9
Cancer-Related Impairments Leading to Functional Limitations
Improved diagnosis and more effective treatments are helping decrease cancer mortality, with a corresponding increase in the number of cancer survivors. Many people are living with and beyond cancer, with implications for their long-term ability to adequately perform normal activities, including work. This chapter provides an overview of cancer-related impairments that may be caused by the disease itself or produced by cancer treatments. These impairments may be clustered or overlap, and in many cases are both acute and chronic. For each impairment, the committee discusses interventions that, with adequate implementation, can help mitigate it.
CONCEPTS IN IMPAIRMENT AND FUNCTION
The committee uses the term “cancer-related impairment” to refer to the adverse physiological or psychological sequelae of cancer or its treatment that may or may not result in limitations in activities. Given the U.S. Social Security Administration’s (SSA’s) requirement that an impairment be expected to last at least 12 months in order for an individual to qualify for benefits (see Chapter 2), in this chapter the committee specifically considers an impairment to be any loss or abnormality of physiological, psychological, or anatomical structure or function that can last 12 months or longer and that is of sufficient severity to alter the performance of activities and tasks required to carry out employment or self-care activities. The committee recognizes that some of the impairments discussed in this chapter may occur for periods of less than 12 months, but these episodes would not meet SSA’s eligibility requirements for disability benefits. The term
“limitation” or “functional limitation” is reserved for those instances when the committee specifically refers to a published model of disability or to SSA policy regarding disability determinations. The World Health Organization’s International Classification of Functioning, Disability and Health biopsychosocial framework suggests that a medical diagnosis may not be the sole—or even the best—determinant of eligibility for benefits (see Figure 9-1; see Chapter 2 for more information on the International Classification of Functioning, Disability and Health framework). SSA does not require a diagnosis for disability eligibility but instead relies on objective medical evidence to establish the existence of a medically determinable impairment.
The National Academies of Sciences, Engineering, and Medicine (the National Academies) has conducted several studies for SSA that explore various aspects of health conditions and disability, including the 2019 report Functional Assessment for Adults with Disabilities (NASEM, 2019). Most recently, the National Academies completed a new report, Selected Health Conditions and Likelihood of Improvement with Treatment (NASEM, 2020), which identified disabling long-term medical conditions (including cancer) in adults that are likely to improve with treatment. That
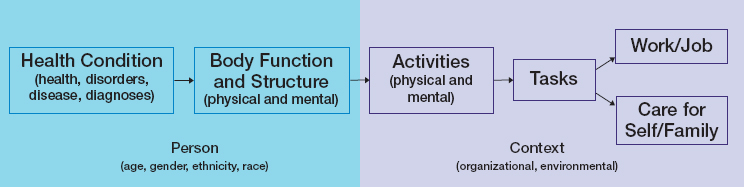
SOURCES: Adapted from WHO, 2001, and NASEM, 2019.
committee was further asked to describe treatments used to improve functioning, the settings in which such treatments are provided, how people are identified for the treatments, the duration of the treatment necessary so that the condition in no longer disabling, what laboratory or other methods (e.g., patient reports or alternatives) are used to assess improvement, and, finally, whether pain is associated with the condition and, if so, the types of treatment prescribed to alleviate the pain. The report addressed several cancers of relevance to this committee, including breast cancer, lung cancer, lymphoma, and head and neck cancers. The committee carefully considered the findings and conclusions of that National Academies report when preparing this chapter, and some of the information presented here is derived from that report.
Assessing Functional Impairments
Assessing a person’s ability to engage in substantial gainful activities can be a complex task. In 2019, at the request of SSA, the National Academies report Functional Assessment for Adults with Disabilities reviewed the literature on the functional assessment of physical and mental abilities relevant to work requirements and the function and impairment trajectories in individuals with back disorders, cardiac impairments, depression, and traumatic brain injury (NASEM, 2019). Although that report did not assess cancer-specific impairments, many of the concepts and assessment tools are applicable to individuals with cancer (see Figure 9-1). The report stated that “assessments that integrate information about impairments and abilities, including multiple tests of different types, repeated over time, provide the most useful information about work-related function” (NASEM, 2019, p. 9). In particular, the report concluded that an individual’s “functional abilities relevant to work requirements when assessed outside of actual work settings may be insufficient to establish [his or her] capacity to perform full-time work on a regular and continuing basis” (NASEM, 2019, p. 8). The many instruments available to assess both physical and mental functional abilities in the research and clinical setting were reviewed in detail.
A common measurement used to help determine cancer treatments is performance status. The performance status tools primarily used in cancer treatment are the Karnofsky Performance Scale (Karnofsky et al., 1948) and the Eastern Cooperative Oncology Group performance status scale (Oken et al., 1982) (described in Chapter 4). Both scales are intended to help clinicians know whether it is safe to treat the patient, but they also have relevance to understanding cancer-related impairments and functional limitations. These clinical performance status values are distinct from well-validated objective measures of physical performance capabilities, such as the Short Physical Performance Battery (Guralnik et al., 1994), the
6-minute walk test (ATS/ACCP, 2003), or a cardiopulmonary stress test (ACSM, 2017). Objective measures can more precisely define cancer-related impairments than simple clinical performance scales.
Patient-Reported Outcomes
An assessment approach that is gaining importance in clinical practice is patient-reported outcomes (PROs). The U.S. Food and Drug Administration report Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims defined PROs as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else” (FDA, 2009).
There are several reasons to incorporate PROs into the assessment of functional status. Primary among these is that some health conditions, such as pain, depression, or fatigue, can only be determined by patient self-report as there are no adequate diagnostic tests for these symptoms. When used as a complement to clinical assessments of functional status, PROs provide deeper and richer information on the health conditions and contextual factors shown in Figure 9-1, adding details that may be missed with clinical assessments alone. Furthermore, PROs identify more data than clinician-standard reporting in the health record, data that would otherwise be missed (Neben-Wittich et al., 2011). There is a robust literature documenting the discrepancy between clinicians and patients when rating symptoms, with many clinicians either over-rating or under-rating patient-reported symptoms that are subjective and lack diagnostic tests (Atherton et al., 2015; Montemurro et al., 2016; Tetreault et al., 2019). Patients report that the clinician outcome measures do not fully capture factors that are relevant to them, such as how symptoms affect their ability to perform desired life activities (Borghuis et al., 2020).
Impairments from Diagnosis to Long-Term Survivorship
The detrimental effects of cancer can begin prior to diagnosis when the patient may experience pain or other symptoms caused by the cancer itself. However, for many cancer survivors, cancer treatments themselves are often associated with significant physical and psychosocial effects, many of which persist for years or even decades after treatment is complete. Furthermore, poor functional capacity at diagnosis (due to cancer or underlying chronic medical conditions) may alter the treatments given, and the toxicities experienced during treatment may lead to a modification of treatment strategies. Adverse effects that occur during or shortly after treatment (e.g., mouth sores from chemotherapy) are often referred
to as acute toxicities, and many of these may resolve after therapy ends. Adverse effects that begin during treatment and persist for years or decades after treatment ends are called long-term, or persistent, effects (e.g., chemotherapy-induced peripheral neuropathy [CIPN]), whereas effects that do not appear during treatment but may be manifest months or years after treatment (e.g., heart failure) are termed late-onset effects (IOM, 2006). Cancer recurrence or another new primary cancer (which may be due to prior treatment) diagnosed years after the initial cancer may also result in impairments.
In the cancer care community, the role of disease- and treatment-related impairments in functional status and work ability is gaining attention. For example, in 2018 the National Coalition for Cancer Survivorship determined that cancer care quality measures reflecting “return” to functional status after cancer diagnosis did not resonate with cancer survivors because after a cancer diagnosis, life is permanently changed. Survivors felt that “redefining” functional status more accurately captured the dynamic nature of functional status during and after cancer diagnosis and treatment (NCCS, 2019). The National Comprehensive Cancer Network (NCCN) subsequently recommended core cancer care quality measures for survivorship care that highlight survivors’ ability to perform meaningful life activities, such as work. These measures also take into account various aspects of quality of life (QOL) (i.e., physical and mental health, physical function, pain interference, fatigue interference, cognitive function, and the psychosocial impact of cancer) (NCCN, 2020f). It is important to note that performance status, a numeric rating described in Chapter 4, often correlates poorly with individuals’ actual functional status obtained using PRO measures (Atkinson et al., 2015).
SELECTED CANCER-RELATED IMPAIRMENTS
This section provides an overview of the incidence and time course of substantial long-term (i.e., 12 months or longer) impairments associated with cancer and its treatment (see Table 9-1), with specific reference to breast cancer and lung cancer; other cancers are discussed as applicable. The committee considers impairments such as pain, fatigue, CIPN, and lymphedema to be among the most common, disabling, and difficult to manage when the goal is returning a person to work. Each impairment is discussed briefly in terms of risk factors, prevalence, diagnosis, management, and prognosis. The committee emphasizes that this list is not comprehensive, that other impairments exist, and that cancer survivors’ experience of these impairments is highly variable: some may experience none of them, while others experience several, and the severity of the impairments varies considerably, as does survivors’ reaction to the impairments.
TABLE 9-1 Impairments Considered in This Chapter, Their Causes, and When They Might Occur
| Impairment | Causes | Occurrencea | |||
|---|---|---|---|---|---|
| Disease Process | Cancer Treatment | Acute | Long-Term | Late-Onset | |
| Pain | • | • | • | • | |
| Cancer-related fatigue | • | • | • | • | |
| Chemotherapy-induced peripheral neuropathyb | • | • | • | ||
| Lymphedema | • | • | • | ||
| Cachexia | • | • | |||
| Cardiotoxicity | • | • | • | • | |
| Cognitive impairments | • | • | • | • | • |
| Depression and anxiety | • | • | • | • | |
| Gastrointestinal impairments | • | • | • | • | • |
| Graft-versus-host disease | • | • | • | ||
| Musculoskeletal impairments | • | • | • | • | • |
| Pulmonary toxicity | • | • | • | • | |
| Sleep disturbances | • | • | • | • | |
NOTE: Other sensory impairments were not included in the table because they are so diverse.
a Acute refers to impairments that may occur during or immediately after treatment; long-term refers to impairments that may begin during or immediately after treatment but that persist for an extended period of time; and late-onset impairments are those that may occur months or years after treatment is complete.
b Although some cancers such as myeloma may cause peripheral neuropathy, the committee focuses on chemotherapy-induced peripheral neuropathy in this report.
Pain
Prevalence, Etiology, and Risk Factors
The treatment of cancer pain can span the cancer continuum from before diagnosis through end of life. Pain often impairs function and occurs in many forms, including acute, singular events; chronic episodic conditions; and chronic persistent pain (Stewart et al., 2003). Chronic pain can physically affect the cancer survivor, causing reduced physical activity and function, fatigue, lethargy, and peripheral neuropathy; such pain can also affect the psychological, cognitive, social, and spiritual aspects of a survivor’s life (Vernucci et al., 2018). There are several types of cancer-related pain: intermittent or non-breakthrough pain, continuous pain, acute pain, chronic
pain caused or associated with the tumor, nociceptive pain, inflammatory pain, and neuropathic pain (Vernucci et al., 2018). Pain is often clustered with fatigue and depression in cancer survivors.
An estimated 40% or more of cancer survivors have persistent disease- or treatment-related pain, which can adversely affect QOL (van den Beuken-van Everdingen et al., 2016). In a Danish study, 47% of disease-free breast cancer survivors experienced pain 2–3 years following the completion of surgery and adjuvant therapy (Gartner et al., 2009). Other studies have shown that even years after breast cancer surgery, between 35% and 60% of women report postoperative pain (Juhl et al., 2016; Mejdahl et al., 2013; van Helmond et al., 2016). Additionally, the number of breast cancer survivors who report severe pain may actually increase over time (Forsythe et al., 2013). Both radiation treatment and endocrine therapy are also associated with long-term pain in breast cancer survivors (Hidding et al., 2014).
Lung cancer is associated with pain resulting from disease progression as well as from treatments such as thoracic surgery or radiation. Mercadante and Vitrano reported that “chest and lumbar spine are the most common sites of pain localization, with 38% of patients with lung cancer having two or more anatomically distinct pains” (2010, p. 10). In particular, lung cancer metastases to the bones are a frequent cause of pain. Chemotherapy with platinum-based drugs or taxanes may also result in painful distal paresthesias.
For other cancers, the data are more limited. For example, among head and neck cancer survivors 45% reported pain, and 12% reported severe pain more than 6 years after diagnosis (Cramer et al., 2018).
Patients with metastatic disease are especially prone to pain, with bone metastases being the most common pain generator (Falk et al., 2014; Milgrom et al., 2017); however, metastatic cancer pain is inadequately characterized. Cancer patients may experience pain in a variety of other organs, such as pain from liver capsule distension in the liver, brain-tumor-related headache, neural compression, and abdominal pain (Cherny et al., 2015). While most pain syndromes in late-stage disease can be treated, typically with opioid medications, many patients experience residual pain and remain prone to the development of new pain generators.
Diagnosis and Assessment
In 2016 the American Society of Clinical Oncology (ASCO) released its evidence-based guideline Management of Chronic Pain in Survivors of Adult Cancers, which covered the screening and treatment of cancer-related pain. The guideline recommends that all patients be screened for pain at each visit and that a team approach be used to determine the optimal treatment plan. In the clinic, the NCCN Clinical Practice Guidelines for Adult
Cancer Pain recommend a numeric pain measurement system similar to that for fatigue (discussed below) ranging from 0 = no pain to 10 = worst pain imaginable. Threshold scores are 1–3 for mild pain, 4–6 for moderate pain, and 7–10 for severe pain (Swarm et al., 2019). However, there are many other types of scales that may be used to assess the intensity and other attributes of pain based on patient reports. Self-report measures are strongly influenced by an individual’s perception of pain, which is currently the only valid measure of pain. Individuals’ perceptions of their pain can, in turn, affect their perception of their functional ability (Reiman and Manske, 2011).
Treatment and Prognosis
The management of cancer pain includes both pharmacologic and nonpharmacologic approaches. The ASCO and NCCN guidelines for the treatment of cancer pain include palliative and end-of-life pain management strategies. A multidisciplinary, multimodal approach to treating chronic cancer pain can be effective (NCCN, 2020b). Chronic pain may develop and persist even when the underlying cause of the initial acute pain, such as a surgical wound, is resolved (Mills et al., 2019), and having more than one cause of chronic pain and having pain of longer duration are both associated with poorer QOL (Pagé et al., 2018). Management of co-occurring symptoms such as depression and anxiety are also important, as they may be contributing to the pain reported by some individuals.
Pharmacological approaches include the use of over-the-counter analgesics such as aspirin, acetaminophen, and nonsteroidal anti-inflammatory drugs (NSAIDs); for more refractory pain, prescription NSAIDs and narcotics such as opioids may be used. Other prescription drugs that may be used to alleviate cancer pain include antidepressants (e.g., selective serotonin reuptake inhibitors [SSRIs], serotonin–norepinephrine reuptake inhibitors [SNRIs]), muscle relaxants, corticosteroids, anticonvulsants, and benzodiazepines. The choice of analgesic agent depends on the type of pain syndrome, the severity of the pain, and the patient’s tolerance and preferences, among other factors. Potential side effects of the opioid analgesics that can affect a person’s ability to work or conduct other activities include drowsiness, nausea, vomiting, dizziness, effects on driving, and deficits in memory processing and learning (Els et al., 2017; Gomes et al., 2013; Kibaly et al., 2019; Manchikanti et al., 2012; Porreca and Ossipov, 2009).
Nonpharmacologic management strategies for cancer pain include interventional approaches such as dorsal column stimulation, and kyphoplasty (injecting cement into the vertebrate) for vertebral compression fractures (Health Quality Ontario, 2016). Other integrative therapies such as massage, aromatherapy, imagery, complementary medical approaches,
and mind-body interventions (e.g., acupuncture) might also be helpful for cancer pain. Incremental pedometer exercise and resistance training accompanied by physical and occupational therapy to mitigate impairments have been shown to decrease cancer pain and improve function (Cheville et al., 2019). Psychological treatments such as relaxation, biofeedback, and cognitive behavioral therapy (CBT)1 may also be effective in reducing chronic cancer pain (Vernucci et al., 2018). CBT teaches patients to use adaptive behaviors including engaging in distracting activities, pacing activities, and appropriate use of medications or physical modalities such as heat, ice, or movement (Syrjala et al., 2014). Pain related to bone metastasis can successfully be treated with a single dose of radiotherapy, as recommended by American Society of Therapeutic Radiation Oncology Guidelines (Lutz et al., 2017; see Chapter 4).
Cancer-Related Fatigue
Prevalence, Etiology, and Risk Factors
Fatigue is among the most prevalent and disabling symptoms associated with cancer, regardless of the type of cancer. Cancer-related fatigue differs from general fatigue in that, rather than being a product of overexertion or lack of sleep, the tiredness and weakness of cancer-related fatigue occur despite receiving adequate or additional sleep and rest (Cella et al., 2001; Wu and McSweeney, 2007). Fatigue interferes with cancer survivors’ ability to participate in meaningful life activities, including work (Islam et al., 2014), and studies have shown that the greater the fatigue severity, the greater its interference with a survivor’s ability to work (Behringer et al., 2016; Crom et al., 2005; Janda et al., 2000; Wang et al., 2014).
The precise etiology of cancer-related fatigue is unknown, as it is commonly found in patients irrespective of the treatment modalities they receive. Although a variety of biologic mechanisms have been proposed for cancer-related fatigue, most research suggests a role for proinflammatory cytokines (Bower, 2014). There is a high degree of variability in the onset, severity, and trajectory of cancer-related fatigue, which is thought to be a
___________________
1 Cognitive behavioral therapy (CBT) is based on the principles that psychological problems are based, in part, on faulty or unhelpful ways of thinking and learned patterns of unhelpful behavior; people can learn better ways of coping with psychological problems, thereby relieving their symptoms and becoming more effective in their lives. CBT treatment usually involves efforts to change thinking patterns by learning to recognize one’s distortions in thinking that are creating problems and reevaluating them in light of reality; gaining a better understanding of the behavior and motivation of others; using problem-solving skills to cope with difficult situations; and learning to develop a greater sense of confidence is one’s own abilities (APA, 2017).
function of moderating factors such as genetic variations (e.g., in the cytokines involved in inflammation), psychological factors (e.g., depression, anxiety, coping style), and anthropometric factors (e.g., body mass index, physical activity level) (Bower, 2014).
Cancer-related fatigue is extremely common during active cancer therapy, with prevalence rates ranging from 25% to 100% of patients depending on the fatigue measure used (Weis, 2011). Post-treatment cancer-related fatigue is also widespread (see Chapters 5 and 6); one study found that among disease-free breast cancer survivors, two-thirds reported ongoing fatigue (Kim et al., 2008). One study noted that survivors who had lung cancer had the highest rate of fatigue (59%) compared with all other cancers; among post-treatment lung cancer survivors, the fatigue prevalence was 35% (Wang et al., 2014).
Diagnosis and Assessment
Cancer-related fatigue is one of the most underreported, undertreated, and difficult-to-treat symptoms (Bower, 2014), which may in part be due to the lack of widely used diagnostic criteria. Although the International Classification of Diseases, 10th Revision (ICD-10), includes diagnostic criteria for cancer-related fatigue (Cella et al., 2001; Kuhnt et al., 2019; Mustian et al., 2017), they may be too lengthy for use in clinical practice, and, of the many instruments for measuring cancer-related fatigue in the research setting, few are available for routine clinical use.
In the clinical setting, in addition to selected items from the ICD-10, NCCN guidelines (2020a) recommend a screening approach for identifying cancer-related fatigue, with recommendations for interventions based on the stage of treatment. A numeric rating scale from 0 (no fatigue) to 10 (worst fatigue imaginable) has been found to be valid, reliable, and feasible in the clinical setting (NCCN, 2020c). The recommended threshold scores are 1–3 for mild fatigue, 4–6 for moderate fatigue, and 7–10 for severe fatigue (Berger et al., 2015).
Treatment and Prognosis
NCCN guidelines (2020c) recommend several evidence-based, nonpharmacologic and pharmacologic treatments for cancer-related fatigue. The management of co-occurring symptoms such as insomnia and depression is also important, as these symptoms may be contributing to the fatigue reported by some individuals. However, if they are not associated with the complaint of fatigue, then interventions that enhance physical activity are recommended (see ASCO guideline) (Bower et al., 2014). A 2017 meta-analysis found that physical activity and psychological interventions were
superior to pharmacological interventions in reducing cancer-related fatigue (Mustian et al., 2017).
Non-pharmacologic interventions such as physical activity, psychosocial support, and CBT to improve sleep, are recommended. In particular, there is growing evidence that exercise and physical activity interventions are the most effective treatments for cancer-related fatigue, whether it occurs during treatment, following treatment, or even at end of life, barring medical contraindications (e.g., cardiomyopathy) or safety concerns (e.g., risk of falls). However, there are still many unknowns with regard to recommending physical activity for the relief of cancer-related fatigue, including which specific activities provide the greatest relief. For example, there is evidence that yoga is effective, but less evidence for walking, other low-impact cardiovascular exercise, and resistance training. Other interventions that are effective include psychological interventions such as general and sleep-specific CBT and mindfulness-based stress reduction; the evidence is not strong for bright white-light therapy, acupuncture, or nutrition consultation. Pharmacologic interventions include psychostimulants such as methylphenidate, and for patients with advanced cancer, short-term use of corticosteroids such as dexamethasone or prednisone (NCCN, 2020c).
As with cancer-related pain, cancer-related fatigue may persist for months or even years after cancer treatment ends (NCCN, 2019c). As a result of improvements in cancer treatment, health care professionals are now more likely to see patients with prolonged cancer-related fatigue as a late-onset effect of their treatment. Impairment-related issues are especially relevant and challenging for patients with cancer who are cured of their malignancy but have continued fatigue (Berger et al., 2015; Morrow et al., 2002).
Chemotherapy-Induced Peripheral Neuropathy
Prevalence, Etiology, and Risk Factors
CIPN is caused by exposure to the neurotoxic agents used to treat a variety of cancers, such as vincristine, taxanes (e.g., docetaxel), platinum-based agents (e.g., oxaliplatin), immunomodulatory drugs (e.g., thalidomide), and treatments for myeloma such as bortezomib (Zajac̨zkowska et al., 2019). Although CIPN most frequently presents with pain, motor and other sensory symptoms may also be present (Miltenburg and Boogerd, 2014), and the signs and symptoms of CIPN range from mild to disabling, with significant implications for function and QOL (Stubblefield et al., 2009). Abnormal sensations including tingling, numbness, and burning, shooting, and throbbing pain are common in the sensory nerves. Weakness, difficulty with gait, and falls can occur. Patients may have trouble with
normal activities. Autonomic dysfunction, including bowel and bladder dysfunction and orthostatic hypotension, can be seen in the more severe cases.
CIPN is one of the most common side effects of cancer treatment (an estimated prevalence of 68% at 1 month after chemotherapy), and although its prevalence decreases with time after treatment ends, it has been estimated that as many as 30% of patients have CIPN at 6 months to 1 year after finishing chemotherapy (Colvin, 2019; Seretny et al., 2014). The risk of developing CIPN increases with higher doses, multiple courses, and combination chemotherapy (e.g., cisplatin and paclitaxel). In addition to the pain and functional impairments it causes, CIPN can be a major dose-limiting toxicity for many chemotherapeutic agents (Stubblefield et al., 2009). The etiology of CIPN is multifactorial and may involve microtubule disruption, oxidative stress and mitochondrial damage, altered ion channel activity, myelin sheath damage, DNA damage, immunological processes, and neuroinflammation (Areti et al., 2014; Zajac̨zkowska et al., 2019).
Diagnosis and Assessment
Guidelines from the International Association for the Study of Pain suggest that CIPN be screened with patient questionnaires, although screening cannot replace a clinical examination with appropriate sensory testing. It is important to assess for the presence of other medical conditions known to be associated with peripheral neuropathy, such as diabetes. A clinical examination that includes quantitative sensory testing can be used to diagnose small-fiber neuropathies, while the measurement of trigeminal reflexes and laser-evoked potentials may also be useful diagnostic tools. For patients with clinical signs of small-fiber dysfunction, a skin biopsy may also be used. The intensity of pain and treatment effects can be assessed with numerical rating scales or visual analog scales, such as the neuropathic pain scale and the neuropathic pain symptom inventory (Haanpää et al., 2011).
Treatment and Prognosis
There are no proven strategies to prevent CIPN. Complete alleviation of CIPN does not typically occur with many of the treatments prescribed for it, although some relief is possible. Some CIPN may resolve when the chemotherapy is discontinued, but it may persist, particularly following the use of platinum-based drugs. First-line therapies for painful CIPN include over-the-counter analgesics, anticonvulsants (e.g., gabapentin), tricyclic antidepressants, topical anesthetic agents (e.g., lidocaine and capsaicin), NSAIDs, anti-arrhythmics, narcotic analgesics, and opioids (Brooks and Kessler, 2017), although the effectiveness of all of these therapies is
limited. The ASCO guideline on the management of CIPN indicates that it may respond to duloxetine to a limited extent (Loprinzi et al., 2020). Multidisciplinary approaches may be more effective for CIPN, including psychotherapy, physiotherapy, exercise, acupuncture, and massage therapy (Bates et al., 2019; Li et al., 2019).
The symptoms of CIPN are sometimes reversible, but they can also persist for years and even worsen over time (ACS, 2019). One study found that at an average of 6 years after treatment, 47% of breast cancer survivors still reported CIPN (Winters-Stone et al., 2017). CIPN can also lead to functional impairments such as loss of balance, which can lead to gait instability and falls. Women with CIPN were 1.8 times more likely to fall than those without CIPN (Winters-Stone et al., 2017). Physical therapy intervention for gait and balance training can improve standing and walking ability and decrease the risk of falls (Kneis et al., 2019).
Lymphedema
Prevalence, Etiology, and Risk Factors
The lymphatic system—often referred to as the second circulation system—consists of lymph vessels, lymph nodes, and two collecting ducts in the chest. The lymphatic system serves as a surveillance system for cancer and is also crucial to the intestinal absorption of fat. The treatment of several cancers, such as breast cancer, head and neck, prostate, and gynecological cancers, among others, may require the surgical removal of lymph nodes should they contain or be suspected to contain cancerous cells (see Chapter 4). Impairments resulting from lymph node removal may include swelling of the affected limb (i.e., lymphedema) as well as poor wound healing, inflammatory responses, decreased oxygenation of tissues, slowed tissue healing time, pain, and fibrosis. There is a linear relationship between the number of lymph nodes removed from the body in the upper or lower extremities (cancerous or not) and the severity of these effects. Thus, lymph node removal may alter the body’s ability to respond to inflammation, infection, overuse, injury, and trauma, which in turn can affect a person’s QOL (Deng et al., 2013). Radiation therapy for cancer may also damage the lymphatic system in the exposed areas (Beesley et al., 2015). Lymphedema specific to breast cancer and to lung cancer is discussed in Chapter 5 and Chapter 6, respectively.
Lymphedema may occur shortly after treatment with surgery or radiation therapy, but it also may occur months or years after treatment (DiSipio et al., 2013; Rupp et al., 2019). Estimates of lymphedema incidence vary widely, likely due to variability among studies in terms of the length of follow-up, threshold for diagnosis, population under study, and surgical procedure. Risk factors for lymphedema include high preoperative body
mass index, axillary lymph node dissection (ALND), and regional lymph node radiation (Abouelazayem et al., 2020; see also Chapter 5). Lymphedema typically occurs in the extremity adjacent to the dissection site. Recent data suggest that the overall 5-year cumulative incidence of lymphedema is about 13.7% after breast cancer treatment (McDuff et al., 2019). Full ALND is associated with a greater risk of lymphedema (20–22%) compared with women who undergo sentinel node biopsy (5–6%) (DiSipio et al., 2013; Shaitelman et al., 2015).
The incidence of lymphedema secondary to head and neck cancer is estimated to be approximately 75% (Deng et al., 2012). Lower-extremity lymphedema can occur after treatment for bladder, gastrointestinal, prostate, or gynecologic cancers as well as melanoma. Many patients (e.g., those with gynecologic and prostate cancers) undergo the removal of their pelvic lymph nodes and are therefore at risk for lymphedema involving both legs, their lower trunks, and genitalia. The accelerated lymphedema progression seen in legs versus arms is due to the increased demands of vertically transporting metabolic waste from the large lower extremity muscle groups against gravity (Vaqas and Ryan, 2003).
Diagnosis and Assessment
The most common measurements for lymphedema assess swelling by comparing the affected body part to an unaffected body part. This works well for upper and lower extremities, but not for the head, neck, or torso. The most common approach in clinical settings is to compare the circumference of the affected limb with that of the unaffected limb, although more sophisticated methods are available (Dylke et al., 2013). Using circumferential measures, lymphedema is diagnosed if the interlimb differences are greater than 5–10%, 100–500 mL in volume, or 1–5 cm in circumference (Smoot et al., 2011). There are also valid and reliable clinical assessments (Spinelli et al., 2019) and surveys for lymphedema (Norman et al., 2001). Bioelectrical impedance analysis is also used diagnostically. There is currently no gold standard for diagnosing lymphedema.
Treatment and Prognosis
One effective treatment for lymphedema, regardless of affected site, is complete decongestive therapy, usually administered by physical or occupational therapists, including manual lymphatic drainage, compression, exercises, and breathing exercises. Resistance exercise is helpful for preventing flare-ups of breast cancer–related lymphedema (Schmitz et al., 2009), and a progressive program of aerobic and resistance exercise does not appear
to exacerbate the risk for it (Kwan et al., 2011). As discussed in Chapters 5 and 8, new surgical techniques are available to manage lymphedema.
In comparison with breast cancer–related lymphedema, there is little evidence to help determine what treatments are most effective for head and neck cancer-related lymphedema. This is due in part to a lack of validated tools to assess lymphedema severity secondary to head and neck cancer.
The treatments for lower-extremity lymphedema are similar to those for breast cancer–related lymphedema. The larger column of fluid in the leg compared with that in the arm makes lower-extremity lymphedema more complicated to prevent and treat.
Cachexia
Prevalence, Etiology, and Risk Factors
Cachexia, a complex metabolic syndrome seen in serious illnesses such as cancer, is characterized by a loss of muscle mass, weight loss, anorexia, asthenia, and anemia. Cancer cachexia occurs mainly during active or palliative treatment, and its signs and symptoms are considered to be indicative of advanced disease (Dhanapal et al., 2011). While it is often associated with anorexia, adequate nutrition supplementation (e.g., through total parenteral nutrition) is ineffective at stopping the loss of weight and muscle mass (Argilés et al., 2019). Studies suggest that between 11% (Vagnildhaug et al., 2018) and 40% (Peixoto da Silva et al., 2020) of women with breast cancer exhibit cachexia, while between 36% (Vagnildhaug et al., 2018) and 50% (Peixoto da Silva et al., 2020) of patients with lung cancer exhibit the syndrome. In fact, individuals with lung cancer were six times more likely to have cachexia than those with hematologic malignancies (Vagnildhaug et al., 2018).
The pathophysiology of cachexia is complex and often begins with anorexia and metabolic changes (Fearon et al., 2011). As with other cancer- and treatment-related effects, increased levels of pro-inflammatory cytokines, such as tumor necrosis factor-α and interleukin-6, are a primary etiologic feature of cachexia; however, a number of other molecular mechanisms also appear to be involved in its development (Peixoto da Silva et al., 2020).
Diagnosis and Assessment
Table 9-2 presents the clinically accepted diagnostic criteria for cachexia (Fearon et al., 2011). Rather than simple adipose tissue loss, which characterizes other types of weight loss, cachexia is marked by sarcopenia (i.e., loss of skeletal muscle mass and function) in addition to loss of weight or body mass.
TABLE 9-2 Diagnostic Levels of Cachexia
| Precachexia | Cachexia | Refractory Cachexia |
|---|---|---|
| Weight loss ≤5% | Weight loss >5%, or | Cancer both procatabolic and not responsive to anticancer treatment |
| Anorexia | Body mass index <20 and weight loss >2%, or | Low performance score |
| Metabolic change | Sarcopenia with weight loss >2% | <3 months expected survival |
SOURCE: Adapted from Fearon et al., 2011, with permission.
Treatment and Prognosis
The complex etiology of cachexia underlies the difficulty in treating this syndrome. The most obvious treatment is to cure the underlying disease (Peixoto da Silva et al., 2020), but in cases where complete remission is not achieved, the inflammatory processes that underlie cachexia will likely contribute to ongoing muscle wasting and poor physical performance. Megesterol acetate, a synthetic derivative of progesterone, improves appetite, caloric intake, and nutritional status (Argilés et al., 2019). It is widely used to treat cachexia, although its mechanism of action is not fully understood.
One promising treatment for cancer cachexia is physical activity, either alone or in combination with other treatments. Exercise decreases systemic inflammation and protein catabolism and improves endocrine function, muscle oxidative capacity, and protein synthesis to promote the maintenance of skeletal muscle mass and weight (Hardee et al., 2017). A multimodal approach includes medications, nutraceuticals, and exercise (Argilés et al., 2019), but there are no clear data on the length of time needed for improvement.
A number of other potential treatments are under investigation but are not in wide use. Nutritional counseling regarding improved intake is not effective for cachexia, although the administration of specialized nutritional supplements can improve weight and nutritional status (Argilés et al., 2019). While medications and supplements may improve weight and decrease muscle wasting, no concurrent improvement in functional status has been found (Advani et al., 2018). Thus survivors with a history of cachexia may experience continued impairments even after the resolution of the syndrome itself.
Cardiotoxicity
Prevalence, Etiology, and Risk Factors
The cardiotoxic effects of cancer treatment are often permanent and progressive (Virizuela et al., 2019), leading to significant morbidity and
negative effects on function. The precise prevalence of cancer treatment-related cardiotoxicity is not known, although it is known to vary according to treatment (Curigliano et al., 2016). Cardiotoxicity is associated with a range of cancer treatments, from chemotherapy and immunotherapy to radiation therapy. Cardiotoxic effects include heart failure, pericarditis, coronary heart disease, hypertension, arrhythmias, and valve disease. For example, chemotherapy may be associated with early-onset congestive heart failure and arrhythmias, while immunotherapy may be associated with myocarditis.
Cardiomyopathy may be persistent and long-term. Radiation therapy is associated with late-onset issues such as restrictive cardiomyopathy, coronary artery disease, valvular disease, and heart block. These effects may be more pronounced for cancers occurring on the left side of the body, where the heart may receive more radiation exposure (Darby et al., 2013). Long-term cancer survivors may experience autonomic dysfunction, particularly if they received chest wall irradiation (Coumbe and Groarke, 2018). This condition is characterized by tachycardia (rapid heart rate), fatigue, blood pressure variability resulting in dizziness, and other symptoms.
Although cardiotoxicity is relatively rare, it can be life-threatening and may occur years after treatment ends (Anderson et al., 2019; Zaorsky et al., 2017), with the greatest risk at 5 years or more after treatment is completed (NCCN, 2020f). These effects may be more likely to develop in older patients, women, and cancer patients who have comorbidities, particularly those who had signs of heart trouble before their cancer (NCCN, 2020f).
A number of chemotherapy agents, including anthracyclines, trastuzumab and other HER2 receptor blockers, antimetabolites, alkylating agents, tyrosine kinase inhibitors, angiogenesis inhibitors, immune checkpoint inhibitors, and thoracic irradiation, are associated with significant cardiotoxicity (Jain et al., 2017). Anthracycline-based chemotherapy with doxorubicin is a common cancer treatment and is associated with a significant dose-dependent cardiotoxicity. Most cardiotoxicity appears to occur in the first year following treatment, and while some patients recover fully, many have only a partial recovery (Cardinale et al., 2015). The targeted therapy trastuzumab is also associated with cardiotoxicity (e.g., heart failure and cardiomyopathy), especially when administered with anthracyclines; however, the effect is usually reversible with cessation of therapy (Bowles et al., 2012). Aromatase inhibitors used for up to 10 years for estrogen-positive breast cancers are also associated with hyperlipidemia, hypercholesterolemia, and hypertension, all of which can lead to cardiovascular disease (Foglietta et al., 2017). The alkylating agent cyclophosphamide (Iqubal et al., 2019), tyrosine kinase inhibitors (Orphanos et al., 2009), angiogenesis inhibitors such as the vascular endothelial growth factor inhibitors that block the growth of blood vessels to tumors (Tocchetti et al.,
2013), and immune checkpoint inhibitors, either alone or in combination (Varricchi et al., 2017), have all been associated with some degree of early (in treatment) cardiotoxicity, ranging from asymptomatic subclinical abnormalities to death from heart failure.
Radiation therapy is also associated with a variety of cardiotoxic effects, often presenting years after the completion of treatment. Radiation therapy for Hodgkin lymphoma can lead to the development of coronary heart disease, valvular heart disease, heart failure, and pericarditis (van Leeuwen and Ng, 2016), and for breast cancer there is a dose-dependent risk of coronary heart disease (Jacobse et al., 2019). Arrhythmias, such as heart block, may also occur. These late-onset complications of cancer treatment are disabling, particularly as they often occur among survivors younger than 65 years who were previously treated for cancer during childhood, adolescence, or as young adult. Treatment of non-small-cell lung cancer using thoracic radiation has been associated with major adverse cardiac effects and death even 2 years after treatment (Atkins et al., 2019).
Diagnosis and Assessment
There is growing recognition of the need to identify and manage cancer treatment–related cardiotoxicity. A cardiovascular evaluation with clinical history and physical examination, electrocardiogram, exercise stress testing, radionuclide imaging, echocardiography, and magnetic resonance imaging can detect cardiotoxicity (Jain et al., 2017).
Oncology clinicians, including medical oncologists, surgical oncologists, and radiation oncologists, as well as non-oncologic clinicians such as internists, primary care physicians, nurse practitioners, and rehabilitation physicians are instrumental in the identification of cardiac risk factors and dysfunction in cancer survivors (Alfano et al., 2016). NCCN guidelines (2020f) recommend that high-risk patients who receive anthracyclines be thoroughly screened for heart failure 1 year after the treatment is complete. The ASCO guidelines indicate an increased risk of adverse cardiac effects for patients receiving high-dose anthracyclines (e.g., doxorubicin). An increased risk is also associated with high-dose radiotherapy (≥30 Gy) or lower-dose anthracyclines in combination with lower-dose radiotherapy (<30 Gy), where the heart is in the treatment field. The guideline also delineates some cardio-protective approaches for at-risk patients (Armenian et al., 2017).
Treatment and Prognosis
Treatment approaches depend on the type of cardiotoxicity and may involve cardiologists, cardio-oncologists, and primary care physicians. A
comprehensive program of cardiac rehabilitation should include risk factor modification and patient education in addition to exercise and strengthening and psychosocial support. The management of hypertension and stable heart failure or coronary artery disease may include medications such as aspirin, angiotensin-converting-enzyme inhibitors or beta-blockers (Jerusalem et al., 2019), and cholesterol-lowering medications (Curigliano et al., 2016). However, more invasive treatment such as angioplasty, open heart surgery, and valve replacement may be needed for the treatment of significant coronary artery disease and valvular disease. As arrhythmias may occur, including complete heart block, a pacemaker or implantable cardioversion device may also be needed.
The management of cardiotoxic impairments may also involve a combination of rehabilitation physicians, physical therapists, occupational therapists, and exercise physiologists (Alfano et al., 2016). The effectiveness of rehabilitation for coronary artery disease in the non-cancer population is well established (Simon et al., 2018), and effectiveness has also been shown in women with breast cancer and treatment-related heart failure (Bonsignore et al., 2017). The emergence of home-based cardiac rehabilitation may improve access and availability.
It is likely that cardiac rehabilitation confers benefits to cancer survivors exposed to cardiotoxic therapies that are similar to those experienced by other cardiac disease patients (Bonsignore et al., 2017). In one study of 152 breast cancer patients who completed a cardiac rehabilitation program, cardiorespiratory fitness improved by 14%, and significant improvements were also noted for QOL and depression (Dolan et al., 2018). Breast cancer survivors with treatment-related heart failure who participated in a cardiac rehabilitation program experienced aerobic benefits comparable to those seen in women with coronary heart disease who participated in such a program (Bonsignore et al., 2017). There is strong evidence that exercise training improves cardiovascular outcomes in cancer survivors (Campbell et al., 2019; Scott et al., 2018); however, cancer treatment with cardiotoxic drugs does not qualify patients for cardiac rehabilitation programs (at least not with third-party insurance coverage). Whether the existing cardiac rehabilitation infrastructure may benefit patients with the cardiotoxic effects from cancer treatment is unknown.
Cognitive Impairment
Prevalence, Etiology, and Risk Factors
Cognition is the mental process of acquiring knowledge and understanding through thought, experience, and the senses. Cognitive impairment, therefore, is defined as negative changes in higher-order mental
processing and may include one or more of the following cognitive domains identified by the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; APA, 2013): attention and concentration, executive function, information processing speed, visuospatial skill, language, learning, and memory (Sachdev et al., 2014). It is a prevalent, bothersome, and potentially debilitating symptom after cancer diagnosis and treatment (Wefel et al., 2015). Up to 25% of cancer survivors demonstrate cognitive impairment on a neuropsychological exam, and greater than 50% report cognitive decline across the cancer care trajectory, including impairment that appears many years after treatment (Ahles et al., 2012). Meta-analyses of cancer survivors have demonstrated cognitive deficits in memory, speed of processing, and executive functioning when compared with non-cancer controls (Anderson-Hanley et al., 2003; Jansen et al., 2005).
Cognitive impairment affects patients with both central nervous system and non-central nervous system cancer sites, including breast (Anderson-Hanley et al., 2003; Falleti et al., 2005; Hutchinson et al., 2012; Jansen et al., 2005), lung (Simo et al., 2015), hematologic (Kelly et al., 2018; Phillips et al., 2013; Williams et al., 2016), colorectal (Vardy et al., 2015; Visovatti et al., 2016), and head and neck (Piai et al., 2019; Williams et al., 2017). The term “chemo brain” or “chemo fog” has been used to describe cognitive impairment experienced after chemotherapy (Hurria et al., 2007). However, more recent studies have shown that cognitive impairment may also occur prior to treatment (Jansen et al., 2011a; Vardy et al., 2015) as well as in patients receiving radiation therapy (de Boer et al., 2018; Gao et al., 2015; Halbsguth et al., 2009; Leung et al., 2011; J. Lin et al., 2017; N. Lin et al., 2013; Marconi et al., 2019; Rossi et al., 2008; Shaw and Ball, 2013; Shibayama et al., 2014, 2019), endocrine therapy (Bender et al., 2015; Ganz and Van Dyk, 2020), or stem cell transplant, especially allogenic transplants (Sharafeldin et al., 2018; Syrjala et al., 2011). Regardless of whether cognitive impairment stems from the cancer or its treatment, it can have substantial impact on functional ability, work ability, and QOL for patients and their families (Von Ah et al., 2018).
Diagnosis and Assessment
Cognitive impairment may be measured using subjective patient self-report measures or objective neuropsychological exam, both of which are important for characterizing cognitive impairment. NCCN supports the use of prompting questions regarding the onset, trajectory, and nature of a patient’s impairment (NCCN, 2020f).
PRO questionnaires can provide information regarding a person’s subjective experiences of cognitive deficits and an indication of whether the person requires further assessment. According to both survivors’ complaints
of cognitive impairments and brain imaging studies, these individuals have to work much harder to respond to tasks in experimental settings, and slowed psychomotor speed is often documented on neurocognitive testing. These individuals do not demonstrate a pattern of dementia, but rather more subtle deficits primarily in executive function and psychomotor speed, similar to what might be seen with normal aging (Carroll et al., 2019). To some extent this appears to be a manifestation of what is increasingly believed to be accelerating aging in various organs as a result of cancer therapies (Guida et al., 2019). Objective neuropsychological assessment has been identified as the gold standard for identifying cognitive impairment (Cheung et al., 2012; Hutchinson et al., 2012); however, there is heterogeneity among published studies regarding the cognitive domains assessed, instruments used, and the thresholds and cut scores used.
Treatment and Prognosis
The NCCN guidelines on survivorship (2020f) identify how to assess and optimize treatment for correlated symptoms such as depression, emotional distress, pain, and sleep disturbance. The recommendations also include practical solutions to enhance recall (e.g., organizational strategies) and to reduce stress (e.g., enhancing coping strategies) (Denlinger et al., 2017). Other nonpharmacological interventions to improve cognitive impairments include cognitive rehabilitation (Von Ah and Crouch, 2020), cognitive training (Von Ah and Crouch, 2020; Von Ah et al., 2012), physical activity/exercise (Myers et al., 2018; Zimmer et al., 2016), and mindfulness-based stress reduction (Hoffman et al., 2012; Johns et al., 2016). The use of pharmacologic agents to address cognitive impairment in non-central nervous system cancers is an area of limited research; studies of stimulants (e.g., methylphenidate and modafinil), medications used for Alzheimer’s disease (e.g., donepezil and memantine), SSRIs (e.g., sertraline and paroxetine), ginkgo biloba, and vitamin E (Von Ah, 2015) have shown mixed results. Memantine in particular is the only medication that has been found to improve cognitive function in patients treated with radiotherapy for brain tumors or for prevention of brain metastasis (Brown et al., 2013; Duman et al., 2018).
Cognitive impairment has been identified across the cancer trajectory (Lange et al., 2019), and it can be long term (Koppelmans et al., 2012). Cognitive decline may occur at differing rates, with some survivors incurring a decline similar to that seen in normal aging, while others decline pathologically at an accelerated rate (Lange et al., 2016; Mandelblatt et al., 2016). Factors that increase the risk of cognitive impairment include advanced age, poor cognitive reserve, genetics (apolipoprotein E), and the presence of comorbidities (e.g., heart disease or diabetes), each of which
should be considered in identifying and managing cognitive impairment in cancer survivors.
Depression and Anxiety
Prevalence, Etiology, and Risk Factors
A diagnosis of cancer may generate high levels of mental anguish and distress that, if prolonged, may lead to anxiety, depression, or both (Linden et al., 2012). This mixed symptomatology is very common, with two-thirds of cancer patients with depression also expressing clinically significant levels of anxiety (Brintzenhofe-Szoc et al., 2009). Cancer survivors are also twice as likely as the general population to commit suicide, and those with advanced cancer are three times as likely to do so (Misono et al., 2008).
The prevalence of depression in cancer survivors, occurring during treatment and lasting for years afterward, may be up to three times higher than among the general population (Linden et al., 2012). Some studies have shown the prevalence of major depressive disorder to be greater than 40% (Okamura et al., 2005; Sneeuw et al., 1993), whereas others have found lower rates (11–13%), with an additional 16% displaying disruptive depressive symptoms (Ng et al., 2011; Smith, 2015). Levels of distress and depression may vary over the cancer trajectory, but are highest during times of vulnerability such as around diagnosis, during chemotherapy, following termination of treatment (re-entry), or at cancer recurrence/progression (Linden et al., 2012). At 5 years after diagnosis, rates of depression are comparable to those in the general population; however, anxiety about recurrence, sexual health and fertility, financial burden, and long-term effects of cancer treatment such as fatigue and lymphedema are not uncommon, particularly among younger survivors (Bloom et al., 2007; Boyes et al., 2009; Jansen et al., 2011b; Smith, 2015). Cancer patients with depression have poorer outcomes, including a poorer QOL, and higher rates of mortality (Pinquart and Duberstein, 2010).
A number of factors may influence the rates of depression and anxiety in cancer survivors, including the cancer site (they are more common for lung, pancreatic, brain, and hematological cancers and least common for invasive skin cancer) (Linden et al., 2012; Zabora et al., 2001), age (younger adults have greater risk of depression), gender (females have a greater risk than males for some cancers) (Linden et al., 2012), metastases (Ciaramella and Poli, 2001), cancer pain (Ciaramella and Poli, 2001; Spiegel et al., 1994), and physical symptom burden (Lo et al., 2010). Other risk factors include a previous history of depression or anxiety, family history of these disorders, the use and misuse of some medications, and prior traumatic life events (Smith, 2015).
Diagnosis and Assessment
ASCO recommends the use of the Patient Health Questionnaire-9 (PHQ-9), a self-report measure, to screen cancer patients for depression in the clinic (Andersen et al., 2014). The PHQ-9 scores each of nine criteria for major depression in the DSM-5 (APA, 2013) on a 4-point Likert scale from “0” (not at all) to “3” (nearly every day) over the previous 2 weeks. ASCO recommends screening and making a psychiatric/psychological referral when the cancer patient–reported PHQ-9 score is 8 or greater (out of a maximum of 27). ASCO further recommends that cancer survivors be periodically reassessed for depression and anxiety over the course of the cancer trajectory, beginning when cancer is first diagnosed (Anderson et al., 2014) (see Figure 4-1).
Cancer survivors may also experience anxiety (Andersen et al., 2014), including specific phobias and social phobia, panic and agoraphobia, generalized anxiety disorder, and other related disorders, such as obsessive compulsive disorder, and post-traumatic stress disorder. Generalized anxiety disorder is the most prevalent anxiety disorder and is commonly comorbid with others anxiety disorders, such as panic disorders and social anxiety disorder (Kessler et al., 2005). Screening should identify the level and nature (problems and concerns) of the distress as a red flag indicator. Screening for anxiety may be done with validated questionnaires such as the Generalized Anxiety Disorder-7 scale, with a score of 6–10 indicating moderate symptomatology and ≥11 indicating moderately severe symptomatology. Patients identified by screening with probable clinically significant depression or anxiety should receive a comprehensive psychosocial assessment by a qualified behavioral health specialist (Andersen et al., 2014; NCCN, 2020a).
Treatment and Prognosis
Both pharmacologic and non-pharmacologic interventions are available for the treatment of depression and anxiety. Treatments may include medications, psychotherapy, or both (Andersen et al., 2014). Pharmacologic therapies for depression and anxiety include first-line antidepressants, such as SSRIs and SNRIs (Andersen et al., 2014; NCCN, 2020f), and other medications such as the antidepressants bupropion or mirtazapine, benzodiazepines, tricyclic antidepressants, and, rarely, monoamine oxidase inhibitors. Buspirone may be prescribed for anxiety disorders. The use of benzodiazepines, other than for short-term (2–4 weeks) acute anxiety, has decreased due to their potential for abuse, dependence, withdrawal, and diversion (Guina and Merrill, 2018); however, they may be effective for other cancer-related adverse effects such as nausea, vomiting, and insomnia (Triozzi et al., 1988).
CBT and related cognitive psychotherapies (e.g., behavioral activation, problem-solving therapy, mindfulness-based cognitive therapy) for cancer patients have also been shown to be effective in reducing both depression and anxiety (Osborn et al., 2006; Sheard and Maguire, 1999), alleviating emotional stress, and improving QOL. Individual interventions were found to be more effective than group therapy (Semple et al., 2006). The team-based collaborative care model, which provides a population-based, stepped-care approach to integrated psychosocial care, can be effective in treating depression and improving anxiety symptoms and QOL in cancer patients (Li et al., 2017; Sharpe et al., 2014). ASCO also recommends a stepped-care approach to psychological or psychosocial interventions for cancer survivors who meet the criteria for depression on the PHQ-9 or for anxiety on the Generalized Anxiety Disorder-7 questionnaire (Anderson et al., 2014). Many cancer patients with significant distress do not receive adequate treatment (IOM, 2008).
Regular exercise and exercise interventions also improve common cancer-related health outcomes, including anxiety, depressive symptoms, fatigue, physical functioning, and health-related QOL (Campbell et al., 2019; Schmitz et al., 2010). Exercise programs that are at least 30 minutes in duration, supervised or partially supervised, and not in the home show the greatest benefit for improving depression (Craft et al., 2012). The American College of Sports Medicine extolled the benefits of exercise on physical functioning and QOL in cancer survivors, recommending that all survivors, even those undergoing treatment, “avoid inactivity” to promote positive health outcomes (Schmitz et al., 2010).
Gastrointestinal Impairments
Prevalence, Etiology, and Risk Factors
Gastrointestinal (GI) symptoms, specifically nausea and vomiting, occur commonly during cancer treatment (Coates et al., 1983), with incidence rates ranging from 30% to 90% (Hesketh et al., 2012; Schwartzberg, 2014; Sekine et al., 2013). These symptoms are acutely debilitating but usually resolve after completion of the chemotherapy.
Constipation is another common and distressing symptom (NCI, 2020), although it is largely a subjective sensation with few objective diagnostic criteria (McQuade et al., 2016). Its incidence increases in patients receiving opioid analgesics or medications with anticholinergic properties, although constipation may also occur with chemotherapy (McQuade et al., 2016). Constipation can range from annoying discomfort to a life-threatening impact with cardiac and respiratory symptoms (Clemens et al., 2013). There
is accumulating evidence that constipation leads to significant disruption in QOL (Dennison et al., 2005; Talley, 2004).
Other symptoms, such as diarrhea, may also result from chemotherapy, abdominal or pelvic radiation (Andreyev, 2016; Shadad et al., 2013), or bone marrow transplant (chronic graft-versus-host disease). Moreover, the diarrhea may last as long as 10 years after treatment and can be associated with significant malnutrition, dehydration, cachexia, fatigue, renal failure, hemorrhoids, and perianal skin breakdown. These long-term consequences may contribute to difficulty in returning to work. Other acute toxicities from systemic chemotherapy agents may include dyspepsia (indigestion), colitis, GI hemorrhage, and perforation/fistula formation. Immunologic agents similarly cause dyspepsia, nausea, vomiting, diarrhea, stomatitis, and liver toxicity (Boussios et al., 2012). Serious GI sequelae of cancer treatment (e.g., GI perforation, hemorrhagic colitis, and cirrhosis) are also common (Boussios et al., 2012).
Radiation and surgical resection of the colon or rectum may lead to bowel incontinence (lack of ability to control release of fecal contents). The incidence of bowel or fecal incontinence is often underreported, but estimates range from 5% to 18%. The occurrence of bowel incontinence may result in secondary morbidities (e.g., decubitus ulcerations, infections), direct and indirect costs (e.g., diapers, incontinence pads, loss of productivity, and missed work), and substantial impact on personal self-esteem and mood (depressive symptoms). Adhesions (fibrous bands that form between tissues and organs as a result of injury during surgery) occur in more than half of individuals undergoing abdominal surgery (Okabayashi et al., 2014) and can lead to lifelong complications from chronic pain and bowel obstruction (ten Broek et al., 2013).
Chronic enteritis (inflammation of the intestines marked by pain, cramping, nausea, or diarrhea) may present months to years after the completion of therapy, or it may begin as acute enteritis and persist after the cessation of treatment (PDQ® Supportive and Palliative Care Editorial Board, 2020). Of people treated with radiation to the abdomen, 5–15% will develop chronic problems that may interfere with their ability to work (Yeoh and Horowitz, 1987).
In general, susceptibility to GI toxicities has been hypothesized to be correlated with older age (Repetto, 2003), but susceptibility may also depend on a complex interplay of genetic variation, comorbidities, and behavioral factors such as level of physical activity, and some GI toxicities, including nausea, may occur in younger individuals (Tonato et al., 1991). The cancer patients who are at greatest risk of severe long-term GI toxicity are those receiving radiotherapy for rectal, anal, gynecological, or urological tumors in the pelvic area (Andreyev, 2016).
In addition, some cancers themselves (e.g., GI and gynecologic cancers) precipitate unpleasant and disabling GI symptoms such as nausea, vomiting, constipation, diarrhea, and bowel incontinence.
Diagnosis and Assessment
The identification of GI toxicity typically begins with patient reports of troublesome symptoms or a perceived abnormal bowel pattern. Normal bowel pattern is typically based on having at least three stools per week and no more than three per day; however, these criteria may be inappropriate for cancer patients. Constipation is viewed as a subjective symptom involving complaints of decreased frequency with incomplete passage of dry, hard stool, while chronic diarrhea is defined as three or more loose or watery stools per day for at least 4 weeks (PDQ® Supportive and Palliative Care Editorial Board, 2020).
The management of chronic GI symptoms begins with taking a careful history and performing a complete physical examination to identify any medical causes of constipation (e.g., the use of opioids; conditions such as diabetes or hypothyroidism). A thorough history of the patient’s bowel pattern, dietary changes, and medications, along with a physical examination, can identify possible causes of constipation or diarrhea. The evaluation also includes an assessment of associated symptoms such as distention, flatus, cramping, and rectal fullness. A digital rectal examination is done to rule out fecal impaction at the level of the rectum. A test for occult blood will be helpful in determining a possible intraluminal lesion. If small bowel obstruction is diagnosed, laparoscopic exploration can be used to identify the presence of adhesions that can be surgically removed (PDQ® Supportive and Palliative Care Editorial Board, 2020).
Treatment and Prognosis
Many treatments for chemotherapy-induced constipation or diarrhea reduce the severity of symptoms but may exacerbate already chronic GI symptoms or result in other adverse effects such as respiratory depression, uneven heartbeat, seizures, and neurotoxicity (McQuade et al., 2016). NCCN has outlined approaches to acute GI symptoms as part of its palliative care guidelines. These approaches may include anti-emetics, dietary changes, reducing the use of narcotics, and stool softeners (NCCN, 2020e). A small, randomized controlled trial has recently shown that olanzapine is effective for controlling chronic nausea and vomiting in patients with advanced cancer (Navari et al., 2020). Dietary changes such as increasing fluid and fiber intake are important management approaches for constipation
(De Giorgio et al., 2015; McQuade et al., 2016). Chronic diarrhea may be managed with antidiarrheal medications and an adjustment of food and fluid intake to increase bulk and prevent dehydration with its resultant electrolyte imbalances (ACS, 2020).
Pelvic floor physical therapy designed to strengthen pelvic floor and core abdominal muscles is a promising intervention for chronic diarrhea. One study found that participants’ diarrhea symptoms and physical function were significantly improved after a 4-week pelvic floor physical therapy intervention (Yang et al., 2012).
Graft-Versus-Host Disease
Prevalence, Etiology, and Risk Factors
Graft-versus-host disease (GVHD) is a serious and potentially life-threatening complication that can occur after allogeneic stem cell transplants. In this complication of hematopoietic stem cell transplant (HSCT), a patient’s tissues are seen as foreign by the donor’s nonidentical stem cells, leading to an immune (inflammatory) response and then disease in the patient. Tissues that may be involved in GVHD include the skin, liver, lungs, and GI tract. GVHD may be classified as acute (occurring within 100 days after transplant), delayed acute (occurring more than 100 days after transplant), or chronic (lasting for years after treatment completion).
There are few reliable data about the prevalence of GVHD. Well-established risk factors for both acute and chronic GVHD include the degree of compatibility between donor and patient, including sex compatibility, treatments given before transplant, the type of immunosuppression used to prevent GVHD, and the source of the stem cells (i.e., blood, bone marrow, or umbilical cord) (Flowers et al., 2011; Gale et al., 1987; Hahn et al., 2008; Higman and Vogelsang, 2004). Other risk factors may include age, comorbidity, viral seropositivity, and prior pregnancy or transfusions. Furthermore, prior acute GVHD increases the risk of chronic GVHD.
Diagnosis and Assessment
A diagnosis of GVHD, particularly acute GVHD of the GI tract, skin, or liver (Firoz et al., 2006), may be made based on clinical judgment. In patients who have undergone allogenic stem cell transplants and whose donor cells begin to attack their own cells, noticeable symptoms of acute GVHD may occur in several body systems, including skin (e.g., rash, itching, dark patches), GI (e.g., profuse diarrhea, nauseas, vomiting, abdominal cramping), and liver (e.g., jaundice, increasing bilirubin). Chronic GVHD
may manifest as scleroderma-like skin involvement (i.e., pathologic hardening and tightening of the skin), lung involvement, dry eyes, or changes in oral mucosa. Although GVHD is a clinical diagnosis, a biopsy is often done when feasible to confirm the diagnosis.
GVHD is graded based on the severity of its presentation in the skin (severity of maculopapular rash and erythroderma), liver (bilirubin), and GI system (diarrhea or abdominal pain) (Chao et al., 2020). Acute GVHD is mostly commonly graded by the International Marrow Transplant Registry grading system (Rowlings et al., 1997), with a range of grades A–D, with D being the most severe with the poorest prognosis. Chronic GVHD is assessed based on the National Institutes of Health consensus criteria (Filipovich et al., 2005).
Treatment and Prognosis
The NCCN guidelines for HSCT identify assessment criteria and an algorithm for the clinical management of acute and chronic GVHD (NCCN, 2020d). The mainstay of GVHD treatment is suppression of the patient’s immune response to donor cells, which may put the patient at additional risk for infection beyond the risk he or she already has from the transplant itself. This risk may be further exacerbated if the patient also received radiation as part of his or her pretransplant treatment, as radiation increases the risk for additional organ damage. For chronic GVHD there may be other therapeutic options, such as topical steroids and extracorporeal photopheresis (skin), artificial saliva (oral mucosa), artificial tears (eyes), and inhaled steroids and bronchodilators (lungs), depending on the site of disease. Ruxolitinib, an oral drug, is a recent opioid alternative that may be an option for some patients with chronic GVHD (Zeiser et al., 2015).
Chronic GVHD in long-term transplant survivors may lead to functional impairments such as chronic fatigue, changes in cognition, chronic pain, anxiety, and GI effects (Baker and Fraser, 2008; Pallua et al., 2010). As treatment may include long-term immunosuppression, these patients are also at risk for serious secondary infections. Patients with organ-specific chronic GVHD are at risk for additional debilitating complications. For example, those with pulmonary GVHD may have significant dyspnea, have limited ability to walk or exercise, depend on supplemental oxygen, and experience progressive lung damage and death. For patients with chronic skin GVHD, specific impairments from scleroderma-like changes include a significantly reduced range of motion. As chronic GVHD affects the skin, women with the condition may also experience vaginal pain, dryness, and other related complications (Filipovich et al., 2005).
Musculoskeletal Impairments
Prevalence, Etiology, and Risk Factors
Cancer survivors may experience various musculoskeletal impairments, including decreased bone density leading to osteopenia and osteoporosis, fractures, fall risks, arthritis, arthralgias, myalgias, and muscular functional impairments. These impairments may result from both cancer (e.g., sarcoma of the bone or soft tissue, bone metastasis) and cancer treatments.
In estrogen-receptor-positive breast cancer, two kinds of endocrine therapies may be used: aromatase inhibitors, used in postmenopausal women only, which lower the amount of estrogen in the body, and tamoxifen, used in both pre- and postmenopausal women, which blocks estrogen receptors on breast cancer cells (see Chapter 5 for more discussion of these therapies); both drugs may be prescribed for as long as a decade after the end of active cancer treatment. Aromatase inhibitors are associated with osteoporosis and bone fractures, whereas tamoxifen does not appear to affect bone health but does have more serious side effects such as blood clots, stroke, and endometrial cancer (Morales et al., 2005; Ramchand et al., 2019). Aromatase inhibitors are also associated with an arthralgia syndrome that affects more than 50% of women who use them (Roberts et al., 2017). This syndrome commonly presents as symmetrical pain or soreness in the hands, feet, lower back, shoulders, hips, or knees, and it often occurs as early morning stiffness and difficulty sleeping. Symptoms develop within the first few months of treatment and persist throughout the treatment period. The bone and muscle pains common with aromatase inhibitors can have negative effects on a woman’s QOL and, in severe cases, may lead to a discontinuation of medication even though continued use may improve the woman’s survival chances (Burstein, 2007; Roberts et al., 2017).
Among breast cancer survivors there are multiple other potential adverse treatment effects on muscle health, including muscle changes resulting from mastectomies, such as a decreased range of motion, rotator cuff disease, adhesive capsulitis, axillary web syndrome, and postmastectomy pain syndrome (Stubblefield and Keole, 2014). Breast reconstruction after a mastectomy may result in shoulder dysfunction, particularly following the use of the latissimus dorsi flap (Blackburn et al., 2018).
Chemotherapeutic agents and hormone treatments for cancer are associated with muscle pain. Musculoskeletal pain induced by taxane, which is used for solid tumors such as breast, ovarian, and non-small-cell lung cancer, is dose dependent and has a very broad prevalence that ranges from 2% to 94%, depending on the definition (Davis et al., 2016). Newer systemic cancer therapies, such as immune checkpoint inhibitors, are also associated with myalgias, arthralgias, and arthritis (Abdel-Rahman et al.,
2017; Cappelli et al., 2017). Depending on the specific agent and duration of use, the frequency of arthritis ranges from 1% to 22% and for myositis from 0.4% to 6% (Abdel-Rahman et al., 2017; Cappelli et al., 2017).
Glucocorticoid therapy, which is used in many cancer regimens, has numerous muscle and skeletal adverse effects, including osteoporosis and muscle weakness. Bisphosphonates and denosumab, which are used to treat multiple myeloma and metastatic bone disease, are both associated with osteonecrosis of the jaw, with a prevalence of 3–18% for bisphosphonates (Migliorati et al., 2011) and 1.9% for denosumab (Qi et al., 2014); however, a Cochrane Collaborative review found the prevalence of this adverse effect to be only 0.5% across 44 randomized controlled trials of bisphosphonates or denosumab involving 37,302 women (O’Carrigan et al., 2017).
Finally, among head and neck cancer patients, surgery and the effects of radiation adversely affect function of the sternocleidomastoid, scalene, and trapezius muscles in 1–13% of patients (Gane et al., 2017). Head and neck cancer may also result in trismus (i.e., limited range of motion in the jaw), mostly due to muscle spasms; trismus occurs in about 28% of patients within 1 year after treatment (Pauli et al., 2013).
Diagnosis and Assessment
The National Osteoporosis Foundation has published guidelines for the diagnosis of osteoporosis (Watts et al., 2010). Osteoporosis is commonly diagnosed with dual-energy x-ray absorptiometry of the hip and lumbar spine or quantitative ultrasonography of the calcaneus (USPSTF, 2018). There are published thresholds for normal bone versus bone at risk for fracture (Watts et al., 2010).
Arthralgias associated with aromatase inhibitors are generally diagnosed by patient self-report of symptoms and are dichotomized as being present or absent (Beckwée et al., 2017). Symptoms are most commonly reported in the wrists or knees (Beckwée et al., 2017).
The clinical presentation of osteonecrosis of the jaw has not been categorized beyond gross presentation.
Muscular issues are diagnosed based on patient-reported symptoms and clinical evaluation. There are no published clinical thresholds for the diagnosis of muscular issues in cancer patients. The most common clinical measurements for muscular issues are range of motion, strength, and a visual analogue scale for pain (0–10, with 10 being the worst pain possible).
Treatment and Prognosis
Several interdisciplinary cancer and bone societies involved in the management of aromatase inhibitor–associated bone loss have developed
recommendations for treating bone health in postmenopausal women receiving breast cancer therapy (Hadji et al., 2017). Effective treatments for aromatase inhibitor–associated arthralgia in breast cancer patients may include acupuncture, exercise, outpatient rehabilitation, and the use of NSAIDs (Roberts et al., 2017; Stubblefield, 2017). Bisphosphonates for the prevention of bone loss have been successful in postmenopausal breast cancer survivors who take aromatase inhibitors, but these are not without risk, as described above. Preliminary studies in postmenopausal women with breast cancer suggest that bisphosphonate use of 8 or more years was associated with higher risk of any bone fracture compared with 2–3 years of use (Drieling et al., 2016). The need for estrogen suppression while protecting bone in premenopausal women can be addressed by adding a gonadotrophin-releasing hormone analogue (e.g., goserelin). With the withdrawal of aromatase inhibitors, bone density recovers over a period of 3–6 months. Arthralgias resolve after discontinuation of treatment, which is typically recommended for 10 years.
As discussed in Chapter 5, in premenopausal women treatments for breast cancer and chemotherapy-induced ovarian failure commonly result in menopause occurring up to a decade earlier than would naturally occur. This premature menopause places these women at an increased risk for adverse bone health; furthermore, these women may then be eligible for aromatase inhibitors, which may also result in bone health challenges. Women who have a return of menses after treatment may have improved bone density.
Treating the adverse effects of glucocorticoid use on bone can take 1 month or longer; the treatment includes a tapering discontinuation of glucocorticoids, optimization of calcium and vitamin D intake, and lifestyle modifications such as eating a balanced diet, maintaining weight in the recommended range, smoking cessation, regular weight-bearing or resistance training exercises, and limiting alcohol intake to one to two alcoholic beverages/day (Buckley et al., 2017). Treatment with bisphosphonates has also been shown to be effective, but this can be associated with adverse effects, including osteonecrosis of the jaw.
Treatments for osteonecrosis of the jaw include conservative approaches to cleaning the wound, surgical removal of bone, and surgical flaps to cover the bone. It is unclear whether stopping bone-modulating therapy will reverse the disease (Migliorati et al., 2011).
The adverse effects of head and neck cancer surgery and radiation on muscular function can be progressive and are often debilitating, but they may be responsive to physical therapy interventions, such as proprioceptive retraining, myofascial release, and restoring range of motion. There are also effective medications, such as gabapentin. Finally, trismus (limited jaw range of motion) may be amenable to physical therapy interventions.
Pulmonary Toxicity
Prevalence, Etiology, and Risk Factors
A variety of cancer treatments, including chemotherapy, radiation therapy, and immunotherapy, have negative pulmonary effects that can cause acute, long-term, or late-onset functional impairments even years following treatment; this is particularly true of radiation therapy. The acute effects include thromboembolism, pleural effusions, bronchospasm, hypersensitivity, and radiation-induced pneumonitis (Shannon, 2020), and chronic toxicities include restrictive lung disease, pulmonary fibrosis, and pulmonary hypertension.
Several chemotherapy agents are associated with pulmonary toxicities. Bleomycin has been used to treat a number of cancers, including head and neck cancers, cancer of the testes and penis in men and cancers of the cervix and vulva in women, and non-Hodgkin and Hodgkin lymphomas, and is associated with pneumonitis and pulmonary fibrosis (NLM, 2019). Tyrosine kinase inhibitors are associated with pulmonary toxicity with an incidence of 0.2–10.9% (Peerzada et al., 2011). Alkylating and platinum-based agents, such as cyclophosphamide, chlorambucil, carmustine, streptozocin, procarbazine, busulfan, cisplatin, and carboplatin, which are used to treat a number of hematologic, breast, and lung cancers, may also cause early pneumonitis. Numerous other chemotherapeutic agents are associated with early-onset pulmonary toxicity, including methotrexate, taxanes, gemcitabine, monoclonal antibodies, and immune checkpoint inhibitors (Li et al., 2018; Shannon, 2020).
Radiation therapy can cause extrinsic (kyphoscoliosis, chest wall fibrosis, phrenic nerve paralysis) or intrinsic lung disease. Acute radiation-induced lung injury manifests as radiation pneumonitis in approximately 5–15% of patients who receive high-dose radiation for lung cancer and in 10–20% of patients receiving chest radiation for other tumors, including breast cancer (Garipagaoglu et al., 1999; Roach et al., 1995). Radiation pneumonitis may develop within a few weeks after the completion of therapy (Li et al., 2018). For example, late radiation-induced lung injury among survivors of Hodgkin lymphoma, non-Hodgkin lymphoma, or osteosarcoma typically presents as restrictive lung disease and pulmonary fibrosis (Hanania et al., 2019). As these cancers tend to occur among younger individuals, debilitating pulmonary complications can result in significant functional declines. The incidence of serious radiation-induced pulmonary complications has decreased as a result of advances in radiation delivery techniques (see Chapters 4 and 8); however, many long-term survivors treated with older techniques remain at risk. An understanding of the relationship between when and
how radiation was delivered and the clinical manifestations of pulmonary toxicity may help distinguish radiation-induced injury from other etiologies (Li et al., 2018).
Diagnosis and Assessment
The diagnosis of pulmonary toxicity following cancer treatment is primarily exclusionary (Shannon, 2020). Patients may present with a variety of symptoms including dyspnea, decreased oxygen saturation on pulse oximetry, cough, shortness of breath, and fatigue. Diagnosis requires information on the patient’s age, the radiation dose administered, the volume of lung irradiated, any co-treatment with chemotherapy, the onset and duration of the symptoms outlined above, and abnormal radiologic features. Additional evaluation with pulmonary function testing and computed tomography imaging may be recommended. More invasive techniques such as bronchoscopy, thoracentesis, or lung biopsy may be used (Li et al., 2018).
Treatment and Prognosis
The time course of pulmonary toxicity is generally divided into early (immediate to 2 months after chemotherapy) and late (2 or more months following completion of chemotherapy) (Garipagaoglu et al., 1999). The treatment of acute pneumonitis depends on its clinical severity, and the pneumonitis typically responds completely to corticosteroids. Although radiation pneumonitis can spontaneously resolve in cases where lung injury is limited, the vast majority of cases (up to 75%) progress to chronic scarring of the lungs (i.e., fibrosis) at the irradiation site within 6–12 months (Li et al., 2018). Identifying and effectively treating patients who may progress to fibrosis remains a challenge (Hanania et al., 2019).
Restrictive lung disease may occur during and following chest wall radiation. It may remain mild and stable or progress to pulmonary fibrosis. Pulmonary fibrosis is a deadly disease characterized by scarring (collagen deposition) and stiffening of the lungs that lead to persistent shortness of breath (dyspnea) and eventual death within 3–4 years of diagnosis. The process is not reversible, and the treatment consists of palliative care (Li et al., 2018).
Regardless of radiation or chemotherapeutic etiology, multi-component pulmonary rehabilitation can improve function, endurance, and QOL among individuals with pulmonary toxicity. Exercise is used to rebuild endurance by improving gas exchange. Breathing retraining, self-management skills, airway clearance techniques, and oxygen therapy are also used (Tiep et al., 2015) to improve oxygenation and decrease the symptoms of breathlessness.
Sleep Disturbances
Prevalence, Etiology, and Risk Factors
Sleep disturbances are a prevalent and bothersome impairment for cancer survivors (Cleeland et al., 2013; Howell et al., 2014; Otte et al., 2015). Sleep disorders can be classified using two main classification systems: the DSM-5 (APA, 2013) or the International Classification of Sleep Disorders (AASM, 2014). The disorders include insomnia, sleep-related breathing disorders (obstructive sleep apnea), hypersomnia, circadian rhythm disorders, parasomnias, sleep-related movement disorders (restless leg disorder), other isolated symptoms, and non-specified disorders (Goblin, 2004). A patient may have one or more sleep disorders at any particular time.
Insomnia is one of the most prevalent sleep disturbances in cancer survivors, with up to 60% reporting problems (Savard et al., 2001, 2011). Insomnia initially presents as difficulty falling asleep, difficulty staying asleep, and frequent and prolonged nighttime awakenings (Ancoli-Israel, 2009). Insomnia syndrome (chronic insomnia) is characterized by insomnia that occurs more than three nights per week, difficulty falling asleep or night time awakenings (>30 minutes), a ratio of sleep time to time spent in bed <85% (sleep efficiency), impaired daytime functioning, and marked distress (Ancoli-Israel, 2009; Savard et al., 2011). Insomnia syndrome is clinically significant as it affects daily functioning, produces daytime fatigue, and impairs cognition (i.e., results in poor concentration and memory), with negative consequences on employment, relationships, QOL, and it may also lead to other health problems (Berger, 2009; Bower et al., 2011; Graci, 2005).
Diagnosis and Assessment
Sleep disturbances, although prevalent, are often under-assessed and subsequently remain undertreated. Most clinical guidelines recommend a two-step process to identify sleep problems, with initial screening questions followed by a more comprehensive examination in cancer patients who screen positive. Common assessment tools to identify sleep disturbance in cancer survivors include sleep diaries, the insomnia severity index, and the PROMIS sleep disturbance and sleep-related impairment questionnaires (Berger et al., 2017; Howell et al., 2014).
The National Institutes of Health identified two screening questions for identifying sleep problems that can be used in the clinical setting (Buysse et al., 2010). The American Academy of Sleep Medicine (AASM, 2014) recommends the completion of a patient history, a general medical/psychiatric questionnaire, the Epworth Sleepiness Scale (Johns, 1991), and a
2-week self-report sleep log. Sleep logs include reports of sleep quality, sleep parameters, napping, daytime impairment, medications, activities, time of evening meal, caffeine and alcohol consumption, and stress level before bedtime. A detailed psychosocial and medication history, including questions about recent stressors, can help identify potential underlying causes and comorbidities, including depression and anxiety. For cancer survivors, other symptoms that can precipitate insomnia, such as pain and fatigue, should also be thoroughly assessed (Howell et al., 2014). Diagnosing some specific sleep disorders may also require objective evaluation, including the use of actigraphy or an overnight evaluation of objective sleep measures with polysomnography (Otte et al., 2015).
Treatment and Prognosis
Sleep disturbances are multifactorial, and disorders can occur simultaneously, which makes assessment and treatment difficult. Sleep specialists can aid in this determination. The NCCN Guidelines for Survivorship provides screening, diagnosis, and management recommendations for sleep disorders in survivors (Denlinger et al., 2016). Effective management of sleep disturbances should address the underlying etiology; for example, management of obstructive sleep apnea includes the use of continuous positive airway pressure, weight reduction, and exercise. The management of restless leg syndrome may include the use of dopamine agonists (e.g., benzodiazepines or gabapentin). For other types of sleep disturbances, nonpharmacological interventions focus on sleep hygiene; CBT, including stress management and relaxation practice; and physical activity or exercise (Denlinger et al., 2016; Mishra et al., 2012; Murawski et al., 2018); psychoeducational therapy and supportive expressive therapy are also recommended (Denlinger et al., 2016). CBT for insomnia in cancer survivors has shown mixed results (Howell et al., 2014).
Clinicians may be reluctant to discuss sleep medications with cancer patients; one study found that when patients with cancer report sleeping problems, clinicians assessed and or made recommendations about medications only about 50% of the time, changed the subject about 19% of the time, and offered to follow-up with the patient only about 5% of the time (Siefert et al., 2014). Pharmacological treatments for insomnia include psychostimulants (e.g., modafinil, methylphenidate) and hypnotics (e.g., zolpidem, ramelteon, methylphenidate). Antidepressants, antihistamines, antiepileptics, and antipsychotics have also been used off-label for the treatment of insomnia (NCCN, 2020f). These medications all have potential adverse effects, and, while they are recommended for sleep disturbances in the general population, their efficacy and safety in adult cancer survivors are not established.
Sleep disturbances are a persistent problem across the cancer care trajectory, from diagnosis to end of life (Berger et al., 2005; Savard et al., 2001). Cancer survivors were 60% more likely to report daytime sleepiness than the general population (Forsythe et al., 2012). Although sleep disturbances tend to diminish over the course of treatment for some, they may persist in a subset of patients who also have fatigue and decreased daytime physical activity. Sleep disturbances are also closely linked with other common cancer impairments, such as fatigue, anxiety, and depression (Albusoul et al., 2017; Bower et al., 2011), and they result in poorer overall QOL (Berger et al., 2017).
Other Sensory Impairments
Prevalence, Etiology, and Risk Factors
In addition to the impairments described above, other impairments may also occur as a result of cancer or its treatment, including changes in hearing (hearing loss, tinnitus, vertigo, imbalance), vision (loss of vision, cataracts), and taste. Hearing deficits have been associated with chemotherapy and may also be related to radiation therapy to the brain. Such radiation therapy may be used for head and neck cancers, childhood and adult leukemias, and cancers that have metastasized to the brain. Vision changes may result from radiation therapy and may also be due to prolonged treatment with corticosteroids or tamoxifen. Changes in taste sensation may be due to chemotherapy but may also result from chronic sinusitis due to surgery or radiation for head and neck cancers (Deshpande et al., 2018). Autonomic dysfunction, described in the section on cardiotoxicity, may also occur. This condition may be due to cancer treatment, but may also occur with cancer involvement of the nerves such as in primary or metastatic brain and other nerve-related malignancies.
Diagnosis and Assessment
An assessment for ototoxicity should be initiated early, as delayed diagnosis may lead to significant impairments and functional limitations, and the ototoxicity may be misdiagnosed as other conditions, such as cognitive dysfunction or depression (Ganesan et al., 2018). Vestibular dysfunction may lead to imbalance and falls.
Similarly, it is important to assess for visual deficits using standard ophthalmic measures. A loss of taste or altered taste, which may lead to weight loss, presents diagnostic challenges. Evaluating autonomic dysfunction is also challenging and may require consultation with cardiology and neurology specialists with the appropriate expertise.
Treatment and Prognosis
The treatment of ototoxicity is complex and requires a multidisciplinary team with expertise in otolaryngology, audiology, vestibular therapy, and neuro-oncology, among others. Hearing aids may be used for hearing loss, though these are often uncomfortable and also expensive. Treatment approaches for autonomic dysfunction may include vestibular therapy, medications such as beta-blockers, and behavioral therapy (Lakoski et al., 2015). In all, these conditions may be debilitating in themselves and may lead to other impairments described earlier.
COMORBIDITIES AND SYMPTOM CLUSTERS
The likelihood that a long-term impairment from cancer treatment will persist may be influenced by a patient’s comorbidities—for example, diabetes—some of which may predate the cancer diagnosis. These comorbidities are discussed below. The assessment of cancer-related impairments resulting from a variety of morbidities, including cancer itself, has been examined by the National Academies in two recent studies done for SSA: Functional Assessment for Adults with Disabilities (NASEM, 2019) and Selected Health Conditions and Likelihood of Improvement with Treatment (NASEM, 2020). Also discussed below is the phenomenon of “symptom clusters,” which may underlie many cancer-related impairments.
Comorbidity, also known as multi-morbidity, is defined as the coexistence of more than one distinct health condition or disease in an individual (Valderas et al., 2009). Having multiple chronic health conditions affects a range of survivor outcomes, including mortality, health-related QOL, and physical and mental functioning (Fortin et al., 2007). Negative outcomes related to comorbidity may be synergistic, as chronic diseases tend to interact with each other in ways that may lead to new clinical presentations (Vetrano et al., 2018). The number and severity of comorbidities and the presence of impairments such as pain potently mediate cancer-related impairment to a greater degree than the presence of any specific health condition (Cheville et al., 2011; Sarfati et al., 2016). Each comorbidity tends to further erode the distance between a cancer survivor’s functional level and the minimal functional level required to perform work or care for self and family, often making it difficult for the survivor to engage in or benefit from treatment. For example, fatigue and pain often amplify the effect of weakness and thus may limit a patient’s participation in rehabilitation treatments (Alfano et al., 2016).
Comorbidities, including clinical depression and anxiety (Bodurka-Bevers et al., 2000), are common among cancer survivors, and those with such comorbidities experience poorer survival, poorer QOL, and higher health
care costs (Sarfati et al., 2016). Although older cancer survivors (i.e., aged 65 and older) frequently have comorbidities, there is growing evidence that comorbid conditions such as depression, anxiety, asthma, high cholesterol, and hypertension are also common in young adults with cancer, particularly those aged 15–39 years (Smitherman et al., 2018). Young adults with cancer are more likely than their cancer-free peers to be frail and to experience a high level of comorbidities, a phenomenon known as accelerated aging (Smitherman et al., 2018). Cancers and several comorbid conditions, such as heart disease, diabetes, and chronic obstructive pulmonary disease, share many risk factors, such as older age, smoking, poor diet, obesity, and alcohol abuse (Sarfati et al., 2016; Sarna et al., 2002).
Symptom clusters—defined as two or more concurrent symptoms that are related and may or may not have a common cause (Fan et al., 2007)—can have significant and synergistic effects on cancer-related impairments and functional limitations. Thus, there is a need to identify treatments that efficiently address the entire cluster, rather than treating each symptom separately. One such symptom cluster is the psychoneurologic cluster, which includes cognitive disturbance, depression, fatigue, insomnia, and pain (Aktas, 2013). Patients with advanced cancer and physical function impairments often have a symptom cluster of depression, fatigue, and pain (Laird et al., 2011; Steel et al., 2010).
While most studies addressing factors related to work are disease-specific, a single “review of reviews” across multiple studies of common mental health disorders, cardiovascular disease, and cancer identified six barriers related to a patient’s ability to work. Those barriers were anxiety, depression, job strain, other comorbidities, older age, and low education (Gragnano et al., 2018). These common factors support the validity of a cross-disease approach when addressing recovery and return-to-work interventions. Therefore, an assessment of an individual’s ability to sustain paid work outside the home on a regular and continuing basis that is based entirely on physical function may overestimate the individual’s capacity if a psychologic comorbidity is present. The identification and treatment of co- and multi-morbidities along with primary diagnoses may help alleviate patients’ impairments and increase their ability to return to work.
Recent models of function and disability, such as the World Health Organization’s International Classification of Functioning, Disability and Health (WHO, 2001; see Chapter 2), acknowledge the web of dynamic, interacting factors that drive disability, and they highlight the difficulty in attributing functional loss to a single toxicity or impairment. The 2019 National Academies study on functional assessments for adults with disabilities acknowledged that the presence of multiple impairments and comorbidities can further limit functioning (NASEM, 2019).
MULTIMODAL INTERVENTIONS
Throughout this report the committee has shown that there are a variety of interventions available to treat many of the symptoms, cancer-related impairments, and adverse effects of cancer and its treatment. Prior sections of this chapter have addressed treatment and management approaches for specific impairments, such as fatigue. It is important to note, however, that cancer-related impairments rarely occur as single problems; as noted in the previous section, impairments often cluster. Thus, for many impairment clusters a multi-component intervention that addresses several impairments may be more effective than interventions that address each impairment separately. In this section the committee considers interventions that extend beyond the pharmacy to address impairments secondary to cancer and its treatments as well as interventions that address multiple impairments.
Although multimodal interventions improve function and QOL for people living with and beyond cancer, not all of the interventions are provided to all cancer survivors who may benefit from them. The barriers to implementation of multimodal interventions include a lack of clinician time, lack of reimbursement for the programs, the need for a clinic champion for practice change (Beidas et al., 2014), the lack of cancer clinician knowledge of the benefits and availability of exercise or cancer rehabilitation services, a care delivery system in which cancer care and exercise or rehabilitation care exist in separate silos, and the varying abilities of patients to follow a treatment program (Alfano et al., 2012; Stout et al., 2016). Several of these barriers are addressed in Chapter 10.
The general categories of nonpharmacologic interventions that may help restore a cancer survivor’s daily functioning and that may be included in a multi-component cancer treatment and survivorship care plan include the following:
- Exercise/Physical Activity. Recently, a consortium that included NCCN, the American College of Sports Medicine, the American Cancer Society, the American Society of Clinical Oncology, the American Physical Therapy Association, and the National Cancer Institute published updated exercise guidance for people living with and beyond cancer (Campbell et al., 2019; Patel et al., 2019) that concluded that exercise reduces the risk for recurrence of breast, colon, and prostate cancer (McTiernan et al., 2019; Patel et al., 2019). The amount of exercise recommended to prevent recurrence of these cancers is 150–300 minutes of moderate intensity aerobic activity or 75–150 minutes of vigorous intensity activity
- per week in addition to twice weekly resistance exercise. In addition, the panel identified eight cancer health-related outcomes for which a program of three times weekly aerobic exercise of 30 minutes and twice weekly resistance exercise is recommended. The eight outcomes include fatigue, QOL, physical function, anxiety, depression, bone health, sleep, and lymphedema (for breast cancer–related lymphedema only) (Campbell et al., 2019; Schmitz et al., 2019). An international, multidisciplinary approach to improving physical function and QOL among people living with and beyond cancer is Exercise Is Medicine (ACSM, 2020). This approach calls for a clinician to ask the cancer patient two questions about current physical activity as a vital sign (Schmitz et al., 2019).
-
Rehabilitation emphasizes a comprehensive approach to the assessment and management of physical, psychologic, vocational, and social needs related to cancer and cancer treatment as well as to comorbidities. Besides the specific effects already addressed in this chapter, rehabilitation also integrates care for additional cancer-related issues such as sexual dysfunction, balance and gait problems, mobility issues, stoma care, swallowing dysfunction, and communication difficulty (Alfano et al., 2012). In recent years, evidence across cancer types suggests that multimodal prehabilitation—rehabilitation that occurs before cancer treatment begins to improve tolerance to surgery or other interventions (Carli et al., 2017)—is associated with improved post-treatment function (Faithfull et al., 2019) and disease-free survival (Trépanier et al., 2019). The components of prehabilitation vary but generally include nutritional supplementation, physical activity, and psychological support. Other components may include comprehensive functional evaluation to establish a pretreatment baseline, physical and occupational therapy for existing and anticipated impairments, and education about the prevention and management of treatment-related adverse effects and impairments (Bolshinsky et al., 2018).
Cancer rehabilitation involves an interdisciplinary approach that should be delivered by trained rehabilitation professionals who are knowledgeable about cancer and the morbidities associated with the disease and its treatments (ARN, 2017). These rehabilitation professionals are able to diagnose and treat the physical, psychological, and cognitive impairments to help cancer survivors maintain and restore function, reduce symptom burden, maximize their independence, and improve their QOL (ARN, 2017; Silver et al., 2015). Depending on each survivor’s identified needs, the cancer rehabilitation
-
team may include a physiatrist, advanced practice nurse, nurse, care coordinator/navigator, social worker, physical therapist, occupational therapist, speech and language pathologist, psychologist, and vocational rehabilitation specialist. As with rehabilitation for any diagnosis, the team composition may change throughout the survivorship trajectory based on the survivor’s changing needs.
Examples of rehabilitative interventions include
- Physical and occupational therapy. Physical and occupational ° therapists are skilled in the assessment and treatment of many of the common adverse effects described in this chapter, including fatigue, lymphedema, and deconditioning (a decrease in physical fitness after a prolonged period of illness or inactivity). Physical and occupational therapists assess and treat the musculoskeletal, neuromuscular, integumentary, and cardiopulmonary rehabilitative needs of patients living with and beyond cancer.
- Physiatry. Physiatrists are physicians with training in physi- ° cal medicine and rehabilitation. They have specific training in diagnosing and treating symptoms in nerves, muscles, bones, joints, and brain. Their treatments may include injections for pain as well as partnering with physical and occupational therapists to develop comprehensive treatment plans to restore physical function to the extent possible after cancer.
- Speech and language pathology. The support and guidance of ° an audiologist or speech and language pathologist or therapist helps promote recovery and also helps a patient cope with the difficult speech and swallowing symptoms that may occur during and after cancer treatment. Ideally, the speech and language pathologist becomes involved when the patient has been identified as having a cancer that may alter speech or eating, such as happens with many head and neck cancers, before surgery or before any chemotherapy or radiation treatments. Counseling and education are provided regarding the functions of voice, speech, and swallowing. An assessment is made to determine the patient’s baseline and to provide guidance as to the patient’s role in his or her rehabilitation. A speech and language pathologist frequently works with patients who have difficulty eating and drinking. The treatment is based on the cause of the problem, such as anatomical changes from surgery, decreased saliva, changes in taste, difficulty opening the mouth due to trismus, and problems protecting the airway, which can result in coughing and choking during meals (Hansen et al., 2018).
- Psychological and Emotional Interventions may include CBT, mindfulness-based stress reduction, family or couples therapy, interpersonal therapy, problem solving/skill building, psycho-educational interventions, and palliative care. The collaborative care model of delivering psychosocial care to cancer patients has been shown to be an effective and efficient organizational approach for integrating evidence-based psychosocial care into routine cancer care (Fann et al., 2012). Complementary and alternative medicine approaches such as massage, guided imagery, reflexology, acupuncture, yoga, and reiki can also be used to reduce emotional distress and symptom burden.
The functional deficits secondary to cancer surgeries, chemotherapy, radiation, and other curative treatments can prevent some patients from returning to their usual activities, including gainful employment and caring for themselves and others. Finding ways to connect cancer survivors and their caregivers with effective, evidence-based interventions (including those described above) in order to restore function and QOL in the setting of limited health care resources is an ongoing challenge. This challenge has grown as the number of survivors living long past the end of their initial course of curative therapy has increased (Campbell et al., 2019).
FINDINGS AND CONCLUSIONS
Findings
- Impairments in people with cancer may be caused by cancer-related symptoms, the adverse effects of cancer treatment, or the interaction of these factors with co-occurring conditions. Moreover, individuals may experience cancer-related effects, treatment-related effects, and effects of co-occurring conditions simultaneously.
- Despite the importance of cancer-related impairments, there is a paucity of evidence on the prevalence and impact of these impairments on patients’ long-term functioning.
- Impairments contributing to functional limitations can be assessed with appropriate standardized, validated tools whenever available. For many impairments, including pain, depression, and fatigue, validated self-report instruments are the primary assessment tools.
- The common causes of functional limitations related to cancer itself, cancer treatment, or multiple factors (e.g., comorbidities) include such things as pain, fatigue, peripheral neuropathy, lymphedema, and cognitive impairments as well as impairments of the heart, lungs, gastrointestinal tract, and musculoskeletal system.
- These impairments may occur prior to, during, and after cancer treatment, depending on individual patient factors, the specific cancer, and the cancer treatments that are used.
- Non-pharmacologic interventions that can reduce impairments include structured exercise, rehabilitation therapy, and psychological/emotional/behavioral interventions; however, these are underused for the treatment of cancer-related impairments.
- Comorbidities often exacerbate cancer-related impairments and functional limitations.
Conclusions
- In determining the ability of a person to engage in substantial gainful activity, it is necessary to consider cancer-related impairments arising from the disease itself, those arising from cancer treatments, and those associated with or exacerbated by pre-existing and treatment-related comorbidities.
- Regular assessment and re-assessment of functional status throughout the cancer care trajectory are warranted, given the dynamic impacts of cancer-related acute, long-term, and late-onset impairments that may result in functional limitations.
- There are various evidence-based exercise and rehabilitation interventions that can mitigate these impairments, but, in general, they are underprescribed and underused.
REFERENCES
AASM (American Academy of Sleep Medicine). 2014. International classification of sleep disorders. Darien, IL: American Academy of Sleep Medicine.
Abdel-Rahman, O., M. Eltobgy, H. Oweira, A. Giryes, A. Tekbas, and M. Decker. 2017. Immune-related musculoskeletal toxicities among cancer patients treated with immune checkpoint inhibitors: A systematic review. Immunotherapy 9(14):1175–1183.
Abouelazayem, M., M. Elkorety, and S. Monib. 2020. Breast lymphedema after conservative breast surgery: An up-to-date systematic review. Clinical Breast Cancer. https://doi.org/10.1016/j.clbc.2020.11.017.
ACS (American Cancer Society). 2019. What is peripheral neuropathy? https://www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/peripheral-neuropathy/what-is-peripherial-neuropathy.html (accessed August 13, 2020).
ACS. 2020. Diarrhea. https://www.cancer.org/treatment/treatments-and-side-effects/physical-side-effects/stool-or-urine-changes/diarrhea.html (accessed September 15, 2020).
ACSM (American College of Sports Medicine). 2017. ACSM’s guidelines for exercise testing and prescription, 10th edition. Philadelphia, PA: Wolters Kluwer.
Advani, S.M., P.G.Advani, H.M. VonVille, and S.H. Jafri. 2018. Pharmacological management of cachexia in adult cancer patients: A systematic review of clinical trials. BMC Cancer 18(1):1174.
Ahles, T.A., J.C. Root, and E.L. Ryan. 2012. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. Journal of Clinical Oncology 30(30):3675–3686.
Aktas, A. 2013. Cancer symptom clusters: Current concepts and controversies. Current Opinions in Supportive and Palliative Care 7(1):38–44.
Albusoul, R.M., A.M. Berger, C.L. Gay, S.L. Janson, and K.A. Lee. 2017. Symptom clusters change over time in women receiving adjuvant chemotherapy for breast cancer. Journal of Pain and Symptom Management 53(5):880–886.
Alfano, C.M., P.A. Ganz, J.H. Rowland, and E.E. Hahn. 2012. Cancer survivorship and cancer rehabilitation: Revitalizing the link. Journal of Clinical Oncology 30(9):904–906.
Alfano, C.M., A.L. Cheville, and K. Mustian. 2016. Developing high-quality cancer rehabilitation programs: A timely need. 2016 ASCO Educational Book. https://meetinglibrary.asco.org/record/50678/edbook#fulltexthttps://meetinglibrary.asco.org/record/50678/edbook#fulltext (accessed November 5, 2020).
Ancoli-Israel, S. 2009. Recognition and treatment of sleep disturbances in cancer. Journal of Clinical Oncology 27(35):5864–5866.
Andersen, B.L., R.J. DeRubeis, B.S. Berman, J. Gruman, V.L. Champion, M.J. Massie, J.C. Holland, A.H. Partridge, K. Bak, M.R. Somerfield, and J.H. Rowland. 2014. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: An American Society of Clinical Oncology guideline adaptation. Journal of Clinical Oncology 32(15):1605–1619.
Anderson, C., J.L. Lund, M.A. Weaver, W.A. Wood, A.F. Olshan, and H.B. Nichols. 2019. Noncancer mortality among adolescents and young adults with cancer. Cancer 125(12):2107–2114.
Anderson-Hanley, C., M.L. Sherman, R. Riggs, V.B. Agocha, and B.E. Compas. 2003. Neuropsychological effects of treatments for adults with cancer: A meta-analysis and review of the literature. Journal of the International Neuropsychology Society 9(7):967–982.
Andreyev, H.J.N. 2016. GI consequences of cancer treatment: A clinical perspective. Radiation Research 185(4):341–348.
APA (American Psychiatric Association). 1994. Diagnostic and statistical manual of mental disorders, 4th edition: DSM–IV. Washington, DC: American Psychiatric Association.
APA. 2013. Diagnostic and statistical manual of mental disorders, 5th edition. Arlington, VA: American Psychiatric Association.
APA. 2017. What is cognitive behavioral therapy? https://www.apa.org/ptsd-guideline/patients-and-families/cognitive-behavioral (accessed September 17, 2020).
Argilés, J.M., F.J. López-Soriano, B. Stemmler, and S. Busquets. 2019. Therapeutic strategies against cancer cachexia. European Journal of Translational Myology 29(1):7960.
Armenian, S.H., C. Lacchetti, A. Barac, J. Carver, L.S. Constine, N. Denduluri, S. Dent, P.S. Douglas, J.B. Durand, M. Ewer, C. Fabian, M. Hudson, M. Jessup, L.W. Jones, B. Ky, E.L. Mayer, J. Moslehi, K. Oeffinger, K. Ray, K. Ruddy, and D. Lenihan. 2017. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology 35(8):893–911.
ARN (Association of Rehabilitation Nurses). 2017. Rehabilitation of people with cancer. https://rehabnurse.org/about/position-statements/rehabilitation-of-people-with-cancer (accessed December 28, 2020).
Atherton, P.J., D.W. Watkins-Bruner, C. Gotay, C.M. Moinpour, D.V. Satele, K.A. Winter, P.L. Schaefer, B. Movsas, and J.A. Sloan. 2015. The complementary nature of patient-reported outcomes and adverse event reporting in cooperative group oncology clinical trials: A pooled analysis (NCCTG N0591). Journal of Pain and Symptom Management 50(4):470–479.
Atkins, K.M., B. Rawal, T.L. Chaunzwa, N. Lamba, D.S. Bitterman, C.L. Williams, D.E. Kozono, E.H. Baldini, A.B. Chen, P.L. Nguyen, A.V. D’Amico, A. Nohria, U. Hoffmann, H.J.W.L. Aerts, and R.H. Mak. 2019. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. Journal of the American College of Cardiology 73(23):2976–2987.
Atkinson, T.M., C.F. Andreotti, K.E. Roberts, R.M. Saracino, M. Hernandez, and E. Basch. 2015. The level of association between functional performance status measures and patient-reported outcomes in cancer patients: A systematic review. Supportive Care in Cancer 23(12):3645–3652.
ATS/ACCP (American Thoracic Society/American College of Chest Physicians). 2003. ATS/ACCP statement on cardiopulmonary exercise testing. American Journal of Respiratory and Critical Care Medicine 167(2):211–277.
Baker, K.S., and C.J. Fraser. 2008. Quality of life and recovery after graft-versus-host disease. Best Practice and Research in Clinical Haematology 21(2):333–341.
Bates, D., B.C. Schultheis, M.C. Hanes, S.M. Jolly, K.V. Chakravarthy, T.R. Deer, R.M. Levy, and C.W. Hunter. 2019. A comprehensive algorithm for management of neuropathic pain. Pain Medicine 20(Suppl 1):S2–S12.
Beckwée, D., L. Leysen, K. Meuwis, and N. Adriaenssens. 2017. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: A systematic review and meta-analysis. Supportive Care in Cancer 25(5):1673–1686.
Beesley, V.L., I.J. Rowlands, S.C. Hayes, M. Janda, P. O’Rourke, L. Marquart, M.A. Quinn, A.B. Spurdle, A. Obermair, A. Brand, M.K. Oehler, Y. Leung, L. McQuire, and P.M. Webb. 2015. Incidence, risk factors and estimates of a woman’s risk of developing secondary lower limb lymphedema and lymphedema-specific supportive care needs in women treated for endometrial cancer. Gynecologic Oncology 136(1):87–93.
Behringer, K., H. Goergen, H. Müller, I. Thielen, C. Brillant, S. Kreissl, T.V. Halbsguth, J. Meissner, R. Greil, P. Moosmann, O. Shonukan, J.U. Rueffer, H.H. Flechtner, M. Fuchs, V. Diehl, A. Engert, and P. Borchmann. 2016. Cancer-related fatigue in patients with and survivors of Hodgkin lymphoma: The impact on treatment outcome and social reintegration. Journal of Clinical Oncology 34(36):4329–4337.
Beidas, R.S., B. Paciotti, F. Barg, A.R. Branas, J.C. Brown, K. Glanz, A. DeMichele, L. Di-Giovanni, D. Salvatore, and K.H. Schmitz. 2014. A hybrid effectiveness-implementation trial of an evidence-based exercise intervention for breast cancer survivors. JNCI Monographs 2014(50):338–345.
Bender, C.M., J.D. Merriman, A.L. Gentry, G.M. Ahrendt, S.L. Berga, A.M. Brufsky, F.E. Casillo, M.M. Dailey, K.I. Erickson, F.M. Kratofil, P.F. McAuliffe, M.Q. Rosenzweig, C.M. Ryan, and S.M. Sereika. 2015. Patterns of change in cognitive function with anastrozole therapy. Cancer 121(15):2627–2636.
Berger, A.M. 2009. Update on the state of the science: Sleep-wake disturbances in adult patients with cancer. Oncology Nursing Forum 36(4):E165–E177.
Berger, A.M., K.P. Parker, S. Young-McCaughan, G.A. Mallory, A.M. Barsevick, S.L. Beck, J.S. Carpenter, P.A. Carter, L.A. Farr, P.S. Hinds, K.A. Lee, C. Miaskowski, V. Mock, J.K. Payne, and M. Hall. 2005. Sleep wake disturbances in people with cancer and their caregivers: State of the science. Oncology Nursing Forum 32(6):E98–E126.
Berger, A.M., K. Mooney, A. Alvarez-Perez, W.S. Breitbart, K.M. Carpenter, D. Cella, C. Cleeland, E. Dotan, M.A. Eisenberger, C.P. Escalante, P.B. Jacobsen, C. Jankowski, T. LeBlanc, J.A. Ligibel, E.T. Loggers, B. Mandrell, B.A. Murphy, O. Palesh, W.F. Pirl, S.C. Plaxe, M.B. Riba, H.S. Rugo, C. Salvador, L.I. Wagner, N.D. Wagner-Johnston, F.J. Zachariah, M.A. Bergman, and C. Smith. 2015. Cancer-related fatigue, version 2.2015. Journal of the National Comprehensive Cancer Network 13(8):1012–1039.
Berger, A.M., E.E. Matthews, and A.M. Kenkel. 2017. Management of sleep–wake disturbances comorbid with cancer. Oncology 31(8):610–617.
Blackburn, N.E., J.G. McVeigh, E. McCaughan, and I.M. Wilson. 2018. The musculoskeletal consequences of breast reconstruction using the latissimus dorsi muscle for women following mastectomy for breast cancer: A critical review. European Journal of Cancer Care 27(2):e12664.
Bloom, J.R., D.M. Petersen, and S.H. Kang. 2007. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psycho-Oncology 16(8):691–706.
Bodurka-Bevers, D., K. Basen-Engquist, C.L. Carmack, M.A. Fitzgerald, J.K. Wolf, C. de Moor, and D.M. Gershenson. 2000. Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gynecologic Oncology 78(3 Pt 1):302–308.
Bolshinsky, V., M.H. Li, H. Ismail, K. Burbury, B. Riedel, and A. Heriot. 2018. Multimodal prehabilitation programs as a bundle of care in gastrointestinal cancer surgery: A systematic review. Diseases of the Colon & Rectum 61(1):124–138.
Bonsignore, A., S. Marzolini, and P. Oh. 2017. Cardiac rehabilitation for women with breast cancer and treatment-related heart failure compared with coronary artery disease: A retrospective study. Journal of Rehabilitative Medicine 49(3):277–281.
Borghuis, E.M.C., M.F. Reneman, and H.R. Schiphorst Preuper. 2020. Assessing discrepancies in outcomes of pain rehabilitation: “These questionnaires don’t measure results that are relevant to me.” Disability and Rehabilitation 42(16):2374–2380.
Boussios, S., G. Pentheroudakis, K. Katsanos, and N. Pavlidis. 2012. Systemic treatment-induced gastrointestinal toxicity: Incidence, clinical presentation and management. Annals of Gastroenterology 25(2):106–118.
Bower, J.E. 2014. Cancer-related fatigue—mechanisms, risk factors, and treatments. Nature Reviews Clinical Oncology 11(10):597–609.
Bower, J.E., P.A. Ganz, M.R. Irwin, L. Kwan, E.C. Breen, and S.W. Cole. 2011. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of Clinical Oncology 29(26):3517–3522.
Bower, J.E., K. Bak, A. Berger, W. Breitbart, C.P. Escalante, P.A. Ganz, H.H. Schnipper, C. Lacchetti, J.A. Ligibel, G.H. Lyman, M.S. Ogaily, W.F. Pirl, and P.B. Jacobsen. 2014. Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. Journal of Clinical Oncology 32(17):1840–1850.
Bowles, E.J., R. Wellman, H.S. Feigelson, A.A. Onitilo, A.N. Freedman, T. Delate, L.A. Allen, L. Nekhlyudov, K.A. Goddard, R.L. Davis, L.A. Habel, M.U. Yood, C. McCarty, D.J. Magid, and E.H. Wagner. 2012. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. Journal of the National Cancer Institute 104(17):1293–1305.
Boyes, A.W., A. Girgis, A.C. Zucca, and C. Lecathelinais. 2009. Anxiety and depression among long-term survivors of cancer in Australia: Results of a population-based survey. Comment. Medical Journal of Australia 191(5):295.
Brintzenhofe-Szoc, K.M., T.T. Levin, Y. Li, D.W. Kissane, and J.R. Zabora. 2009. Mixed anxiety/depression symptoms in a large cancer cohort: Prevalence by cancer type. Psychosomatics 50(4):383–391.
Brooks, K.G., and T.L. Kessler. 2017. Treatments for neuropathic pain. Clinical Pharmacist 9(12).
Brown, P.D., S. Pugh, N.N. Laack, J.S. Wefel, D. Khuntia, C. Meyers, A. Choucair, S. Fox, J.H. Suh, D. Roberge, V. Kavadi, S.M. Bentzen, M.P. Mehta, and D. Watkins-Bruner. 2013. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro-Oncology 15(10):1429–1437.
Buckley, L., G. Guyatt, H.A. Fink, M. Cannon, J. Grossman, K.E. Hansen, M.B. Humphrey, N.E. Lane, M. Magrey, M. Miller, L. Morrison, M. Rao, A.B. Robinson, S. Saha, S. Wolver, R.R. Bannuru, E. Vaysbrot, M. Osani, M. Turgunbaev, A.S. Miller, and T. McAlindon. 2017. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis & Rheumatology 69(8):1521–1537.
Burstein, H.J. 2007. Aromatase inhibitor-associated arthralgia syndrome. Breast 16(3):223–234.
Buysse, D.J., L. Yu, D.E. Moul, A. Germain, A. Stover, N.E. Dodds, K.L. Johnston, M.A. Shablesky-Cade, and P.A. Pilkonis. 2010. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep 33(6):781–792.
Campbell, K.L., K.M. Winters-Stone, J. Wiskemann, A.M. May, A.L. Schwartz, K.S. Courneya, D.S. Zucker, C.E. Matthews, J.A. Ligibel, L.H. Gerber, G.S. Morris, A.V. Patel, T.F. Hue, F.M. Perna, and K.H. Schmitz. 2019. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Medicine & Science in Sports & Exercise 51(11):2375–2390.
Cappelli, L.C., A.K. Gutierrez, C.O. Bingham, 3rd, and A.A. Shah. 2017. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: A systematic review of the literature. Arthritis Care & Research 69(11):1751–1763.
Cardinale, D., A. Colombo, G. Bacchiani, I. Tedeschi, C.A. Meroni, F. Veglia, M. Civelli, G. Lamantia, N. Colombo, G. Curigliano, C. Fiorentini, and C.M. Cipolla. 2015. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 131(22):1981–1988.
Carli, F., C. Gillis, and C. Scheede-Bergdahl. 2017. Promoting a culture of prehabilitation for the surgical cancer patient. Acta Oncologia 56(2):128–133.
Carroll, J.E., K. Van Dyk, J.E. Bower, Z. Scuric, L. Petersen, R. Schiestl, M.R. Irwin, and P.A. Ganz. 2019. Cognitive performance in survivors of breast cancer and markers of biological aging. Cancer 125(2):298–306.
Cella, D., K. Davis, W. Breitbart, and G. Curt. 2001. Cancer-related fatigue: Prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. Journal of Clinical Oncology 19(14):3385–3391.
Chao, N., R. Negrin, and A.G. Rosmarin. 2020. Clinical manifestations, diagnosis, and grading of acute graft-versus-host disease. https://www.uptodate.com/contents/clinical-manifestations-diagnosis-and-grading-of-acute-graft-versus-host-disease (accessed June 29, 2020).
Cherny, N.I., R. Sullivan, U. Dafni, J.M. Kerst, Z. Sobrero, C. Zielinski, E.G. de Vries, and M.J. Piccart. 2015. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Annals of Oncology 26(8):1547–1573.
Cheung, Y.T., E.H.-J. Tan, and A. Chan. 2012. An evaluation on the neuropsychological tests used in the assessment of postchemotherapy cognitive changes in breast cancer survivors. Supportive Care in Cancer 20(7):1361–1375.
Cheville, A.L., A.B. Kornblith, and J.R. Basford. 2011. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. American Journal of Physical Medicine & Rehabilitation 90(5 Suppl 1):S27–S37.
Cheville, A.L., T. Moynihan, J. Herrin, C. Loprinzi, and K. Kroenke. 2019. Effect of collaborative telerehabilitation on functional impairment and pain among patients with advanced-stage cancer: A randomized clinical trial. JAMA Oncology 5(5):644–652.
Ciaramella, A., and P. Poli. 2001. Assessment of depression among cancer patients: The role of pain, cancer type, and treatment. Psycho-Oncology 10(2):156–165.
Cleeland, C.S., F. Zhao, V.T. Chang, J.A. Sloan, A.M. O’Mara, P.B. Gilman, M. Weiss, T.R. Mendoza, J.W. Lee, and M.J. Fisch. 2013. The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns Study. Cancer 119(24):4333–4340.
Clemens, K.E., M. Faust, B. Jaspers, and G. Mikus. 2013. Pharmacological treatment of constipation in palliative care. Current Opinions in Supportive and Palliative Care 7(2):183–191.
Coates, A., S. Abraham, S.B. Kaye, T. Sowerbutts, C. Frewin, R.M. Fox, and M.H. Tattersall. 1983. On the receiving end—patient perception of the side-effects of cancer chemotherapy. European Journal of Cancer and Clinical Oncology 19(2):203–208.
Colvin, L.A. 2019. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain 160(Suppl 1):S1–S10.
Coumbe, B.G.T, and J.D. Groarke. 2018. Cardiovascular autonomic dysfunction in patients with cancer. Current Cardiology Reports 20(8):69.
Craft, L.L., E.H. Vaniterson, I.B. Helenowski, A.W. Rademaker, and K.S. Courneya. 2012. Exercise effects on depressive symptoms in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiology, Biomarkers, and Prevention 21(1):3–19.
Cramer, J.D., J.T. Johnson, and M.L. Nilsen. 2018. Pain in head and neck cancer survivors: Prevalence, predictors, and quality-of-life impact. Otolaryngology—Head and Neck Surgery 159(5):853–858.
Crom, D.B., P.S. Hinds, J.S. Gattuso, V. Tyc, and M.M. Hudson. 2005. Creating the basis for a breast health program for female survivors of Hodgkin disease using a participatory research approach. Oncology Nursing Forum 32(6):1131–1141.
Curigliano, G., D. Cardinale, S. Dent, C. Criscitiello, O. Aseyev, D. Lenihan, and C.M. Cipolla. 2016. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA: A Cancer Journal for Clinicians 66(4):309–325.
Darby, S.C., M. Ewertz, P. McGale, A.M. Bennet, U. Blom-Goldman, D. Brønnum, C. Correa, D. Cutter, G. Gagliardi, B. Gigante, M.-B. Jensen, and A. Nisbet. 2013. Risk of ischemic heart disease in women after radiotherapy for breast cancer. New England Journal of Medicine 368:987–998.
Davis, L.L., J.S. Carpenter, and J.L. Otte. 2016. State of the science: Taxane-induced musculoskeletal pain. Cancer Nursing 39(3):187–196.
de Boer, S.M., M.E. Powell, L. Mileshkin, D. Katsaros, P. Bessette, C. Haie-Meder, P.B. Ottevanger, J.A. Ledermann, P. Khaw, A. Colombo, A. Fyles, M.-H. Baron, I.M. JürgenliemkSchulz, H.C. Kitchener, H.W. Nijman, G. Wilson, S. Brooks, S. Carinelli, D. Provencher, C. Hanzen, L.C.H.W. Lutgens, V.T.H.B.M. Smit, N. Singh, V. Do, R. D’Amico, R.A. Nout, A. Feeney, K.W. Verhoeven-Adema, H. Putter, C.L. Creutzberg, M. McCormack, K. Whitmarsh, R. Allerton, D. Gregory, P. Symonds, P.J. Hoskin, M. Adusumalli, A. Anand, R. Wade, A. Stewart, W. Taylor, R.F.P.M. Kruitwagen, H. Hollema, E. Pras, A. Snyers, L. Stalpers, J.J. Jobsen, A. Slot, J.-W.M. Mens, T.C. Stam, B. Van Triest, E.M. Van der Steen-Banasik, K.A.J. De Winter, M.A. Quinn, I. Kolodziej, J. Pyman, C. Johnson, A. Capp, R. Fossati, S. Gribaudo, A.A. Lissoni, A. Ferrero, G. Artioli, C. Davidson, C.M. McLachlin, P. Ghatage, P.V.C. Rittenberg, L. Souhami, G. Thomas, P. Duvillard, D. Berton-Rigaud, and N. Tubiana-Mathieu. 2018. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. The Lancet Oncology 19(3):295–309.
De Giorgio, R., E. Ruggeri, V. Stanghellini, L.H. Eusebi, F. Bazzoli, and G. Chiarioni. 2015. Chronic constipation in the elderly: A primer for the gastroenterologist. BMC Gastroenterology 15:130.
Deng, J., B.A. Murphy, M.S. Dietrich, N. Wells, K.A. Wallston, R.J. Sinard, A.J. Cmelak, J. Gilbert, and S.H. Ridner. 2013. Impact of secondary lymphedema after head and neck cancer treatment on symptoms, functional status, and quality of life. Head and Neck 35(7):1026–1035.
Deng, J., S.H. Ridner, M.S. Dietrich, N. Wells, K.A. Wallston, R.J. Sinard, A.J. Cmelak, and B.A. Murphy. 2012. Prevalence of secondary lymphedema in patients with head and neck cancer. Journal of Pain and Symptom Management 43(2):244–252.
Denlinger, C.S., J.A. Ligibel, M. Are, K.S. Baker, G. Broderick, W. Demark-Wahnefried, D.L. Friedman, M. Goldman, L.W. Jones, A. King, G.H. Ku, E. Kvale, T.S. Langbaum, M.S. McCabe, M. Melisko, J.G. Montoya, K. Mooney, M.A. Morgan, J.J. Moslehi, T. O’Connor, L. Overholser, E.D. Paskett, J. Peppercorn, M.A. Rodriguez, K.J. Ruddy, T. Sanft, P. Silverman, S. Smith, K.L. Syrjala, S.G. Urba, M.T. Wakabayashi, P. Zee, N.R. McMillian, and D.A. Freedman-Cass. 2016. NCCN guidelines insights: Survivorship, version 1. Journal of the National Comprehensive Cancer Network 14(6):715–724.
Denlinger, C.S., T. Sanft, K.S. Baker, S. Baxi, G. Broderick, W. Demark-Wahnefried, D.L. Friedman, M. Goldman, M. Hudson, N. Khakpour, A. King, D. Koura, E. Kvale, R.M. Lally, T.S. Langbaum, M. Melisko, J.G. Montoya, K. Mooney, J.J. Moslehi, T. O’Connor, L. Overholser, E.D. Paskett, J. Peppercorn, M.A. Rodriguez, K.J. Ruddy, P. Silverman, S. Smith, K.L. Syrjala, A. Tevaarwerk, S.G. Urba, M.T. Wakabayashi, P. Zee, D.A. Freedman-Cass, and N.R. McMillian. 2017. Survivorship, version 2.2017, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network 15(9):1140–1163.
Dennison, C., M. Prasad, A. Lloyd, S.K. Bhattacharyya, R. Dhawan, and K. Coyne. 2005. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 23(5):461–476.
Deshpande, T.S., P. Blanchard, L. Wang, R.L. Foote, X. Zhang, and S.J. Frank. 2018. Radiation-related alterations of taste function in patients with head and neck cancer: A systematic review. Current Treatment Options in Oncology 19(12):72.
Dhanapal, R., T. Saraswathi, and R.N. Govind. 2011. Cancer cachexia. Journal of Oral and Maxillofacial Pathology 15(3):257–260.
DiSipio, T., S. Rye, B. Newman, and S. Hayes. 2013. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. The Lancet Oncology 14(6):500–515.
Dolan, L.B., D. Barry, T. Petrella, L. Davey, A. Minnes, A. Yantzi, S. Marzolini, and P. Oh. 2018. The cardiac rehabilitation model improves fitness, quality of life, and depression in breast cancer survivors. Journal of Cardiopulmonary Rehabilitation and Prevention 38(4):246–252.
Drieling, R.L., A.Z. LaCroix, S.A. Beresford, D.M. Boudreau, C. Kooperberg, R.T. Chlebowski, M. Gass, C.J. Crandall, C.R. Womack, and S.R. Heckbert. 2016. Long-term oral bisphosphonate use in relation to fracture risk in postmenopausal women with breast cancer: Findings from the Women’s Health Initiative. Menopause 23(11):1168–1175.
Duman, J.G., J. Dinh, W. Zhou, H. Cham, V.C. Mavratsas, M. Paveškovic, S. Mulherkar, S.L. McGovern, K.F. Tolias, and D.R. Grosshans. 2018. Memantine prevents acute radiation-induced toxicities at hippocampal excitatory synapses. Neuro-Oncology 20(5):655–665.
Dylke, E.S., L.C. Ward, and S.L. Kilbreath. 2013. Standardized approach to lymphedema screening. Oncologist 18(11):1242.
Els, C., T.D. Jackson, D. Kunyk, V.G. Lappi, B. Sonnenberg, R. Hagtvedt, S. Sharma, F. Kolahdooz, and S. Straube. 2017. Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: An overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2017(10):CD012509.
Faithfull, S., L. Turner, K. Poole, M. Joy, R. Manders, J. Weprin, K. Winters-Stone, and J. Saxton. 2019. Prehabilitation for adults diagnosed with cancer: A systematic review of long-term physical function, nutrition and patient-reported outcomes. European Journal of Cancer Care 28(4):e13023.
Falk, S., K. Bannister, and A.H. Dickenson. 2014. Cancer pain physiology. British Journal of Pain 8(4):154–162.
Falleti, M.G., A. Sanfilippo, P. Maruff, L. Weih, and K.A. Phillips. 2005. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: A meta-analysis of the current literature. Brain and Cognition 59(1):60–70.
Fan, G., L. Filipczak, and E. Chow. 2007. Symptom clusters in cancer patients: A review of the literature. Current Oncology 14(5):173–179.
Fann, J.R., K. Ell, and M. Sharpe. 2012. Integrating psychosocial care into cancer services. Journal of Clinical Oncology 30(11):1178–1186.
FDA (U.S. Food and Drug Administration). 2009. Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims. Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims (accessed July 25, 2020).
Fearon, K., F. Strasser, S.D. Anker, I. Bosaeus, E. Bruera, R.L. Fainsinger, A. Jatoi, C. Loprinzi, N. MacDonald, G. Mantovani, M. Davis, M. Muscaritoli, F. Ottery, L. Radbruch, P. Ravasco, D. Walsh, A. Wilcock, S. Kaasa, and V.E. Baracos. 2011. Definition and classification of cancer cachexia: An international consensus. The Lancet Oncology 12(5):489–495.
Filipovich, A.H., D. Weisdorf, S. Pavletic, G. Socie, J.R. Wingard, S.J. Lee, P. Martin, J. Chien, D. Przepiorka, D. Couriel, E.W. Cowen, P. Dinndorf, A. Farrell, R. Hartzman, J. Henslee-Downey, D. Jacobsohn, G. McDonald, B. Mittleman, J.D. Rizzo, M. Robinson, M. Schubert, K. Schultz, H. Shulman, M. Turner, G. Vogelsang, and M.E.D. Flowers. 2005. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biology of Blood and Marrow Transplantation 11(12):945–956.
Firoz, B.F., S.J. Lee, P. Nghiem, and A.A. Qureshi. 2006. Role of skin biopsy to confirm suspected acute graft-vs-host disease: Results of decision analysis. Archives of Dermatology 142(2):175–182.
Flowers, M.E., Y. Inamoto, P.A. Carpenter, S.J. Lee, H.P. Kiem, E.W. Petersdorf, S.E. Pereira, R.A. Nash, M. Mielcarek, M.L. Fero, E.H. Warren, J.E. Sanders, R.F. Storb, F.R. Appelbaum, B.E. Storer, and P.J. Martin. 2011. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 117(11):3214–3219.
Foglietta, J., A. Inno, F. de Iuliis, V. Sini, S. Duranti, M. Turazza, L. Tarantini, and S. Gori. 2017. Cardiotoxicity of aromatase inhibitors in breast cancer patients. Clinical Breast Cancer 17(1):11–17.
Forsythe, L.P., K.J. Helzlsouer, R. MacDonald, and L. Gallicchio. 2012. Daytime sleepiness and sleep duration in long-term cancer survivors and non-cancer controls: Results from a registry-based survey study. Supportive Care in Cancer 20(10):2425–2432.
Forsythe, L.P., C.M. Alfano, S.M. George, A. McTiernan, K.B. Baumgartner, L. Bernstein, and R. Ballard-Barbash. 2013. Pain in long-term breast cancer survivors: The role of body mass index, physical activity, and sedentary behavior. Breast Cancer Research and Treatment 137(2):617–630.
Fortin, M., H. Soubhi, C. Hudon, E.A. Bayliss, and M. van den Akker. 2007. Multimorbidity’s many challenges. BMJ (Clinical Research Edition) 334(7602):1016–1017.
Gale, R.P., M.M. Bortin, D.W. van Bekkum, J.C. Biggs, K.A. Dicke, E. Gluckman, R.A. Good, R.G. Hoffmann, H.E. Kay, and J.H. Kersey. 1987. Risk factors for acute graft-versus-host disease. British Journal of Haematology 67(4):397–406.
Gane, E.M., Z.A. Michaleff, M.A. Cottrell, S.M. McPhail, A.L. Hatton, B.J. Panizza, and S.P. O’Leary. 2017. Prevalence, incidence, and risk factors for shoulder and neck dysfunction after neck dissection: A systematic review. European Journal of Surgical Oncology 43(7):1199–1218.
Ganesan, P., J. Schmiedge, V. Manchaiah, S. Swapna, S. Dhandayutham, and P.P. Kothandaraman. 2018. Ototoxicity: A challenge in diagnosis and treatment. Journal of Audiology & Otology 22(2):59–68.
Ganz, P.A., and K. Van Dyk. 2020. Cognitive impairment in patients with breast cancer: Understanding the impact of chemotherapy and endocrine therapy. Journal of Clinical Oncology 38(17):1871–1874.
Gao, Y., F. Gao, J.-L. Ma, and D.-L. Zhao. 2015. Palliative whole-brain radiotherapy and health- related quality of life for patients with brain metastasis in cancer. Neuropsychiatric Disease and Treatment 11:2185–2190.
Garipagaoglu, M., M.T. Munley, D. Hollis, J.M. Poulson, G.C. Bentel, G. Sibley, M.S. Anscher, M. Fan, R.J. Jaszczak, and R.E. Coleman. 1999. The effect of patient-specific factors on radiation-induced regional lung injury. International Journal of Radiation Oncology, Biology, Physics 45(2):331–338.
Gartner, R., M.B. Jensen, J. Nielsen, M. Ewertz, N. Kroman, and H. Kehlet. 2009. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 302(18):1985–1992.
Goblin, A.Z. 2004. Sleep psychiatry. New York: Taylor and Francis.
Gomes, T., D.A. Redelmeier, D.N. Juurlink, I.A. Dhalla, X. Camacho, and M.M. Mamdani. 2013. Opioid dose and risk of road trauma in Canada: A population-based study. JAMA Internal Medicine 173(3):196–201.
Graci, G. 2005. Pathogenesis and management of cancer-related insomnia. Journal of Supportive Oncology 3(5):349–359.
Gragnano, A., A. Negrini, M. Miglioretti, and M. Corbière. 2018. Common psychosocial factors predicting return to work after common mental disorders, cardiovascular diseases, and cancers: A review of reviews supporting a cross-disease approach. Journal of Occupational Rehabilitation 28(2):215–231.
Guida, J.L., T.A. Ahles, D. Belsky, J. Campisi, H.J. Cohen, J. DeGregori, R. Fuldner, L. Ferrucci, L. Gallicchio, L. Gavrilov, N. Gavrilova, P.A. Green, C. Jhappan, R. Kohanski, K. Krull, J. Mandelblatt, K.K. Ness, A. O’Mara, N. Price, J. Schrack, S. Studenski, O. Theou, R.P. Tracy, and A. Hurria. 2019. Measuring aging and identifying aging phenotypes in cancer survivors. Journal of the National Cancer Institute 111(12):1245–1254.
Guina, J., and B. Merrill. 2018. Benzodiazepines I: Upping the care on downers: The evidence of risks, benefits and alternatives. Journal of Clinical Medicine 7(2):17.
Guralnik, J.M., E.M. Simonsick, L. Ferrucci, R.J. Glynn, L.F. Berkman, D.G. Blazer, P.A. Scherr, and R.B. Wallace. 1994. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology 49(2):M85–M94.
Haanpää, M., N. Attal, M. Backonja, R. Baron, M. Bennett, D. Bouhassira, G. Cruccu, P. Hansson, J.A. Haythornthwaite, G.D. Iannetti, T.S. Jensen, T. Kauppila, T.J. Nurmikko, A.S. Rice, M. Rowbotham, J. Serra, C. Sommer, B.H. Smith, and R.D. Treede. 2011. NEPSIG guidelines on neuropathic pain assessment. Pain 152(1):14–27.
Hadji, P., M.S. Aapro, J.J. Body, M. Gnant, M.L. Brandi, J.Y. Reginster, M.C. Zillikens, C.C. Gluer, T. de Villiers, R. Baber, G.D. Roodman, C. Cooper, B. Langdahl, S. Palacios, J. Kanis, N. Al-Daghri, X. Nogues, E.F. Eriksen, A. Kurth, R. Rizzoli, and R.E. Coleman. 2017. Management of aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESECO IMS, and SIOG. Journal of Bone Oncology 7:1–12.
Hahn, T., P.L. McCarthy, Jr., M.J. Zhang, D. Wang, M. Arora, H. Frangoul, R.P. Gale, G.A. Hale, J. Horan, L. Isola, R.T. Maziarz, J.J. van Rood, V. Gupta, J. Halter, V. Reddy, P. Tiberghien, M. Litzow, C. Anasetti, S. Pavletic, and O. Ringdén. 2008. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. Journal of Clinical Oncology 26(35):5728–5734.
Halbsguth, T., H. Müller, H. Flechtner, C. Brillant, K. Behringer, T. Schober, H. Nisters-Backes, A. Engert, and P. Borchmann. 2009. Quality of life in a 2-year follow-up: Signs of PNP predicting fatigue in survivors treated in the HD10-12 trials of the German Hodgkin study group (GHSG). Blood 112(11):1455.
Hanania, A.N., W. Mainwaring, Y.T. Ghebre, N.A. Hanania, and M. Ludwig. 2019. Radiation-induced lung injury: Assessment and management. Chest 156(1):150–162.
Hansen, K., M. Chenoweth, H. Thompson, and A. Strouss. 2018. Role of the speech-language pathologist (SLP) in the head and neck cancer team. Cancer Treatment and Research 174:31–42.
Hardee, J.P., B.R. Counts, and J.A. Carson. 2017. Understanding the role of exercise in cancer cachexia therapy. American Journal of Lifestyle Medicine 13(1):46–60.
Health Quality Ontario. 2016. Vertebral augmentation involving vertebroplasty or kyphoplasty for cancer-related vertebral compression fractures: A systematic review. Ontario Health Technology Assessment Series 16(11):1–202.
Hesketh, P.J., P. Sanz-Altamira, J. Bushey, and A.M. Hesketh. 2012. Prospective evaluation of the incidence of delayed nausea and vomiting in patients with colorectal cancer receiving oxaliplatin-based chemotherapy. Supportive Care Cancer 20(5):1043–1047.
Hidding, J.T., C.H. Beurskens, P.J. van der Wees, H.W. van Laarhoven, and M.W. Nijhuis-van der Sanden. 2014. Treatment related impairments in arm and shoulder in patients with breast cancer: A systematic review. PLOS ONE 9(5):e96748.
Higman, M.A., and G.B. Vogelsang. 2004. Chronic graft versus host disease. British Journal of Haematology 125(4):435–454.
Hoffman, C.J., S.J. Ersser, J.B. Hopkinson, P.G. Nicholls, J.E. Harrington, and P.W. Thomas. 2012. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: A randomized, controlled trial. Journal of Clinical Oncology 30(12):1335–1342.
Howell, D., T.K. Oliver, S. Keller-Olaman, J.R. Davidson, S. Garland, C. Samuels, J. Savard, C. Harris, M. Aubin, K. Olson, J. Sussman, J. MacFarlane, and C. Taylor. 2014. Sleep disturbance in adults with cancer: A systematic review of evidence for best practices in assessment and management for clinical practice. Annals of Oncology 25(4):791–800.
Hurria, A., G. Somlo, and T. Ahles. 2007. Renaming “chemobrain.” Cancer Investigations 25(6):373–377.
Hutchinson, A.D., J.R. Hosking, G. Kichenadasse, J.K. Mattiske, and C. Wilson. 2012. Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treatment Reviews 38(7):926–934.
IOM (Institute of Medicine). 2006. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press.
IOM. 2008. Cancer care for the whole patient: Meeting psychosocial health needs. Washington, DC: The National Academies Press.
Iqubal, A., M.K. Iqubal, S. Sharma, M.A. Ansari, A.K. Najmi, S.M. Ali, J. Ali, and S.E. Haque. 2019. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sciences 218:112–131.
Jacobse, J.N., F.K. Duane, N.B. Boekel, M. Schaapveld, M. Hauptmann, M.J. Hooning, C.M. Seynaeve, M.H.A. Baaijens, J.A. Gietema, S.C. Darby, F.E. van Leeuwen, B.M.P. Aleman, and C.W. Taylor. 2019. Radiation dose-response for risk of myocardial infarction in breast cancer survivors. International Journal of Radiation Oncology, Biology, Physics 103(3):595–604.
Jain, D., R.R. Russell, R.G. Schwartz, G.S. Panjrath, and W. Aronow. 2017. Cardiac complications of cancer therapy: Pathophysiology, identification, prevention, treatment, and future directions. Current Cardiology Reports 19(5):36.
Janda, M., N. Gerstner, A. Obermair, A. Fuerst, S. Wachter, K. Dieckmann, and R. Pötter. 2000. Quality of life changes during conformal radiation therapy for prostate carcinoma. Cancer: Interdisciplinary International Journal of the American Cancer Society 89(6):1322–1328.
Jansen, C.E., C. Miaskowski, M. Dodd, G. Dowling, and J. Kramer. 2005. A meta-analysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer 104(10):2222–2233.
Jansen, C.E., B.A. Cooper, M.J. Dodd, and C.A. Miaskowski. 2011a. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Supportive Care in Cancer 19(10):1647–1656.
Jansen, L., A. Herrmann, C. Stegmaier, S. Singer, H. Brenner, and V. Arndt. 2011b. Health-related quality of life during the 10 years after diagnosis of colorectal cancer: a population-based study. Journal of Clinical Oncology 29(24):3263–3269.
Jerusalem, G., P. Lancellotti, and S.B. Kim. 2019. HER2+ breast cancer treatment and cardiotoxicity: Monitoring and management. Breast Cancer Research and Treatment 177(2):237–250.
Johns, M.W. 1991. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14(6):540–545.
Johns, S.A., L.F. Brown, K. Beck-Coon, T.L. Talib, P.O. Monahan, R.B. Giesler, Y. Tong, L. Wilhelm, J.S. Carpenter, D. Von Ah, C.D. Wagner, M. de Groot, K. Schmidt, D. Monceski, M. Danh, J.M. Alyea, K.D. Miller, and K. Kroenke. 2016. Randomized controlled pilot trial of mindfulness-based stress reduction compared to psychoeducational support for persistently fatigued breast and colorectal cancer survivors. Supportive Care in Cancer 24(10):4085–4096.
Juhl, A.A., P. Christiansen, and T.E. Damsgaard. 2016. Persistent pain after breast cancer treatment: A questionnaire-based study on the prevalence, associated treatment variables, and pain type. Journal of Breast Cancer 19(4):447–454.
Karnofsky, D.A., W.H. Abelmann, L.F. Craver, and J.H. Burchenal. 1948. The use of the nitrogen mustards in the palliative treatment of carcinoma—with particular reference to bronchogenic carcinoma. Cancer 1(4):634–656.
Kelly, D.L., D. Buchbinder, R.F. Duarte, J.J. Auletta, N. Bhatt, M. Byrne, Z. DeFilipp, M. Gabriel, A. Mahindra, M. Norkin, H. Schoemans, A.J. Shah, I. Ahmed, Y. Atsuta, G.W. Basak, S. Beattie, S. Bhella, C. Bredeson, N. Bunin, J. Dalal, A. Daly, J. Gajewski, R.P. Gale, J. Galvin, M. Hamadani, R.J. Hayashi, K. Adekola, J. Law, C.J. Lee, J. Liesveld, A.K. Malone, A. Nagler, S. Naik, T. Nishihori, S.K. Parsons, A. Scherwath, H.L. Schofield, R. Soiffer, J. Szer, I. Twist, A. Warwick, B.M. Wirk, J. Yi, M. Battiwalla, M.E. Flowers, B. Savani, and B.E. Shaw. 2018. Neurocognitive dysfunction in hematopoietic cell transplant recipients: Expert review from the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and Complications and Quality of Life Working Party of the European Society for Blood and Marrow Transplantation. Biology of Blood Marrow Transplant 24(2):228–241.
Kessler, R.C., W.T. Chiu, O. Demler, K.R. Merikangas, and E.E. Walters. 2005. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry 62(6):617–627.
Kibaly, C., C. Xu C, C.M. Cahill, C.J. Evans, and P.Y. Law. 2019. Non-nociceptive roles of opioids in the CNS: opioids’ effects on neurogenesis, learning, memory and affect. Nature Reviews Neuroscience 20(1):5–18.
Kim, S.H., B.H. Son, S.Y. Hwang, W. Han, J.-H. Yang, S. Lee, and Y.H. Yun. 2008. Fatigue and depression in disease-free breast cancer survivors: Prevalence, correlates, and association with quality of life. Journal of Pain and Symptom Management 35(6):644–655.
Kneis, S., A. Wehrle, J. Müller, C. Maurer, G. Ihorst, A. Gollhofer, and H. Bertz. 2019. It’s never too late—Balance and endurance training improves functional performance, quality of life, and alleviates neuropathic symptoms in cancer survivors suffering from chemotherapy-induced peripheral neuropathy: Results of a randomized controlled trial. BMC Cancer 19(1):414.
Koppelmans, V., M.M. Breteler, W. Boogerd, C. Seynaeve, C. Gundy, and S.B. Schagen. 2012. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. Journal of Clinical Oncology 30(10):1080–1086.
Kuhnt, S., M. Friedrich, T. Schulte, D. Cella, and A. Hinz. 2019. Screening properties of the diagnostic criteria for cancer-related fatigue. Oncology Research and Treatment 42(9):440–447.
Kwan, M.L., J.C. Cohn, J.M. Armer, B.R. Stewart, and J.N. Cormier. 2011. Exercise in patients with lymphedema: A systematic review of the contemporary literature. Journal of Cancer Survivorship 5(4):320–336.
Laird, B.J.A., A.C. Scott, L.A. Colvin, A.-L. McKeon, G.D. Murray, K.C.H. Fearon, and M.T. Fallon. 2011. Pain, depression, and fatigue as a symptom cluster in advanced cancer. Journal of Pain and Symptom Management 42(1):1–11.
Lakoski, S.G., L.W. Jones, R.J. Krone, P.K. Stein, and J.M. Scott. 2015. Autonomic dysfunction in early breast cancer: Incidence, clinical importance, and underlying mechanisms. American Heart Journal 170(2):231–241.
Lange, M., N. Heutte, O. Rigal, S. Noal, J.E. Kurtz, C. Levy, D. Allouache, C. Rieux, J. Lefel, B. Clarisse, C. Veyret, P. Barthelemy, N. Longato, H. Castel, F. Eustache, B. Giffard, and F. Joly. 2016. Decline in cognitive function in older adults with early-stage breast cancer after adjuvant treatment. Oncologist 21(11):1337–1348.
Lange, M., N. Heutte, S. Noal, O. Rigal, J. E. Kurtz, C. Levy, D. Allouache, C. Rieux, J. Lefel, B. Clarisse, A. Leconte, C. Veyret, P. Barthelemy, N. Longato, L. Tron, H. Castel, F. Eustache, B. Giffard, and F. Joly. 2019. Cognitive changes after adjuvant treatment in older adults with early-stage breast cancer. Oncologist 24(1):62–68.
Leung, A., K. Lien, L. Zeng, J. Nguyen, A. Caissie, S. Culleton, L. Holden, and E. Chow. 2011. The EORTC QLQ-BN20 for assessment of quality of life in patients receiving treatment or prophylaxis for brain metastases: A literature review. Expert Review of Pharmacoeconomics & Outcomes Research 11(6):693–700.
Li, F., Z. Zhou, A. Wu, Y. Cai, H. Wu, M. Chen, and S. Liang. 2018. Preexisting radiological interstitial lung abnormalities are a risk factor for severe radiation pneumonitis in patients with small-cell lung cancer after thoracic radiation therapy. Radiation Oncology 13(1):82.
Li, K., D. Giustini, and D. Seely. 2019. A systematic review of acupuncture for chemotherapy-induced peripheral neuropathy. Current Oncology 26(2):e147–e154.
Li, M., E.B. Kennedy, N. Byrne, C. Gérin-Lajoie, M.R. Katz, H. Keshavarz, S. Sellick, and E. Green. 2017. Systematic review and meta-analysis of collaborative care interventions for depression in patients with cancer. Psycho-Oncology 26(5):573–587.
Lin, J., X. Lv, M. Niu, L. Liu, J. Chen, F. Xie, M. Zhong, S. Qiu, L. Li, and R. Huang. 2017. Radiation-induced abnormal cortical thickness in patients with nasopharyngeal carcinoma after radiotherapy. NeuroImage: Clinical 14:610–621.
Lin, N.U., E.Q. Lee, H. Aoyama, I.J. Barani, B.G. Baumert, P.D. Brown, D.R. Camidge, S.M. Chang, J. Dancey, L.E. Gaspar, G.J. Harris, F.S. Hodi, S.N. Kalkanis, K.R. Lam-born, M.E. Linskey, D.R. Macdonald, K. Margolin, M.P. Mehta, D. Schiff, R. Soffietti, J.H. Suh, M.J. van den Bent, M.A. Vogelbaum, J.S. Wefel, and P.Y. Wen. 2013. Challenges relating to solid tumour brain metastases in clinical trials, part 1: Patient population, response, and progression. A report from the RANO group. The Lancet Oncology 14(10):e396–e406.
Linden, W., A. Vodermaier, R. Mackenzie, and D. Greig. 2012. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. Journal of Affective Disorders 141(2–3):343–351.
Lo, C., C. Zimmermann, A. Rydall, A. Walsh, J.M. Jones, M.J. Moore, F.A. Shepherd, L. Gagliese, and G. Rodin. 2010. Longitudinal study of depressive symptoms in patients with metastatic gastrointestinal and lung cancer. Journal of Clinical Oncology 28(18):3084–3089.
Loprinzi, C.L., C. Lacchetti, J. Bleeker, G. Cavaletti, C. Chauhan, D.L. Hertz, M.R. Kelley, A. Lavino, M.B. Lustberg, J.A. Paice, B.P. Schneider, E.M.L. Smith, M.L. Smith, T.J. Smith, N. Wagner-Johnston, and D.L. Hershman. 2020. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. Journal of Clinical Oncology 38(28):3325–3348.
Lutz, S., T. Balboni, J. Jones, S. Lo, J. Petit, S.E. Rich, R. Wong, and C. Hahn. 2017. Palliative radiation therapy for bone metastases: Update of an ASTRO evidence-based guideline. Practical Radiation Oncology 7(1):4–12.
Manchikanti, L., S. Abdi, S. Atluri, C.C. Balog, R.M. Benyamin, M.V. Boswell, K.R. Brown, B.M. Bruel, D.A. Bryce, P.A. Burks, A.W. Burton, A.K. Calodney, D.L. Caraway, K.A. Cash, P.J. Christo, K.S. Damron, S. Datta, T.R. Deer, S. Diwan, I. Eriator, F.J. Falco, B. Fellows, S. Geffert, C.G. Gharibo, S.E. Glaser, J.S. Grider, H. Hameed, M. Hameed, H. Hansen, M.E. Harned, S.M. Hayek, S. Helm, 2nd, J.A. Hirsch, J.W. Janata, A.D. Kaye, A.M. Kaye, D.S. Kloth, D. Koyyalagunta, M. Lee, Y. Malla, K.N. Manchikanti, C.D. McManus, V. Pampati, A.T. Parr, R. Pasupuleti, V.B. Patel, N. Sehgal, S.M. Silverman, V. Singh, H.S. Smith, L.T. Snook, D.R. Solanki, D.H. Tracy, R. Vallejo, B.W. Wargo, and the American Society of Interventional Pain Physicians. 2012. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2—guidance. Pain Physician 15(3 Suppl):S67–S116.
Mandelblatt, J.S., J.D. Clapp, G. Luta, L.A. Faul, M.D. Tallarico, T.D. McClendon, J.A. Whitley, L. Cai, T.A. Ahles, R.A. Stern, P.B. Jacobsen, B.J. Small, B.N. Pitcher, E. Dura-Fernandis, H.B. Muss, A. Hurria, H.J. Cohen, and C. Isaacs. 2016. Long-term trajectories of self-reported cognitive function in a cohort of older survivors of breast cancer: CALGB 369901 (Alliance). Cancer 122(22):3555–3563.
Marconi, R., A. Serafini, A. Giovanetti, C. Bartoleschi, C.M. Pardini, G. Bossi, and L. Strigari. 2019. Cytokine modulation in breast cancer patients undergoing radiotherapy: A revision of the most recent studies. International Journal of Molecular Sciences 20(2):382.
McDuff, S.G.R., A.I. Mina, C.L. Brunelle, L. Salama, L.E.G. Warren, M. Abouegylah, M. Swaroop, M.N. Skolny, M. Asdourian, T. Gillespie, K. Daniell, H.E. Sayegh, G.E. Naoum, H. Zheng, and A.G. Taghian. 2019. Timing of lymphedema after treatment for breast cancer: When are patients most at risk? International Journal of Radiation Oncology, Biology, Physics 103(1):62–70.
McQuade, R.M., V. Stojanovska, R. Abalo, J.C. Bornstein, and K. Nurgali. 2016. Chemotherapy-induced constipation and diarrhea: Pathophysiology, current and emerging treatments. Frontiers in Pharmacology 7:414.
McTiernan, A., C.M. Friedenreich, P.T. Katzmarzyk, K.E. Powell, R. Macko, D. Buchner, L.S. Pescatello, B. Bloodgood, B. Tennant, A. Vaux-Bjerke, S.M. George, R.P. Troiano, and K.L. Piercy. 2019. Physical activity in cancer prevention and survival: A systematic review. Medicine & Science in Sports & Exercise 51(6):1252–1261.
Mejdahl, M.K., K.G. Andersen, R. Gärtner, N. Kroman, and H. Kehlet. 2013. Persistent pain and sensory disturbances after treatment for breast cancer: Six year nationwide follow-up study. BMJ 346:f1865.
Mercadante, S., and V. Vitrano. 2010. Pain in patients with lung cancer: Pathophysiology and treatment. Lung Cancer 68(1):10–15.
Migliorati, C.A., J.B. Epstein, E. Abt, and J.R. Berenson. 2011. Osteonecrosis of the jaw and bisphosphonates in cancer: A narrative review. Nature Reviews in Endocrinology 7(1):34–42.
Milgrom, D.P., N.L. Lad, L.G. Koniaris, and T.A. Zimmers. 2017. Bone pain and muscle weakness in cancer patients. Current Osteoporosis Reports 15(2):76–87.
Mills, S.E.E., K.P. Nicolson, and B.H. Smith. 2019. Chronic pain: A review of its epidemiology and associated factors in population-based studies. British Journal Anaesthesiology 123(2):e273–e283.
Miltenburg, N.C., and W. Boogerd. 2014. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treatment and Review 40:872–882.
Mishra, S.I., R.W. Scherer, P.M. Geigle, D.R. Berlanstein, O. Topaloglu, C.C. Gotay, and C. Snyder. 2012. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database of Systematic Reviews 2012(8):CD007566.
Misono, S., N.S. Weiss, J.R. Fann, M. Redman, and B. Yueh. 2008. Incidence of suicide in persons with cancer. Journal of Clinical Oncology 26(29):4731–4738.
Montemurro, F., G. Mittica, C. Cagnazzo, V. Longo, P. Berchialla, G. Solinas, P. Culotta, R. Martinello, M. Foresto, S. Gallizioli, A. Calori, B. Grasso, C. Volpone, G. Bertola, G. Parola, G. Tealdi, P.L. Giuliano, M. Aglietta, and A.M. Ballari. 2016. Self-evaluation of adjuvant chemotherapy-related adverse effects by patients with breast cancer. JAMA Oncology 2(4):445–452.
Morales, L., P. Neven, and R. Paridaens. 2005. Choosing between an aromatase inhibitor and tamoxifen in the adjuvant setting. Current Opinion in Oncology 17(6):559–565.
Morrow, G.R., P.L. Andrews, J.T. Hickok, J.A. Roscoe, and S. Matteson. 2002. Fatigue associated with cancer and its treatment. Supportive Care in Cancer 10(5):389–398.
Murawski, B., L. Wade, R.C. Plotnikoff, D.R. Lubans, and M.J. Duncan. 2018. A systematic review and meta-analysis of cognitive and behavioral interventions to improve sleep health in adults without sleep disorders. Sleep Medicine Reviews 40:160–169.
Mustian, K.M., C.M. Alfano, C. Heckler, A.S. Kleckner, I.R. Kleckner, C.R. Leach, D. Mohr, O.G. Palesh, L.J. Peppone, B.F. Piper, J. Scarpato, T. Smith, L.K. Sprod, and S.M. Miller. 2017. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncology 3(7):961–968.
Myers, J.S., K.I. Erickson, S.M. Sereika, and C.M. Bender. 2018. Exercise as an intervention to mitigate decreased cognitive function from cancer and cancer treatment: An integrative review. Cancer Nursing 41(4):327–343.
NASEM (National Academies of Sciences, Engineering, and Medicine). 2019. Functional assessment for adults with disabilities. Washington, DC: The National Academies Press.
NASEM. 2020. Selected health conditions and likelihood of improvement with treatment. Washington, DC: The National Academies Press.
Navari, R.M., C.M. Pywell, J.G. Le-Rademacher, P. White, A.B. Dodge, C. Albany, and C.L. Loprinzi. 2020. Olanzapine for the treatment of advanced cancer–related chronic nausea and/or vomiting: A randomized pilot trial. JAMA Oncology 6(6):895–899.
NCCN (National Comprehensive Cancer Network). 2020a. Distress during cancer care. https://www.nccn.org/patients (accessed October 5, 2020).
NCCN. 2020b. Adult cancer pain, version 1.2020. https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf (accessed October 5, 2020).
NCCN. 2020c. Cancer-related fatigue, version 2.2020. https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf (accessed April 26, 2020).
NCCN. 2020d. Hematopoietic cell transplant (HCT): Pre-transplant recipient evaluation and management of graft-versus-host disease. NCCN guidelines, version 2.2020. https://www.nccn.org/professionals/physician_gls/pdf/hct.pdf (accessed August 27, 2020).
NCCN. 2020e. Palliative care, version 1.2020. https://www.nccn.org/professionals/physician_gls/pdf/palliative.pdf (accessed October 5, 2020).
NCCN. 2020f. Survivorship, version 2.2020. https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf (accessed June 17, 2020).
NCCS (National Coalition for Cancer Survivorship). 2019. Redefining functional status: A patient-led quality measurement effort. https://canceradvocacy.org/wp-content/uploads/2020/02/RFS-Project-Final-Overview-12-17-19.pdf (accessed April 15, 2020).
NCI (National Cancer Institute). 2020. Gastrointestinal complications (PDQ®)–Health professional version. https://www.cancer.gov/about-cancer/treatment/side-effects/constipation/gi-complications-hp-pdq#_8 (accessed June 29, 2020).
Neben-Wittich, M.A., P.J. Atherton, D.J. Schwartz, J.A. Sloan, P.C. Griffin, R.L. Deming, J.C. Anders, C.L. Loprinzi, K.N. Burger, J.A. Martenson, and R.C. Miller. 2011. Comparison of provider-assessed and patient-reported outcome measures of acute skin toxicity during a phase III trial of mometasone cream versus placebo during breast radiotherapy: The North Central Cancer Treatment Group (N06C4). International Journal of Radiation Oncology, Biology, Physics 81(2):397–402.
Ng, C.G., M.P. Boks, N.Z. Zainal, and N.J. de Wit. 2011. The prevalence and pharmacotherapy of depression in cancer patients. Journal of Affective Disorders 131(1–3):1–7.
NLM (U.S. National Library of Medicine). 2019. LABEL: BLEOMYCIN—bleomycin sulfate injection, powder, lyophilized, for solution. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b5806c40-12ce-48e3-8abd-9f8997ef4428&audience=consumer (accessed December 8, 2020).
Norman, S.A., L.T. Miller, H.B. Erikson, M.F. Norman, and R. McCorkle. 2001. Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Physical Therapy 81(6):1192–1205.
O’Carrigan, B., M.H. Wong, M.L. Willson, M.R. Stockler, N. Pavlakis, and A. Goodwin. 2017. Bisphosphonates and other bone agents for breast cancer. Cochrane Database of Systematic Reviews 10(10):CD003474.
Okabayashi, K., H. Ashrafian, E. Zacharakis, H. Hasegawa, Y. Kitagawa, T. Athanasiou, and A. Darzi. 2014. Adhesions after abdominal surgery: A systematic review of the incidence, distribution and severity. Surgery Today 44(3):405–420.
Okamura, M., S. Yamawaki, T. Akechi, K. Taniguchi, and Y. Uchitomi. 2005. Psychiatric disorders following first breast cancer recurrence: Prevalence, associated factors, and relationship to quality of life. Japanese Journal of Clinical Oncology 35(6):302–309.
Oken, M.M., R.H. Creech, D.C. Tormey, J. Horton, T.E. Davis, E.T. McFadden, and P.P. Carbone. 1982. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 5(6):649–655.
Orphanos, G.S., G.N. Ioannidis, and A.G. Ardavanis. 2009. Cardiotoxicity induced by tyrosine kinase inhibitors. Acta Oncologica 48(7):964–970.
Osborn, R.L., A.C. Demoncada, and M. Feuerstein. 2006. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: Meta-analyses. International Journal of Psychiatry in Medicine 36(1):13–34.
Otte, J.L., J.S. Carpenter, S. Manchanda, K.L. Rand, T.C. Skaar, M. Weaver, Y. Chernyak, X. Zhong, C. Igega, and C. Landis. 2015. Systematic review of sleep disorders in cancer patients: Can the prevalence of sleep disorders be ascertained? Cancer Medicine 4(2):183–200.
Pagé, M.G., M. Fortier, M.A. Ware, and M. Choinière. 2018. As if one pain problem was not enough: Prevalence and patterns of coexisting chronic pain conditions and their impact on treatment outcomes. Journal of Pain Research 11:237–254.
Pallua, S., J. Giesinger, A. Oberguggenberger, G. Kemmler, D. Nachbaur, J. Clausen, M. Kopp, B. Sperner-Unterweger, and B. Holzner. 2010. Impact of GVHD on quality of life in long-term survivors of haematopoietic transplantation. Bone Marrow Transplant 45(10):1534–1539.
Patel, A.V., C.M. Friedenreich, S.C. Moore, S.C. Hayes, J.K. Silver, K.L. Campbell, K. Winters-Stone, L.H. Gerber, S.M. George, and J.E. Fulton. 2019. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Medicine & Science in Sports & Exercise 51(11):2391–2402.
Pauli, N., J. Johnson, C. Finizia, and P. Andréll. 2013. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncologica 52(6):1137–1145.
PDQ® Supportive and Palliative Care Editorial Board. PDQ gastrointestinal complications. Bethesda, MD: National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/side-effects/constipation/GI-complications-hp-pdq (accessed December 28, 2020).
Peerzada, M.M., T.P. Spiro, and H.A. Daw. 2011. Pulmonary toxicities of tyrosine kinase inhibitors. Clinical Advances in Hematology & Oncology 9(11):824–836.
Peixoto da Silva, S., J.M.O. Santos, M.P. Costa e Silva, R.M. Gil da Costa, and R. Medeiros. 2020. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. Journal of Cachexia, Sarcopenia and Muscle 11(3):619–635.
Phillips, K.M., H.L. McGinty, J. Cessna, Y. Asvat, B. Gonzalez, M.G. Cases, B.J. Small, P.B. Jacobsen, J. Pidala, and H.S. Jim. 2013. A systematic review and meta-analysis of changes in cognitive functioning in adults undergoing hematopoietic cell transplantation. Bone Marrow Transplant 48(10):1350–1357.
Piai, V., J.B. Prins, I.M. Verdonck-de Leeuw, C.R. Leemans, C.H.J. Terhaard, J.A. Langendijk, R.J. Baatenburg de Jong, J.H. Smit, R.P. Takes, and R.P.C. Kessels. 2019. Assessment of neurocognitive impairment and speech functioning before head and neck cancer treatment. JAMA Otolaryngology—Head & Neck Surgery 145(3):251–257.
Pinquart, M., and P.R. Duberstein. 2010. Depression and cancer mortality: A meta-analysis. Psychological Medicine 40(11):1797–1810.
Porreca, F., and M.H. Ossipov. 2009. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: Mechanisms, implications, and management options. Pain Medicine 10:654–662.
Qi, W.-X., L.-N. Tang, A.-N. He, Y. Yao, and Z. Shen. 2014. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: A meta-analysis of seven randomized controlled trials. International Journal of Clinical Oncology 19(2):403–410.
Ramchand, S.K., Y.M. Cheung, B. Yeo, and M. Grossmann. 2019. The effects of adjuvant endocrine therapy on bone health in women with breast cancer. Journal of Endocrinology 241(3):R111–R124.
Reiman, M.P., and R.C. Manske. 2011. The assessment of function: How is it measured? A clinical perspective. Journal of Manual & Manipulative Therapy 19(2):91–99.
Repetto, L. 2003. Greater risks of chemotherapy toxicity in elderly patients with cancer. Journal of Supportive Oncology 1(4 Suppl 2):18–24.
Roach 3rd, M., D.R. Gandara, H.-S. Yuo, P.S. Swift, S. Kroll, D.C. Shrieve, W.M. Wara, L. Margolis, and T.L. Phillips. 1995. Radiation pneumonitis following combined modality therapy for lung cancer: Analysis of prognostic factors. Journal of Clinical Oncology 13(10):2606–2612.
Roberts, K., K. Rickett, R. Greer, and N. Woodward. 2017. Management of aromatase inhibitor induced musculoskeletal symptoms in postmenopausal early breast cancer: A systematic review and meta-analysis. Critical Reviews in Oncology/Hematology 111:66–80.
Rossi, J.F., L. Laurier, J.-L. Misset, D. Spaeth, F. Uwer, P. Soubeyran, R. Largiller, J.-P. Metges, J.-Y. Blay, M. Attal, T. Facon, O. Hermine, R. Bugat, J.-P. Spano, L. Cals, H. Cure, D. Atlan, and M. Gjoklaj. 2008. Prospective multicenter study for evaluating cognitive disorders in hematology–oncology: A pilot study of 118 patients. Blood 112(11):1309.
Rowlings, P.A., D. Przepiorka, J.P. Klein, R.P. Gale, J.R. Passweg, P.J. Henslee-Downey, J.Y. Cahn, S. Calderwood, A. Gratwohl, G. Socié, M.M. Abecasis, K.A. Sobocinski, M.J. Zhang, and M.M. Horowitz. 1997. IBMTR severity index for grading acute graft-versus-host disease: Retrospective comparison with Glucksberg grade. British Journal of Haematology 97(4):855–864.
Rupp, J., C. Hadamitzky, C. Henkenberens, H. Christiansen, D. Steinmann, and F. Bruns. 2019. Frequency and risk factors for arm lymphedema after multimodal breast-conserving treatment of nodal positive breast cancer—A long-term observation. Radiation Oncology 14(1):39.
Sachdev, P.S., D. Blacker, D.G. Blazer, M. Ganguli, D.V. Jeste, J.S. Paulsen, and R.C. Petersen. 2014. Classifying neurocognitive disorders: The DSM-5 approach. Nature Reviews Neurology 10(11):634–642.
Sarfati, D., B. Koczwara, and C. Jackson. 2016. The impact of comorbidity on cancer and its treatment. CA: A Cancer Journal for Clinicians 66(4):337–350.
Sarna, L., G. Padilla, C. Holmes, D. Tashkin, M.L. Brecht, and L. Evangelista. 2002. Quality of life of long-term survivors of non–small-cell lung cancer. Journal of Clinical Oncology 20(13):2920–2929.
Savard, J., S. Simard, J. Blanchet, H. Ivers, and C.M. Morin. 2001. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep 24(5):583–590.
Savard, J., H. Ivers, J. Villa, A. Caplette-Gingras, and C.M. Morin. 2011. Natural course of insomnia comorbid with cancer: An 18-month longitudinal study. Journal of Clinical Oncology 29(26):3580–3586.
Schmitz, K.H., R.L. Ahmed, A. Troxel, A. Cheville, R. Smith, L. Lewis-Grant, C.J. Bryan, C.T. Williams-Smith, and Q.P. Greene. 2009. Weight lifting in women with breast-cancer-related lymphedema. New England Journal of Medicine 361(7):664–673.
Schmitz, K.H., K.S. Courneya, C. Matthews, W. Demark-Wahnefried, D.A. Galvao, B.M. Pinto, M.L. Irwin, K.Y. Wolin, R.J. Segal, A. Lucia, C.M. Schneider, V.E. von Gruenigen, A.L. Schwartz, and American College of Sports. 2010. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine & Science in Sports & Exercise 42(7):1409–1426.
Schmitz, K.H., A.M. Campbell, M.M. Stuiver, B.M. Pinto, A.L. Schwartz, G.S. Morris, J.A. Ligibel, A. Cheville, D.A. Galvão, C.M. Alfano, A.V. Patel, T. Hue, L.H. Gerber, R. Sallis, N.J. Gusani, N.L. Stout, L. Chan, F. Flowers, C. Doyle, S. Helmrich, W. Bain, J. Sokolof, K.M. Winters Stone, K.L. Campbell, and C.E. Matthews. 2019. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA: A Cancer Journal for Clinicians 69(6):468–484.
Schwartzberg, L. 2014. Addressing the value of novel therapies in chemotherapy-induced nausea and vomiting. Expert Reviews in Pharmacoeconomics & Outcomes Research 14(6):825–834.
Scott, J.M., T.S. Nilsen, D. Gupta, and L.W. Jones. 2018. Exercise therapy and cardiovascular toxicity in cancer. Circulation 137(11):1176–1191.
Sekine, I., Y. Segawa, K. Kubota, and T. Saeki. 2013. Risk factors of chemotherapy-induced nausea and vomiting: Index for personalized antiemetic prophylaxis. Cancer Science 104(6):711–717.
Semple, C.J., L. Dunwoody, K. Sullivan, and W.G. Kernohan. 2006. Patients with head and neck cancer prefer individualized cognitive behavioural therapy. European Journal of Cancer Care 15(3):220–227.
Seretny, M., G.L. Currie, E.S. Sena, S. Ramnarine, R. Grant, M.R. MacLeod, L.A. Colvin, and M. Fallon. 2014. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 155(12):2461–2470.
Shadad, A.K., F.J. Sullivan, J.D. Martin, and L.J. Egan. 2013. Gastrointestinal radiation injury: Symptoms, risk factors, and mechanisms. World Journal of Gastroenterology 19(2):185–198.
Shaitelman, S.F., K.D. Cromwell, J.C. Rasmussen, N.L. Stout, J.M. Armer, B.B. Lasinski, and J.N. Cormier. 2015. Recent progress in the treatment and prevention of cancer-related lymphedema. CA: A Cancer Journal for Clinicians 65(1):55–81.
Shannon, V.R. 2020. Cancer treatment-related lung injury. In J.L. Nates and K.J. Price (eds.), Oncologic critical care. Cham, Switzerland: Springer Nature. Pp. 531–556.
Sharafeldin, N., A. Bosworth, S.K. Patel, Y. Chen, E. Morse, M. Mather, C. Sun, L. Francisco, S.J. Forman, F.L. Wong, and S. Bhatia. 2018. Cognitive functioning after hematopoietic cell transplantation for hematologic malignancy: Results from a prospective longitudinal study. Journal of Clinical Oncology 36(5):463–475.
Sharpe, M., J. Walker, C. Holm-Hansen, P. Martin, S. Symeonides, C. Gourley, L. Wall, D. Weller, G. Murray, and SMaRT (Symptom Management Research Trials) Oncology-2 Team. 2014. Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology-2): A multicentre randomised controlled effectiveness trial. The Lancet 384(9948):1099–1108.
Shaw, M.G., and D.L. Ball. 2013. Treatment of brain metastases in lung cancer: Strategies to avoid/reduce late complications of whole brain radiation therapy. Current Treatment Options in Oncology 14(4):553–567.
Sheard, T., and P. Maguire. 1999. The effect of psychological interventions on anxiety and depression in cancer patients: Results of two meta-analyses. British Journal of Cancer 80(11):1770–1780.
Shibayama, O., K. Yoshiuchi, M. Inagaki, Y. Matsuoka, E. Yoshikawa, Y. Sugawara, T. Akechi, N. Wada, S. Imoto, K. Murakami, A. Ogawa, A. Akabayashi, and Y. Uchitomi. 2014. Association between adjuvant regional radiotherapy and cognitive function in breast cancer patients treated with conservation therapy. Cancer Medicine 3(3):702–709.
Shibayama, O., K. Yoshiuchi, M. Inagaki, Y. Matsuoka, E. Yoshikawa, Y. Sugawara, T. Akechi, N. Wada, S. Imoto, K. Murakami, A. Ogawa, and Y. Uchitomi. 2019. Long-term influence of adjuvant breast radiotherapy on cognitive function in breast cancer patients treated with conservation therapy. International Journal of Clinical Oncology 24(1):68–77.
Siefert, M.L., F. Hong, B. Valcarce, and D.L. Berry. 2014. Patient and clinician communication of self-reported insomnia during ambulatory cancer care clinic visits. Cancer Nursing 37(2):E51–E59.
Silver, J.K., V.S. Raj, J.B. Fu, E.M. Wisotzky, S.R. Smith, and R.A. Kirch. 2015. Cancer rehabilitation and palliative care: Critical components in the delivery of high-quality oncology services. Supportive Care in Cancer 23(12):3633–3643.
Simo, M., J.C. Root, L. Vaquero, P. Ripolles, J. Jove, T. Ahles, A. Navarro, F. Cardenal, J. Bruna, and A. Rodriguez-Fornells. 2015. Cognitive and brain structural changes in a lung cancer population. Journal of Thoracic Oncology 10(1):38–45.
Simon, M., K. Korn, L. Cho, G.G. Blackburn, and C. Raymond. 2018. Cardiac rehabilitation: A class 1 recommendation. Cleveland Clinical Journal of Medicine 85(7):551–558.
Smith, H.R. 2015. Depression in cancer patients: Pathogenesis, implications, and treatment (review). Oncology Letters 9(4):1509–1514.
Smitherman, A.B., C. Anderson, J.L. Lund, J.T. Bensen, D.L. Rosenstein, and H.B. Nichols. 2018. Frailty and comorbidities among survivors of adolescent and young adult cancer: A cross-sectional examination of a hospital-based survivorship cohort. Journal of Adolescent and Young Adult Oncology 7(3):374–383.
Smoot, B.J., J.F. Wong, and M.J. Dodd. 2011. Comparison of diagnostic accuracy of clinical measures of breast cancer–related lymphedema: Area under the curve. Archives of Physical Medicine and Rehabilitation 92(4):603–610.
Sneeuw, K.C.A., N.K. Aaronson, M.C.C. Vanwouwe, J.A. Sergeant, J.A. Vandongen, and H. Bartelink. 1993. Prevalence and screening of psychiatric-disorder in patients with early-stage breast-cancer. Quality of Life Research 2(1):50–51.
Spiegel, D., S. Sands, and C. Koopman. 1994. Pain and depression in patients with cancer. Cancer 74(9):2570–2578.
Spinelli, B., M.J. Kallan, X. Zhang, A. Cheville, A. Troxel, J. Cohn, L. Dean, K. Sturgeon, M. Evangelista, Z. Zhang, D. Ebaugh, and K.H. Schmitz. 2019. Intra- and interrater reliability and concurrent validity of a new tool for assessment of breast cancer-related lymphedema of the upper extremity. Archives of Physical Medicine & Rehabilitation 100(2):315–326.
Steel, J.L., K.H. Kim, M.A. Dew, M.L. Unruh, M.H. Antoni, M.C. Olek, D.A. Geller, B.I. Carr, L.H. Butterfield, and T.C. Gamblin. 2010. Cancer-related symptom clusters, eosinophils, and survival in hepatobiliary cancer: An exploratory study. Journal of Pain and Symptom Management 39(5):859–871.
Stewart, W.F., J.A. Ricci, E. Chee, D. Morganstein, and R. Lipton. 2003. Lost productive time and cost due to common pain conditions in the U.S. workforce. JAMA 290(18):2443–2454.
Stout, N.L., J.K. Silver, V.S. Raj, J. Rowland, L. Gerber, A. Cheville, K.K. Ness, M. Radomski, R. Nitkin, M.D. Stubblefield, G.S. Morris, A. Acevedo, Z. Brandon, B. Braveman, S. Cunningham, L. Gilchrist, L. Jones, L. Padgett, T. Wolf, K. Winters-Stone, G. Campbell, J. Hendricks, K. Perkin, and L. Chan. 2016. Toward a national initiative in cancer rehabilitation: Recommendations from a subject matter expert group. Archives of Physical Medicine & Rehabilitation 97(11):2006–2015.
Stubblefield, M.D. 2017. The underutilization of rehabilitation to treat physical impairments in breast cancer survivors. PM&R 9(9S2):S317–S323.
Stubblefield, M.D., and N. Keole. 2014. Upper body pain and functional disorders in patients with breast cancer. PM&R 6(2):170–183.
Stubblefield, M.D., H.J. Burstein, A.W. Burton, C.M. Custodio, G.E. Deng, M. Ho, L. Junck, G.S. Morris, J.A. Paice, S. Tummala, and J.H. Von Roenn. 2009. NCCN task force report: Management of neuropathy in cancer. Journal of the National Comprehensive Cancer Network (Suppl 5):S1–S26.
Swarm, R.A., A.P. Abernethy, D.L. Anghelescu, C. Benedetti, S. Buga, C. Cleeland, O.A. Deleon-Casasola, J.G. Eilers, B. Ferrell, M. Green, N.A. Janjan, M.M. Kamdar, M.H. Levy, M. Lynch, R.M. McDowell, N. Moryl, S.A. Nesbit, J.A. Paice, M.W. Rabow, K.L. Syrjala, S.G. Urba, S.M. Weinstein, M. Dwyer, and R. Kumar. 2013. Adult cancer pain. Journal of the National Comprehensive Cancer Network 11(8):992–1022.
Syrjala, K.L., S.B. Artherholt, B.F. Kurland, S.L. Langer, S. Roth-Roemer, J.B. Elrod, and S. Dikmen. 2011. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. Journal of Clinical Oncology 29(17):2397–2404.
Syrjala, K.L., M.P. Jensen, M.E. Mendoza, J.C. Yi, H.M. Fisher, and F.J. Keefe. 2014. Psychological and behavioral approaches to cancer pain management. Journal of Clinical Oncology 32(16):1703–1711.
Talley, N.J. 2004. Definitions, epidemiology, and impact of chronic constipation. Review of Gastroenterology Disorders 4(Suppl 2):S3–S10.
ten Broek, R.P.G., Y. Issa, E.J.P. van Santbrink, N.D. Bouvy, R.F.P.M. Kruitwagen, J. Jeekel, E.A. Bakkum, M.M. Rovers, and H. van Goor. 2013. Burden of adhesions in abdominal and pelvic surgery: Systematic review and met-analysis. British Medical Journal 347:f5588.
Tetreault, L.A., M.P. Zhu, R.M. Howard, F. Sorefan-Mangou, A.A. Patel, G.D. Schroeder, E.M. Massicotte, J.H. Bhadiwala, M.G. Fehlings, and J.R. Wilson. 2019. The discrepancy between functional outcome and self-reported health status after surgery for degenerative cervical myelopathy. The Spine Journal 19(11):1809–1815.
Tiep, B., V. Sun, M. Koczywas, J. Kim, D. Raz, A. Hurria, and J. Hayter. 2015. Pulmonary rehabilitation and palliative care for the lung cancer patient. Journal of Hospice & Palliative Nursing 17(5):462–468.
Tocchetti, C.G., G. Gallucci, C. Coppola, G. Piscopo, C. Cipresso, C. Maurea, A. Giudice, R.V. Iaffaioli, C. Arra, and N. Maurea. 2013. The emerging issue of cardiac dysfunction induced by antineoplastic angiogenesis inhibitors. European Journal of Heart Failure 15(5):482–489.
Tonato, M., F. Roila, and A. Del Favero. 1991. Methodology of antiemetic trials: A review. Annals of Oncology 2(2):107–114.
Trépanier, M., E.M. Minnella, T. Paradis, R. Awasthi, P. Kaneva, K. Schwartzman, F. Carli, G.M. Fried, L.S. Feldman, and L. Lee. 2019. Improved disease-free survival after prehabilitation for colorectal cancer surgery. Annals of Surgery 270(3):493–501.
Triozzi, P.L., D. Goldstein, and J. Laszlo. 1988. Contributions of benzodiazepines to cancer therapy. Cancer Investigations 6(1):103–111.
USPSTF (U.S. Preventive Services Task Force). 2018. Final recommendation statement: Osteoporosis to prevent fractures: Screening. https://www.uspreventiveservicestaskforce.org/uspstf/document/RecommendationStatementFinal/osteoporosis-screening (accessed November 5, 2020).
Vagnildhaug, O.M., T.R. Balstad, S.S. Almberg, C. Brunelli, A.K. Knudsen, S. Kaasa, M. Thronæs, B. Laird, and T.S. Solheim. 2018. A cross-sectional study examining the prevalence of cachexia and areas of unmet need in patients with cancer. Supportive Care in Cancer 26(6):1871–1880.
Valderas, J.M., B. Starfield, B. Sibbald, C. Salisbury, and M. Roland. 2009. Defining comorbidity: Implications for understanding health and health services. Annals of Family Medicine 7(4):357–363.
van den Beuken-van Everdingen, M.H., L.M. Hochstenbach, E.A. Joosten, V.C. Tjan-Heijnen, and D.J. Janssen. 2016. Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. Journal of Pain and Symptom Management 51(6):1070–1090.
van Helmond, N., M.A. Steegers, G.P. Filippini-de Moor, K.C. Vissers, and O.H. Wilder-Smith. 2016. Hyperalgesia and persistent pain after breast cancer surgery: A prospective randomized controlled trial with perioperative COX-2 inhibition. PLOS ONE 11(12):e0166601.
van Leeuwen, F.E., and A.K. Ng. 2016. Long-term risk of second malignancy and cardiovascular disease after Hodgkin lymphoma treatment. Hematology. American Society of Hematology. Education Program 2016(1):323–330.
Vaqas, B., and T.J. Ryan. 2003. Lymphoedema: Pathophysiology and management in resource-poor settings—relevance for lymphatic filariasis control programmes. Filaria Journal 2(1):4.
Vardy, J.L., H.M. Dhillon, G.R. Pond, S.B. Rourke, T. Bekele, C. Renton, A. Dodd, H. Zhang, P. Beale, S. Clarke, and I.F. Tannock. 2015. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal, controlled study. Journal of Clinical Oncology 33(34):4085–4092.
Varricchi, G., M.R. Galdiero, G. Marone, G. Criscuolo, M. Triassi, D. Bonaduce, G. Marone, and C.G. Tocchetti. 2017. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2(4):e000247.
Vernucci, E., S. Agrawal, and V. Rai. 2018. Role of multidisciplinary approach in the management of cancer pain. Annals of Psychiatry and Clinical Neuroscience 1(1):1005.
Vetrano, D.L., D. Rizzuto, A. Calderón-Larrañaga, G. Onder, A.-K. Welmer, R. Bernabei, A. Marengoni, and L. Fratiglioni. 2018. Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: A Swedish cohort study. PLOS Medicine 15(3):e1002503.
Virizuela, J., A.M. García, R. De las Penas, A. Santaballa, R. Andrés, C. Beato, S. De La Cruz, J. Gavila, S. Gonzalez-Santiago, and T.L. Fernández. 2019. Some clinical guidelines on cardiovascular toxicity (2018). Clinical and Translational Oncology 21(1):94–105.
Visovatti, M.A., P.A. Reuter-Lorenz, A.E. Chang, L. Northouse, and B. Cimprich. 2016. Assessment of cognitive impairment and complaints in individuals with colorectal cancer. Oncology Nursing Forum 43(2):169–178.
Von Ah, D. 2015. Cognitive changes associated with cancer and cancer treatment: State of the science. Clinical Journal of Oncology Nursing 19(1):47–56.
Von Ah, D., and A. Crouch. 2020. Cognitive rehabilitation for cognitive dysfunction after cancer and cancer treatment: Implications for practice. Cancer Nursing 36(1):150977.
Von Ah, D., J.S. Carpenter, A. Saykin, P. Monahan, J. Wu, M. Yu, G. Rebok, K. Ball, B. Schneider, M. Weaver, E. Tallman, and F. Unverzagt. 2012. Advanced cognitive training for breast cancer survivors: A randomized controlled trial. Breast Cancer Research & Treatment 135(3):799–809.
Von Ah, D., S. Storey, and A. Crouch. 2018. Relationship between self-reported cognitive function and work-related outcomes in breast cancer survivors. Journal of Cancer Survivorship 12(2):246–255.
Wang, X.S., F. Zhao, M.J. Fisch, A.M. O’Mara, D. Cella, T.R. Mendoza, and C.S. Cleeland. 2014. Prevalence and characteristics of moderate to severe fatigue: A multicenter study in cancer patients and survivors. Cancer 120(3):425–432.
Watts, N.B., J.P. Bilezikian, P.M. Camacho, S.L. Greenspan, S.T. Harris, S.F. Hodgson, M. Kleerekoper, M.M. Luckey, M.R. McClung, R.P. Pollack, and S.M. Petak. 2010. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocrine Practice 16(Suppl 3):1–37.
Wefel, J.S., S.R. Kesler, K.R. Noll, and S.B. Schagen. 2015. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA: A Cancer Journal for Clinicians 65(2):123–138.
Weis, J. 2011. Cancer-related fatigue: Prevalence, assessment and treatment strategies. Expert Reviews in Pharmacoeconomics & Outcomes Research 11(4):441–446.
WHO (World Health Organization). 2001. International classification of functioning, disability and health. Geneva, Switzerland: World Health Organization.
Williams, A.M., C.S. Zent, and M.C. Janelsins. 2016. What is known and unknown about chemotherapy-related cognitive impairment in patients with haematological malignancies and areas of needed research. British Journal of Haematology 174(6):835–846.
Williams, A.M., J. Lindholm, F. Siddiqui, T.A. Ghanem, and S.S. Chang. 2017. Clinical assessment of cognitive function in patients with head and neck cancer: Prevalence and correlates. Otolaryngology—Head & Neck Surgery 157(5):808–815.
Winters-Stone, K.M., F. Horak, P.G. Jacobs, P. Trubowitz, N.F. Dieckmann, S. Stoyles, and S. Faithfull. 2017. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. Journal of Clinical Oncology 35(23):2604–2612.
Wu, H.S., and M. McSweeney. 2007. Cancer-related fatigue: “It’s so much more than just being tired.” European Journal of Oncology Nursing 11(2):117–125.
Yang, E.J., J.Y. Lim, U.W. Rah, and Y.B. Kim. 2012. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: A randomized controlled trial. Gynecologic Oncology 125(3):705–711.
Yeoh, E.K., and M. Horowitz. 1987. Radiation enteritis. Surgery Gynecology & Obstetrics 165(4):373–379.
Zabora, J., K. BrintzenhofeSzoc, B. Curbow, C. Hooker, and S. Piantadosi. 2001. The prevalence of psychological distress by cancer site. Psycho-Oncology 10(1):19–28.
Zajac̨zkowska, R., M. Kocot-Kępska, W. Leppert, A. Wrzosek, J. Mika, and J. Wordliczek. 2019. Mechanisms of chemotherapy-induced peripheral neuropathy. International Journal of Molecular Science 20(6):1451.
Zaorsky, N.G., T.M. Churilla, B.L. Egleston, S.G. Fisher, J.A. Ridge, E.M. Horwitz, and J.E. Meyer. 2017. Causes of death among cancer patients. Annals of Oncology 28(2):400–407.
Zeiser, R., A. Burchert, C. Lengerke, M. Verbeek, K. Maas-Bauer, S.K. Metzelder, S. Spoerl, M. Ditschkowski, M. Ecsedi, K. Sockel, F. Ayuk, S. Ajib, F.S. de Fontbrune, I.K. Na, L. Penter, U. Holtick, D. Wolf, E. Schuler, E. Meyer, P. Apostolova, H. Bertz, R. Marks, M. Lübbert, R. Wäsch, C. Scheid, F. Stölzel, R. Ordemann, G. Bug, G. Kobbe, R. Negrin, M. Brune, A. Spyridonidis, A. Schmitt-Gräff, W. van der Velden, G. Huls, S. Mielke, G.U. Grigoleit, J. Kuball, R. Flynn, G. Ihorst, J. Du, B.R. Blazar, R. Arnold, N. Kröger, J. Passweg, J. Halter, G. Socié, D. Beelen, C. Peschel, A. Neubauer, J. Finke, J. Duyster, and N. von Bubnoff. 2015. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: A multicenter survey. Leukemia 29(10):2062–2068.
Zimmer, P., F.T. Baumann, M. Oberste, P. Wright, A. Garthe, A. Schenk, T. Elter, D.A. Galvao, W. Bloch, S.T. Hubner, and F. Wolf. 2016. Effects of exercise interventions and physical activity behavior on cancer related cognitive impairments: A systematic review. Biomedical Research International 2016:1820954 (Epub April 10, 2016).
































































