DOT during construction (2000). Samples taken from within median while corrosion- monitoring stations were being installed 1 year after construction (2016). GB placed as cap over expanded clay LWF. Sample obtained from quarry that was source during construction. North Carolina DOT installed corrosion- monitoring stations within MSE wall fill during construction (2016). Samples taken from borings advanced beneath shoulder of pavement and into MSE fill near corrosion- monitoring station installed after construction (1980) by New York State DOT in 2000. Drainage inlet nearby Subdrains and drainage inlets located within MSE fill at some locations. Subdrains located within median carrying stormwater to low end of approach (away from abutments). Subdrains and drainage inlets located within MSE fill at some locations. No drainage inlets observed along MSE walls. Drainage inlets located behind wall face. Water runoff Subdrains installed within median direct stormwater to low end of approach (away from abutments). Surrounding area is relatively flat. Surface water directed to paved shoulders and into drainage inlets. Viaduct crosses a creek and bases of MSE walls are within stream banks. Subdrains installed within median direct stormwater to low end of approach (away from abutments). Surrounding area is relatively flat. Stormwater directed toward MSE wall face due to pavement superelevation. Surrounding area is relatively flat. Waterway is in front of abutments, and edges of approach are within a coastal wetland. Subdrains direct stormwater away from viaduct and abutments. Runoff from superelevated bridge decks will travel toward drain inlets or embankment slopes along bases of walls. Surrounding area is relatively flat. Location on wall MSE walls support approaches to viaduct and include a facing that spans median separating this divided highway. MSE walls support approaches and serve as facing at viaduct abutments. A creek flows in front of the abutments. MSE walls support approaches to viaduct and include a facing that spans median separating this divided highway. Sample came from a quarry (Terra Firma Materials) and was sampled from a stock pile. MSE walls support approach to viaduct crossing intercoastal waterway. MSE walls support approach and serve as facing to abutments for viaduct that crosses several highways at an intersection. Chloride/sulfate levels Extremely high sulfate content and low chloride content. High chloride content, over acceptance limit. Low sulfate content. High sulfate content, over acceptance limit, and extremely low chloride content. Extremely low chloride content and low sulfate content. Extremely high chloride content and low sulfate content. Note: SW = well-graded sand; GB = granular base. Table 3-2. Summary of sample sources: Medium.
20 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials Characteristic Site (sample type/USCS designation) El Paso, TX (limestone/SW) Calgary, AB, Canada (sand/GW) Prince George, BC, Canada (glacial till/GW) Ashdown, AR (sandstone/GW) Temple, TX (limestone/GW) Sprain Brook, NY (limestone/GW) Raleigh, NC (granite/GP) Garden City, TX (limestone/GP) Latitude 31°56'15.7"N 50°53'33.50"N 53°38'35.60"N 33°46'18.5"N 31°06'11.0"N 41°3'44.43"N 35°52'24.89"N 31°51'39.9"N Longitude 106°32'38.8"W 114°3'15.88"W 122°39'54.35"W 94°10'54.0"W 97°21'32.2"W 73°48'25.95"W 78°34'6.30"W 101°35'32.2"W Under asphalt/ concrete Sample came from a quarry (Jobe Avispa Quarry) and was sampled from a stock pile. Corrosion rate measurements were obtained from laboratory measurements on steel specimens embedded within material from stockpile sample. Samples obtained from test embankment constructed from same sources of materials used to construct MSE walls. Samples taken from side hill cut near corrosion- monitoring stations installed to monitor performance of hollow bar soil nails that serve to stabilize the cut. Sample came from quarry (Hanson Aggregates) and was sampled from a stock pile. Corrosion rate measurements were obtained from laboratory measurements on steel specimens embedded within material from stockpile sample. Sample taken from MSE wall that was deconstructed. Samples taken from test pits advanced beneath paved shoulder. Metal loss and corrosion rates were observed from reinforcement samples exhumed and examined after wall failure. Samples taken from test pits advanced behind sloping wing walls of abutment for viaduct, near locations of corrosion- monitoring stations installed behind abutments by North Carolina DOT during construction (2004). Sample came from quarry (Laredo Paving, Garden City) and was sampled from a stock pile. Corrosion rate measurements were obtained from laboratory measurements on steel specimens embedded within material from stockpile sample. Drainage inlet nearby Subsurface drainage is installed behind MSE wall face. Drainage inlets are installed at several locations along tops of the soil nail walls. Drainage inlets are included within embankment behind wall and thought to have contributed to the failure in 2016. Drainage inlets are located within the median. Water runoff Stormwater directed down side slopes of approach embankment or collected at a point behind abutment and directed into pavement subdrain. Stormwater and meltwater run down surface of hillside behind soil nail wall and into swale at top of wall. Swale directs water to drain inlets that run down face of soil nail wall and discharge into Frazier River in front of wall. Stormwater runoff follows along superelevated paved shoulders to drainage inlets located within median or at shoulder. Stormwater runoff collected by drain inlets is directed away from the wall. Location on wall MSE wall serves as abutment to viaduct that crosses highway. Soil nail wall 2,000 ft long supports a side hill cut parallel to Frazier River below wall. MSE walls support approaches and abutment to viaduct. MSE walls serve as abutments to viaduct with sloping wing walls. Chloride/sulfate levels High sulfate and chloride content over acceptance limit. Extremely high sulfate content and low chloride content. Extremely low chloride and sulfate content. Extremely low chloride content and low sulfate content. Low sulfate and chloride content. High chloride content over acceptance limit and low sulfate content. Extremely low sulfate and chloride ion content. High sulfate content just below acceptance limit and high chloride content above acceptance limit. Note: BC = British Columbia; GW = well-graded gravel; GP = poorly graded gravel. Table 3-3. Summary of sample sources: Coarse.
Laboratory Measurements 21 Characteristic Site (sample type/USCS designation) Maple Rd., NY (limestone/GW) Wake Forest, NC (granite/GP) Round Rock, TX (limestone/GP) El Paso, TX, Coarse MSE (limestone/GP) Louisiana LWF Crushed (expanded clay/SW) Waco, TX (limestone/GW) Bastrop, TX (limestone/GP) Latitude 42°59'28.56"N 35°57'55.56"N 31°47'16.8"N Longitude 78°47'18.76"W 78°32'30.93"W 106°31'13.6"W Under asphalt/ concrete Samples taken by New York State DOT in 1988 from test pits advanced behind sloping wing walls of abutment for viaduct, near locations of corrosion- monitoring stations installed behind abutments after wall was constructed in 1986. Samples taken from test pits advanced behind sloping wing walls of abutment for viaduct, near locations of corrosion- monitoring stations installed behind abutments by North Carolina DOT during construction (2005). Sample taken from MSE wall that was deconstructed. Sample taken from existing MSE wall and UTEP- installed corrosion- monitoring stations at this site. Sample sent to UTEP from producer in Louisiana. This sample was tested as is and after crushing. Drainage inlet nearby No noticeable drainage inlet behind MSE wall. Drainage inlets capture runoff from viaduct and direct water away from walls. Water runoff Stormwater runoff collected from end of bridge deck and directed down embankment slopes via subdrain. Topography surrounding site is relatively flat. Stormwater runoff captured by paved ditch behind sloping wing wall. This is a raised embankment and topography of surrounding area is flat. Location on wall MSE walls serve as abutments to viaduct with sloping wing walls. MSE walls serve as abutments to viaduct with sloping wing walls. Chloride/sulfate levels High sulfate and low chloride ion content. Extremely low chloride and sulfate content. Extremely low chloride content and low sulfate content. Extremely low chloride and sulfate content. Extremely low chloride and sulfate content. Extremely low chloride and sulfate content. Extremely low chloride and sulfate content. Table 3-3. (Continued). The grading number (GN) expresses the coarseness of the sample with a number ranging from 0 to 7 and is computed with Equation 3-1 (Oman 2004): GN 1 100 PP PP PP PP PP PP PP (3-1)1 in. in in # 4 #10 # 40 # 2003 4 3 8( )= à + + + + + + where PP signifies percentage passing. The value of GN increases with respect to the fineness of the sample. For example, GN = 0 represents a very coarse sample (>1 in.), and GN = 7 represents a sample in which 100% of the material passes the No. 200 sieve. Values of GN in this study ranged between 0.15 and 5.65, with an approximate median of 3 (i.e., about half of the samples had a GN of >3 and the rest had a GN of <3). In this study, samples with GN < 3 were gravels in which less than 12% of the sample (percentage by weight) passed the No. 40 sieve (i.e., the sample included little fine sand).
22 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials Fi ne M ed iu m 0 20 40 60 80 10 0 egatnecreP Fines Fine Sand Coarse Sand Gravel C oa rs e Florida El Paso, TX, MSE M-U-D, NY South Carolina LWF PIP, NY @ 15ft PIP, NY @ 10ft South Carolina GB PIP, NY @ 5ft Pharr, TX Lousiana LWF Crushed Rochester, NY El Paso, TX Calagary, AB Prince George, BC Ashdown, AR Temple, TX Sprain Brook, NY Raleigh, NC Garden City, TX Maple Rd., NY Wake Forest, NC Round Rock, TX Lousiana LWF Uncrushed Waco, TX El Paso, TX, Coarse MSE San Antonio, TX Bastrop, TX (a) Figure 3-1. Characteristics of the sample domain used in the laboratory investigations: (a) sample composition.
Laboratory Measurements 23 0 1 2 3 4 5 6 Fi ne M ed iu m C oa rs e Grading Number (GN) Florida El Paso, TX, MSE M-U-D, NY South Carolina LWF PIP, NY @ 15ft PIP, NY @ 10ft South Carolina GB PIP, NY @ 5ft Pharr, TX Lousiana LWF Crushed Rochester, NY El Paso, TX Calagary, AB Prince George, BC Ashdown, AR Temple, TX Sprain Brook, NY Raleigh, NC Garden City, TX Maple Rd., NY Wake Forest, NC Round Rock, TX Lousiana LWF Uncrushed Waco, TX El Paso, TX, Coarse MSE San Antonio, TX Bastrop, TX (b) Figure 3-1. (Continued) Characteristics of the sample domain used in the laboratory investigations: (b) grading number. (continued on next page)
24 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials 0 10 20 30 40 50 60 70 80 90 10 0 Fi ne M ed iu m C oa rs e Percentage passing #10 sieve Florida El Paso, TX, MSE M-U-D, NY South Carolina LWF PIP, NY @ 15ft PIP, NY @ 10ft South Carolina GB PIP, NY @ 5ft Pharr, TX Lousiana LWF Crushed Rochester, NY El Paso, TX Calagary, AB Prince George, BC Ashdown, AR Temple, TX Sprain Brook, NY Raleigh, NC Garden City, TX Maple Rd., NY Wake Forest, NC Round Rock, TX Lousiana LWF Uncrushed Waco, TX El Paso, TX, Coarse MSE San Antonio, TX Bastrop, TX (c) Figure 3-1. (Continued) Characteristics of the sample domain used in the laboratory investigations: (c) percentage passing No. 10 sieve.
Laboratory Measurements 25 The percentage passing the No. 10 sieve (2 mm) is of particular interest because the current test procedures specified by AASHTO for measurement of electrochemical properties are per- formed on the samples after they have been separated on a No. 10 sieve. The percentage passing the No. 10 sieve in the sample domain is shown in Table 3-4. 3.3 Comparison of Results from Different Test Methods The results obtained from different test procedures were compared in terms of ⢠Precision and repeatability, ⢠Bias relative to those obtained from the current AASHTO tests, and ⢠Trends identified from the data. These comparisons were made to check whether any of the procedures performed better than others in terms of repeatability, precision, and bias. Cases in which the results from different test methods were similar and in which the results were different were also identified. In cases in which differences in results were observed, further analyses were performed to identify the best result for characterizing the potential for steel corrosion. This section first describes the precision and bias from measurements of resistivity for different samples/specimens and then describes measurements of salt content and pH. [Note: This report considers samples to be the material that was retrieved from the source in bulk (i.e., including all particle sizes) and speci- mens to be the portions of the samples that are prepared (e.g., passed through a sieve of a given size) for testing as prescribed by a given test standard.] 3.3.1 Resistivity Figure 3-2 summarizes the statistics describing the precision observed in testing replicates and the bias of each test procedure with respect to AASHTO T 288. The bar graphs and error bars shown in Figure 3-2 represent the mean and standard deviation, respectively (mean values are presented in ascending order). The five test procedures shown on the left-hand side of Figure 3-2 directly measure the resistivity of a compacted soil specimen in a soil box; the three test procedures shown on the right-hand side of the figure measure the conductivity of a leachate extracted from a soilâwater solution (the resistivity of this solution is the reciprocal of conductivity). Soil box tests allow for the effects on resistivity from level of compaction, moisture con- tent, and texture of the soil being investigated. Test methods performed on the specimens com- pacted in a soil box include AASHTO T 288, ASTM G187, Tex-129-E, Tex-129-M, and ASTM WK24621. These test procedures vary in terms of the particle sizes included in the test specimen, the specimen preparations prior to testing, the size of the test box (depends on the maximum particle size), and the moisture content at which the minimum resistivity is reported. Percentage Passing No. 10 Sieve Number of Samples PP#10 > 60 5 25 < PP#10 < 60 12 10 < PP#10 < 25 3 PP#10 < 10 7 Table 3-4. Percentage passing the No. 10 sieve in the sample domain.
26 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials The process for determining the resistivity from a soil box test is described in Section 1.3.3 (see Figure 1-1). Tests involving measurements of conductivity from leachates extracted from the solids (Tex-620-J, Tex-620-M and SC-T-143) include ⢠Preparing a measured amount of dry material for testing, ⢠Adding a measured volume of distilled or DI water, and ⢠Measuring the conductivity of the extracts. In leaching tests, the differences include dilution ratios (i.e., mass of water per mass of soil), methods of agitation, and resting times, at which no agitation is applied before starting the conductivity measurements. The resistivity of the leachate is computed as the reciprocal of conductivity. Results from leaching tests cannot be directly correlated with those obtained from compacted specimens. This is because other factors, including the tortuosity of the path of the electrical current through an actual compacted specimen, significantly affect the resis- tivity measurements. Resistivity of compacted soil specimens Conductivity of aqueous solutions 3.2 4.6 4.8 5.3 7.4 4.5 4.9 4.9 0 2 4 6 8 10 12 14 Tex-129-E AASHTO T 288 Tex-129-M ASTM G187 ASTM WK24621 Tex-620-M Tex-620-J SC-T-143 P re ci si on C V (µ C V ) (a) (b) Note: w.r.t. = with respect to. 1.1 1.4 2.3 3.8 1.6 1.9 5.2 0 2 4 6 8 10 12 14 Tex-129-E ASTM G187 Tex-129-M ASTM WK24621 Tex-620-J SC-T-143 Tex-620-M B ia s w .r. t. A A S H T O T 2 88 ( µ) Resistivity of compacted soil specimens Conductivity of aqueous solutions Figure 3-2. Summary of the test results for measuring resistivity/conductivity: (a) precision, (b) bias with respect to AASHTO T 288.
Laboratory Measurements 27 Details about the precision and bias of the test results obtained from different test procedures are discussed in the following subsections. 3.3.1.1 Precision/Repeatability for Individual Test Methods Three to five replicates from nine different samples were tested with each test method. The nine samples represent a range of characteristics in terms of coarseness (gradation), source, mineralogy, and corrosivity (range of resistivity). Five replicates were tested with samples from Florida; South Carolina (LWF); and El Paso, Texas. Three replicates were tested from six samples from Marcy-Utica-Deerfield (M-U-D), New York; South Carolina (GB samples); Pharr, Texas; Rochester, New York; Raleigh, North Carolina; and Wake Forest, North Carolina. Consistency between replicates was maintained by controlling the gradation of each replicate. Each sample was broken down into individual grain-size components and then recombined into replicates such that each satisfied the overall gradation of the sample. This was done to minimize the effects of sampling error on the test results, such that the variation in results was mostly related to differences between the test procedures. The mean (m), standard deviation (s), and coefficient of variation (CV = s/m) were computed from the results obtained from each set of replicates. Further statistics were generated from the replicate CVs to obtain the mean (mCV) and standard deviation (sCV) of the CVs observed between samples. The coefficient of variation between measurements was used to describe the precision of each test method with a ranking index (RI) as shown in Equation 3-2. RI (3-2)CV CV= m + s Lower RI values corresponded to better repeatability of the results for a given test method. In Figure 3-2a, the RI values correspond to the upper limit of the error bars. The obtained RI values of the resistivity test methods ranged between 6.8 and 13.2%. The following results were observed in the laboratory resistivity test data: ⢠The lowest RI values (best repeatability) were observed in Tex-620-J, Tex-129-E, Tex-129-M, and Tex-620-M, with RI values ranging from 6.8% to 7.6%. ⢠The RI values from the other test methods were higher, ranging from 9.1% to 13.2%. The repeatability of the results from ASTM WK24621, with an RI of 13.2%, was the poorest, as compared with the other test methods for resistivity. ⢠The repeatability of the results from tests performed on leachate extracted from a soilâwater mixture (Tex-620-J, Tex-620-M, and SC-T-143) was comparable to what was achieved from the soil box tests (Tex-129-M, Tex-129-E, ASTM G187, AASHTO T 288, and ASTM WK24621). ⢠The repeatability of the results obtained from the Texas modified procedures for measure- ment of resistivity/conductivity (Tex-129-M and Tex-620-M) was better than that of the results obtained from AASHTO T 288, SC-T-143, ASTM G187, and ASTM WK24621. The next section compares the resistivity measurements from alternative test procedures with those from the AASHTO tests and identifies data trends. 3.3.1.2 Comparison of Different Resistivity Tests with AASHTO T 288 The research team plotted resistivity test results from Tex-129-E, ASTM G187, Tex-129-M, and ASTM WK24621 against the resistivity values obtained from the AASHTO T 288 standard in Figure 3-3. In general, the data appear to be positivity correlated; that is, materials with rela- tively higher resistivity values according to AASHTO T 288 also showed high resistivity values according to the alternative test procedures. An important observation is that the resistivity measurements from Tex-129-E and ASTM G187 were more strongly correlated with those
28 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials obtained from AASHTO T 288 (Figure 3-3, a and b as compared with the resistivity measure- ments obtained with Tex-129-M and ASTM WK24621 (Figure 3-3, c and d). Data from alternative test methods were normalized with respect to results obtained from the same samples tested according to AASHTO T 288. The ratio of results from an alternative test method to those from AASHTO T 288 was defined as the bias for the alternative test method. Figure 3-4 summarizes the bias statistics from the alternative test procedures, where the bars represent the mean bias (mbias) in Figure 3-4a and the CV of the bias (CVbias) in Figure 3-4b. The whiskers in Figure 3-4a represent the standard deviation of the biases (sbias). The following general conclusions were made on the basis of the presentation of data in Figure 3-4: ⢠The mean bias is approximately 1 for Tex-129-E, with a CV of 22%. These statistics are mani- fested in the relatively narrow band and proximity to the line of equal values depicted in Figure 3-3a. Results from Tex-129-E and AASHTO T 288 are expected to be close because of the similarities between these test methods. The two tests differ in terms of the sieve size used to separate the specimen from the sample (No. 8 versus No. 10, respectively) and the 12-hour curing period prescribed by AASHTO T 288 for the first moisture increment. For the materials tested in this study, these differences did not have a significant impact on the results. Other test procedures showed mean bias values noticeably higher than 1.00 (as high as 5.22 for Tex-620-M), with CVs generally higher than 50%. (a) (c) (b) (d) 0 5,000 10,000 15,000 20,000 0 5,000 10,000 15,000 20,000 A S T M G 18 7 ( â¢c m ) AASHTO T 288 ( â¢cm) 0 10,000 20,000 30,000 40,000 50,000 0 10,000 20,000 30,000 40,000 50,000 T ex -1 29 -M ( -c m ) AASHTO T 288 ( â¢cm) 0 5,000 10,000 15,000 20,000 0 5,000 10,000 15,000 20,000 T ex -1 29 -E ( cm ) AASHTO T 288 ( â¢cm) Line of equal values ⢠0 10,000 20,000 30,000 40,000 50,000 0 10,000 20,000 30,000 40,000 50,000 AASHTO T 288 ( â¢cm) A S T M W K 24 62 1 ( -c m ) Figure 3-3. Comparison of soil box test results relative to AASHTO T 288: (a) Tex-129-E, (b) ASTM G187, (c) Tex-129-M, and (d) ASTM WK24621.
Laboratory Measurements 29 ⢠The mean bias is greater for test procedures that involve coarser gradations (i.e., ASTM G187, Tex-129-M, and ASTM WK24621). ASTM G187 includes particle sizes up to ¼ in., but Tex-129-M and ASTM WK24621 both include particle sizes up to 1¾ in. This is reflected in the mean bias values, which are higher for results obtained from ASTM WK24621 and Tex-129-M than for those from ASTM G187. The bias from ASTM WK24621 is higher than that from Tex-129-M due to the manner in which measurements are taken after the sample is drained for ASTM WK24621. ⢠The mean bias overall for the tests on compacted soil specimens and tests performed with leachates was 2.13 and 2.95, respectively. Differences between results obtained with these techniques are expected because the effects from tortuosity cannot be included when conduc- tivity measurements from leachate are being used. ⢠Biases from tests on leachate are all greater than 1, even for samples that are separated into finer components (e.g., for Tex-620-J the sample is separated on a No. 40 sieve). This is due to the different dilution ratios and methods of mixing and extracting leachates used in different leaching tests as compared with soil box tests. (a) (b) 1.07 1.41 2.28 3.75 1.69 1.95 5.22 0 1 2 3 4 5 6 7 8 9 10 Tex-129-E ASTM G187 Tex-129-M ASTM WK24621 Tex-620-J SC-T-143 Tex-620-M M ea n bi as ( µ bi as ) Resistivity of compacted soil specimens Conductivity of aqueous solutions Resistivity of compacted soil specimens Conductivity of aqueous solutions 22 31 86 136 57 65 88 0 20 40 60 80 100 120 140 160 Tex-129-E ASTM G187 Tex-129-M ASTM WK24621 Tex-620-J Tex-620-M SC-T-143 C V b ia s (% ) Figure 3-4. Statistics of resistivity test bias with respect to AASHTO T 288: (a) bias mean and standard deviation and (b) coefficient of variation (CV = rbias /lbias).
30 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials The data from each test method were grouped according to the fineness of the samples (fine sand, coarse sand, and gravel), as previously illustrated in Figure 3-1. These data are summarized in Figure 3-5 including the mean (Figure 3-5a) and CV (Figure 3-5b) of the bias observed from all samples included in each fineness group. SC-T-143 could not be performed with fine samples because of the lack of settlement of the finer particles during the specified standing time. The research team concluded that, as the coarseness of the sample increases, the mean bias and CV increase. When the materials characterized as fine sand and the results from soil box tests are considered, the average bias is close to 1 with a relatively low CV (average CV = 8%). How- ever, the biases for coarse sand and gravel were 1.6 and 3.1, respectively, when the results from Tex-129-M, which included coarse particles within the test specimen, were considered. Also, the CVbias increased incrementally for materials characterized as coarse sands and gravels, for which CVs in excess of 30% were observed. The means of the biases of the results from leaching tests performed on fine samples were higher than 1, due to the manner in which the samples were diluted for leaching, as com- pared with the moisture contents that prevail for compacted, saturated samples. For compacted samples, the moisture contents were generally less than 50% by weight, but dilution ratios as high as 10:1 (water:solids) are commonly used for leaching tests. The bias from Tex-620-M was more than twice the bias from Tex-129-M. Since the same specimen gradation was used in both tests, the observed differences are due to the manner in which leachate is prepared and tested for Tex-620-M as compared with tests conducted on compacted specimens (Tex-129-M). The mean biases of the test results from Tex-620-J do not trend with respect to coarseness of the (a) (b) 1 1 0.9 0.9 1.3 2.2 1.2 1.6 1.6 2 1.8 0.9 4.6 1 1.4 3.1 5.8 1.7 2.6 6.5 0 1 2 3 4 5 6 7 Tex-129-E ASTM G187 Tex-129-M ASTM WK24621 Tex-620-J SC-T-143 Tex-620-M M ea n bi as ( µ bi as ) Fine sand Coarse sand Gravel Resistivity of compacted soil specimens Conductivity of aqueous solutions 11 12 3 6 56 44 18 25 56 40 58 96 72 26 35 77 116 59 72 53 0 20 40 60 80 100 120 Tex-129-E ASTM G187 Tex-129-M ASTM WK24621 Tex-620-J SC-T-143 Tex-620-M C V ( % ) Fine sand Coarse sand Gravel Resistivity of compacted soil specimens Conductivity of aqueous solutions Figure 3-5. Resistivity measurements from samples with different textures: (a) mean bias and (b) coefficient of variation.
Laboratory Measurements 31 sample. This is because the sample is separated on a No. 40 sieve, and only the finer portion is included in the test specimen. Trends within the data were identified to reconcile the variability (high CVs) of the bias from testing coarse sand and gravel materials. These trends distinguish parameters that influence the resistivity of geomaterials and describe how parameters correlate with the measured values. The bias of Tex-129-M and ASTM WK24621 with respect to AASHTO T 288 were investigated in this study with a model developed at the University of Texas at El Paso (Arciniega et al. 2018, 2019). The model shows how resistivity of soil can be described in terms of sample gradations and salt ion concentrations. Nondimensional scaling parameters that show how results from dif- ferent resistivity test procedures may be compared and correlated with one another were derived from this model. These correlation values were reviewed to identify trends in the data that were then related to easily observed material characteristics, as shown in Table 3-5. The following conclusions are based on the information presented in Table 3-5: ⢠If the sample has more than 60% of particles passing the No. 10 sieve, then bias is close to 1 and results from testing in accordance with Tex-129-M and AASHTO T 288 are similar. ⢠When the grading number of the sample is greater than 3 and less than 40% of the particles pass the No. 10 sieve, the bias in resistance measurements is greater than 3 (higher bias). Thus, the difference in the results obtained from materials with these characteristics with Tex-129-M as compared with AASHTO T 288 is relatively large. In general, these materials can be described as gravels with significant amounts of coarse and fine sand, where the percentage of gravel is approximately 50% and the coarse sand component is approximately 30%. Tex-129-M appears to be a good alternative to AASHTO T 288 for evaluating the effect of the coarseness and gradation of the sample on measurements of resistivity. The test procedure is like AASHTO T 288 and considers how moisture content and degree of saturation affect the resistivity of a compacted sample. 3.3.2 Salt Content Figure 3-6 summarizes the precision of measurements of salt content observed in tests of replicates. The samples and replicates are the same as those used in evaluating the precision of resistivity tests (Section 3.3.1). The bar graphs and whiskers in Figure 3-6 represent mCV and sCV, respectively, and include measurements of salt content according to AASHTO T 290, AASHTO T 291, Tex-620-J, and Tex-620-M. A common measurement technique, IEC, was applied for all test standards, such that the comparisons presented herein depict differences due to sample preparations including dilution ratios and methods of mixing. For the comparison of results from different test methods described here, chloride and sulfate ion concentrations were measured with the IEC method for all of the test procedures. Sample Type Bias < 1.5 1.5 < Bias < 3.0 Bias > 3 GN PP#10 GN PP#10 GN PP#10 Gravel â â 2.0â3.0 6â40 3.0â3.6 24â40 Coarse sand 4.5â4.8 60â70 3.9â4.5 50â60 â â Fine sand 5.0â6.7 >80 â â â â Table 3-5. Resistivity test bias correspondence with grading number and PP#10.
32 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials ASTM D4327 was employed to measure chloride and sulfate concentrations simultaneously as well as other anions (e.g., bicarbonate anion) by using the ion exchange chromatograph. Ion chroma tography is a more accurate and reproducible technique for measuring salt concentrations as compared with traditional methods (e.g., titration, photospectrometry). The IEC method is more automated, less expensive, and indicates potential interference that is not identified by the current AASHTO tests. However, sample treatments described in AASHTO T 290 and T 291 are needed to prepare an extract for IEC measurements. Only samples with salt content greater than 10 mg/kg (ppm) were included for computations of the CV statistics (mCV and sCV). These included two samples from South Carolina (LWF and GB), one sample from Rochester, New York, and one sample from El Paso, Texas. Measure- ments less than 10 mg/kg are not reliable because the resolution of the measurement device is large compared with the measurements. The following observations are based on the CV statistics depicted in Figure 3-6: ⢠Differences between precision and repeatability across the tests are more distinct between the tests for chloride than between those for measurements of sulfate. ⢠For sulfate measurements, AASHTO T 290 rendered repeatability equal to or better than the other test methods that were evaluated. ⢠Measurements of chloride from Tex-620-M are less repeatable than measurements from other methods. Salt content measured according to Tex-620-J was high as compared with that measured according to Tex-620-M and the AASHTO tests because of the method used for the sample preparation, which includes pulverizing the sample to pass a No. 40 sieve and heating it to 150°F before extracting the leachate. Tex-620-J was originally used by the Texas DOT for mea- surements of chloride contents in concrete samples. Although the Texas DOT has applied this test in evaluating fills for MSE walls, it does not appear to be applicable. Therefore, data from Tex-620-J are not included in the forthcoming comparisons. The following sections discuss and compare results for sulfate obtained from AASHTO T 290 and results for chloride obtained from AASHTO T 291 with results from Texas modified procedures (Tex-620-M). 10.1 10.7 11.8 3.7 7.5 12.9 0 2 4 6 8 10 12 14 16 18 20 AASHTO T 290 Tex-620-M Tex-620-J Tex-620-J AASHTO T 291 Tex-620-M Sulfate Content Chloride Content P re ci si on C V ( % ) Figure 3-6. Results of tests for measurement of salt content and observations of precision [includes only results from samples with sulfate and chloride content greater than 10 mg/kg (ppm), n = 4].
Laboratory Measurements 33 The research team tested 26 samples: 21 according to AASHTO and Texas modified pro- cedures and five according to Tex-620-M only. The five coarse samples were not tested accord- ing to the AASHTO standards because of the lack of sufficient constituents passing the No. 10 sieve. Figure 3-7 compares the salt content obtained from Tex-620-M and the AASHTO pro cedures. Except for samples with a high content of particles passing the No. 10 sieve, the Tex-620-M procedure rendered lower salt content as compared with the AASHTO tests. This was due to the larger particle sizes included in the Tex-620-M tests as compared with the AASHTO tests, which are performed on the finer fraction of the sample (that passing the No. 10 sieve). The black dashed line, which shows the best fit for sulfate measurements by Tex-620-M (R2 = 0.79), indicates that the sulfate content measured by Tex-620-M was approximately 70% of that measured by the AASHTO tests. Similarly, the chloride content measured by Tex-620-M (R2 = 0.76) was approximately 50% of that measured by the AASHTO tests. For measurements of low salt content (â¤10 mg/kg), the results from Tex-620-M agreed well with those from the AASHTO tests. There were also a few measurements of higher salt content in which the results from the three tests were approximately the same. There were four observations in which the sulfate content measured according to Tex-620-M was significantly lower than that measured by AASHTO T 290 (about one-fifth, or 20%, of that measured by AASHTO T 290). Bias was computed as the ratio of equivalent total salt content obtained from Tex-620-M divided by the equivalent total salt content computed from the results of AASHTO T 290 and T 291. Equivalent total salt content considers the combining power of chloride and sulfate in solution in terms of their milliequivalent units and is useful in checking trends between salt content and resistivity, as described in the next section. In general, lower salt content are mea- sured according to the modified test procedures, so the bias is less than 1. The lowest values of bias were from samples with a low percentage of particles passing the No. 10 sieve. The trend in bias with respect to the percentage of particles in each sample passing the No. 10 sieve was as follows: ⢠The lowest biases were from samples with less than 25% of the particles passing the No. 10 sieve. 620-M = 0.71 (T 290) R ² = 0.79 620-M = 0.51 (T 291) R ² = 0.76 0 200 400 600 800 1,000 0 200 400 600 800 1,000 T ex -6 20 -M ( m g/ kg ) AASHTO T 290 & T 291 (mg/kg) Chloride Sulfate Line of equal values Figure 3-7. Correlation between measurements of salt content according to Tex-620-M and AASHTO T 290 and T 291.
34 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials ⢠For samples with more than 60% of the particles passing the No. 10 sieve, the bias was close to 1. In these samples, the fines and fine sand components dominated the leaching of salts from samples tested according to either the AASHTO or Texas modified procedures. ⢠In addition to the percentage of particles passing the No. 10 sieve, other factors related to the test technique may also affect the bias. Higher dilution ratios and different methods of mixing may render measurements of salt content from Tex-620-M that are higher compared with AASHTO T 290 and T 291, even for samples with a large fraction passing the No. 10 sieve. 3.3.3 Correlation Between Salt Content and Resistivity Salts affect the electrical resistivity of an aqueous solution because salts dissociate into their components (ions) when dissolved in water and create an electrically conductive solution. The resistivity decreases as the solution becomes more concentrated with ions (higher ion mobility). Thus, the measurement of resistivity is negatively correlated with the measurement of salt content. The research team evaluated the correlation between salt content and resistivity measurements to assess the veracity of these measurements. If the results do not show a con- sistent trend between the salt content and resistivity, then the tests used to measure salt content or electrical resistivity may not provide a true measurement. This may also be related to the unknown presence of other ions that affect the measured resistivity in addition to chloride or sulfate ions. Resistivity measurements were paired with the measurements of salt content. Data pair- ings between resistivity and salt content include the AASHTO test series (AASHTO T 288 for resistivity and AASHTO T 290 and T 291 for salt content), the current Texas DOT test pro- cedures (Tex-129-E and Tex-620-J), and the proposed Texas DOT modified test procedures (Tex-129-M and Tex-620-M). ASTM tests for measurements of resistivity, including ASTM G187 and ASTM WK24621, were paired with test for salt content measured according to AASHTO T 290 and T 291 and Tex-620-M, respectively. In this manner, tests for resistivity and salt concentrations were performed on specimens that had been separated from the sample into similar particle sizes before testing. For Tex-620-M, conductivity/resistivity and salt content measurements were performed on the same specimen. This is unique as compared with measurements of resistivity from compacted soil specimens paired with salt content measured from leachate. Regression analysis was performed to assess the coefficient of determination (R2) between the resistivity and salt content measurements (mg/kg). A power law, as shown in Equation 3-3, was found to provide the best fit to the data. For Equation 3-3, chloride and sulfate ion contents were combined to render equivalent salt content in terms of mg/kg: cm mg kg (3-3)A B ( )r Ω =  ï£ï£¬   â ⢠where A and B are regression coefficients relating salt content to resistivity, where the salt con- tent is expressed in milligrams/kilogram. A represents the resistivity in the limit when the salt content is 1 mg/kg; âB describes how quickly resistivity decreases as salt content increases. The model parameters and coefficients of determination for each of the pairings are sum- marized in Table 3-6. The highest coefficient of determination (R2 = 0.79) was achieved by comparing the results from salt content and conductivity measurements with the Tex-620-M procedure. The coefficients of determination from measurements of resistivity on compacted soil specimens were lower and ranged from 0.36 to 0.64. The best correlation from resistivity
Laboratory Measurements 35 measurements observed on compacted soil specimens was obtained with the AASHTO test series (R2 = 0.64) and the worst with ASTM WK24621 and Tex-620-M (R2 = 0.36). Subsequently, AASHTO test procedures were applied to samples with at least 22% of particles passing the No. 10 sieve, and the Texas modified procedures were applied to samples with less than 22% passing the No. 10 sieve. This resulted in improved correlation between resistivity and salt content. Similar analyses were performed using milliequivalents to express the salt content instead of parts per million. Measurements of alkalinity in terms of part per million of calcium carbonate were included for the calculation of milliequivalents, in which the carbonate ions are con- sidered as another salt component in the sample mixture. Alkalinity is commonly determined as the capacity of water to buffer acids (i.e., the acid-neutralizing capacity of water), where the major acid-buffering constituents in water are bicarbonate (HCO3 â) and carbonate (CO3 2â) ions. Table 3-7 shows the correlation obtained using milliequivalents. Comparing the coefficients of determination in Tables 3-6 and 3-7 shows that utilizing milliequivalents results in signifi- cant improvements in correlation. For the data parings from Tex-129-M and Tex-620-M, R2 increases from 0.43 (Table 3-6) to 0.59 (Table 3-7); for the Tex-620-M measurements of both conductivity and salt, R2 increases from 0.79 (Table 3-6) to 0.88 (Table 3-7). The following conclusions are drawn from the results presented in this section: ⢠The precision and repeatability of test methods for measurements of salt content are similar. ⢠Salt content measured according to Tex-620-J is higher than that measured according to the AASHTO tests; salt content measurements from Tex-620-M are generally lower than those from the other tests. Parameter (ppm salts) Test Procedure A B R2 Leachate Tex-620-M conductivity and salt 122,000 0.53 0.79 Soil Box AASHTO T 288, T 290, T 291 27,000 0.47 0.64 ASTM G187 and AASHTO T 290 and T 291 22,000 0.35 0.50 Tex-129-E and Tex-620-J 48,000 0.49 0.52 Tex-129-M and Tex-620-M 41,000 0.47 0.43 ASTM WK24621 and Tex-620-M 57,000 0.44 0.36 Table 3-6. Resistivity model parameters. Parameter (mEq chloride, sulfate and alkalinity) Test Procedure A B R2 Leachate Tex-620-M conductivity and salt 142,105 1.13 0.88 Soil Box AASHTO T 288, T 290, and T 291 13,500 0.82 0.64 Tex-129-M and Tex-620-M 57,000 1.12 0.59 Note: mEq = milliequivalents. Table 3-7. Resistivity model parameters.
36 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials ⢠The best correlation between salt content and resistivity measured on a compacted specimen is obtained with the AASHTO test standards. ⢠Milliequivalents are the best way to express salt content and to allow consideration of the effects from other salts besides chloride and sulfate on the resistivity measurements. 3.3.4 Measurements of pH Figure 3-8 summarizes the statistics describing the precision observed in testing replicates and the bias of each test procedure with respect to AASHTO T 289. Precision was observed in testing replicates from the same nine sources as those described for resistivity testing and measure ment of salt content. The test procedures for measuring pH vary in terms of ⢠Whether or not the sample is air-dried; ⢠Particle sizes included in the test specimen; ⢠Dilution ratios used in the sample preparation; ⢠Methods of mixing water with the sample, including whether or not the mixture is heated; and ⢠The period that the soilâwater mixture is allowed to stand before the first measurement is made. (a) (b) 1 0.7 0.9 0.9 1.1 1.2 0 0.5 1 1.5 2 2.5 3 AASHTO T 289 NCHRP 21-06 Tex-128-E Tex-620-J ASTM D4972 Tex-620-M P re ci si on C V ( µ C V ) 0.99 0.98 0.97 1.03 1.1 0 0.2 0.4 0.6 0.8 1 1.2 ASTM D4972 NCHRP 21-06 Tex-620-J Tex-128-E Tex-620-M B ia s w .r. t. A A S H T O T 2 89 ( µ) Figure 3-8. Summary of test results for measuring pH: (a) precision and (b) bias with respect to AASHTO T 289.
Laboratory Measurements 37 The bar graphs and whiskers in Figure 3-8 represent the mean and standard deviation, respec- tively, in pH units. The pH measurements from all samples range between 7.6 and 9.5. The mean of the measurements from AASHTO T 289 was 8.34, which means that a CV of 1% corresponds to an average standard deviation difference of approximately 0.08 pH units. Higher pH values were measured according to Tex-620-M, with a mean pH value of 8.95. On the basis of the data shown in Figure 3-8, the repeatability of the different measurement techniques is similar to that between the test procedures described by NCHRP Project 21-06 (Vilda 2009), Tex-620-J, and Tex-128-E. Tex-620-J and Tex-128-E are similar in terms of the particle sizes included in the test specimens, the use of higher dilution ratios, and the heat applied during the mixing procedure. Application of heat appears to improve the repeatability of the test results. However, there are significant differences between these procedures and the NCHRP 21-06 procedure. In the NCHRP 21-06 procedure, heat is not applied during mixing, and this is the only procedure whereby the sample is not air-dried as a part of sample prepara- tions. Including moisture, which has been a part of the mixture over time, may result in more consistent extractions and corresponding measurements of pH from NCHRP 21-06. The repeatability of ASTM D4972 and AASHTO T 289 was lower, but similar. These proce- dures are similar except for the methods of mixing. However, the two procedures differ from previously discussed methods in terms of the dilution ratio (1:1) and mixing the sample without the application of heat. The poorest repeatability was observed in measurements of pH obtained with Tex-620-M, which incorporates gravel-sized particles within the test specimen. Figure 3-8b shows that, except for Tex-620-M, the biases of results from all of the test methods with respect to data from AASHTO T 289 were close to 1. The biases of the results from Tex-620-M were observed to increase with respect to sample coarseness; that is, the bias from testing a gravel sample was higher than that from a sample that included coarse or fine sand. The results of measurements of pH are plotted in Figure 3-9. pH values obtained from Tex-620-J, NCHRP 21-06 (Vilda 2009), ASTM D4972, Tex-128-E, and Tex-620-M are compared with those from AASHTO T 289. Figure 3-9 shows that the pH values measured by Tex-620-J (Figure 3-9a) and NCHRP 21-06 (Figure 3-9b) were lower, those from ASTM D4972 (Figure 3-9c) were nearly equal, and those from Tex-128-E (Figure 3-9d) and Tex-620-M (Figure 3-9e) were higher than measurements from AASHTO T 289. Other methods rendered a stronger cor- relation with results from AASHTO T 289 (R2 > 0.6), as compared with the correlation with Tex-620-M (R2 = 0.33). The following conclusions are drawn from the results presented in this section: ⢠Measurements of pH from Tex-620-M are less repeatable as compared with measurements from other test methods investigated in this study. ⢠In general, Tex-620-M renders pH values that are higher than those obtained from the other test methods investigated in the study. ⢠Results from NCHRP 21-06 (Vilda 2009) are more repeatable as compared with AASHTO T 289 and do not have a significant bias with respect to results obtained from AASHTO T 289. 3.4 Characterization of Corrosion Potential and Correlation with Corrosion Rates The previous sections compare results from testing samples in accordance with current AASHTO standards and alternative procedures for preparing specimens and making measure- ments. Given the better precision observed from results with the Tex-129-M test and its simi- larities with the AASHTO T 288 test procedure, the research team considered Tex-129-M as an
38 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials alternative to AASHTO T 288 for testing coarse materials. The following observations were made on the basis of the distribution of bias in the results: ⢠When more than 60% of the sample passes the No. 10 sieve, similar results are obtained from measurements of resistivity and salt content made with current test standards or the modified Texas procedures (Tex-129-M for resistivity and Tex-620-M for salt content). ⢠Significant differences in measurements of resistivity and salt content were observed for materials having less than approximately 25% passing the No. 10 sieve. ⢠The largest differences were observed in testing sandy gravels with 3.0 < GN < 4.0 and less than 40% passing the No. 10 sieve. 3.4.1 Correlation Between Resistivity and Performance Data Up to this point, this study focused on identifying differences between test results and the factors affecting these differences. The next step was to determine whether and when measure- ments different from those achieved with AASHTO T 288 might render a better result. Perfor- mance was modeled by relating observations of performance to site conditions and identifying parameters that provide the best correlation with performance data. Performance was defined in 5 6 7 8 9 10 5 6 7 8 9 10 T ex -6 20 -J AASHTO T 289 620-J = 0.96 (T 289) R2 = 0.48 Line of equal values 21-06 = 0.98 (T 289) 5 6 7 8 9 10 5 6 7 8 9 10 N C H R P 2 1- 06 AASHTO T 289 R2 = 0.70 128-E = 1.03 (T 289) 5 6 7 8 9 10 5 6 7 8 9 10 T ex -1 28 -E AASHTO T 289 R2 = 0.63 D4972 = 0.99 (T 289) 5 6 7 8 9 10 5 6 7 8 9 10 A S T M D 49 72 AASHTO T 289 R2 = 0.69 5 6 7 8 9 10 5 6 7 8 9 10 T ex -6 20 -M AASHTO T 289 620-M =1.08 (T 289) R2 = 0.33 (a) (b) (c) (d) (e) Figure 3-9. Comparisons of pH measurements relative to AASHTO T 289: (a) Tex-620-J, (b) NCHRP 21-06 (Vilda 2009), (c) ASTM D4792, (d) Tex-128-E, and (e) Tex-620-M.
Laboratory Measurements 39 terms of the durability of earth reinforcements as quantified by observations of metal losses and corrosion rates (CRs). Site conditions include the environment surrounding the earth reinforce- ments, most notably, the resistivity of the fills or native soils. The coefficient of determination, R2, between the corrosion rate and resistivity measurements was used as an index to rank the accuracy of the results from each of the resistivity tests included in the test program. Resistivity is often considered to be an indicator of corrosivity, as this single parameter is correlated with numerous factors that affect corrosion reactions, including salt and moisture content (King 1977; Romanoff 1957). The data set for the regression analysis included measurements from 19 sample sources incor- porating 28 measurements of corrosion rates. Observed corrosion rates include 18 data points from galvanized steel specimens and 10 data points from plain steel specimens. The measure- ments presented herein are the maximum observed from each site/source. The maximums were used to consider the durability of the most vulnerable elements. The data set includes in situ corrosion rate measurements from the field and corrosion rates measured from laboratory tests. In situ measurements of corrosion rates from field studies involved variable moisture con- tents and corrosion rate measurements from locations near the tops and bases of the MSE walls. The linear polarization resistance technique (Jones 1996; Tait 1994) was used for in situ mea- surements of corrosion rates. As many as 30 samples were monitored at a given site, and the maximum values were observed from sample locations where conditions for corrosion were more severe (e.g., higher moisture content, cycles of wetting and drying, availability of oxygen). Laboratory tests included samples embedded within fills under the most severe conditions that may be encountered in the field. Moisture content was maintained near the optimum moisture content for compaction as well as saturated conditions. The observed corrosion rates are consid- ered to be extremes/maximums as compared with what is likely to occur in the field. In general, corrosion rates tend to attenuate with respect to time when conditions favor the development of a protective scale on the steel surface; however, the majority of the attenua- tion occurs within the first year (Romanoff 1957). Corrosion rate measurements presented herein are from samples that have been embedded in fill for at least 1 year (i.e., with relatively stabilized corrosion rates). Considering each of the resistivity test methods, the research team plotted measurements of corrosion rates versus measurements of resistivity for plain steel and galvanized elements separately. The best fit to the data was obtained with the power law shown in Equation 3-4. Table 3-8 summarizes the regression coefficients (C and D) and the coefficient of determination (R2) obtained from each regression analysis. These data are dis- cussed by grouping them according to the gradation of the specimens prepared for testing. Test Method Galvanized Steel Plain Steel C D R2 C D R2 Group 1 AASHTO T 288 9,945 0.93 0.46 9,073 0.90 0.20 Tex-129-E 12,380 0.95 0.38 1,492 0.70 0.08 ASTM G187 24,613 1.00 0.40 386,844 1.30 0.16 Group 2 Tex-129-M 1,102 0.67 0.19 55,542 1.05 0.31 ASTM WK24621 9,169 0.88 0.33 88,746 1.08 0.27 Tex-620-M 140,664 1.11 0.32 467,000 1.16 0.30 Table 3-8. Regression of observed corrosion rates and resistivity measurements.
40 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials CR m year cm (3-4)C D( )m ï£ï£¬   = à r Ω â⢠where C represents the corrosion rate in the limit when the resistivity is equal to 1 Ω ⢠cm. Because the parameter D is a negative power, it shows how quickly the corrosion rates increase as resistivity decreases. Group I includes results from AASHTO T 288, Tex-129-E and ASTM G187. For Group I, samples were separated, and the finer portion (passing the No. 8, No. 10, or ¼-in. sieve) was included in the specimen for measurement of resistivity. Group II included tests in which the specimen was more representative of the source, including particle sizes up to 1¾ in. (Tex 129-M, ASTM WK24621, Tex-620-M). The Group I tests rendered better correlation for galvanized steel, and the Group II tests rendered better correlation for plain steel specimens. This may be because galvanized surfaces are more uniform as compared with the surfaces of plain steel specimens. Correlation between resistivity and corrosion rates is affected by the variability of the soil samples, and the specimens included in Group II have more variability as compared with those in Group I. The characteris- tics of the metal along the surface of plain steel are more variable, and, compared with those with galvanized steel, the correlation is less affected by the uniformity of the finer specimens included in Group I. The correlation with respect to the performance of galvanized steel and plain steel is discussed in the following subsections. 3.4.1.1 Performance of Galvanized Steel For Group I, the corrosion rates of galvanized elements were negatively correlated with resis- tivity (0.38 < R2 < 0.46). The regression coefficient, âD, was very similar for the different test methods, ranging from â0.93 to â1.00. The C coefficient was more than twice as high for ASTM G187 as compared with those from AASHTO T 288 or Tex-129-E (i.e., results from ASTM G187 correlate with higher corrosion rates). This is directly related to the bias of the resistivity measurements from ASTM G187 compared with those of AASHTO T 288. For Group II, corrosion rates did not correlate as well with corrosion rate measurements as compared with the correlation obtained from Group I. These correlation values can be described as low to moderate (0.19 < R2 < 0.33). The lower degrees of correlation are because the tests in Group IâAASHTO T 288 in particularâare suited to a broader range of materials than the range of materials for which results from Tex-129-M and other tests in Group II are applicable. Better correlation is obtained by culling the data set to include only materials that have more than 22% of particles passing the No. 10 sieve in the test results from AASHTO T 288 and those with less than 22% passing the No. 10 sieve in the test results from Tex-129-M. The selection of a threshold of 22% is consistent with ⢠The observation that trends between the salt content and resistivity measurements are more prevalent when materials are grouped on the basis of whether more than 22% of particles pass the No. 10 sieve, as discussed in Section 3.3.2, and ⢠The high bias values that are observed in measurements of resistivity and salt content when the percentage passing the No. 10 sieve is less than 22%, as discussed in Sections 3.3.1.2 and 3.3.2. In the proposed protocol, the threshold of 22% is rounded up to 25% (see Section 3.5 and the appendix). There are seven samples within the Phase II test program with less than 22% of particles passing the No. 10 sieve and corresponding measurements of corrosion rate. These include samples from Wake Forest, North Carolina; San Antonio, Texas; Bastrop, Texas; Maple Road, New York; Waco, Texas; Garden City, Texas; and samples of coarse aggregate from an
Laboratory Measurements 41 MSE wall in El Paso, Texas. The correlation corresponding to AASHTO T 288 and Tex-129-M is shown in Figure 3-10 and Figure 3-11, respectively. Figure 3-10 depicts data from AASHTO T 288 that do not include the coarse samples. The data collected for the LWF from South Carolina, which was primarily expanded clay, were also removed. LWF samples were considered different from natural materials because of their absorption and chemical compositions. The regression showed some improvement (R2 = 0.50) compared with the case in which all of the samples were included (R2 = 0.46). The regression coefficients were changed (C = 2,733 and D = 0.73) such that the computed corrosion rates were different. It was observed that corrosion rate measurements became more dispersed with decreasing resistivity, which caused the regression to decrease. R2 = 0.50 0 5 10 15 20 25 30 35 40 0 2,000 4,000 6,000 8,000 10,000 12,000 14,000 16,000 18,000 20,000 C or ro si on r at e (µ m /y ea r) Resistivity ( ·cm) CR = (2,877)Ï-0.73 Figure 3-10. Galvanized steel corrosion rates and resistivity measurements from samples with more than 22% of particles passing the No. 10 sieve, as tested according to AASHTO T 288. 0 5 10 15 20 25 30 35 40 0 10,000 20,000 30,000 40,000 50,000 60,000 C or ro si on ra te (µ m /y ea r) Resistivity ( ·cm) Figure 3-11. Galvanized steel corrosion rates and measurements of resistivity from samples with less than 22% of particles passing the No. 10 sieve, as tested according to Tex-129-M.
42 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials Figure 3-11 depicts data from application of Tex-129-M to samples with less than 22% of particles passing the No. 10 sieve. Compared with Figure 3-10 and the resistivity measurements from AASHTO T 288 (with >22% passing the No. 10 sieve), the corrosion rates depicted in Figure 3-11 are lower for all levels of resistivity measured according to Tex-129-M (with <22% passing the No. 10 sieve). Corrosion rate measurements from Waco, Texas, and an MSE wall in El Paso, Texas, with coarse fill were very low (<<1 mm/year). The low corrosion rate measure- ments were likely because these were field measurements from sites that were very dry (desert locations) at the time the measurements were made. Thus, resistivity measurements obtained from samples that are saturated do not apply very well to these data. More data including measurements of corrosion rates and resistivity from sites located throughout North America and Europe are available from a database cataloged as part of NCHRP 24-28 (Fishman and Withiam, 2011). These data include the maximum corrosion rates observed from each site. However, the resistivity measurements from Tex-129-M are not avail- able for this data set. These data were culled such that coarse samples with less than 22% passing the No. 10 sieve were removed from the data set. The culled data are presented in Figure 3-12 with 36 data points including 10 data points coincident with the samples included in the Phase II laboratory testing for the present study. Regression analysis using the data from Figure 3-12 showed regression coefficients and cor- relation similar to the data presented in Figure 3-10, which includes only data collected from Phase II of this study. The regression from the broader database renders C = 5,267 compared with C = 2,733, D = 0.84 compared with D = 0.73, and R2 = 0.62 compared with R2 = 0.50. These sets of coefficients were used with Equation 3-4 and resistivity between 200 Ω ⢠cm and 50,000 Ω ⢠cm as input. Differences in computed corrosion rates were within 3 mm/year for computed corrosion rates in excess of 15 mm/year, and the difference decreased to 0.5 mm/year for computed corrosion rates of approximately 1 mm/year. These similarities indicate that the model is robust and fits well to the data that were not included in the set initially used to determine regression coefficients. This provides confidence that the model obtained from regression analysis is not limited to describing those data collected in Phase II of this project and has a broader application. CR = (5,267)Ï-0.84 R² = 0.62 0 5 10 15 20 25 30 35 40 0 5,000 10,000 15,000 20,000 25,000 30,000 35,000 40,000 45,000 C or ro si on r at e (m m /y ea r) Resistivity ( ·cm) Figure 3-12. Galvanized steel corrosion rates and measurements of resistivity from worldwide data. Testing was done according to AASHTO T 288 on samples with more than 22% of particles passing the No. 10 sieve.
Laboratory Measurements 43 3.4.1.2 Performance of Plain Steel The correlation between test results from Group I and corrosion rates measured on plain steel specimens was considered to be low (0.08 < R2 < 0.20). This may be due to the paucity of corrosion rate measurements available from plain steel specimens placed within MSE wall fill. The correlation using results from Tex-129-M was better (R2 = 0.31) and considered moderately correlated (0.25 < R2 < 0.49). Figure 3-13 depicts data from correlating results of AASHTO T 288 with measurements of corrosion rate on plain steel specimens for samples with more than 22% of particles passing the No. 10 sieve. Good correlation (R2 = 0.4) was achieved with outliers removed, whereby higher corrosion rates were observed (Prince George, British Columbia, Canada, and M-U-D, New York). The correlation coefficients from this regression were C = 1,470 and D = 0.68. There were only two data points with corrosion rate measurements and resistivity measured according to Tex-129-M for materials with less than 22% of particles passing the No. 10 sieve. For these two points, the higher corrosion rate corresponds to the lower measurement of resistivity. It was concluded that results from Tex-129-M apply well to materials with less than approx- imately 22% of particles passing the No. 10 sieve. For materials with more than 22% passing the No. 10 sieve, AASHTO T 288 is appropriate for measurement of resistivity. These observa- tions were used to develop the proposed protocol discussed in Section 3.5 and are presented in the appendix. 3.4.2 Classification of Soil Corrosivity 3.4.2.1 Characterization Scheme Proper characterization of corrosion potential needs to consider the nature and physical char- acteristics of the material, its electrochemical properties, and various factors related to the site conditions. Characterization of corrosion potential may be done by setting threshold limits for individual electrochemical parameters (e.g., electrical resistivity, pH, and sulfate and chloride content), similar to the AASHTO standard, or may involve ranking according to a multi variate CR = (1,470)Ï-0.68 R² = 0.39 0 5 10 15 20 25 30 35 40 45 0 2,000 4,000 6,000 8,000 10,000 12,000 14,000 16,000 18,000 20,000 C or ro si on r at e (µ m /y ea r) Resistivity ( ·cm) Figure 3-13. Plain steel corrosion rates and measurements of resistivity from AASHTO T 288 and samples with more than 22% of particles passing the No. 10 sieve.
44 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials approach. Several schemes exist for screening and characterizing the corrosion potential of earthen materials. These schemes are often developed for specific applications (e.g., MSE walls, piles, culverts, and pipelines) that may involve aspects of the installations, site conditions, and electrochemical properties. Following are some of the most common schemes that use a multi- variate approach: ⢠The German Technical and Scientific Association for Gas and Water standard DVGW GW 9 is one of the earliest corrosion assessment methods applied to pipeline construction in Europe (Shreir et al. 1994). ⢠Eyre and Lewis (1987) modified the German scheme, which was then adopted in a slightly revised form by the UK Highways Agency in its Design Manual for Roads and Bridges (2000). However, this revised scheme does not consider the beneficial effect of the presence of carbonates on corrosivity, which was originally included in DVGW GW 9. ⢠Jones (1985) developed a method for characterizing the corrosivity of soils that considered a number of variables related to soil type, site conditions, and electrochemical properties. ⢠The standard method used in Great Britain to assess the design life requirements of buried galvanized steel structuresâculverts in particularâis based on a multivariate classification system that rates different environments in contact with the structure according to their corrosivity (Brady and McMahon, 1994). The classification scheme used in Great Britain considers characteristics of the soil, including mechanical properties described in terms of particle size and plasticity and electrochemical parameters including resistivity, pH, and the presence of sulfate, chloride, and sulfide. This study focused on the application of AASHTO criteria to characterize corrosion potential (Table 1-1) and on DVGW GW 9, which considers the protective effects associated with the presence of carbonates. Table 3-9 presents the characterization scheme of DVGW GW 9, in which several factors are involved in the corrosivity assessment, including physical and elec- trochemical properties of the earthen material (soil), site conditions, groundwater levels, and the presence of industrial fills. Points/marks are assigned for each factor and are summed to calculate an overall score. This score is used to assess corrosivity; lower (more negative) scores indicate more severe corrosion conditions. The scheme considers the benefits (positive score) from the presence of carbonates on the corrosion behavior of buried metals. The sum of the points assigned to each category can range from a best of +4 to a worst case of â25. Corrosivity and expected corrosion rates can be evaluated on the basis of this overall score and the informa- tion in Table 3-10 and Table 3-11. The DVGW GW 9 scheme was used to compute corrosion indices for all sample sources included in Phase II of the test program. Multiple electrochemical measurements from earth materials, including pH, resistivity, and soluble chloride and sulfate ion contents, were incorpo- rated to compute a corrosivity index. Correspondingly, corrosivity indices were computed with results from the AASHTO test series, current Texas DOT test procedures, proposed modified Texas DOT test procedures, and ASTM test procedures. Indices were also computed according to the following criteria for application of the appro- priate electrochemical test methods. The appropriate test standards were selected on the basis of the character of the material being tested and the percentage of particles passing the No. 10 sieve: ⢠If the sample had greater than 25% of particles passing the No.10 sieve or GN < 3, then AASHTO T 288 was applied. ⢠If the sample had less than 25% of particles passing the No. 10 sieve and GN > 3, then Tex-129-M was applied. The grading number was included with the screening to restrict the use of Tex-129-M to coarse-textured samples with a relatively high gravel content.
Item and Measured Value Marks Soil Composition Calcareous, marly limestone, sandy marl, not stratified sand +2 Loam, sandy loam (loam content 75% or less), marly loam, sandy clay soil (silt content 75% or less) 0 Clay, marly clay, humus â2 Peat, thick loam, marshy soil â4 Groundwater Level at Buried Position None 0 Exist â1 Vary â2 Resistivity (â¦Â·cm) >10,000 0 5,000â10,000 â1 2,300â5,000 â2 1,000â2,300 â3 <1,000 â4 Moisture Content (%) <20 0 >20 â1 pH >6 0 <6 â2 Sulfide and Hydrogen Sulfide None 0 Trace â2 Exist â4 Carbonate (%) >5 +2 1â5 +1 <1 0 Chloride (mg/kg) <100 0 >100 â1 Sulfate (mg/kg) <200 0 200â500 â1 500â1,000 â2 >1,000 â3 Cinder and Coke None 0 Exist â4 Table 3-9. Characterization scheme from DVGW GW 9. Total Score Category Soil Corrosivity Risk of Deep/Wide Pitting Risk of General Corrosion 0 Ia Virtually not corrosive Very low Very low 0 to â4 Ib Slightly corrosive Low Very low â5 to â10 II Corrosive Medium Low < â10 III Highly corrosive High Medium ⥠Table 3-10. Soil corrosivity/aggressiveness (for carbon steel), DIN 50 929, Part 3.
46 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials The characterization of corrosivity was compared with the measurement of the corrosion rate from galvanized and plain steel elements. The following sections compare correlation with performance considering the characterization of corrosivity by DVGW GW 9. Characterization based solely on resistivity, as discussed in Section 3.4.1, is also included in the comparison. 3.4.2.2 Correlation Between Results of Characterization Scheme and Performance Data Formulas determined by regression analysis do not depict how corrosion rates vary within selected ranges of resistivity or other material characteristics. Alternatively, data clusters are use- ful in quantifying the variations and uncertainties associated with data within selected regions of a sample domain. A data cluster is a group of objects that are more similar to each other as compared with those in other groups (clusters). In this study, clusters were defined in terms of similar material characteristics (e.g., gradation, maximum particle size, electrochemistry, or corrosivity index). Distinct ranges of performance were identified, as measured by corrosion rates that are associated with each data cluster. Cluster analysis was used to demonstrate the advantage of the proposed protocol over exist- ing test standards for characterizing the steel corrosivity of earthen materials. Table 3-12 pre- sents data clusters arranged according to resistivity measured with AASHTO T 288, Tex-129-M or ASTM WK24621, as prescribed in the previous section. ASTM WK24621 was applied to expanded clay, the particles of which are porous. The 24-hour soaking period included in the ASTM WK24621 procedure allowed moisture to be absorbed before the test, which ensured that all measurements were made with moisture occupying the pore spaces within the solid particles. Resistivity measurements (r) were grouped into three clusters: r > 10,000 Ω ⢠cm, 3,000 Ω ⢠cm < r < 10,000 Ω ⢠cm; and 1,000 Ω ⢠cm < r < 3,000 Ω ⢠cm. Distinctly different ranges of corrosion rate measurements were observed within these defined clusters for galvanized and plain steel elements. Corresponding ranges of corrosion rates, shown in Table 3-13, are similar to those described in DIN 50 929, Part 3 (Brady and McMahon, 1994), corresponding to noncorrosive, slightly corrosive, and corrosive conditions. Two exclusions, indicated by shaded cells, are evident in the 28 measurements shown in Table 3-12. Table 3-14 presents data clusters arranged according to corrosivity index as determined from DVGW GW 9. The corrosivity indices, Σ(I), are grouped in three clusters: ⢠Σ(I) ⥠0, ⢠â3 ⤠Σ(I) < 0, and ⢠â5 ⤠Σ(I) < â3. General Corrosion Localized Corrosion (pitting) Total Score Category Rate (µm/year) Range (µm/year) Rate (µm/year) Range (µm/year) 0 Ia 5 2.5â10 30 15â60 0 to â4 Ib 10 5â20 60 30â120 â5 to â10 II 20 10â40 200 100â400 < â10 III 60 30â120 400 200â800 ⥠Table 3-11. Expected corrosion forms/rates (for carbon steel), DIN 50 929, Part 3.
Laboratory Measurements 47 Sample GN PP#10 Test Method (proposed protocol) Ï (â¦Â·cm) CR (µm/year) Galvanized Plain Ï > 10,000 â¦Â·cm San Antonio, TX 0.18 2 Tex-129-M 42,666 1.0 NAa Wake Forest, NC 2.21 8 Tex-129-M 31,651 0.3 < 0.1 Bastrop, TX 0.15 2 Tex-129-M 24,155 0.4 NA Ocala, FL 5.65 91 AASHTO T 288 16,535 1.8 3.8 Ashdown, AR 2.88 36 AASHTO T 288 13,958 1.8b NA 3,000 â¦Â·cm < Ï < 10,000 â¦Â·cm M-U-D, NY 5.24 82 AASHTO T 288 9,064 4.8 39 South Carolina LWF 4.83 68 ASTM WK24621 7,045 1.2 8.4 Maple Rd., NY 2.50 22 Tex-129-M 3,817 3.7 16 Triangle Town Center, NC 3.51 24 AASHTO T 288 5,056 5.8 1.6 Waco, TX 1.26 7 Tex-129-M 4,499 0.3 NA Prince George, BC, Canada 2.89 32 AASHTO T 288 4,527 NA 20 Garden City, TX 2.52 22 Tex-129-M 3,613 4.3 NA El Paso, TX, coarse/MSE 0.22 2 Tex-129-M 3,307 0.2 NA El Paso, TX, fine/MSE 5.52 87 AASHTO T 288 3,026 21c NA South Carolina GB 4.48 56 AASHTO T 288 2,486 3.2d 5.8 1,000 â¦Â·cm < Ï < 3000 â¦Â·cm PIP, NY 4.62 61 AASHTO T 288 1,872 37 30 Sprain Brook, NY 2.54 27 AASHTO T 288 1,720 33 NA Quarry; El Paso, TX 3.64 41 AASHTO T 288 914 14.8 NA Rochester, NY 3.85 49 AASHTO T 288 679 9.6 20 a NA = not available. b CR measured from moist and saturated samples. Results are from moist samples to be consistent with other measurements presented in this table. Measurement from saturated sample is 7.7 µm/year. c This reading is from the top of the MSE wall. The CR measured near the base of the MSE wall was much lower (2.1 µm/year). d One outlier equal to 35 µm/year that appears to be dubious. Next highest is 3.2 µm/year. Table 3-12. Data clustering according to resistivity and observed rates of corrosion. Resistivity Cluster Observed CR Galvanized Steel Plain Steel Ï > 10,000 â¦Â·cm CR < 2 µm/year CR < 5 µm/year 3,000 â¦Â·cm < Ï < 10,000 â¦Â·cm 0 µm/year < CR < 6 µm/year 1.0 µm/year < CR < 20 µm/year 1,000 â¦Â·cm < Ï < 3,000 â¦Â·cm 10 µm/year < CR < 35 µm/year 10 µm/year < CR < 40 µm/year Table 3-13. Range of corrosion rates according to resistivity. Table 3-15 describes the ranges of corrosion rates corresponding to each cluster. Com- pared to the clustering with resistivity measurements, these clusters result in a tighter range of observed performance within each cluster. Four exclusions are indicated in shaded cells in Table 3-14. After clustering was applied to these data, the benefits of the proposed protocol were apparent. Distinct clusters of data were observed that can be useful for relating characterizations of cor- rosivity to performance.
48 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials 3.5 Recommended Protocol Recommendations based on the results of the analyses of the data collected in Phase II of this study were incorporated into the proposed protocol (see appendix), which is shown as a flowchart in Figure 3-14. In general, the proposed protocol describes application of the current AASHTO test series for samples with GN > 3 or in which the percentage passing the No. 10 sieve is greater than 25%. If GN < 3 and the percentage passing the No. 10 sieve is less than 25%, the Texas modified procedures are recommended (i.e., Tex-129-M and Tex-620-M). Sample GN PP#10 Test Method (proposed protocol) Corrosivity Ranka [â(I)] CR (µm/year) Galvanized Plain Not Corrosive: â(I) ⥠0 San Antonio, TX 0.18 2 Tex-129-M 1.0 NAb Wake Forest, NC 2.21 8 Tex-129-M 2 0.3 <0.1 Bastrop, TX 0.15 2 Tex-129-M 2 0.4 NA Ashdown, AR 2.88 36 AASHTO T 288 2 1.8c NA Triangle Town Center, NC 3.51 24 AASHTO T 288 1 5.8 1.6 Ocala, FL 5.65 91 AASHTO T 288 0 1.8 3.8 South Carolina LWF 4.83 68 ASTM WK24621 0 1.2 8.4 El Paso, TX, coarse/MSE 0.22 2 Tex-129-M 0 0.2 NA Waco, TX 1.26 7 Tex-129-M 0 0.3 NA Slightly Corrosive : â3 ⤠â(I) < 0 M-U-D, NY 5.24 82 AASHTO T 288 â1 4.8 39 Garden City, TX 2.52 22 Tex-129-M â1 4.3 NA South Carolina GB 4.48 56 AASHTO T 288 â1 3.2d 5.8 Maple Rd., NY 2.50 22 Tex-129-M â2 3.7 16 El Paso, TX, fine/MSE 5.52 87 AASHTO T 288 â2 21e NA Prince George, BC, Canada 2.89 32 AASHTO T 288 â3 NA 20 Corrosive: â5 ⤠â(I) < â3 PIP, NY 4.62 61 AASHTO T 288 â4 37 30 Sprain Brook, NY 2.54 27 AASHTO T 288 â4 33 NA Quarry; El Paso, TX 3.64 41 AASHTO T 288 â4 14.8 NA Rochester, NY 3.85 49 AASHTO T 288 â5 9.6 20 a German method DVGW GW 9. b NA = not available. c CR measured from moist and saturated samples. Results are from moist samples to be consistent with other measurements presented in this table. Measurement from saturated sample is 7.7 µm/year. d One outlier equal to 35 µm/year that appears to be dubious. Next highest is 3.2 µm/year. e This reading is from the top of the MSE wall. The CR measured near the base of the MSE wall was much lower (2.1 µm/year). Table 3-14. Data clustering relating corrosivity rankings to observed rates of corrosion. Corrosivity Cluster Observed CR Galvanized Steel Plain Steel â(I) ⥠0 CR < 2 µm/year CR < 5 µm/year â3 ⤠â(I) < 0 2 µm/year < CR < 5 µm/year 5 µm/year < CR < 20 µm/year â5 ⤠â(I) < â3 10 µm/year < CR < 35 µm/year 20 µm/year < CR < 40 µm/year Table 3-15. Range of corrosion rates and corresponding ranges of corrosivity indices.
Note: Clâ = chloride; SO4 = sulfate; H2O = water; CaCO3 = calcium carbonate. Figure 3-14. Flow chart of proposed AASHTO Standard Practice: Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials.
50 Electrochemical Test Methods to Evaluate the Corrosion Potential of Earthen Materials Four factors were considered in selecting methods for the proposed protocol: ⢠Precision and repeatability of the test methods, ⢠Compatibility between parameters (e.g., salt content and resistivity), ⢠Correlation between geochemical and electrochemical propertiesâcorrosivity and corrosion rates, and ⢠Utility of the test results. All of these factors support the implementation of the AASHTO and Texas modified proce- dures within the proposed protocol. The statistics included in the evaluation of the test methods describe the repeatability of the test results and correlation between measurements of corrosivity/resistivity and corrosion rates. These statistics demonstrate that the proposed protocol renders results that correlate best with observations of corrosion rates, although observed differences are not large. Also, the repeat- ability of the Texas modified test series is the best as compared with the other test methods. This is part of the justification for recommending the AASHTO test series and the Texas modified test series in the proposed protocol. Other considerations include the observed trends between parameters such as salt content and resistivity and the utility of the test results, which favors use of the AASHTO or Texas modified tests. The coefficient of correlation and the statistics of the measurements are not the only factors considered in the selection of test recommendations. There are benefits to obtaining the rela- tionship between moisture content and resistivity. These benefits include the ability to relate laboratory and field measurements of resistivity where the field moisture content is known. Correlation between laboratory and field measurements also requires that the gradation of the material tested in the laboratory and in the field is similar. Tex-129-M satisfies these needs because the test specifies that resistivity measurements be obtained for a range of moisture content up to saturation, and the test includes all particle sizes up to 1¾ in. ASTM G187 is only performed at moisture content corresponding to as-received or saturated and only includes particle sizes up to ¼ in. Therefore, for the MSE wall application, ASTM G187 is not as desirable as Tex-129-M. ASTM G187 may be desirable for other applications where water content is not variable with respect to time or is maintained at particular levels, such as always saturated. ASTM WK24621 is also not applicable to a range of moisture content but may apply to some materials that drain freely. ASTM WK24621 may be particularly applicable for materials that absorb moisture, because saturation requires soaking, and a 24-hour soaking period is specified by WK24621.
